Abstract
Background/Objective:
In an animal model of spinal cord injury, a latent respiratory motor pathway can be pharmacologically activated through central adenosine A1 receptor antagonism to restore respiratory function after cervical (C2) spinal cord hemisection that paralyzes the hemidiaphragm ipsilateral to injury. Although respiration is modulated by central and peripheral mechanisms, putative involvement of peripheral adenosine A2 receptors in functional recovery in our model is untested. The objective of this study was to assess the effects of peripherally located adenosine A2 receptors on recovery of respiratory function after cervical (C2) spinal cord hemisection.
Methods:
Respiratory activity was electrophysiologically assessed (under standardized recording conditions) in C2-hemisected adult rats with the carotid bodies intact (H-CBI; n =12) or excised (H-CBE; n =12). Animals were administered the adenosine A2 receptor agonist, CGS-21680, followed by the A1 receptor antagonist, 1, 3-dipropyl-8-cyclopentylxanthine (DPCPX), or administered DPCPX alone. Recovered respiratory activity, characterized as drug-induced activity in the previously quiescent left phrenic nerve of C2-hemisected animals in H-CBI and H-CBE rats, was compared. Recovered respiratory activity was calculated by dividing drug-induced activity in the left phrenic nerve by activity in the right phrenic nerve.
Results:
Administration of CGS-21680 before DPCPX (n = 6) in H-CBI rats induced a significantly greater recovery (58.5 ± 3.6%) than when DPCPX (42.6 ± 4.6%) was administered (n = 6) alone. In H-CBE rats, prior administration of CGS-21680 (n = 6) did not enhance recovery over that induced by DPCPX (n = 6) alone. Recovery in H-CBE rats amounted to 39.7 ± 3.7% and 38.4 + 4.2%, respectively.
Conclusions:
Our results suggest that adenosine A2 receptors located in the carotid bodies can enhance the magnitude of adenosine A1 receptor–mediated recovery of respiratory function after C2 hemisection. We conclude that a novel approach of targeting peripheral and central adenosine receptors can be therapeutically beneficial in alleviating compromised respiratory function after cervical spinal cord injury.
Keywords: Cervical spinal cord hemisection, Carotid bodies, Adenosine A1 and A2 receptors, Respiratory function
INTRODUCTION
In the mammalian respiratory system, brain stem bulbospinal neurons within the dorsal and rostral ventral respiratory groups in the medulla send respiratory inputs to the phrenic motor nucleus (1). Monosynaptic connections have been shown between the bulbospinal neurons and phrenic motoneurons (2). These connections mediate respiratory drive to the respiratory motoneurons (3,4). Interruption of central respiratory drive (after high cervical spinal injury) invariably leads to respiratory distress, paresis, or even death if mechanical respiratory support is not provided (5).
In previous studies, our laboratory has shown that, in a rat model of spinal cord injury (SCI), a latent respiratory motor pathway can be pharmacologically activated to restore respiratory motor function to a hemidiaphragm paralyzed by upper cervical (C2) spinal cord hemisection (6). Briefly, in this model, a C2 spinal cord hemisection (rostral to the phrenic motoneurons at C3-C5) interrupts the major descending bulbospinal respiratory motor pathway and paralyzes the hemidiaphragm ipsilateral to the hemisection (7). Enhanced descending central drive (under hypoxia), hypercapnia, asphyxia, or pharmacologic intervention can activate a latent respiratory motor pathway and restore respiratory motor function to the paralyzed hemidiaphragm. The axons of the latent respiratory motor pathway are not injured by the hemi-section because they descend into the cord contralateral to the hemisection, cross the midline, and innervate phrenic motoneurons ipsilateral and caudal to the hemisection. The axons of the latent respiratory motor pathway have been referred to as the crossed phrenic pathway (8–11).
Functional recovery after activation of the latent respiratory pathway can be expressed under hypoxic, hypercapnic, or asphyxic conditions, as well as pharmacologically with the systemic administration of the methylxanthine, theophylline, and serotonergic compounds (6,12–20). While central antagonism of adenosine A1 receptors is implicated in the effect of theophylline, the phosphodiesterase inhibitory effect of the drug may not be involved (14,15). In follow-up studies with specific adenosine A1 and A2 receptor compounds, we showed quantitatively that the specific adenosine A1 receptor antagonist, 1, 3-dipropyl-8-cyclopentylxanthine, (DPCPX) was more efficacious than theophylline in inducing functional recovery, and furthermore, the A2 receptor antagonist, 3,7-dimethyl-1-propargylxanthine was ineffective. However, in the same study, we showed that prior activation of A2 receptors (probably located in the carotid bodies) with a specific agonist, CGS-21680, enhanced the magnitude of DPCPX-induced recovery. CGS-21680 is an adenosine A2 agonist that has been characterized as peripherally specific, although it has been reported to exert delayed central effects in sheep (21–23). We concluded that, in rats, antagonism of central adenosine A2 receptors does not induce functional recovery in our model; however, activation of adenosine A2 receptors purportedly located in the carotid bodies may subserve the central adenosine A1 receptor–mediated functional recovery (16).
Adenosine is a neuromodulator, and in particular, the A1subtype is inhibitory, whereas the A2A subtype is excitatory (23). In the respiratory system, A1 receptors depress and A2A receptors excite respiratory activity (21,24–26). In many species, adenosine receptors are located in the carotid bodies and modulate respiratory activity (21,27,28).
In this study, we tested the hypothesis that adenosine receptors located in the carotid bodies influence recovery of respiratory activity in adult rats subjected to a left C2 hemisection. The hypothesis is predicated on the reported excitatory actions of adenosine A2 receptors on respiratory activity and our previous observation that activation of adenosine A2 receptors enhances adenosine A1 receptor–mediated recovery in our model of SCI.
METHODS
All surgical procedures applied in this study followed the guidelines set out by the National Institutes of Health and followed by the Division of Laboratory Animal Research at Wayne State University. Efforts were made to minimize pain and suffering and to reduce the total number of animals. Adult Sprague-Dawley female rats (260–340 g) were used in the study. Animals were classified as hemisected with the carotid body intact (H-CBI; n = 12) or hemisected with the carotid body excised (H-CBE; n = 12). H-CBI and H-CBE animals were further categorized into 2 equal groups of 6 animals each based on the sequence in which test drugs were administered. H-CBI group 1 animals were administered the peripherally acting adenosine A2 receptor agonist, CGS-21680, followed 15 minutes later by the specific adenosine A1 receptor antagonist, DPCPX. H-CBI group 2 animals were administered DPCPX only. H-CBE animals were similarly categorized into 2 groups with the same order of drug administration. Control animals were categorized as carotid body sham-operated plus C2 hemisection (n = 4) and sham C2 hemisection plus carotid body excision (n = 4).
Before excision of the carotid bodies, animals were anesthetized with ketamine (70 mg/kg) and xylazine (7 mg/kg, intramuscularly [IM]) approximately 10 minutes after injection with atropine sulfate (0.1 mg/kg, IM) to minimize mucus secretions. To excise the carotid bodies, a ventral midline incision was made in the neck to expose and visualize the carotid artery bifurcation and related structures with the aid of a Zeiss compound microscope (OpMi-1, Goettingen, West Germany). Tissue within the carotid bifurcation was carefully excised with a pair of microscissors according to the method of Olsen et al (29). The excised tissue was placed in 4% paraformaldehyde for processing of adenosine A1 and A2A receptors using immunohistochemical techniques as described elsewhere (30). Functional assessment of a complete bilateral carotid body excision was later conducted by the intravenous (IV) administration of sodium cyanide (20 μg/kg) at the conclusion of all electrophysiologic recordings as described in the next section.
After carotid body excision, animals were returned to clean litter-lined cages to recover. They were allowed to survive postoperatively for 7 days before any other procedures. On the eighth day after carotid body excision, all H-CBE animals were subjected to a left C2 spinal cord hemisection (31). Twenty-four hours later, they were prepared for electrophysiologic assessment of respiratory-related activity.
H-CBI animals were subjected to a left C2 hemisection only and allowed to recover in a clean litter-lined cage. Electrophysiologic assessment of respiratory activity in the phrenic nerves was conducted 24 hours after hemisection in H-CBI rats. Control animals were similarly assessed for activity in the phrenic nerves.
Electrophysiology
Before electrophysiologic experiments, animals were anesthetized with chloral hydrate (400 mg/kg, intraperitoneally) approximately 10 minutes after IM administration of atropine sulfate to minimize mucus secretions. A tracheotomy was performed, an endotracheal tube (PE 160) was inserted into the trachea, and the femoral vein and femoral artery were cannulated (PE 50) to administer drugs and monitor blood pressure, respectively. Body temperature was monitored with a rectal probe and maintained with a thermostatic blanket (FHC Inc., Bowdoinham, ME) at 37.0°C to 37.2°C. Blood pressure was maintained at 80 to 110 mmHg by IV administration of a 5% dextrose and dextran solution (Baxter Laboratories, Round Lake, IL).
The left phrenic nerve (LPN) and right phrenic nerve (RPN) were exposed in the neck, desheathed, and placed on bipolar platinum recording electrodes. The nerves were immersed in mineral oil to prevent desiccation. Electrophysiologic recordings were conducted under the following standardized conditions: (a) paralysis with pancuronium bromide (0.5 mg/kg IV) to eliminate afferent activity associated with muscle contraction, (b) bilateral vagotomy to prevent entrainment of respiratory motor output with the small animal ventilator, (c) artificial ventilation on a small animal ventilator (Harvard Rodent Ventilator, Holliston, MA), and (d) end-tidal CO2 was monitored continuously and maintained at a constant level (38–43 mmHg) with a flow-through capnograph (Novametrix, Wallingford, CT). All electrophysiologic recordings were conducted with care to ensure appropriate contact between the electrode and nerves. However, this approach can have limitations, such as the exact diameter of the electrodes, the actual contact between the nerves and electrodes, and the certainty of continued immersion of the nerve in oil.
Signals from the LPN and RPN were monitored continuously and amplified (502 AM preamplifier; Tektronix, Elgin, IL) at 50,000×, filtered (0.1–3 kHz), and analyzed with a Cambridge Electronic Design data acquisition system and a spike 2 computer program (Cambridge Electronic Design Ltd, Cambridge, UK). All recordings were rectified and integrated with a moving averager (CWE, MA-821 CWE, Ardmore, PA) set at 0.1 s.
Quantification of Respiratory-Related Activity
The mean areas under the integrated waveform (AUC: ±SEM) of 10 consecutive respiratory bursts in the RPN and LPN were calculated before and after drug administration. In all hemisected animals, a total absence of respiratory-related activity in the LPN was the criterion for a functionally complete hemisection. Only animals that satisfied this criterion were included in the study. Inspiratory burst duration (TI), interburst interval (TE), and respiratory frequency (f) were also assessed before and after drug administration. All data are expressed as means ± SEM. In H-CBI (group 1) animals, the adenosine A2 agonist CGS-21680 (1 mg/kg) was administered approximately 15 minutes before the administration of the A1 antagonist, DPCPX (0.05 mg/kg). The respective doses of DPCPX and CGS-21680 chosen were based on previous studies (16). In H-CBI (group 2) animals, only DPCPX (0.05 mg/kg) was administered.
H-CBE animals were similarly treated with respect to the order of drug administration. Changes in TI, TE, and f after drug administrations were quantitatively analyzed. It has been shown in cats that the integrated phrenic waveform most closely linked with respiratory function is amplitude (32). However, in this study, we used the AUC analysis as a measure of respiratory activity because this approach takes into account activity of all components of the respiratory burst (ie, early, mid-, and late units) to provide a more comprehensive index of phrenic motor output as a measure of respiratory activity. Previous studies in our laboratory have shown that using both parameters to assess phrenic nerve activity showed that changes in amplitude correlated with significant changes in area (33).
Statistical Analysis
Data for drug-induced actions are expressed as means ± SEM and compared using a repeated analysis of variance test (Newman-Keuls). Significance was set at P <0.05.
Drugs
DPCPX (RBI, Natick, MA) was dissolved in 0.1 mol/L NaOH and made fresh each day. CGS-21680 was dissolved in water. 8-(p-sulphophenyl) theophylline (RBI) was dissolved in 0.9% saline with a little warming. CGS-21680 was made fresh daily and used on the day of the experiment. Sodium cyanide (Sigma, St Louis, MO) was prepared as a stock solution in physiologic saline and refrigerated. Adequate volumes were dispensed and used as necessary during experiments.
RESULTS
H-CBI (Group 1)
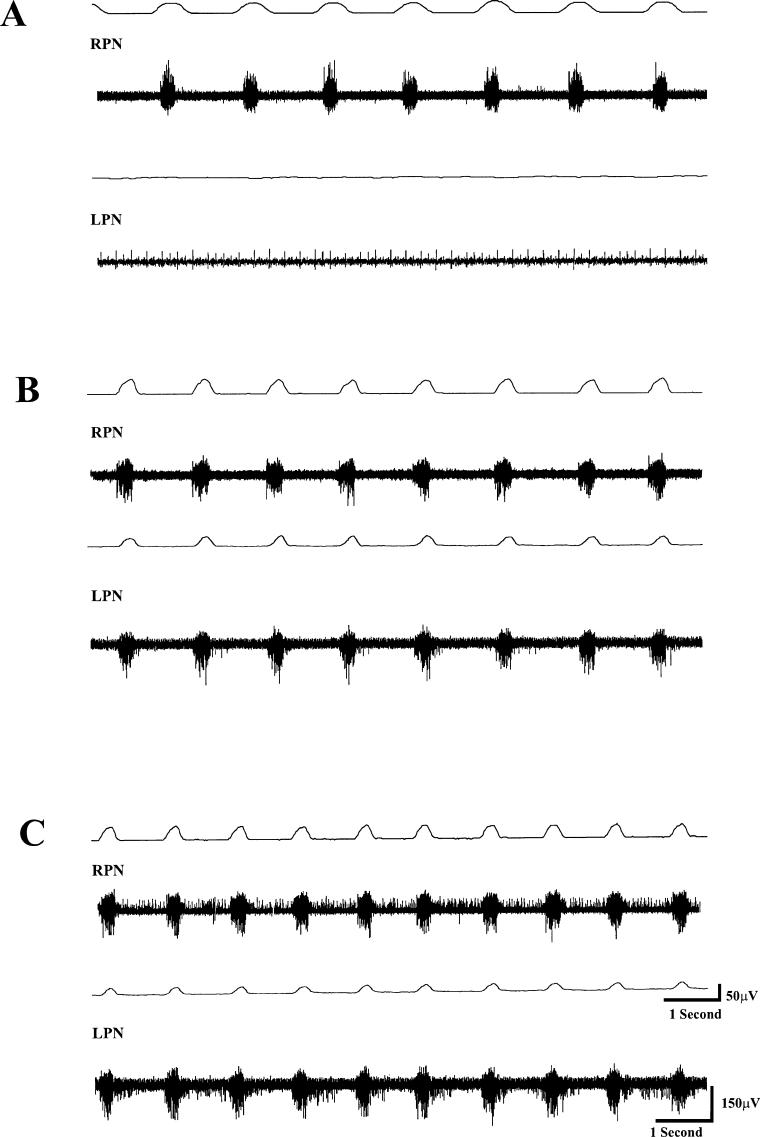
Before CGS-21680 administration, TI, TE, and f values were 0.45 ± 0.01 seconds, 0.9 ± 0.04 seconds, and 41.0 ± 3.6 breaths/min, respectively. The value for the integrated waveform of the RPN for all animals in this group was 58.7 ± 4.9 μV/s. No respiratory-related activity was detected in the LPN, confirming that the hemisection was functionally complete (Figure 1A). CGS-21680 (1.0 mg/kg) administration did not significantly alter TI, TE, and f. Subsequent administration of DPCPX (0.05 mg/kg) approximately 15 minutes after CGS-21680 induced respiratory-related activity in the previously quiescent LPN in all cases (Figure 1B and C). The onset of DPCPX-induced changes was evident 3 to 5 minutes after DPCPX administration, and the maximal effect lasted more than 2 hours. The area under the integrated waveform for the LPN amounted to 40.5 ± 3.9 μV/s. DPCPX administration also significantly (P < 0.05) enhanced the integrated waveform of the RPN to 98.6 ± 6.3 μV/s. In addition, TI and f values were significantly (P < 0.05) changed to 0.35 ± 0.03 seconds and 51.3+3.6 breaths/min, respectively, from pre-DPCPX values. Furthermore, tonic activity was evident in the RPN and LPN (Figure 1C). TE did not significantly change from predrug levels. The magnitude of recovered activity in the LPN expressed as a percent of predrug activity in the RPN amounted to 58.5 ± 3.6% (Figure 2). When expressed as a function of activity in the RPN after drug administration, the magnitude of recovery was 42.6 ± 4.2%. In this group, the mean end-tidal CO2 was 41.0 ± 0.9 mmHg.
Figure 1. A representative set of tracings from an animal (H-CBI, group 1) 24 hours after a left C2 hemisection. In this and all other tracings, the top tracing in each pair is an integrated waveform, and the bottom tracing shows the raw compound action potentials from each nerve. (A) Activity in the RPN is evident, whereas the LPN is devoid of respiratory-related activity, indicating that the hemi-section was functionally complete. (B) Prior administration of the CGS-21680 did not induce recovery of respiratory activity in the LPN. However, 10 minutes after administration of DPCPX (0.05 mg/kg), RPN activity is enhanced, whereas the previously quiescent LPN shows robust respiratory-related activity synchronous with RPN activity. In addition, there is onset of enhanced tonic activity, particularly in the LPN. (C) Fifteen minutes after DPCPX administration, respiratory frequency is further enhanced. Note that tonic activity is also evident in the LPN and RPN. DPCPX-induced respiratory-related changes in H-CBI animals lasted more than 30 minutes.
Figure 2. A summary of the quantitative analysis of the magnitude of recovered activity in the LPN in H-CBI and H-CBE after administration of DPCPX (only) or sequential administration of CGS-21680 followed by DPCPX is shown in this histogram. In both H-CBI and H-CBE animals, DPCPX induced recovery of respiratory-related activity in the LPN. The magnitude of recovery in H-CBI animals (42.6 ± 4.6%), whereas greater than that in H-CBE animals (38.4 ± 4.2%), was not statistically significant. However, the magnitude of DPCPX-induced recovery in H-CBI animals after prior CGS-21680 administration was significantly (P < 0.05) enhanced (compare H-CBI DPCPX only and H-CBI CGS-21680 + DPCPX). In contrast, the magnitude of DPCPX-induced recovery was not enhanced further by prior CGS-21680 administration in H-CBE animals. Statistical significance is shown by *P < 0.05 (Newman-Keuls comparison test).
Mean blood pressure for all animals in this group before administration of drugs was 102.5 ± 8.5 mmHg. Administration of CGS-21680 induced a transient (<0.5 minutes) decrease (to 70–75 mmHg) in blood pressure; however, the values returned to predrug levels for the duration of the recordings. DPCPX did not induce significant changes in blood pressure.
At the conclusion of the electrophysiologic recordings, sodium cyanide (20 μg/kg) was administered to test the response of peripheral chemoreceptors in the carotid bodies. The effect of the sodium cyanide test was characterized by enhanced amplitude and f (Table 1).
TABLE 1.
Respiratory Parameters in H-CBI and H-CBE Animals Before and After Administration of Sodium Cyanide (20μg/kg) *
H-CBI (Group 2)
The integratd waveform of the RPN in this group amounted to 52.5 ± 4.6 μV/s, whereas no activity was detected in the LPN—a confirmation that the left C2 hemisection was functionally complete. TI, TE, and f were 0.40 ± 0.01 seconds, 0.96 ± 0.04 seconds, and 42.0 ± 2.6 breaths/min, respectively, before drug (ie, DPCPX only) administration. DPCPX (0.05 mg/kg) induced recovery of respiratory activity in the LPN in all cases. The onset of DPCPX-induced changes was evident 5 to 10 minutes after administration, and the maximal effect lasted more than 2 hours. After DPCPX administration, the integrated waveform in the RPN was significantly (P < 0.05) enhanced to 75.9 ± 5.6 μV/s. The integrated waveform of the respiratory bursts in the LPN amounted to 25.9 ± 4.7 μV/s. TI decreased significantly (P < 0.05) to 0.31 ± 0.03 seconds, and f increased significantly (P < 0.05) to 49.9 ± 3.2 breaths/min after DPCPX administration. The magnitude of recovered activity in the LPN expressed as a percent of activity in the RPN before drug administration was 42.6 ± 4.6% (Figure 2). When expressed as a percent of activity in the RPN after drug administration, the magnitude of recovery amounted to 30.4 ± 3.6%. TE was not significantly altered after DPCPX administration. End tidal CO2 was 40.2 ± 0.8 mmHg.
Mean blood pressure for this group was 95.6 ± 6.5 mmHg before drug administration. DPCPX administration did not significantly affect blood pressure for the duration of the recording.
H-CBE (Group 1)
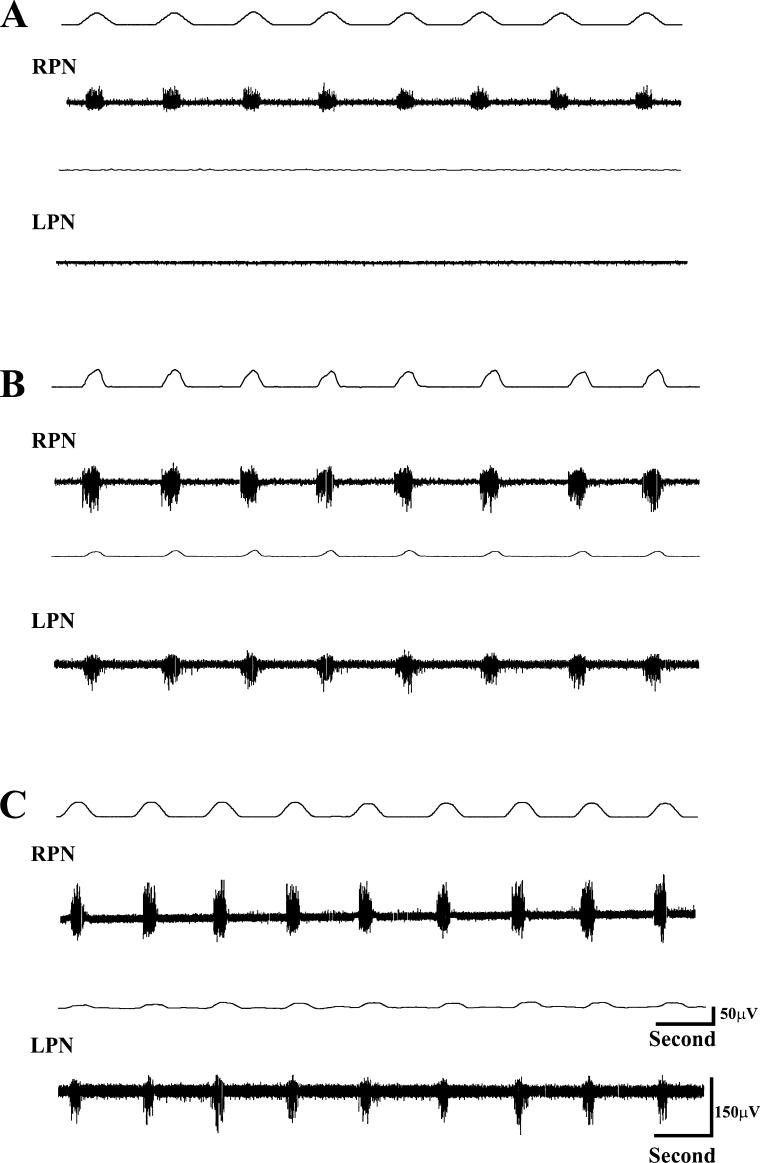
TI, TE, and f were 0.59 ± 0.03 seconds, 1.03 ± 0.02 seconds, and 38.5 ± 3.5 breaths/min, respectively, before drug administration. The integrated waveform in the RPN was 60.3 ± 5.7 μV/s before drug administration. CGS-21680 (1.0 mg/kg) administration did not induce respiratory-related activity in the previously quiescent LPN and did not significantly change predrug levels of TI, TE, or f. Subsequent administration of DPCPX enhanced the RPN waveform to 70.6 ± 6.3 μV/s, and in addition, induced activity in the previously quiescent LPN. The integrated waveform detected in the latter nerve amounted to 23.7 ± 4.3 μV/s. The magnitude of recovered activity in the LPN when expressed as a function of predrug activity in the RPN was 39.7 ± 3.7% (Figure 2). When the magnitude of recovery was expressed as a percent of activity in the RPN after drug administration, it amounted to 34.3 ± 4.7%. TI, TE, and f after DPCPX were 0.40 + 0.03 seconds, 0.86 + 0.4 seconds, and 46.8 + 3.2 breaths/min, respectively. Interestingly, CGS 21680 administration in H-CBE animals did not induce tonic activity in either the LPN or RPN, in marked contrast to H-CBI animals (compare Figures 3 and 1C). End tidal CO2 was 39.6 ± 0.9 mmHg.
Figure 3. The effects of the systemically administered adenosine A2 receptor agonist, CGS-21680, and the A1 antagonist DPCPX on respiratory-related activity were assessed in an animal (H-CBE, group 1) 24 hours after hemisection. (A) Activity in the contralateral RPN is evident, whereas the LPN is totally devoid of respiratory-related activity, indicating a functionally complete hemi-section. Approximately 10 minutes after administration of CGS-21680 (no respiratory activity was induced), DPCPX was administered. (B) Activity in the previously quiescent LPN is induced, whereas activity in the RPN is further enhanced. (C) Ten to 15 minutes after DPCPX administration, the respiratory-related changes induced are even more evident. However, in marked contrast to H-CBI animals, there was no induced tonic effect in the LPN or RPN. The DPCPX-induced activation lasted more than 2 hours.
Mean blood pressure values amounted to 88.5 ± 6.5 mmHg before drug administration. CGS-21680 induced an initial transient (<0.5 minutes) depression (65–72 mmHg). DPCPX did not alter blood pressure.
At the conclusion of the electrophysiologic recordings, sodium cyanide (20 μg/kg) was administered to test the response of peripheral chemoreceptors in the carotid bodies. The effects of the sodium cyanide test are summarized in Table 1. Furthermore, immunohistochemical analysis (data not shown) showed the immunoreactivity of adenosine A1 and A2 receptors in the carotid bodies similar to a previous study in our laboratory (30).
H-CBE (Group 2)
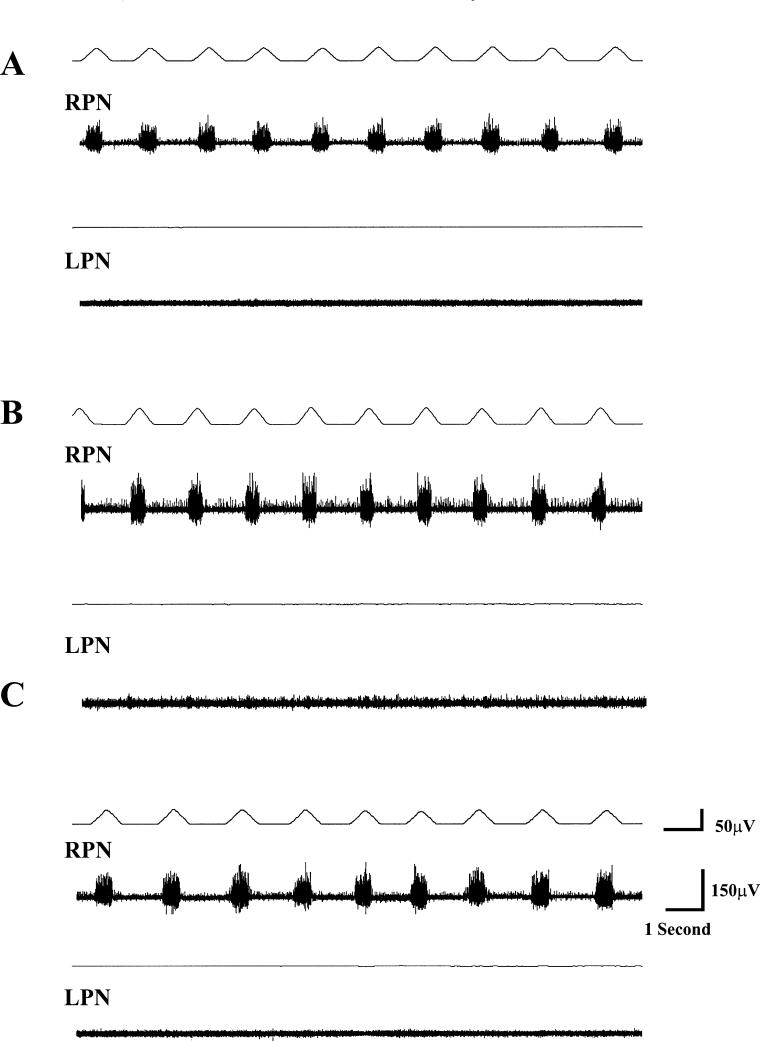
Before DPCPX administration, TI, TE, and f were 0.62 ± 0.02 seconds, 0.95 ± 0.08 seconds, and 39.6 ± 3.3 breaths/min, respectively. The integrated waveform in the RPN was 52.6 ± 5.6 μV/s, and the LPN was devoid of respiratory-related activity (confirmation of a physiologically complete hemisection; Figures 1A, 3A, and 4A). DPCPX induced respiratory activity in the LPN in all cases (Figure 3).
Figure 4. Temporal effects of CGS-21680 in a carotid body sham control animal 24 hours after C2 hemisection are shown. (A) The total absence of respiratory-related activity in the LPN is indicative of a functionally complete hemisection, whereas activity in the RPN is evident. (B) Ten minutes after administration of CGS-21680, there is no induced activity in the LPN; there is, however, mild onset of tonic activity that increases progressively, and by 30 minutes after administration (C), is rather obvious. Administration of the peripherally specific adenosine antagonist 8-(p-sulphophenyl)theophylline (10 mg/kg; based on previous studies) obviated the CGS-21680–induced tonicity approximately 15 minutes after administration (D), indicating that the enhanced tonic effect after CGS-21680 is mediated by A2 receptors located in the periphery.
Onset of DPCPX-induced effects was evident 5 to 10 minutes after administration. After DPXPC injection, TI, TE, and f changed significantly (P < 0.05) to 0.47 ± 0.03 seconds, 0.72 ± 0.07 seconds, and 50.4 ±3.3 breaths/min, respectively. The integrated waveform for the RPN and LPN amounted to 56.8 ± 4.9 and 21.78 ± 5.3 μV/s, respectively. The magnitude of recovered respiratory activity in the LPN expressed as a function of predrug activity in the RPN was 38.4 ± 4.2% (Figure 2). When expressed as a function of postdrug activity in the RPN, the magnitude of recovery was 37.5 ± 3.6%. End tidal CO2 was 40.4 ±0.8 mmHg, which was not significantly different (P >0.05) from either HCBI group.
Mean blood pressure values for all animals in this group amounted to 88.5 ± 6.5 mmHg before drug administration. DPCPX did not significantly alter blood pressure.
Sodium Cyanide Test
H-CBE animals did not show any remarkable changes in respiratory parameters after NaCN. The results are summarized in Table 1.
Carotid Body Sham-Operated Plus C2 Hemisection Animals
In this control group, the adenosine A1 receptor antagonist DPCPX induced recovery of respiratory activity (data not shown). When the adenosine A2 receptor agonist was administered alone, it induced tonic activity that progressively enhanced; however, administration of the peripherally specific antagonist 8-(p-sulfophenyl)theophylline (20 mg/kg) obviated the tonic activity confirming a peripheral adenosine receptor–mediated action (Figure 4C). The dose of 8-(p-sulfophenyl)theophylline was chosen based on previous studies (16). The end tidal CO2 levels for the control animals was 40.5 ±0.6 mmHg, which was not significantly (P > 0.05) different from either experimental group.
Sham C2 Hemisected Plus Carotid Body Excision Animals
The effect of DPCPX in this group was similar to the effects of the drug reported in naïve noninjured animals from our previous study. In sham C2 hemisection plus carotid body excision animals, DPCPX induced significant changes in respiratory parameters (Table 2). CGS-21680 administration did not induce any observed tonic activity.
TABLE 2.
Respiratory–Related Changes Following DPCPX (0.05mg/kg) Administration in Sham-Operated C2 Plus Carotid Body Excision Animals
Mortality After Carotid Body Excision
In H-CBE animals (n = 16), 4 (25%) were excluded from the study because of postsurgical complications. In 2 instances, the animals died as a result of apnea, an irregular pattern of breathing, or from complications of excessive bleeding or laryngopharyngeal impairment. In 2 other cases, the animals recovered from the surgery itself but thereafter exhibited difficulty in swallowing. After consultation with and approval from the Division of Laboratory Animal Research, the latter 2 animals were killed by the systemic administration of 4% chloral hydrate (500 mg/kg) followed by a bilateral pneumothorax surgical procedure. H-CBI animals underwent a left C2 hemisection and were allowed to recover in clean litter-lined cages. Weight loss after the left C2 hemisection was minimal (5–15 g).
DISCUSSION
The results from this study show that the specific adenosine A1 receptor antagonist DPCPX restores respiratory activity in adult rats subjected to an upper cervical (C2) hemisection with the carotid bodies intact or bilaterally excised. More importantly, prior administration of the peripherally specific adenosine A2 receptor agonist CGS-21680 enhanced the magnitude of DPCPX-induced recovered activity in animals with intact carotid bodies. In animals with bilaterally excised carotid bodies, prior administration of the A2 agonist did not influence the magnitude of DPCPX-induced recovered activity. The results of this study are discussed in terms of adenosine receptors and the putative interaction of central and peripheral adenosine receptors in the restoration of respiratory activity after upper cervical SCI.
It is generally known that adenosinergic mechanisms are involved in respiratory control in many species, including humans (34–38). Our laboratory showed previously that the pharmacologic activation of a latent respiratory motor pathway with the methylxanthine, theophylline, restores respiratory function in C2-hemi-sected rats through adenosine A1 receptor antagonism (14,15). However, the exact mechanism of theophylline- induced activation remains to be elucidated. In a subsequent study, we showed the efficacy of the specific adenosine A1 receptor antagonist DPCPX in functional restoration in our model and suggested that activation of A2 receptors (probably located in the carotid bodies) may further enhance the effect of the A1 antagonist–mediated action (16). Our suggestion from the above-referenced study was predicated on the observation that systemic administration of CGS-21680, a peripherally specific adenosine A2 receptor agonist (that has shown a delayed central effect), enhanced respiratory activity in non-injured rats and seemed to further improve the magnitude of recovered activity induced by the specific A1 antagonist DPCPX in animals subjected to a left C2 spinal cord hemisection (16).
In this study, prior CGS-21680 administration did not enhance the magnitude DPCPX-induced actions in animals with bilateral carotid body excision, suggesting that the effect of CGS-21680 is mediated through the carotid bodies. The observation that the peripherally specific adenosine antagonist 8-(p-sulfophenyl)theophyl-line blocked the effect (enhanced tonicity) of CGS-21680 in sham-operated animals confirms that CGS-21680 modulates respiratory activity through peripheral receptors located in the carotid bodies. Furthermore, Bae et al (30) showed that adenosine A2 receptors are located in the carotid bodies; this demonstration strongly implies that adenosine A2 receptors located in the carotid bodies underlie the CGS-21680–mediated actions. While it can be argued that other substances that stimulate the carotid bodies can influence the magnitude of the DPCPX-induced effect, it must be stressed that the specificity of CGS-21680 for adenosine A2 receptors implies strongly that these receptors are implicated in the enhanced effect.
Although respiration is primarily under central nervous system control, peripherally located chemoreceptors are involved in the modulation of respiration (16,27,35–37). In particular, it has been shown in various species that adenosine receptors located in the carotid bodies modify respiratory activity (16,26,28,29,36,39). Immunohistochemical studies have confirmed the presence of adenosine A1 and A2 receptors in the carotid bodies of rats (28,39,40). The seminal work of McQueen and Ribeiro (27) first suggested that adenosine A2 receptors in the carotid bodies are excitatory. Subsequent investigations have extended the work of McQueen and Ribeiro (27) by further characterizing peripheral adenosine A2 receptors as excitatory (21,36,41). Conversely, peripheral adenosine A1 receptors are inhibitory (17,27).
In this study, we showed that DPCPX can restore respiratory function in C2-hemisected rats with the carotid bodies intact or excised. This finding supports and extends a previous demonstration that theophylline, acting through adenosine A1 receptor antagonism, restores respiratory function in C2-hemisected rats with the carotid bodies intact or excised (30). It further suggests that the carotid bodies per se may not be responsible for restoration of respiratory activity after cervical SCI, although they can modulate the recovered activity. In addition, our results show that prior administration of the A2 agonist CGS-21680 enhanced the magnitude of the DPCPX-induced effect when the carotid bodies were not excised. The magnitude of the enhanced respiratory-related activity was characterized by an increased amplitude in respiratory bursts. In addition, TI was significantly decreased and was reflected as an enhanced f. In marked contrast, CGS-21680 did not enhance the DPCPX-induced effect in animals with the carotid bodies excised. We suggest that excision of the carotid bodies eliminates the putative influence of peripherally located adenosine A2 receptors on respiration. A previous immunohistochemical analysis from our laboratory showed adenosine A1 and A2 receptor immunoreactivity in the carotid bodies and hence supports this position (30). We conclude from these observations that CGS-21680 stimulates adenosine A2 receptors located in the carotid bodies to further modulate the DPCPX-induced actions. The excitatory action of the adenosine A2 receptor–specific agonist CGS-21680 on respiratory activity observed in this study supports and extends previous studies that have described enhanced respiratory frequency and enhanced amplitude mediated by adenosine receptors, in particular the A2 subtype (17,35,36,42). However, it must be stressed that activation of adenosine A2 receptors to enhance adenosine A1 receptor–mediated action shown in this study offers a unique perspective with therapeutic potential in cases of respiratory impairment after upper cervical SCI.
CONCLUSION
The results of this study showed that adenosine-related medicines that combine A1 blockade with A2 receptor activation have therapeutic potential. The differential effect of the adenosine A2 receptor agonist in the presence or absence of the carotid bodies in the magnitude of recovered respiratory activity in C2-hemi-sected rats shows that it is feasible to enhance the magnitude of adenosine A1 receptor–mediated recovery of respiratory function after cervical SCI. The findings of this study are therapeutically relevant particularly in cases of respiratory compromise. In our previous studies with the methylxanthine, theophylline, we emphasized the potential therapeutic benefits of adenosinergic compounds in patients with SCIs. Thus far, results from 2 clinical studies based largely on our animal studies have shown that the drug not only improves inspiratory muscle drive but can also facilitate weaning from mechanical ventilatory support (43,44).
However, we recognize that theophylline has a rather narrow therapeutic window that may limit its clinical efficacy. The demonstration that DPCPX is more efficacious in our model underscores the clinical potential of DPCPX (or other adenosine compounds) in patients with SCIs with respiratory impairments. The findings reported in this study strongly suggest a novel approach of selectively targeting adenosine peripheral receptors (A2) to further enhance the centrally mediated adenosine A1 receptor actions in respiratory function after upper cervical SCI that results in respiratory impairment.
Acknowledgments
The authors thank Drs. Han Bae and Harry Goshgarian for assistance in carotid body excision and critical review of the manuscript, respectively.
Footnotes
This study was supported by grant HD 35766 from the National Institutes of Health to K. D. Nantwi.
REFERENCES
- Goshgarian HG, Rafols JA. The ultrastructure of synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J Neurocytol. 1984;13:85–109. doi: 10.1007/BF01148320. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Smith JC. Cellular mechanisms underlying modulation of breathing pattern in mammals. Ann N Y Acad Sci. 1989;563:114–130. doi: 10.1111/j.1749-6632.1989.tb42194.x. [DOI] [PubMed] [Google Scholar]
- Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. J Neurophysiol. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003;82:803–814. doi: 10.1097/01.PHM.0000078184.08835.01. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Goshgarian HG. Actions of systemic theophylline on hemdiaphragmatic recovery in rats following cervical spinal cord hemisection. Exp Neurol. 1996;140:53–59. doi: 10.1006/exnr.1996.0114. [DOI] [PubMed] [Google Scholar]
- Aserinsky E. Effects of usage of a dormant respiratory nerve pathway upon its subsequent activity. Exp Neurol. 1961;3:467–475. doi: 10.1016/0014-4886(61)90022-x. [DOI] [PubMed] [Google Scholar]
- Guth L. Functional plasticity in the respiratory pathway of the mammalian spinal cord. Exp Neurol. 1976;51:414–420. doi: 10.1016/0014-4886(76)90265-x. [DOI] [PubMed] [Google Scholar]
- Moreno DE, Yu X-J, Goshgarian HG. Identification of axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116:216–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Rosenblueth A, Ortiz T. The crossed respiratory impulses to the phrenic. Am J Physiol. 1936;117:495–513. [Google Scholar]
- Goshgarian HG. The role of cervical afferent nerve fiber inhibition of the crossed phrenic phenomenon. Exp Neurol. 1981;72:211–225. doi: 10.1016/0014-4886(81)90139-4. [DOI] [PubMed] [Google Scholar]
- O'Hara TE, Goshgarian HG. Quantitative assessment of phrenic nerve functional recovery mediated by the crossed phrenic reflex at various time intervals after spinal cord injury. Exp Neurol. 1991;111:244–250. doi: 10.1016/0014-4886(91)90012-2. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. Developmental plasticity in the respiratory pathway of the adult rat. Exp Neurol. 1979;66:547–555. doi: 10.1016/0014-4886(79)90201-2. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Guth L. Demonstration of functionally ineffective synapses in the guinea pig spinal cord. Exp Neurol. 1977;57:211–225. doi: 10.1016/0014-4886(77)90093-0. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Theophylline-induced recovery in a hemidiaphragm paralyzed by hemisection in rats' contribution of adenosine receptors. Neuropharmacology. 1998;37:113–121. doi: 10.1016/s0028-3908(97)00190-1. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Alkylxanthine-induced recovery of respiratory function following cervical spinal cord injury in adult rats. Exp Neurol. 2001;168:123–134. doi: 10.1006/exnr.2000.7581. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Actions of specific adenosine receptor A1 and A2 agonists and antagonists in recovery of phrenic motor output following upper cervical spinal cord injury in adult rats. Clin Exp Pharmacol Physiol. 2002;29:915–923. doi: 10.1046/j.1440-1681.2002.03750.x. [DOI] [PubMed] [Google Scholar]
- Mitchell GS, Sloan HE, Jiang C, Miletic V, Hayashi F, Lipski J. 5-Hydroxytryptophan (5-HTP) augments spontaneous and evoked phrenic motoneurons discharge in spinalized rats. Neurosci Lett. 1992;141:75–78. doi: 10.1016/0304-3940(92)90338-8. [DOI] [PubMed] [Google Scholar]
- Ling L, Bach KB, Mitchell GS. Serotonin reveals ineffective spinal pathways to contralateral phrenic motoneurons in spinally hemisected rats. Exp Brain Res. 1994;101:35–43. doi: 10.1007/BF00243214. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Baker-Herman TL, Golder FJ, Doperalski NJ, Watters JJ, Mitchell GS. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005;22:203–213. doi: 10.1089/neu.2005.22.203. [DOI] [PubMed] [Google Scholar]
- Zhou S-Y, Goshgarian HG. 5-Hydrotryptophan-induced respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol. 2000;89:1528–1536. doi: 10.1152/jappl.2000.89.4.1528. [DOI] [PubMed] [Google Scholar]
- Nikodijevic O, Sarges R, Daly JW, Jacobson A. Behavioral effects of A1- and A2-selective adenosine agonists and antagonists. Evidence for synergism antagonism. J Pharmacol Exp Ther. 1991;259:286–294. [PMC free article] [PubMed] [Google Scholar]
- Hutchinson AJ, Williams M, De Jesus R, et al. 2-(Arylalkylamino)adenosine-5-uronamides: a new class of highly selective adenosine A2 receptor ligands. J Med Chem. 1990;33:1919–1924. doi: 10.1021/jm00169a015. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Chau A. Fetal cardiovascular and breathing responses to an adenosine A2Areceptor agonist in sheep. Am J Physiol. 1998;274:R152–R159. doi: 10.1152/ajpregu.1998.274.1.R152. [DOI] [PubMed] [Google Scholar]
- Carley DW, Radulovacki M. Role of peripheral adenosine A1 receptors in regulation of sleep apneas in rats. Exp Neurol. 1999;159:545–550. doi: 10.1006/exnr.1999.7167. [DOI] [PubMed] [Google Scholar]
- Monteiro EC, Ribeiro JA. Ventilatory effects of adenosine mediated by carotid body chemoreceptors in the rat. Naunyn Schmiedeberg's Arch Pharmacol. 1987;335:143–148. doi: 10.1007/BF00177715. [DOI] [PubMed] [Google Scholar]
- Reid PG, Watt AH, Penny WJ, Newby AC, Smith AP, Routledge PA. Plasma adenosine concentrations during adenosine-induced respiratory stimulation in man. Eur J Clin Pharmacol. 1991;40:175–180. doi: 10.1007/BF00280073. [DOI] [PubMed] [Google Scholar]
- McQueen DS, Ribeiro JA. On the specificity and type of receptor involved in carotid body activation by adenosine in the cat. Br J Pharmacol. 1983;80:347–354. doi: 10.1111/j.1476-5381.1983.tb10040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AH, Reid PG, Stephens MR, Routledge PA. Adenosine-induced respiratory stimulation depends on site of infusion. Evidence for an action on the carotid body? Br J Clin Pharmacol. 1987;23:486–490. doi: 10.1111/j.1365-2125.1987.tb03081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EB, Jr, Vidruk EH, Dempsey JA. Carotid body excision significantly changes ventilatory control in awake rats. Am J Physiol. 1988;64:666–671. doi: 10.1152/jappl.1988.64.2.666. [DOI] [PubMed] [Google Scholar]
- Bae H, Nantwi KD, Goshgarian HG. Recovery of respiratory function following C2 hemi and carotid body denervation in adult rats: influence of peripheral adenosine receptors. Exp Neurol. 2005;91:94–103. doi: 10.1016/j.expneurol.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Liou WW, Goshgarian HG. Quantitative assessment of the effect of chronic phrenicotomy on the induction of the crossed phrenic phenomenon. Exp Neurol. 1994;127:145–153. doi: 10.1006/exnr.1994.1088. [DOI] [PubMed] [Google Scholar]
- Eldridge FL. Relationship between phrenic nerve activity and ventilation. Am J Physiol. 1971;221:535–543. doi: 10.1152/ajplegacy.1971.221.2.535. [DOI] [PubMed] [Google Scholar]
- Hadley SD, Walker PD, Goshgarian HG. Effects of serotonin synthesis inhibitor p-CPA on the expression of the crossed phrenic phenomenon 4h following C2 spinal cord hemi-section. Exp Neurol. 1999;160:479–488. doi: 10.1006/exnr.1999.7240. [DOI] [PubMed] [Google Scholar]
- Eldridge FL, Milhorn DE, Killey JP. Antagonism by theophylline of respiratory inhibition induced by adenosine. J Appl Physiol. 1985;59:1428–1433. doi: 10.1152/jappl.1985.59.5.1428. [DOI] [PubMed] [Google Scholar]
- Rudolphi KA, Schubert P, Parkinson FE, Fredholm BB. Adenosine and brain ischemia. Cerebrovasc Brain Metab Rev. 1992;4:346–349. [PubMed] [Google Scholar]
- Koos BJ, Maeda T, Jan C. Adenosine A1 and A2A receptors modulate sleep state and breathing in sheep. J Appl Physiol. 2001;91:343–350. doi: 10.1152/jappl.2001.91.1.343. [DOI] [PubMed] [Google Scholar]
- McQueen DS, Ribeiro JA. Effect of adenosine on carotid chemoreceptor activity in the cat. Br J Pharmacol. 1981;74:129–136. doi: 10.1111/j.1476-5381.1981.tb09964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW, Maxwell DL, Conradson T-BG, Dixon CMS, Barnes PJ. Circulatory and respiratory effects of infused adenosine in conscious man. Br J Clin Pharmacol. 1987;24:309–317. doi: 10.1111/j.1365-2125.1987.tb03174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlekauf HR, Rivkees SA, Raubould HE, Bitticaca M, Goldhaber JI, Weiss JN. Localization and functional effects of adenosine A1 receptors in cardiac vagal afferents in adult rats. Am J Physiol. 1998;274:H441–H447. doi: 10.1152/ajpheart.1998.274.2.H441. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Conforti L. Millhorn DE. Gene expression and function of adenosine A2A receptor in the rat carotid body. Am J Physiol. 2000;279:L273–L282. doi: 10.1152/ajplung.2000.279.2.L273. [DOI] [PubMed] [Google Scholar]
- McQueen DS, Ribeiro JA. Pharmacological characterization of the receptor involved in chemoexcitation by adenosine. Br J Pharmacol. 1986;88:615–620. doi: 10.1111/j.1476-5381.1986.tb10242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro EC, Ribeiro JA. Adenosine and carotid body chemoreceptor regulation of respiration in the rat. Pflugers Arch Suppl. 1986;1:116P. [Google Scholar]
- Ferguson GT, Narendra K, Lattin CT, Goshgarian HG. Clinical effects of theophylline on inspiratory muscle drive in tetraplegia. Neurorehabil Neural Repair. 1999;13:191–197. [Google Scholar]
- Bascom AT, Lattin CD, Aboussouan LS, Goshgarian HG. Effect of acute aminophylline administration on diaphragm function in high cervical tetraplegia: a case report. Chest. 2005;127:658–661. doi: 10.1378/chest.127.2.658. [DOI] [PubMed] [Google Scholar]