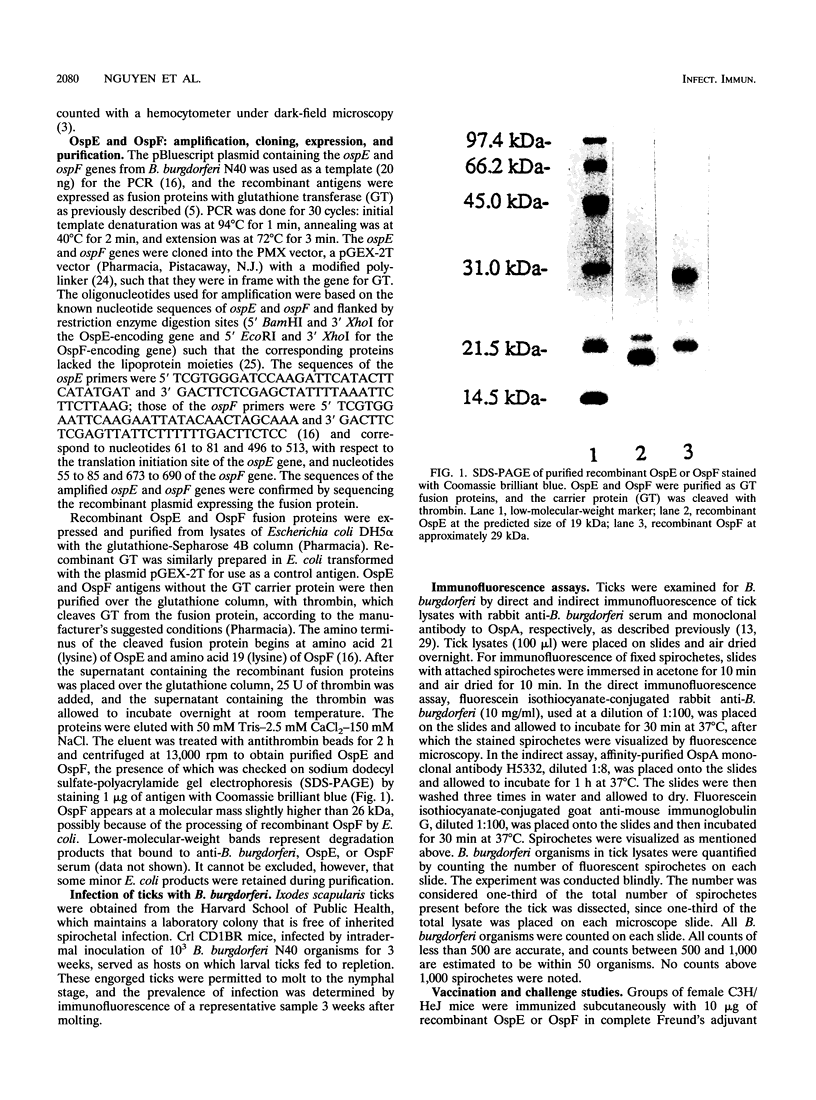
Abstract
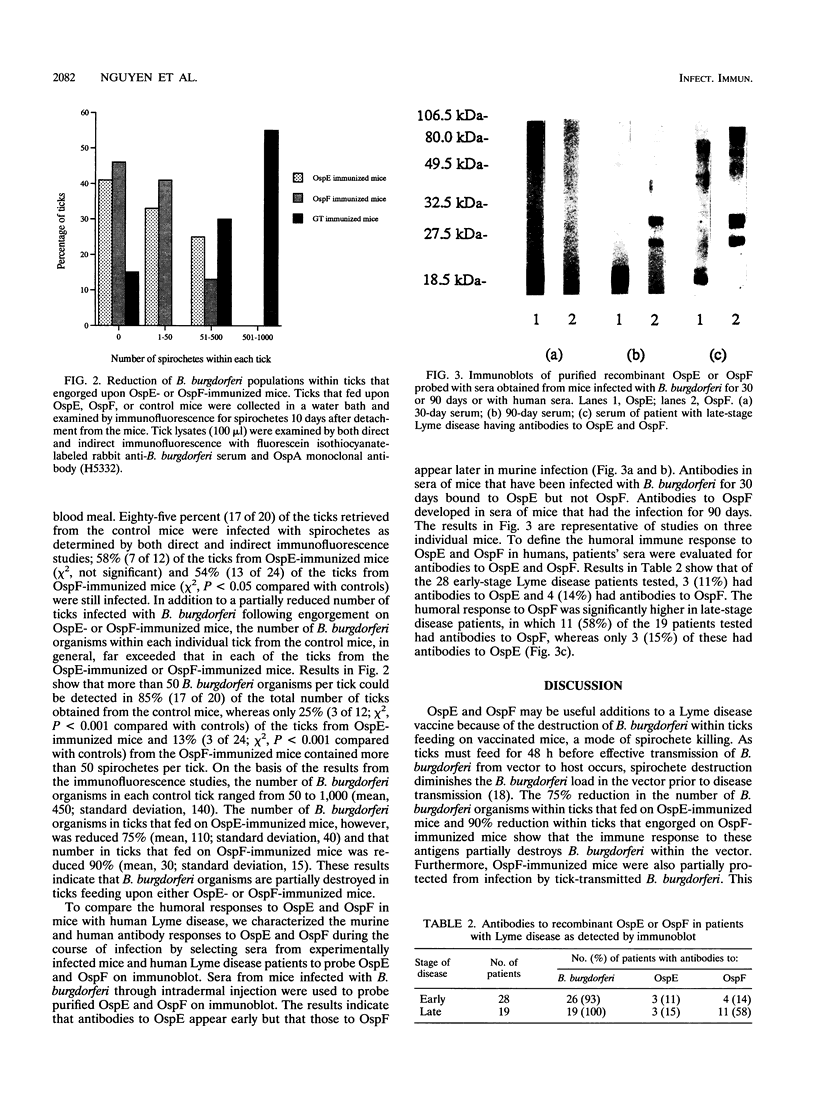
We determined whether Borrelia burgdorferi outer surface proteins (Osps) E and F could elicit immune responses useful for a Lyme disease vaccine. Thirty days after challenge with B. burgdorferi, mice produced antibodies to OspE but not OspF, whereas antibodies to OspF were present in sera of mice obtained 90 days after infection. Examination of sera from patients with Lyme disease revealed antibodies to OspF in a small number (14%) of early-stage disease patients but in a majority (58%) of patients with late-stage disease, while antibodies to OspE were rarely detected in patients. Mice immunized with recombinant OspE or OspF produced high titers of antibodies to OspE or OspF, respectively. OspF-immunized mice were partially protected from both intradermal syringe challenge and tick-mediated transmission of B. burgdorferi while vaccination with OspE did not confer immunity. B. burgdorferi organisms were, however, substantially destroyed within ticks that engorged on either OspE- (75% reduction in the number of spirochetes within the ticks, compared with controls) or OspF (90% reduction in the number of spirochetes within the ticks)-immunized mice.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong A. L., Barthold S. W., Persing D. H., Beck D. S. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am J Trop Med Hyg. 1992 Aug;47(2):249–258. doi: 10.4269/ajtmh.1992.47.249. [DOI] [PubMed] [Google Scholar]
- Baranton G., Postic D., Saint Girons I., Boerlin P., Piffaretti J. C., Assous M., Grimont P. A. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J Syst Bacteriol. 1992 Jul;42(3):378–383. doi: 10.1099/00207713-42-3-378. [DOI] [PubMed] [Google Scholar]
- Barbour A. G. Isolation and cultivation of Lyme disease spirochetes. Yale J Biol Med. 1984 Jul-Aug;57(4):521–525. [PMC free article] [PubMed] [Google Scholar]
- Barthold S. W., Beck D. S., Hansen G. M., Terwilliger G. A., Moody K. D. Lyme borreliosis in selected strains and ages of laboratory mice. J Infect Dis. 1990 Jul;162(1):133–138. doi: 10.1093/infdis/162.1.133. [DOI] [PubMed] [Google Scholar]
- Barthold S. W., Persing D. H., Armstrong A. L., Peeples R. A. Kinetics of Borrelia burgdorferi dissemination and evolution of disease after intradermal inoculation of mice. Am J Pathol. 1991 Aug;139(2):263–273. [PMC free article] [PubMed] [Google Scholar]
- Coleman J. L., Rogers R. C., Benach J. L. Selection of an escape variant of Borrelia burgdorferi by use of bactericidal monoclonal antibodies to OspB. Infect Immun. 1992 Aug;60(8):3098–3104. doi: 10.1128/iai.60.8.3098-3104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdile L. F., Brandt M. A., Warakomski D. J., Westrack G. J., Sadziene A., Barbour A. G., Mays J. P. Role of attached lipid in immunogenicity of Borrelia burgdorferi OspA. Infect Immun. 1993 Jan;61(1):81–90. doi: 10.1128/iai.61.1.81-90.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Kantor F. S., Flavell R. A. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990 Oct 26;250(4980):553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Barthold S. W., Persing D. H., Sun X., Kantor F. S., Flavell R. A. Borrelia burgdorferi strain 25015: characterization of outer surface protein A and vaccination against infection. J Immunol. 1992 Apr 1;148(7):2256–2260. [PubMed] [Google Scholar]
- Fikrig E., Huguenel E. D., Berland R., Rahn D. W., Hardin J. A., Flavell R. A. Serologic diagnosis of Lyme disease using recombinant outer surface proteins A and B and flagellin. J Infect Dis. 1992 Jun;165(6):1127–1132. doi: 10.1093/infdis/165.6.1127. [DOI] [PubMed] [Google Scholar]
- Fikrig E., Tao H., Kantor F. S., Barthold S. W., Flavell R. A. Evasion of protective immunity by Borrelia burgdorferi by truncation of outer surface protein B. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4092–4096. doi: 10.1073/pnas.90.9.4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fikrig E., Telford S. R., 3rd, Barthold S. W., Kantor F. S., Spielman A., Flavell R. A. Elimination of Borrelia burgdorferi from vector ticks feeding on OspA-immunized mice. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5418–5421. doi: 10.1073/pnas.89.12.5418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jauris-Heipke S., Fuchs R., Motz M., Preac-Mursic V., Schwab E., Soutschek E., Will G., Wilske B. Genetic heterogenity of the genes coding for the outer surface protein C (OspC) and the flagellin of Borrelia burgdorferi. Med Microbiol Immunol. 1993 Mar;182(1):37–50. doi: 10.1007/BF00195949. [DOI] [PubMed] [Google Scholar]
- Lam T. T., Nguyen T. P., Montgomery R. R., Kantor F. S., Fikrig E., Flavell R. A. Outer surface proteins E and F of Borrelia burgdorferi, the agent of Lyme disease. Infect Immun. 1994 Jan;62(1):290–298. doi: 10.1128/iai.62.1.290-298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S. J., Carter C. J., Howell J. K., Barbour A. G. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect Immun. 1992 Nov;60(11):4662–4672. doi: 10.1128/iai.60.11.4662-4672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piesman J., Mather T. N., Sinsky R. J., Spielman A. Duration of tick attachment and Borrelia burgdorferi transmission. J Clin Microbiol. 1987 Mar;25(3):557–558. doi: 10.1128/jcm.25.3.557-558.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preac-Mursic V., Wilske B., Patsouris E., Jauris S., Will G., Soutschek E., Rainhardt S., Lehnert G., Klockmann U., Mehraein P. Active immunization with pC protein of Borrelia burgdorferi protects gerbils against B. burgdorferi infection. Infection. 1992 Nov-Dec;20(6):342–349. doi: 10.1007/BF01710681. [DOI] [PubMed] [Google Scholar]
- Rosa P. A., Schwan T., Hogan D. Recombination between genes encoding major outer surface proteins A and B of Borrelia burgdorferi. Mol Microbiol. 1992 Oct;6(20):3031–3040. doi: 10.1111/j.1365-2958.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- Schaible U. E., Kramer M. D., Eichmann K., Modolell M., Museteanu C., Simon M. M. Monoclonal antibodies specific for the outer surface protein A (OspA) of Borrelia burgdorferi prevent Lyme borreliosis in severe combined immunodeficiency (scid) mice. Proc Natl Acad Sci U S A. 1990 May;87(10):3768–3772. doi: 10.1073/pnas.87.10.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U. E., Wallich R., Kramer M. D., Gern L., Anderson J. F., Museteanu C., Simon M. M. Immune sera to individual Borrelia burgdorferi isolates or recombinant OspA thereof protect SCID mice against infection with homologous strains but only partially or not at all against those of different OspA/OspB genotype. Vaccine. 1993;11(10):1049–1054. doi: 10.1016/0264-410x(93)90132-h. [DOI] [PubMed] [Google Scholar]
- Sears J. E., Fikrig E., Nakagawa T. Y., Deponte K., Marcantonio N., Kantor F. S., Flavell R. A. Molecular mapping of Osp-A mediated immunity against Borrelia burgdorferi, the agent of Lyme disease. J Immunol. 1991 Sep 15;147(6):1995–2000. [PubMed] [Google Scholar]
- Simon M. M., Schaible U. E., Kramer M. D., Eckerskorn C., Museteanu C., Müller-Hermelink H. K., Wallich R. Recombinant outer surface protein a from Borrelia burgdorferi induces antibodies protective against spirochetal infection in mice. J Infect Dis. 1991 Jul;164(1):123–132. doi: 10.1093/infdis/164.1.123. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Bartenhagen N. H., Craft J. E., Hutchinson G. J., Newman J. H., Rahn D. W., Sigal L. H., Spieler P. N., Stenn K. S., Malawista S. E. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983 Jul;99(1):76–82. doi: 10.7326/0003-4819-99-1-76. [DOI] [PubMed] [Google Scholar]
- Steere A. C., Grodzicki R. L., Kornblatt A. N., Craft J. E., Barbour A. G., Burgdorfer W., Schmid G. P., Johnson E., Malawista S. E. The spirochetal etiology of Lyme disease. N Engl J Med. 1983 Mar 31;308(13):733–740. doi: 10.1056/NEJM198303313081301. [DOI] [PubMed] [Google Scholar]
- Steere A. C. Lyme disease. N Engl J Med. 1989 Aug 31;321(9):586–596. doi: 10.1056/NEJM198908313210906. [DOI] [PubMed] [Google Scholar]
- Sădziene A., Rosa P. A., Thompson P. A., Hogan D. M., Barbour A. G. Antibody-resistant mutants of Borrelia burgdorferi: in vitro selection and characterization. J Exp Med. 1992 Sep 1;176(3):799–809. doi: 10.1084/jem.176.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford S. R., 3rd, Fikrig E., Barthold S. W., Brunet L. R., Spielman A., Flavell R. A. Protection against antigenically variable Borrelia burgdorferi conferred by recombinant vaccines. J Exp Med. 1993 Aug 1;178(2):755–758. doi: 10.1084/jem.178.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]