Abstract
Background/Objective:
The purpose of the study was to determine whether arterial diameter, flow-mediated dilatation (FMD), and arterial range are affected by spinal cord injury (SCI). We assessed arm (radial) and leg (posteriortibial) arteries that are comparable in size and function to determine whether (a) arterial function is reduced in individuals with SCI vs nondisabled subjects and (b) decrements to SCI arterial function are greater in the legs vs arms.
Participants:
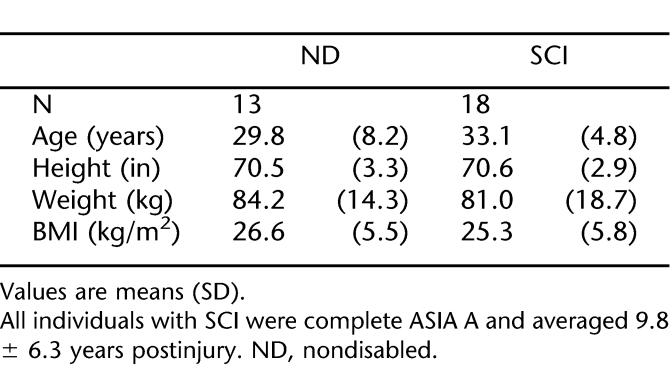
Eighteen men with chronic (9.8 ± 6.3 years) SCI (T2 to T11; American Spinal Injury Association A) and 13 nondisabled subjects matched for age (33.1 ± 4.8 vs 29.8 ± 8.2 years old, respectively), height, and weight (BMI = 25.3 ± 5.8 vs 26.6 ± 5.5 kg/m2, respectively).
Methods:
Radial and posterior tibial artery B-mode ultrasound images were continuously captured to measure resting diameter, occluded diameter, and postischemic diameters. Hierarchical linear modeling accounted for the nested experimental design.
Results:
Individuals with SCI have lower systemic (arm + leg) FMD than nondisabled subjects (9.3% vs 12.3%, respectively; P = 0.035), primarily because of reduced leg FMD (11.5 ± 3.1% vs 7.0 ± 2.8% for SCI arms vs legs, respectively; P = 0.010). Persons with SCI also had lower arterial range than nondisabled subjects (0.79 vs 1.00 mm, respectively; P = 0.043), primarily because of the legs (0.81 ± 0.09 vs 0.56 ± 0.11 mm for SCI arms vs legs, respectively; P = 0.030).
Conclusion:
Leg arterial function seems to deteriorate at greater rates compared to the arms for individuals with SCI. Interventions to improve cardiovascular health should include measurements taken in the legs.
Keywords: Spinal cord injuries, Flow-mediated dilation, Arterial range, Arterial function, Cardiovascular disease
INTRODUCTION
Cardiovascular disease prevalence is approximately 200% greater for persons with spinal cord injury (SCI) compared with nondisabled counterparts (1). Unfortunately, limited research is available to describe the pathogenesis of cardiovascular disease after SCI. There is a particular need to identify whether deterioration of arterial function is more pronounced in arteries below lesion level. Because of paralysis below the lesion and subsequent disuse of these limbs, lower extremity arterial function may be at particular risk for deterioration.
A popular noninvasive assessment of arterial function is brachial artery flow-mediated dilation (FMD) (2). FMD reflects the ability of the endothelium to relax vascular smooth muscle in response to increased blood velocity–induced shear stress (3). Reduced FMD is an early marker of atherosclerosis (2), a surrogate marker of cardiovascular function (2,4), and a predictor of future cardiovascular complications (5,6). Given the incidence of cardiovascular disease (CVD) after SCI, it may be surprising that normal brachial artery FMD has been reported (7). Retention of upper extremity function may explain these discrepant findings. The legs, however, are mostly inactive, which in turn may lead to greater declines in arterial function within these limbs. So why did De Groot el al (7) find normal femoral artery FMD in SCI? Direct comparisons between the femoral and brachial arteries are difficult. Compared with the brachial artery, the notably larger femoral artery has different structural composition and decreased dilatory capacity to increases in shear stress stimuli (8). There remains a need to compare upper vs lower extremity arterial function using arteries similar in size and function.
Arterial function is also reflected using resting arterial size (9). However, assessments solely limited to resting arterial size may lead to erroneous conclusions. Structural remodeling may, in part, explain the 45% to 50% reduction in SCI femoral diameter (10,11), although changes in sympathetic tone after SCI may also contribute (12,13). Arterial size may be more accurately indicated through an arterial “physiological” operating range (14,15) that represents the physiological minimum to maximum diameter range. Decreased arterial range has been shown in nondisabled subjects with peripheral artery disease (14) but remains to be measured in patients with SCI.
For the purpose of this study, we define “arterial function” using 3 surrogate markers: (a) resting diameter, (b) FMD, and (c) arterial range. The purpose of this study was to assess arm (radial) and leg (posteriortibial) arteries that are comparable in size and function and determine whether (a) arterial function is reduced in individuals with SCI compared with nondisabled subjects and (b) decrements to SCI arterial function is greater in the legs vs arms. Our hypotheses were (a) arterial function is reduced in individuals with SCI vs controls and (b) SCI results in greater reductions to arterial function in the legs relative to arms.
METHODS
Subjects
Eighteen men with chronic SCI and 13 nondisabled control subjects were tested. All subjects were non-smokers. None of the subjects suffered from disease or disabilities other than SCI. Patients with SCI had complete American Spinal Injury Association (ASIA) A spinal cord lesions of traumatic origin at levels between T2 and T12. Time since injury was at least 2 years (9.8 ± 6.3 years). Four subjects were medicated with oxybutynin chloride (Ditropan), 3 with baclofen, and one subject took Duragesic (fentanyl transdermal system), Sloniacin, gabapentin (Neurontin), buspirone (BuSpar), Dulcolax, and albuterol. We used a rating scale (range 0–4) to measure spasms “. . .by the number of sustained flexor and extensor muscle spasms over a 1-hour period,” (ie, 0 = none, 4 = > 10 spontaneous spasms per hour). Four subjects reported a 0 rating, 8 had a rating of 1 (vigorous sensory and motor stimulation results in spasms), and 6 had a rating of 2 (occasional spontaneous spasms and easily induced spasms). This study was conducted with the approval of the Institutional Review Boards at the University of Georgia and Shepherd Center (Atlanta), and all subjects provided written informed consent.
Protocol
This cross-sectional study was conducted at the University of Georgia and the Shepherd Center (Atlanta). Protocols, equipment, and the ultrasound technician were identical between locations. Subjects reported for testing after fasting for 12 hours. Subjects were asked to consume water ad libitum. After reading and signing the study protocol and consent forms, subjects rested in supine position for at least 10 minutes. We induced changes in arterial diameter by proximal cuff occlusion of the dominant limb for 5 and then 10 minutes. The 10-minute occlusion period, administered on return to resting diameter, provided the minimum and maximal diameters used to calculate arterial range. Blood pressures and heart rates taken from the opposing arm were measured using a semi-automated device (Data-scope, Montvale, NJ). Recordings were taken after 10 minutes of supine rest, at the end of occlusion, and 5 minutes after occlusion.
Ultrasound Diameter Measurements
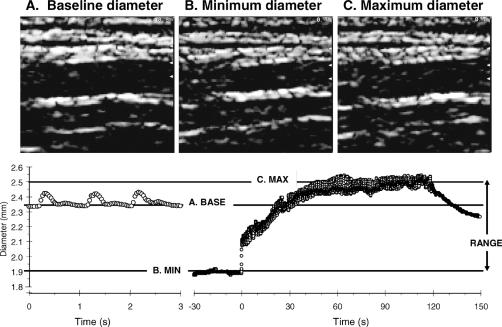
Diameter measurements were made using a high-resolution portable B-mode ultrasound imaging machine (GE Logiq book, GE Medical, Milwaukee, WI) with a 7- to 13-MHz linear probe. The radial artery was imaged at the midpoint of the forearm, and the posterior tibial artery was 3 to 5 cm superior to the calcaneus. Magnification and focal zone settings were adjusted to optimize imaging of the proximal and distal vessel wall. Image focus was maintained throughout the entire experiment using a specialized probe-holding device. High-quality MPEG2 recordings of the entire experiment were made using a Dell Laptop PC equipped with a video capture device (ADS technologies, Cerritos, CA). Video files collected at 30 frames per second were converted into JPEG images. JPEG images provide comparable accuracy for ultrasound image measurements compared to the DICOM (Digital Imaging and Communications in Medicine) standard (16). Images were analyzed off-line using semiautomated edge-detection software custom written to interface with National Instruments Lab view software (Figure 1). Analysis was not done in a blinded manner, but the use of an automated computer program minimized potential bias in analysis. Reported resting diameters represent the average from 2 minutes of rest. Minimum diameters represent the last 30 seconds of ischemia. Maximum diameters were calculated from the average of the 3 largest adjacent diameters after cuff release. In our laboratory, between-day coefficients of variation for measurements of diameter ranging from rest to maximal diameters are 2% to 3% (17).
Figure 1. Examples of the image analysis for measuring arterial diameters. B-mode images of (A) resting, (B) minimum, and (C) maximum diameters are shown. (Bottom) Representative plot of arterial diameter in response to 10 minutes of proximal ischemia. Images were captured at a rate of 30 images per second. Arterial range was calculated by subtracting the minimum diameter from the maximum diameter.
Measures of Arterial Function
FMD was calculated as (5-minute maximum diameter − resting diameter)/resting diameter × 100, where the 5-minute maximum diameter represents the maximum diameter after 5 minutes of proximal ischemia.
Briefly, a pneumatic tourniquet placed around the limb proximal to the insonated artery was rapidly inflated (1–2 seconds) to a pressure of approximately 100 mmHg above systolic blood measured from the opposing arm. Previous studies have shown that use of a proximal cuff provides a larger effect size than using a distal cuff and may be a better predictor of cardiovascular disease than FMD with a distal cuff (18). The determination of minimal diameter when transmural pressure in the artery is near zero is also attainable. Immediately after cuff release, we visualized the hyperemic response using velocity color imaging to ensure that artery focus was maintained during ischemia.
Arterial range was calculated as maximum diameter − minimum diameter, where the maximum diameter was after 10 minutes of proximal ischemia. Our laboratory has shown that the minimal diameter occurs within 3 to 5 minutes after proximal cuff ischemia (15) and that 10 minutes of ischemia induces the maximal physiological diameter (15).
Statistical Analysis
Between-group physical characteristics were analyzed using univariate ANOVA with SPSS 13 for Windows. Arm and leg arterial function outcomes were analyzed through hierarchical linear modeling (HLM) with the HLM6 (SSI, Lincolnwood, IL) statistical package. A key application of HLM relates to the capacity for accounting for correlated measures by recognizing the nested data structure. Briefly, separate 2-level hierarchical linear models were built to compare resting diameters, FMD, and arterial range in individuals with SCI and nondisabled subjects. For each model arm and leg measurement, sites (level 1) were nested within each subject (level 2). The level 1 unconditional (ie, no covariates) model was specified as follows:
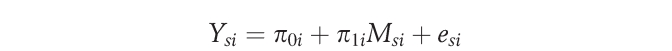 |
where πli is the slope parameter representing the difference between arm and leg scores. The intercept parameter (π0i) represents arterial function for person i when Msi = 0 (ie, the composite [arm + leg] arterial function score), because measurement site was group centered.
These parameters (π0i and π1i) become outcomes at level 2:
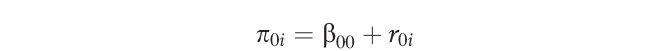 |
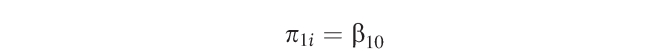 |
where r0i is the unique increment associated with individual i, indicating that the individual means are allowed to randomly vary. Level 2 outcomes were used for hypothesis testing. The variance of the slope was constrained to zero. A limitation to the current data set is that only 2 measures (arm and leg) were made per individual for each outcome, limiting the model to 1 randomly varying coefficient at level 1.
Model construction for each arterial function outcome followed 2 stages: (a) development of a measurement model and (b) inclusion of a grouping variable to test for differences between nondisabled and SCI groups. The grouping variable was subsequently included as uncentered so that each group has their own intercept/composite (arm + leg) arterial function outcome and their own slope (difference between arm and leg).
To evaluate the effect of duration of injury, the best-fit measurement models identified above were used as initial models. Duration of SCI injury was included uncentered at level 2. Nondisabled subjects were coded as 0 years of injury. Subsequently, to partial out the variance caused by age, age was grand-centered at level 2.
Statistical significance is defined as P < 0.05. All P values reported are two-tailed.
RESULTS
Subject Characteristics
One case of dysreflexia occurred during the testing of an individual with SCI, resulting in data exclusion. We excluded 2 additional subjects because of missing leg data. We used the remaining 18 SCI and 13 nondisabled subjects for data analysis. Table 1 shows subject characteristics. We found no significant differences for age, height, or weight between groups.
Table 1.
Subject Characteristics
Resting Arterial Diameters
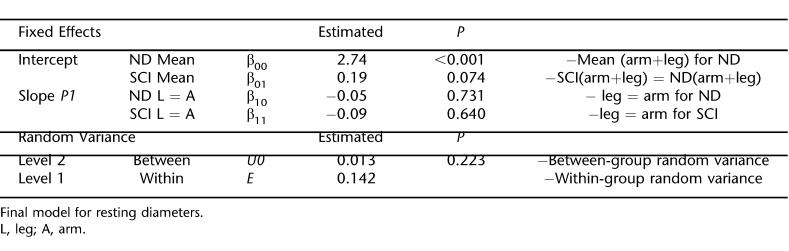
The HLM model for resting diameters (Table 2) shows larger diameters for the SCI than nondisabled controls (P = 0.037). Within individuals, for both groups, arm and leg diameters were not significantly different. There was not significant between-group random variance. Therefore, while we found a small difference between groups for resting diameters, resting diameter could be included as a fixed covariate in subsequent FMD and arterial range models to control for the between-group differences.
Table 2.
HLM Model Fit for Resting Diameters
Flow-Mediated Dilation
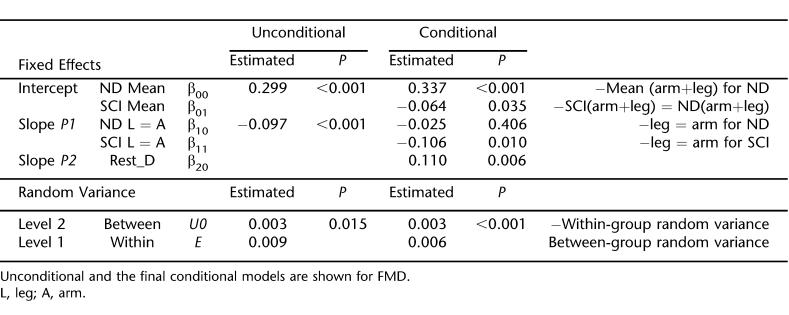
Calculated as a percentage, FMD was 12.9 ± 3.5% and 12.0 ± 5.2% in the arms and legs of nondisabled subjects, respectively. FMD was 11.5 ± 3.1% and 7.0 ± 2.8% in the arms and legs of individuals with SCI. For statistical analysis and for presentation, we use FMD as the absolute diameter adjusted for initial diameter. Figure 2A shows the actual measured values, and Figure 2C shows the model derived FMD values for the arms and legs of SCI and nondisabled subjects. Table 3 shows the results of HLM analysis. Patients with SCI have lower FMD than nondisabled subjects (P = 0.035). The reduced FMD in the individuals with SCI is primarily caused by reduced leg FMD (P = 0.010).
Figure 2. FMD and arterial range measurements as absolute diameters in the arms and legs of individuals with SCI and nondisabled individuals. (A) Original data for FMD represented by the absolute diameter size after 5 minutes of ischemia. (B) Original data for arterial range. (C) HLM FMD transformed data accounting for differences in resting diameter. (D) HLM arterial range transformed data accounting for differences in resting diameter. *P < 0.05, within-group differences between arm and leg scores. Original data shown in A and B are mean and SD. The HLM model-fit values are mean and SE and correspond to models shown in Tables 4 and 5.
Table 3.
HLM Model Fit for Flow-Mediated Dilation
Arterial Range
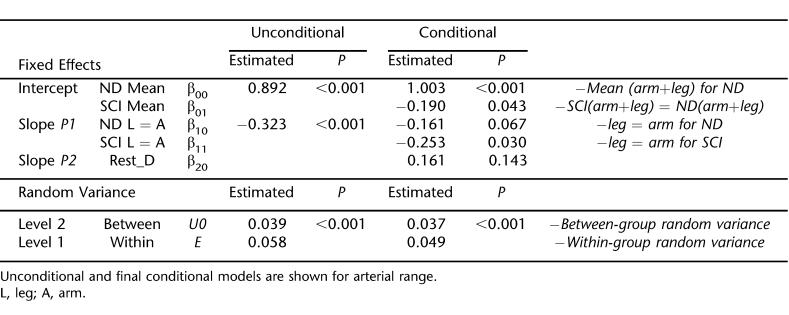
Figure 2B shows the unadjusted measured values, and Figure 2D shows the model-derived arterial range values for the arms and legs of SCI and nondisabled subjects. Table 4 shows the results of HLM analysis. As above for FMD, model 2 normalizes arterial range to group-centered resting diameters. Patients with SCI have lower arterial range values than nondisabled subjects (P = 0.043). The reduced arterial range in the individuals with SCI was primarily caused by reduced leg arterial range (P = 0.030).
Table 4.
HLM Model Fit for Arterial Range
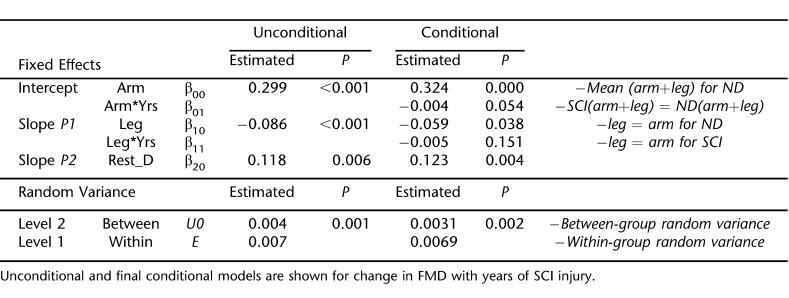
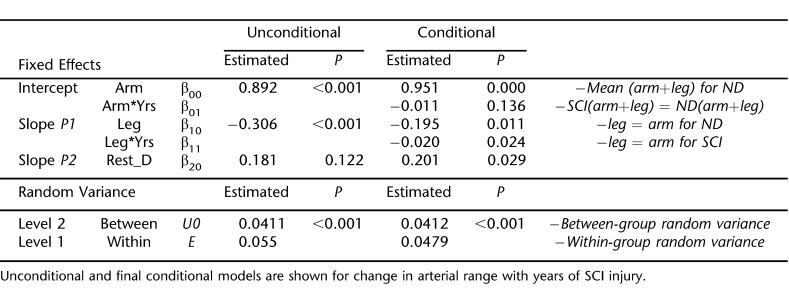
Change in FMD and Arterial Range With Years of SCI
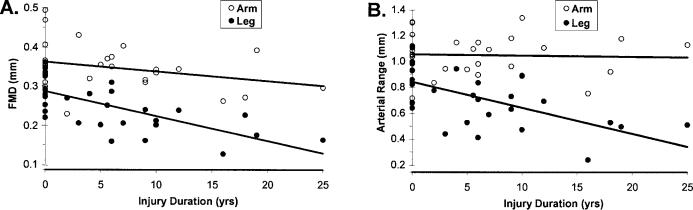
Duration of SCI injury was substituted for the SCI grouping variable at level 2 to explore the relationships between injury time and arterial function. Model 1 for each arterial function measure (FMD and arterial range) was identical to the final FMD and arterial range models presented above. Figure 3 shows the final models, which summarize the predicted change in arm and leg arterial function measures with duration of injury. Model parameters are shown in Tables 5 and 6. Arm FMD (1.4%/year) and arterial range (1.1%/year) decreased at slower rates than leg FMD (1.8%/year) and arterial range (2.6%/year). When included as a level 2 covariate, patient age does not significantly explain any additional variance for either model.
Figure 3. (A) FMD and (B) arterial range plotted against years of SCI injury. Nondisabled subjects are dummy-coded to have 0 years of injury. Values correspond to models shown in Tables 5 and 6.
Table 5.
Estimated Effect of SCI Injury Duration on Flow-Mediated Dilation
Table 6.
Estimated Effect of SCI Injury Duration on Arterial Range
DISCUSSION
This study found reduced FMD and arterial range in individuals with SCI compared with nondisabled subjects. In addition, we found greater decreases in FMD and arterial range below compared with above the SCI lesion.
Flow-Mediated Dilation
For more than a decade, brachial artery FMD has been widely used as a global arterial function index (2,4). Few studies have evaluated FMD in arteries other than the brachial artery. Because we found greater decreases in FMD in the legs compared with the arms for patients with SCI, our results suggest that lower extremity arteries require assessment. The retention of upper extremity function likely explains the lack of deterioration for SCI radial arteries. Patients with paraplegia mostly rely on upper body function for performing daily activities. Because individuals with SCI actively use their upper extremities, blood flow patterns may be such that the blood vessels retain their functional function status because of normal shear stressor activity (19,20).
FMD can be calculated as (a) post-only score, (b) change score, (c) fraction, or (d) covaried for resting diameter. We calculated FMD as absolute diameter change and covaried for resting diameters. The rationale was 3-fold: (a) our 3 measurements (resting diameters, FMD, arterial range) could be easily compared; (b) the data were less likely to become nonnormally distributed; and (c) measurement variation was reduced. FMD is commonly presented as a fraction (the percentage change in diameter), whereas using resting diameter as a covariate is more likely to adjust for bias because of resting values (21–23). A simulation study found the greatest statistical power for the ANCOVA approach out of the 4 methods listed above, with fraction scores resulting in the lowest power (22).
A limitation of our study is that we were unable to measure the hyperemic response in the posterior tibial artery because of restrictions of the portable ultrasound machine used. Previous studies have stressed the importance of correcting for differences in blood flow responses (shear rate) when measuring FMD (8,17,24–26). However, in previous studies, we found no evidence that hyperemic blood velocity after cuff ischemia was reduced in patients with SCI (10,27). We do agree that future studies would benefit from the measurement of blood velocity and calculation of shear rates.
Resting Arterial Diameters
Resting diameters in both extremities were approximately 7% larger for SCI vs nondisabled subjects. Physical characteristic (body size) could not explain this small difference because the groups were not different. The posteriortibial artery seems to contrast with the SCI femoral artery, which undergoes major vascular atrophy (37%–50%) (10,11,28). However, our group has previously found the decrease in femoral arterial diameter to be proportional to leg muscle atrophy in these patients (10). Because the distal posterior tibial artery primarily serves the foot, the relative lack of atrophy of tissues in the foot may help explain why the posterior tibial artery did not show signs of atrophy. Nonetheless, while only small difference were found between groups, resting diameters can still be used to explain a notable portion of variation when statistically comparing groups (21–23). For example, resting diameters have been shown to negatively correlate with FMD, in large part because of the affects of initial diameter size on the shear stress gradient after ischemia (8,26). Results from our HLM model show that there was no random variation between groups, supporting the decision to use resting diameters as a fixed covariate for our FMD and arterial range models.
Arterial Range
Arterial range was reduced by 5.2% (P = 0.043) in the arms and by 35.1% (P = 0.030) in the legs of SCI compared with nondisabled controls. We observed that reduced SCI arterial range was predominantly caused by larger minimum diameters. The same phenomenon was observed in nondisabled persons with peripheral vascular disease (14). The impaired capacity of the arteries to relax during proximal ischemia when transmural pressure is at a minimum likely reflects arterial stiffening. Maximal (physiological) leg diameters, however, did not differ between groups. After 10 minutes of ischemia, there is a resultant maximal physiological blood velocity stimulus for both nondisabled and SCI groups (27), and subsequently, maximal physiological dilation (unpublished observations). Naylor et al (25) found that maximal dilation was achieved with a combination of ischemia, exercise, and the use of an endothelium-independent vasodilator (nitroglycerin). However, they also found 10 minutes of ischemia to induce a near maximal response, supporting the use of a single “noninvasive” physiological intervention. Nonetheless, we also acknowledge that supraphysiological responses (eg, as attained by nitroglycerin) may provide additional information concerning arterial structure.
Arterial Function Rate of Change
While our study used a cross-sectional design, we did find evidence that duration of injury correlated with reduced FMD and arterial range. We found that arm FMD decreases by 1.2% per year, whereas arterial range does not significant decline. The magnitude of decrease for leg artery FMD and arterial range was 1.8% and 2.6% per year, respectively. The addition of subject age as a model covariate did not significantly alter these relationships. These data contrast the findings by De Groot et al (11), who assessed the time-course of vascular adaptations to inactivity and paralyses in humans. They found significantly reduced diameters and blood flow within 6 weeks after SCI, with no further changes up to 13 months. However, these arterial adaptations (to the femoral artery) have been closely linked to muscle atrophy (10) and may not adequately reflect changes to “arterial function.” Furthermore, while structural adaptations may occur within a relatively short period, the capacity of an artery to respond to hemodynamic stimuli may continue to diminish. Interestingly, De Groot et al did find that carotid and brachial artery diameter and flow were similar in SCI vs nondisabled controls, supporting the notion that the lower extremities are at particular risk.
Limitations
A limitation to our analysis of change in arterial function with duration of injury was the use of nondisabled subjects to approximate arterial function before SCI. It is not feasible to test individuals with SCI before injury, but our results support the need for additional studies to evaluate injury duration and arterial function. A limitation to the interpretation of our findings of impaired arterial function in people with SCI is that we were not able to separate out the potential effects of sympathetic nervous system (SNS) dysfunction and disuse caused by paralysis. Disuse is a known cardiovascular disease risk factor, and our results are consistent with those found after lower limb suspension (29) or bed rest (30). While exaggerated SNS activity is thought to result in endothelial dysfunction (13), the effects of impaired SNS function are less clear. In able-bodied humans who undergo thoracic sympathectomy, endothelial function seems to be either not altered or enhanced (31–33). In SCI, injury above the T6 level results in significantly reduced SNS outflow and supraspinal control to the lower extremity blood vessels, whereas with injury below T6, there is generally sufficient sympathetically innervated vasculature to limit the hemodynamic manifestations of SNS dysfunction. Eight of our subjects had SCI below T6 and 10 had SCI above T6. While we found no correlations between injury level and arterial function, our subject pool was limited in number, and the effects of injury level on arterial function warrants further attention.
CONCLUSIONS
This study provides evidence that individuals with SCI have impaired arterial function measured as decreased FMD and arterial range. The legs were found to be impaired to a greater extent than the arms. Therefore, assessments of cardiovascular disease risk should include measurements of arteries below the lesion level. Furthermore, interventions to improve cardiovascular health in persons with SCI should target the lower extremities.
Footnotes
This study was supported in part by NIH HL65179 and a pilot grant from the University of Georgia and the Shepherd Center for Acquired Brain Injury.
REFERENCES
- Kocina P. Body composition of spinal cord injured adults. Sports Med. 1997;23:48–60. doi: 10.2165/00007256-199723010-00005. [DOI] [PubMed] [Google Scholar]
- Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- Murakami T, Arai Y. Relationship between non-invasively evaluated endothelial dysfunction and future cardiovascular events. Presented at the ACC Conference, Orlando, FL, March 2001.
- Schroeder S, Enderle MD, Ossen R, et al. Noninvasive determination of endothelium-mediated vasodilation as a screening test for coronary artery disease: pilot study to assess the predictive value in comparison with angina pectoris, exercise electrocardiography, and myocardial perfusion imaging. Am Heart J. 1999;138(4 Pt 1):731–739. doi: 10.1016/s0002-8703(99)70189-4. [DOI] [PubMed] [Google Scholar]
- De Groot PC, Poelkens F, Kooijman M, Hopman MT. Preserved flow mediated dilation in the inactive legs of spinal cordinjured individuals. Am J Physiol Heart Circ Physiol. 2004;287:H374–H380. doi: 10.1152/ajpheart.00958.2003. [DOI] [PubMed] [Google Scholar]
- Silber HA, Ouyang P, Bluemke DA, Gupta SN, Foo TK, Lima JA. Why is flow-mediated dilation dependent on arterial size? Assessment of the shear stimulus using phase-contrast magnetic resonance imaging. Am J Physiol Heart Circ Physiol. 2005;288:H822–H828. doi: 10.1152/ajpheart.00612.2004. [DOI] [PubMed] [Google Scholar]
- Van Bortel LM, Duprez D, Starmans-Kool MJ, et al. Clinical applications of arterial stiffness, Task Force III: recommendations for user procedures. Am J Hypertens. 2002;15:445–452. doi: 10.1016/s0895-7061(01)02326-3. [DOI] [PubMed] [Google Scholar]
- Olive JL, Dudley GA, McCully KK. Vascular remodeling after spinal cord injury. Med Sci Sports Exer. 2003;35(6):901–907. doi: 10.1249/01.MSS.0000069755.40046.96. [DOI] [PubMed] [Google Scholar]
- De Groot PC, Van Kuppevelt DH, Pons C, Snoek G, Van Der Woude LH, Hopman MT. Time course of arterial vascular adaptations to inactivity and paralyses in humans. Med Sci Sports Exerc. 2003;35:1977–1985. doi: 10.1249/01.MSS.0000099088.21547.67. [DOI] [PubMed] [Google Scholar]
- Owlya R, Vollenweider L, Trueb L, et al. Cardiovascular and sympathetic effects of nitric oxide inhibition at rest and during static exercise in humans. Circulation. 1997;96:3897–3903. doi: 10.1161/01.cir.96.11.3897. [DOI] [PubMed] [Google Scholar]
- Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39:683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- Harris LM, Faggioli GL, Shah R, et al. Vascular reactivity in patients with peripheral vascular disease. Am J Cardiol. 1995;76:207–212. doi: 10.1016/s0002-9149(99)80066-6. [DOI] [PubMed] [Google Scholar]
- Black C, Vickerson B, McCully K. Noninvasive assessment of vascular function in the posterior tibial artery of healthy humans. Dyn Med. 2003;2(1):1. doi: 10.1186/1476-5918-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hangiandreou NJ, James EM, McBane RD, Tradup DJ, Persons KR. The effects of irreversible JPEG compression on an automated algorithm for measuring carotid artery intima-media thickness from ultrasound images. J Digit Imaging. 2002;15(suppl 1):258–260. doi: 10.1007/s10278-002-5022-0. [DOI] [PubMed] [Google Scholar]
- Stoner L, Sabatier M, Edge K, McCully K. The relationship between blood velocity and conduit artery diameter, and the effects of smoking on vascular responsiveness. J Appl Physiol. 2004;96:2139–2145. doi: 10.1152/japplphysiol.01107.2003. [DOI] [PubMed] [Google Scholar]
- Vogel RA, Corretti MC, Plotnick GD. A comparison of brachial artery flow-mediated vasodilation using upper and lower arm arterial occlusion in subjects with and without coronary risk factors. Clin Cardiol. 2000;23:571–575. doi: 10.1002/clc.4960230805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnasso A, Carallo C, Irace C, et al. Association between intima-media thickness and wall shear stress in common carotid arteries in healthy male subjects. Circulation. 1996;94:3257–3262. doi: 10.1161/01.cir.94.12.3257. [DOI] [PubMed] [Google Scholar]
- Zarins CK, Zatina MA, Giddens DP, Ku DN, Glagov S. Shear stress regulation of artery lumen diameter in experimental atherogenesis. J Vasc Surg. 1987;5:413–420. [PubMed] [Google Scholar]
- Tu YK, Blance A, Clerehugh V, Gilthorpe MS. Statistical power for analyses of changes in randomized controlled trials. J Dent Res. 2005;84:283–287. doi: 10.1177/154405910508400315. [DOI] [PubMed] [Google Scholar]
- Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001;1:6. doi: 10.1186/1471-2288-1-6. Epub 2001 June 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twisk J, Proper K. Evaluation of the results of a randomized controlled trial: how to define changes between baseline and follow-up. J Clin Epidemiol. 2004;57:223–228. doi: 10.1016/j.jclinepi.2003.07.009. [DOI] [PubMed] [Google Scholar]
- Silber HA, Bluemke DA, Ouyang P, Du YP, Post WS, Lima JA. The relationship between vascular wall shear stress and flow-mediated dilation: endothelial function assessed by phase-contrast magnetic resonance angiography. J Am Coll Cardiol. 2001;38:1859–1865. doi: 10.1016/s0735-1097(01)01649-7. [DOI] [PubMed] [Google Scholar]
- Naylor LH, Weisbrod CJ, O'Driscoll G, Green DJ. Measuring peripheral resistance and conduit arterial structure in humans using Doppler ultrasound. J Appl Physiol. 2005;98(6):2311–2315. doi: 10.1152/japplphysiol.01047.2004. Epub 2005 Feb 3. [DOI] [PubMed] [Google Scholar]
- Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- Olive JL, McCully KK, Dudley GA. Blood flow response in individuals with incomplete spinal cord injuries. Spinal Cord. 2002;40:640–646. doi: 10.1038/sj.sc.3101379. [DOI] [PubMed] [Google Scholar]
- Nash MS, Jacobs PL, Montalvo BM, Klose KJ, Guest RS, Needham-Shropshire BM. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 ambulation system: part 5. Lower extremity blood flow and hyperemic responses to occlusion are augmented by ambulation training. Arch Phys Med Rehabil. 1997;78:808–814. doi: 10.1016/s0003-9993(97)90192-1. [DOI] [PubMed] [Google Scholar]
- Bleeker MW, De Groot PC, Poelkens F, Rongen GA, Smits P, Hopman MT. Vascular adaptation to 4 wk of deconditioning by unilateral lower limb suspension. Am J Physiol Heart Circ Physiol. 2005;288:H1747–H1755. doi: 10.1152/ajpheart.00966.2004. [DOI] [PubMed] [Google Scholar]
- Bleeker MW, De Groot PC, Rongen GA, et al. Vascular adaptation to deconditioning and the effect of an exercise countermeasure: results of the Berlin Bed Rest study. J Appl Physiol. 2005;99:1293–1300. doi: 10.1152/japplphysiol.00118.2005. [DOI] [PubMed] [Google Scholar]
- Charkoudian N, Eisenach JH, Atkinson JL, Fealey RD, Joyner MJ. Effects of chronic sympathectomy on locally mediated cutaneous vasodilation in humans. J Appl Physiol. 2002;92:685–690. doi: 10.1152/japplphysiol.00758.2001. [DOI] [PubMed] [Google Scholar]
- Lepori M, Sartori C, Duplain H, Nicod P, Scherrer U. Sympathectomy potentiates the vasoconstrictor response to nitric oxide synthase inhibition in humans. Cardiovasc Res. 1999;43:739–743. doi: 10.1016/s0008-6363(99)00084-x. [DOI] [PubMed] [Google Scholar]
- Eisenach JH, Clark ES, Charkoudian N, et al. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol. 2002;92:2019–2025. doi: 10.1152/japplphysiol.01025.2001. [DOI] [PubMed] [Google Scholar]