Abstract
Background/Objective:
A significant fraction of patients with cervical spinal cord injury suffer from respiratory muscle paralysis and dependence on chronic mechanical ventilation. In selected patients, diaphragm pacing (DP) through electrical stimulation of the phrenic nerves provides an alternative to mechanical ventilation with significant advantages in life quality.
Methods:
A case report of an individual who successfully underwent DP using intramuscular diaphragm electrodes. A brief review of the state of the art of DP including the clinical benefits of DP, patient selection and evaluation, description of equipment, methods of transition from mechanical ventilation to DP, potential complications and side effects, long-term outcome, and potential future developments in this field is included.
Results:
Several available DP systems are available, including conventional ones in which electrodes are positioned directly on the phrenic nerves through thoracotomy and less invasive systems in which electrodes are placed within the diaphragm through laparoscopy. For patients with only unilateral phrenic nerve function, a combined intercostal and unilateral diaphragm pacing system is under development.
Conclusions:
In patients with ventilator-dependent tetraplegia, there are alternative methods of ventilatory support, which offer substantial benefits compared to mechanical ventilation.
Keywords: Spinal cord injuries; Tetraplegia; Mechanical respiratory ventilation; Diaphragm pacing; Phrenic nerve pacing; Respiratory insufficiency; Electric stimulation; Respiration, artificial
INTRODUCTION
Of the 11,000 new cases of spinal cord injury (SCI) that occur each year in the United States, approximately one half are sustained at the cervical level (1). More than 80% of injuries are caused by vehicular crashes, falls, and gunshot wounds (2). Many of these patients develop respiratory compromise and require mechanical ventilatory support. Whereas many patients can eventually be weaned off mechanical ventilation, a substantial number (200–400) will require chronic mechanical ventilation (2).
Unlike most other causes of chronic respiratory failure, the lungs, chest wall, and respiratory muscles are usually physiologically normal in patients with ventilator-dependent tetraplegia. Moreover, the spinal cord and spinal nerves below the level of injury are also intact. Consequently, inspiratory muscle function can be partially restored in these patients using electrical stimulation techniques. For these reasons, individuals with ventilator-dependent tetraplegia can be offered an alternative method of ventilatory support by diaphragm pacing (DP), a more natural and physiologic form of breathing.
The concept of phrenic nerve stimulation to provide ventilatory support dates back to the 18th century (3). In the 1940s, Sarnoff et al and Whittenberger et al (4,5) first showed that ventilation could be maintained with percutaneous electrodes in patients with poliomyelitis. In the 1960s, Glenn and colleagues (6–8) made significant technological advances, which led to the development of modern day pacing systems. They developed an implantable electrode/receiver system (6–8) that could be activated by radiofrequency waves generated by a power source external to the body. These investigators also accumulated a significant clinical experience that defined patient evaluation methods, surgical techniques, and safe parameters of stimulation that resulted in optimal diaphragm conditioning (9–17).
Subsequent studies have led to further refinements including improved electrode design (18,19) and less invasive methods of application (20,21). Based on current technology and available pacing systems, DP can be offered to eligible patients with spinal cord injury as a safe and effective method of ventilatory support.
CASE REPORT
L.N., a 32-year-old man, suffered a cervical SCI (C3 level) consequent to a motor vehicle crash that caused tetraplegia and dependence on mechanical ventilatory support. After review of potential risks and benefits, he was highly motivated to undergo DP as an alternative to mechanical ventilation. His family was also highly supportive of this decision.
Preliminary studies included phrenic nerve conduction studies, which indicated normal bilateral phrenic nerve function, and fluoroscopic examination of his diaphragm during cervical phrenic nerve stimulation, which revealed marked descent of each hemidiaphragm.
This subject elected to undergo intramuscular diaphragm pacing rather than conventional phrenic nerve pacing because of the less invasive nature of this procedure. Because this system is only available under an Investigational Device Exemption (IDE) from the Food and Drug Administration (FDA), informed consent was first obtained in accordance with the Institutional Review Board at MetroHealth Medical Center.
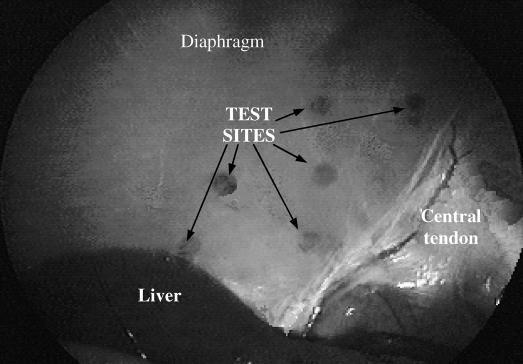
Standard laparoscopic techniques were used to place intramuscular diaphragm electrodes within the costal portion of each hemidiaphragm. After placement of 4 trocars into the abdominal wall (Figure 1) and creation of a pneumoperitoneum, a mapping procedure was performed to determine the region in which the phrenic nerves enter the diaphragm (phrenic nerve motor points; Figure 2). Using a suction electrode, which could be reversibly applied to the abdominal surface of the diaphragm, electrical stimulation was applied at several test sites in the general region of the motor points (Figure 3) to determine the response to stimulation. The strength of diaphragm activation, which reflected the proximity of the electrode to the motor point, was determined by the magnitude of abdominal pressure and visual assessment of the degree of diaphragm descent. Two stainless steel intramuscular diaphragm electrodes (Peterson Electrode; Synapse Biomedical, Oberlin, OH) were inserted within each hemidiaphragm in the region of the phrenic nerve motor points. The electrodes were positioned within 1 to 2 cm of each other. The electrodes were inserted using a specially designed delivery device (Synapse Biomedical) that allowed insertion of the electrodes in the same plane as the diaphragm. During diaphragm stimulation, cardiac monitoring showed no evidence of coincident cardiac stimulation.
Figure 1. Laparoscopic implant procedure. Four laparoscopic ports provided access to the abdominal cavity; ports were used for visualization, insufflation of the abdominal cavity, diaphragm mapping, and insertion of the implant tool. Reprinted with permission from DiMarco AF, Onders RP, Kowalski KE, Miller ME, Ferek S, Mortimer JT. Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med. 2002;166:1604–1606 (20).
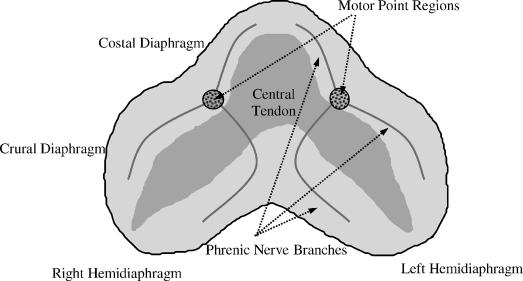
Figure 2. Schematic representation of entrance points of each phrenic nerve into each hemidiaphragm (phrenic nerve motor points). Reprinted with permission from DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest. 2005;127:671–678 (21).
Figure 3. Photograph of several test sites on the abdominal surface of the diaphragm. A suction electrode, which could be reversibly applied to the diaphragm, was used to determine the optimal site for permanent electrode placement. Application of the suction electrode resulted in a small hematoma, which was used to identify each test site.
The electrodes were tunneled subcutaneously to the mid-chest region and attached to a connecting circuit. From this area, each wire was tunneled separately subcutaneously to the left subclavicular region where they exited the chest wall. The patient remained in the hospital overnight for observation.
Two weeks later, the patient was admitted to the General Clinical Research Center and began a reconditioning program to increase the strength and endurance of the diaphragm. A 4-channel electrical stimulator (Synapse Biomedical) was used to activate the diaphragm. Inspired volume generation was evaluated with each of the 4 electrodes individually and in combination. In each instance, 25 milliampere (mA) was required to achieve maximum inspired volume generation. During the initial portion of the reconditioning period, stimulation of both electrodes within each hemidiaphragm resulted in greater inspired volumes compared to stimulation of one electrode alone. Pulse widths greater than 0.1 millisecond did not result in increases in inspired volume generation. Initial applied stimulation was 20 hertz (Hz). Inspiratory time was set at 1.1 seconds; respiratory rate was set between 10 and 12 breaths/min, based on patient comfort and optimization of speech.
Initially, DP was provided for 10 minutes each hour for 5 to 6 h/d. Each week, stimulation time was increased by 10 to 15 min/h, as tolerated. After 5 weeks, continuous pacing was achieved for 8 hours. Subsequently, the number of hours of pacing per day was increased. After 8 weeks, 24-hour continuous pacing was achieved. Because stimulation of one electrode within each hemidiaphragm provided inspired volumes similar to that obtained with both electrodes, DP was achieved with a single electrode in each hemidiaphragm. Oxygen saturation by finger pulse oximetry and end-tidal CO2 at the tracheal opening were monitored during diaphragm reconditioning.
After the conditioning period, the inspired volumes during chronic pacing (11 Hz) and maximum stimulation (50 Hz) were 820 and 1,240 mL, respectively. Changes in esophageal, gastric, and airway pressures during chronic and maximum stimulation are shown in Figure 4. The difference between gastric and esophageal pressures, transdiaphragmatic pressure (Pdi), is a commonly used index of the force of diaphragm contraction. Pdi during chronic and maximum stimulation in this subject were 21 and 79 cmH2O, respectively. Both Pdi and inspired volume production were in the range of values observed during conventional phrenic nerve stimulation, a method by which electrodes are placed directly on the phrenic nerves (22).
Figure 4. Representative gastric, esophageal, and airway pressures during maximum (24 mA, 50 Hz) and chronic (24 mA, 11 Hz) bilateral phrenic nerve stimulation. Pressure measurements are represented in cm H2O. Data were collected after the reconditioning period, approximately 10 weeks after electrode implantation. Reprinted with permission from DiMarco AF. Restoration of respiratory muscle function following spinal cord Injury. Review of electrical and magnetic stimulation techniques. Respir Physiol Neurobiol. 2005;147:273–287.
L.N. described diaphragm pacing as effortless and similar to normal breathing and “like night and day, when compared to mechanical ventilation.” More specific benefits derived from diaphragm pacing included ease of transport from bed to chair and travel out of the home to social events and medical office visits, improved speech, and overall improved sense of well being, characterized by this individual as “feeling more normal.”
This patient exemplifies the ideal candidate for DP; his case report serves as a template for this review. The discussion focuses on the various methodologies of implementing DP and important clinical aspects of this technique.
DISCUSSION
Clinical Benefits of Diaphragm Pacing
All patients with chronic respiratory failure consequent to tetraplegia can be adequately supported by mechanical ventilation. In eligible patients, however, DP has specific advantages that render this technique the preferable modality (23–27). In this regard, it should be noted that the derived clinical benefits can vary significantly among individuals.
As in the case description, patients using DP often describe a sense of more normal breathing and general health (24,28–35). This can be attributable, in part, to the fact that patients are breathing with their own respiratory muscles and do not require any equipment applied to the airway (16,25,36). Some patients also describe an increased level of comfort related to negative pressure ventilation compared with the positive pressure associated with mechanical ventilation. Other potential advantages of negative pressure ventilation include reduction in the incidence of barotrauma and improved cardiovascular function (26,36).
With DP, connection to an external life support system, which involves a ventilator and attached tubing, is eliminated, resulting in increased mobility. This is particularly true of patients unable to sustain themselves for more than a few minutes off mechanical ventilation (27). In such cases, even short-term disconnection from the ventilator is quite stressful. While transport outside the home for occupational or social events is facilitated, even daily routines, such as simple transfers from bed to chair, are much less cumbersome. Without ventilator tubing, discomfort associated with tension on the tracheostomy tube is also eliminated. Moreover, fear of ventilator disconnection, a major concern, is no longer a problem. The subject of this case review resumed his occupation as a financial consultant, and consequent to DP, was much more comfortable visiting clients.
Other potential benefits of DP include improved speech, improved olfactory sensation, and in some cases, elimination of the social embarrassment associated with ventilator noise and attachment to a life support system (34).
Patient Selection and Evaluation
Diaphragm pacing is a costly undertaking, and depending on the specific technique of implantation, variable amounts of risk are associated with the surgical procedure. Consequently, potential candidates should be carefully screened and meet specific eligibility criteria.
The initial evaluation of potential candidates for DP should first include a thorough analysis of psychosocial factors (15,16,21,25,34). Patients should be highly motivated to improve their ability to function independently. A cooperative and supportive caregiver support system is also important. Ideal candidates and their caregivers anticipate the benefits of improved mobility and speech, leading to greater social interaction, expanded participation in rehabilitation activities, and/or improved occupational opportunities. Potential candidates should also be screened for significant underlying lung, chest wall, or primary muscle disease, because these disorders may preclude successful pacing (16,37).
A thorough assessment of phrenic nerve function should be performed in all patients contemplating DP (14,17,21,26,27). Unfortunately, many patients with tetraplegia have sustained injury to the phrenic motoneurons in the spinal cord and/or phrenic rootlets. If phrenic nerve function is absent or significantly reduced, DP should not be undertaken. Phrenic nerve function should be assessed both by measurements of phrenic nerve conduction times (38,39) and by fluoroscopic evaluation of diaphragm movement (14,34) during phrenic nerve stimulation.
The cervical portion of the phrenic nerves can be electrically stimulated through surface electrodes or monopolar needle electrodes at the posterior border of the sternocleidomastoid muscle at the level of the cricoid cartilage (10,38,39). Diaphragmatic electromyography (EMG) can be monitored with surface recording electrodes positioned between the seventh and ninth intercostal spaces. Electrical current is applied with single pulses of gradually increasing intensity until a supra-maximal M-wave is observed. Stimulation is associated with coincident outward movement of the abdominal wall. Phrenic nerve conduction time is measured as the interval between the applied stimulus and onset of the compound muscle action potential (CAP). Normal mean onset latency is 7 to 9 milliseconds in adults (38,39). Successful pacing has been accomplished with mild prolongation of conduction times, up to 14 milliseconds (15). Latencies are significantly shorter in children: 2.2 milliseconds at 6 months of age and increasing to 4.2 milliseconds from 5 to 11 years of age (40,41). Because patients maintained on mechanical ventilation for prolonged periods may have variable degrees of atrophy of the diaphragm, there may be reductions in the magnitude of the CAP. For this reason and associated technical difficulties associated with EMG recordings, such as optimal electrode placement and variable amounts of fatty tissue, which may reduce signal amplitude, the magnitude of the diaphragmatic CAP is a less reliable indicator of phrenic nerve function compared with conduction time (27,38,42).
In our own experience, phrenic nerve stimulation is associated with both false-positive and false-negative results. Therefore, fluoroscopic examination of the diaphragm during electrical stimulation should also be performed. The diaphragm should descend at least 3 to 4 cm during stimulation as visualized fluoroscopically (14,34). In individuals with normal function, the diaphragm descends more than 5 cm.
The status of phrenic nerve function can also be assessed by measurements of transdiaphragmatic pressure (pressure difference across the diaphragm) during phrenic nerve stimulation. This measurement requires the placement of small balloon-tipped catheters into the lower esophagus and stomach to assess intrathoracic and intra-abdominal pressure, respectively. Unilateral single shock stimulation results in trans-diaphragmatic pressure of approximately 10 cmH2O (39). Where available, phrenic nerve function can also be assessed by cervical magnetic stimulation of the phrenic nerves (43).
Equipment and Methods of Application
The goal of any DP system is to provide safe and effective activation of the diaphragm, sufficient to provide adequate ventilation to meet metabolic requirements. Optimal design of a respiratory stimulation system would involve a closed-loop system using command signals from the brain to drive the diaphragm. All currently used respiratory devices and those under development, however, are relatively primitive in this regard because they involve open-loop systems. Consequently, inspired volumes and respiratory rates are fixed at specified levels. Ventilatory pattern and absolute level of ventilation therefore are not amenable to sudden changes in metabolic needs or other requirements such as optimal ventilatory pattern for speech and swallowing. In tetraplegia, however, metabolic needs are relatively constant because of their very limited motor function, and patients learn to adapt their speech and swallowing patterns to accommodate their fixed ventilatory pattern.
While there are a variety of phrenic nerve stimulation systems, each has a number of features in common. The basic configuration consists of an implantable receiver-electrode assembly and external power supply and transmitter (Figure 5). The transmitter generates a radio-frequency signal, which is inductively coupled to the implanted receiver using antenna wires, which must be affixed to the skin over the receiver. The receiver demodulates the signal and transmits electrical current to the phrenic nerves. Activation of the phrenic nerve causes diaphragm contraction, a fall in intrathoracic pressure, and consequent inspiratory airflow.
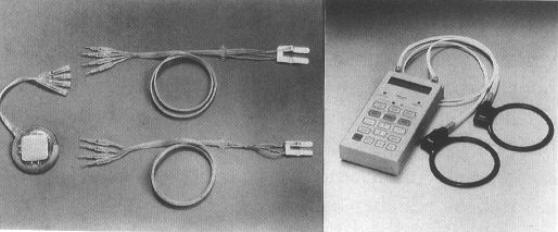
Figure 5. Respiratory pacing system of Atrotech (Tampere, Finland) consisting of two electrode arrays and one implantable stimulator (left) and external controller with two antenna coils (right). Reprinted with permission from Creasey G, Elefteriades J, DiMarco A, et al. Electrical stimulation to restore respiration. J Rehabil Res Dev. 1996;33:123–132 (32).
Stimulus amplitude (mA), stimulus frequency (Hz), and in some instances, pulse width (μs), can be adjusted to modulate the magnitude of tidal volume. Respiratory rate, usually in the range of 8 to 14 breaths/min, can be adjusted by altering train rate. Adjustments in stimulus on-time allow for adjustments in inspiratory time and inspiratory flow rate. Sigh breaths can also be provided.
Available DP Systems. The following is a brief discussion of systems that involve placement of electrodes directly on the phrenic nerves bilaterally (conventional diaphragm pacing systems), newer devices that involve placement of electrodes directly into the diaphragm using less invasive laparoscopic surgery (described in the case report), and combined intercostal and diaphragm stimulation, which can be applied in patients with only unilateral diaphragm function.
Conventional Diaphragm Pacing. —Conventional phrenic nerve pacing generally requires a thoracotomy for electrode placement. Cervical electrode placement, while less invasive surgically, has been used in the past (37,44) but is presently discouraged for several reasons. In the presence of an accessory branch from a lower segment of the cervical spinal cord that joins the main trunk of the phrenic nerve in the lower neck region or thorax, cervical phrenic nerve stimulation may result in incomplete diaphragm activation (12,15). Moreover, other nerves in close vicinity to the phrenic nerve may be activated, resulting in pain or undesirable movement. Finally, neck movement may place significant mechanical stress on the nerve/electrode system increasing the risk of nerve injury.
The second intercostal space is the most commonly used surgical approach for thoracic placement (11,14). Electrodes are often placed on each phrenic nerve during a single procedure. Some centers, however, prefer to place the electrodes in 2 separate procedures. Strict aseptic technique is imperative to prevent infection; prophylactic antibiotics are recommended. It is useful to obtain surveillance cultures before surgery because these patients have chronic tracheostomies and urinary catheters and may be colonized with pathogenic bacteria or fungi.
It should be mentioned that, in a small group of children with central hypoventilation syndrome, phrenic nerve electrodes have also been successfully placed in the thorax using less invasive thoracoscopic surgery (45,46). These patients also developed complications of pneumonia, atelectasis, and pneumothorax postoperatively. This procedure is technically demanding and its potential applicability in patients with SCI is unknown. Thoracoscopic placement of phrenic nerve pacemakers using robotic assistance has also been evaluated in a small group of subjects, 2 of whom had tetraplegia (47). The success of this technique is questionable, however, because neither patient achieved full-time pacing, and one patient was maintained off mechanical ventilation for only a few hours per day.
A potential complication of electrode placement is iatrogenic injury to the phrenic nerve and subsequent pacemaker failure (16,17). It is critical therefore that the phrenic nerves are carefully manipulated to avoid stretching and the development of tension on the nerve during surgery. To prevent ischemic injury, the network of blood vessels within the perineurium must be preserved (16,17).
The electrode wires are connected to a radiofrequency receiver, which is usually positioned superficially over the anterior chest wall. The site of receiver placement should be selected carefully to ensure easy accessibility. Our own preference is placement over the lower anterior rib cage just above the costal margin. This position provides a firm surface on which the receivers can be palpated and external antennas secured in place. The anterior abdominal wall is an alternative position and may be preferable in thin individuals to avoid pressure injury. In systems that require 2 receivers, they should be placed at least 15 cm apart. A more detailed discussion of the surgical procedure can be found in previous reviews (14,34,44).
The pacing system should be tested before closure of the surgical incisions (14). Threshold current values of each electrode should be determined by gradually increasing stimulus amplitude until a diaphragm twitch is observed or palpated. Threshold values should range from 0.1 to 2.0 mA. Suprathreshold current should result in forceful diaphragm contraction. If threshold values are high, the electrode leads may need to be repositioned. With devices that use multiple lead combinations around the nerve, threshold and suprathreshold values should be assessed for each lead combination.
There are 3 commercially available DP systems: (a) Avery Biomedical Devices, (b) Atrotech OY, and (c) Medimplant systems. The Atrotech device is available under a clinical trial in the United States; the Medimplant system is available only in Europe. Each of these systems is designed for lifetime use, and technical support is usually included in the purchase price. Perioperative on-site assistance is also provided. The specific characteristics of each device are reviewed only briefly. Specific characteristics are provided in Table 1. More detailed operational information, design characteristics, and maintenance information can be obtained from the specific manufacturer.
Table 1.
Technical Features of Phrenic Nerve Pacing Systems
Avery Biomedical Devices (Commack, NY).—Based on the technology and expertise developed by Dr. William Glenn and co-investigators, Avery commercialized the first DP system in the 1960s. Since the initial design, this device has undergone significant refinement, especially with receiver technology. The electrode, which contains a partial trough for nerve placement, consists of a semicircular platinum-iridium ribbon embedded in molded silicone rubber. Both monopolar and bipolar electrodes are available (14). The bipolar design is recommended for patients with cardiac pacemakers. The most recently developed Mark IV transmitter (26) allows greater flexibility in terms of stimulus parameters and has a longer battery life compared with previous models. A unique feature of this device is an optional interface that allows biofeedback control from pulse oximetry and CO2 monitoring (26). In addition, trans-telephonic monitoring is available, allowing the electronic output and phrenic nerve-diaphragm neurophysiologic response to be monitored by telephone (48). This system has premarket approval (PMA) from the FDA.
Atrotech OY (Tampere, Finland).—This system differs from the Avery system in the electrode technology. The electrode is made of 2 identical strips of Teflon fabric with 2 platinum buttons mounted onto each strip. This 4-pole arrangement (18,19,48) divides the nerve into 4 stimulation compartments, each of which is designed to activate a quadrant of the phrenic nerve. During a single stimulation sequence, which consists of 4 current combinations, one pole in turn acts as a cathode and one pole on the opposite side as an anode. Consequently, there are 4 excitation compartments around the nerve. Combined stimulation of all quadrants of the nerve, each at 5 to 6 Hz, results in activation of the diaphragm near its optimum fusion frequency of 20 to 25 Hz, resulting in smooth diaphragmatic contraction (18,19,49).
This stimulation pattern is intended to more closely mimic natural activation of the nerve in which all motor units are not synchronously activated. Theoretically, this stimulation pattern should enhance the transformation of muscle fibers into slow-twitch, fatigue-resistant fibers and thereby improve the endurance characteristics of the diaphragm and shorten the conditioning process compared with conventional unipolar stimulation (17–19,49). This system had been available in the United States under an IDE from the FDA. However, as of October 2005, the IDE study has been terminated. Further availability of this device in the United States is unknown.
Medimplant Biotechnisches Labor (Vienna, Austria).—This system is also unique in terms of electrode design (50), involving multiple electrode contacts with the nerve (49,50). A microsurgical technique is required to suture 4 electrode leads to the epineurium of each phrenic nerve. The nerve tissue between each electrode lead provides different stimulation compartments. As many as 16 different electrode combinations can be adjusted individually for each nerve. Only one electrode combination is stimulated during any given inspiration, thereby functioning in similar fashion to the Avery electrode. The various compartments are stimulated in sequence during subsequent inspirations (49). It should be noted that each stimulation is applied at the higher stimulus frequency of 26 Hz compared with the other 2 systems. As with the Atrotech device, only a portion of the nerve is stimulated at any given time, allowing more time for recovery. This form of stimulation, referred to as carousel stimulation, is also thought to reduce the incidence of fatigue compared with the unipolar design (49,50).
Phrenic Nerve Stimulation Through Intramuscular Diaphragm Electrodes.—Recent studies (20,21,51) indicate that phrenic nerve stimulation can also be accomplished with intramuscular electrodes placed directly into the diaphragm, as described in the case report. The advantage of this technique is that phrenic nerve stimulation can be achieved less invasively by laparoscopic surgery rather than a thoracotomy, which is usually required with conventional phrenic nerve pacing. Consequently, this procedure can be performed on an outpatient basis or overnight observational stay, resulting in a significant reduction in costs. In addition, the risk of phrenic nerve injury is eliminated because this procedure does not require manipulation of the nerve. It is important to note, however, that as with conventional phrenic nerve pacing, this technique also requires bilateral intact phrenic nerve function. Therefore, the evaluation procedure for these patients is the same as that for conventional DP.
Using this procedure, 4 laparoscopic ports are required to provide access to the abdominal cavity for visualization, insufflation of the abdominal cavity, diaphragm mapping of the phrenic nerve motor points, and insertion of the electrode implant tool (Figure 1) (20,21,51,52). A mapping procedure (52) is used to determine the precise location of the motor points (region of the phrenic nerve insertion into the diaphragm; Figure 2). Two intramuscular electrodes are implanted into each hemidiaphragm near the phrenic nerve motor points using specially designed surgical tools. In most instances, stimulation of both electrodes in each hemidiaphragm is necessary to achieve optimal inspired volume generation. Because the phrenic nerve is activated through current spread through the muscular diaphragm, stimulus currents in the range of 24 to 25 mA are required. This compares to 1 to 3 mA with direct phrenic nerve pacing. More specific details of the electrode implantation are provided in the case report.
This system is relatively new, with clinical experience of approximately 5 years. This compares with the much longer clinical experience with conventional pacing systems, which in the case of the Avery system is more than 20 years. It is also important to note that with the current intramuscular DP system, there is a small risk of infection because electrode wires exit the skin (35). This DP system is available under an IDE from the FDA. A totally implantable system with radiofrequency coupling similar to conventional pacing devices is under development.
Combined Intercostal and Diaphragm Pacing.—Injury to the phrenic motorneurons in the spinal cord and/or the phrenic rootlets exiting the spinal cord is common in tetraplegia. Because these patients have absent or inadequate phrenic nerve function, they are not candidates for DP. Previous studies (53–56) have shown that electrical stimulation applied to the upper thoracic ventral roots results in activation of the inspiratory intercostal muscles and large inspired volumes. This is accomplished by electrodes positioned on the ventral surface of the upper thoracic spinal cord through a hemilaminectomy. Moreover, ventilation achieved by intercostal muscle stimulation by this technique results in gas exchange comparable with that achieved with DP (56).
In clinical trials (55), an electrode was placed on the ventral surface of the spinal cord at the T2–T3 level through a cervical hemilaminectomy in individuals with ventilator-dependent tetraplegia with absent phrenic nerve function. Activation of the intercostal muscles alone in ventilator-dependent tetraplegia resulted in inspired volumes that approximated volumes achieved with unilateral diaphragm stimulation. Intercostal muscle stimulation by this technique is associated with mild flexion of both hands and contraction of the muscles of the upper torso, which was well tolerated. Ventilation achieved by this technique, however, was not sufficient to maintain long-term ventilatory support.
Subsequent clinical (57–59) trials were performed in individuals with ventilator-dependent tetraplegia who have a single functional phrenic nerve and therefore are not candidates for DP. In this study, combined intercostal and unilateral phrenic nerve stimulation was successful in maintaining long-term ventilatory support. Subjects could be comfortably maintained off mechanical ventilation from 16 to 24 h/d. As with DP, each patient was aware of improvements in mobility, sense of smell, speech quality, and overall sense of well being. The equipment for this technique is under development and therefore is not commercially available. It has received approval by the FDA through an IDE.
Initiation of Pacing
After surgical implantation, a period of approximately 2 weeks (14) should elapse before initiation of pacing. This time is necessary for resolution of edema and inflammation at the nerve/electrode interface and healing of all wounds. Because most patients have been maintained on mechanical ventilation for significant time periods, the transition to DP requires gradual conditioning because of diaphragmatic atrophy (3,16,27). If the initial pacing period is too prolonged, diaphragmatic fatigue and secondary respiratory failure will ensue.
While on mechanical ventilation, many patients are maintained with large tidal volumes, resulting in chronic hyperventilation and secondary reduction in bicarbonate stores. When switched to the pacing system, which is designed to maintain normal Pco2 levels, acidosis may develop secondary to the rise in Pco2. As a result, the patient may experience the sensation of dyspnea despite eucapnia. It is advisable therefore to make gradual ventilator adjustments to restore Pco2 values to near normal levels before initiation of pacing.
During initial pacing sessions, stimulus threshold values (minimum stimulus amplitude that results in visible or palpable diaphragm contraction) and supra-maximal amplitudes and frequencies (lowest stimulus parameters that result in maximum inspired volume production) should be determined. Threshold values are useful to follow, because they provide an index of the integrity of the system. Significant increases in threshold values would indicate poor electrode contact or edema, inflammation, or fibrosis at the nerve/electrode interface. The magnitude of inspired volume during maximum stimulation is also a useful parameter to follow throughout the conditioning phase, because it gradually increases and then plateaus when optimal conditioning has been achieved. With the Atrotech and Medimplant devices, threshold and maximum stimulus parameters should be determined for each lead combination. With the intramuscular device, parameters should be obtained for each electrode lead alone and in combination with the second electrode within the same hemidiaphragm. Minimal or no response indicates that the electrode is not positioned close enough to the phrenic nerve.
Previous studies have shown that chronic low-frequency stimulation results in the transformation of a muscle with a mixed fiber type or predominantly fast fiber type population to one with predominantly slow twitch muscle fibers (60). Compared with fast twitch muscles, slow twitch muscles are characterized by higher capillary density and mitochondrial volume. Slow twitch muscles are highly oxidative and fatigue resistant, characteristics that seem optimal for the diaphragm, a muscle that is expected to contract 10 to 14 times/min for life. High-frequency stimulation should be avoided because there is evidence that this stimulus paradigm may be associated with myopathic-like ultrastructural changes within the diaphragm (61). Chronic DP, therefore, should be undertaken with low-frequency stimulation.
Measurements of inspired volumes should be made in the supine and sitting postures. Because of the higher lung volume and shorter diaphragm length in the sitting posture, diaphragm excursions and consequently inspired volumes will be reduced. However, this effect is alleviated to a large extent by use of a snug-fitting abdominal binder.
Initial parameters for chronic DP should involve use of supramaximal stimulus amplitudes; tidal volume is achieved by changing stimulus frequency. With respiratory rates between 8 and 14 breaths/min, stimulus frequency is adjusted to achieve inspired volumes resulting in Pco2 values in the low normal range. Stimulus frequency should be set at the lowest level possible. With the exception of the Medimplant device, stimulus frequency should not exceed 20 Hz. Tidal volume and respiratory rate are further adjusted to optimize patient comfort and speech.
The transition from mechanical ventilation to chronic full-time DP must be individualized for each patient. Full-time DP should be achieved as quickly as possible but without the development of significant diaphragm fatigue. Several approaches can be applied to achieve this goal. According to an experimental protocol, the patient in the case report was paced initially for a short period (5–10 minutes) each hour for the first week; this time was gradually increased each week, as tolerated, until full-time pacing throughout the day was achieved. After full-time pacing was achieved throughout the day, pacing was extended to full-time. Another approach that may result in more rapid transition to full-time DP, is the initial use of the above mentioned stimulus parameters, until significant blood gas alterations (reductions in oxygen saturation or elevations in end-tidal Pco2), significant reductions in inspired volume (monitored every 5–10 minutes), or patient discomfort are observed. Having determined the duration that initial DP can be comfortably maintained, pacing is initiated for a somewhat shorter period (~5 minutes) every hour during the day for the first week. This assessment is repeated weekly, and a new pacing schedule is applied accordingly.
During sleep, the tracheostomy should be capped with a valve such as the Passy-Muir device, which allows airflow through the tracheostomy, or left open. This is needed to prevent upper airway obstruction. The tracheostomy tube can be capped while the patient is awake.
Monitoring of the Pacing System
Pacemaker function should be monitored on a routine basis and emergently in situations in which patients complain of difficulty breathing. Tidal volume can be easily measured by attachment of a spirometer to the tracheostomy tube. Because stimulus transmitters allow separate stimulation, each hemidiaphragm can be evaluated independently. Pulse oximetry and end-tidal Pco2 measurements, if available, should also be performed to assess the adequacy of ventilation.
It is important to note that reductions in inspired volume can occur despite a normal functioning pacemaker system. Retained secretions, for example, can cause an increase in airway resistance and the development of atelectasis with secondary reductions in lung compliance. These mechanical derangements will reduce inspired volume generation. Fortunately, removal of airway secretions results in prompt improvement in volume generation.
If pacemaker failure is suspected and inspired volume generation is significantly reduced or absent on either side, the function of the external components should be evaluated in sequence (62,63). First, the batteries should be replaced because this is the most common cause of pacemaker malfunction. If function is not restored, the antenna contacts with the skin should be checked. If these are secure, the antenna should be replaced. If these measures are not successful, the transmitter should be replaced with the back-up unit (62,63).
If evaluation of the external components does not resolve the problem, the integrity of the internal components should be assessed (64). This evaluation can be performed by placing surface electrodes at the costal margin to record the pacemaker stimulus pulse and diaphragm action potential. The signals should be amplified and recorded on an oscilloscope. These signals are shown schematically in Figure 6. If the DP system is functioning properly, the radio-frequency signal from the transmitting antennae, stimulus pulse from the phrenic nerve and electrode, and diaphragm action potential are seen on the oscilloscope. If only the radio-frequency signal and stimulus pulse are seen but not the action potential, this indicates that the wire insulation is not intact, the phrenic nerve is not in contact with the electrode, or the phrenic nerve has been damaged. If neither the stimulus pulse nor action potential is seen, the receiver is not functional and will probably need replacement.
Figure 6. (A) Schematic illustration of an oscilloscopic tracing showing a properly functioning diaphragm pacing system. The bottom trace (T) represents the output from an extra receiver that was used to trigger the oscilloscope sweep. The top trace (S) represents the signal obtained from the percutaneous leads on the chest. Shown in sequence are the radiofrequency signal (RF) from the transmitting antenna, the stimulus pulse (SP) from the electrode on the phrenic nerve, and the compound action potential (AP) from the diaphragm. (B) Schematic of a malfunctioning pacing system. The tracing is the same except for the absence of the action potential. Breakage of wire insulation, lack of adequate phrenic nerve/electrode contact, and phrenic nerve damage are consistent with this finding. (C) Schematic of another type of malfunctioning pacing system. In this case, the action potential and radiofrequency signal are absent. This finding suggests receiver malfunction. Reprinted with permission from Weese-Mayer DE, Morrow AS, Brouillette RT, Ilbawi MN, Hunt CE. Diaphragm pacing in infants and children. Am Rev Respir Dis. 1989;139:974–979 (64).
Complications/Side Effects
Over the past several decades, DP has evolved into a safe and effective modality because of technological developments and increased clinical experience. With appropriate patient selection, proper use of stimulus parameters, regular equipment maintenance, and appropriate patient monitoring, the incidence of complications is low. While pacemaker failure is infrequent, mechanical ventilation or other means of ventilatory support such as manual Ambu bagging should be immediately available.
Surgical Complications. The phrenic nerve is quite fragile and easily damaged. Iatrogenic injury to the nerve can occur secondary to direct mechanical trauma or compromise of nerve blood supply (16,17). Therefore, precautions must be taken in handling the nerve and potentially injurious methods such as use of cautery for minor bleeding should be avoided. Late injury can also occur because of the subsequent development of tissue reaction and fibrosis around the electrode (16,17,19). In some instances, the development of fibrosis around the nerve interferes only with electrode contact and is amenable to surgical intervention. Nerve injury was quite common with previous use of the older bipolar cuff electrodes (9,17,19), which encircled the nerve. The incidence of nerve injury was reduced significantly by the introduction of the monopolar electrodes by Avery (17,19,26). The incidence of nerve injury with the Atrotech and Medimplant electrodes is unknown, but thought to be quite low.
All surgical procedures, particularly those involving implantation of a foreign body, carry some risk of infection. Previous reports (16,17,19) have indicated infection rates of approximately 3%, although with modern surgical technique, this rate may be significantly lower. Infection is a very serious complication because this occurrence usually dictates removal of all implanted components. With placement of intramuscular diaphragm electrodes, there is a small risk of pneumothorax. This risk is minimal, however, with an experienced surgical team.
Pacemaker Failure. There are several technical problems that can result in reductions in volume production. Battery function should be checked first as this is the most common cause of mechanical failure. With each of the available systems, regular battery changes are required and adherence to these maintenance schedules prevents battery-related issues. Low battery alarms are also present on most systems, as well. External antenna wires are subject to breakage at stress points either near the connection to the transmitter or, more commonly, near their attachment to the chest wall. The life of antenna wires is highly dependent on individual usage factors.
Failure of the radio-frequency receiver was a fairly common problem with older systems because of leakage of body fluids through the epoxy encapsulation (9,16,17,24,33,38). Wire breakage within the receiver can also occur (24,38). Failure of the receiver often occurred within 5 years with older systems (16,17,24,38). With modern day systems, receiver life has been extended to a 10-year life expectancy.
The durability of the Atrotech quadripolar system was analyzed in 64 patients who had undergone DP for a mean period of 2 years (19). The incidence of electrode and receiver failure was 3.1% and 5.9%, respectively. Failure of 1 or more of the 4 electrode combinations was more common but usually did not interfere with successful pacing.
Upper Airway Obstruction. The state of wakefulness is associated with automatic synchronization of upper airway muscle activation with paced diaphragm activation. Sleep, however, is often associated with a reduction in upper muscle activation and asynchrony between upper airway muscle and diaphragm contraction. During sleep, therefore, there is propensity for the negative inspiratory pressures generated by diaphragm contraction to collapse the upper airway, resulting in a form of obstructive sleep apnea (19,24,38). For most patients, a patent tracheostomy is necessary during sleep. Maintenance of a tracheostomy also allows for ease of suctioning and application of mechanical ventilation on an emergency basis.
Exposure to Magnetic and Radiofrequency Fields. Strong magnetic fields, as occurs with magnetic resonance imaging testing, can override the electronic circuitry of pacing systems. Energy transmission associated with such testing could be transmitted to the electrode causing nerve injury. In addition, displacement of the internal components with secondary malfunction could also occur. Exposure to electrotherapeutic devices, which generate strong radio-frequency fields, may also interfere with pacemaker function and should be avoided.
Long-Term Patient Outcome
L.N. has successfully maintained continuous DP for more than 2 years. His only respiratory concerns relate to the requirement of intermittent daily suctioning for management of airway secretions. There are numerous other reports of successful long-term DP in individual patients for periods of more than 10 years (33,62,63). Previous studies (2,16,17,19,27) of large patient groups, however, describe significant numbers of patients in whom successful ventilatory support could not be achieved. In one of the largest series of patients implanted before 1985 (27), for example, DP was considered successful in only approximately 50% of individuals. These studies, however, are not reflective of modern day experience with DP because they were performed at a time when the technology of DP and patient selection methods were not well defined.
There are very few recent studies analyzing modern day success rates and incidence of side effects. In one more recent study (65), however, 14 people with tetraplegia were followed systematically for as long as 15 years, with a mean use of 7.6 years. With chronic bilateral low-frequency stimulation, threshold and amplitude values required for maximum excursion of the diaphragm and tidal volume generation were unchanged for the duration of follow-up. Moreover, there was no evidence of nerve or diaphragm injury based on analyses of available pathologic specimens.
Future Directions
The ideal DP system would provide complete restoration of normal inspiratory muscle function. In this regard, current systems have significant limitations. As mentioned above, all current systems are of the open-loop design. Therefore, electrical signals, which activate the diaphragm, occur independent of the spontaneous generation of electrical signals from the central nervous system. Consequently, upper airway muscle activation can occur independent of diaphragm activation, placing patients at risk for upper airway obstruction during sleep (19,24,38). In addition, ventilation cannot change in response to changes in metabolic demand. One possible solution would be the use of an upper airway muscle signal to trigger diaphragm activation. This type of device would eliminate the need for a tracheostomy and provide a mechanism for ventilatory adjustment to speech and changes in ventilatory requirements
For several reasons, DP is currently not undertaken in tetraplegia for at least 12 months after injury (17,34). First, there are reports of late recovery of phrenic nerve function (66). In addition, the procedure is expensive and placement of the electrodes on the phrenic nerve carries the risk of nerve injury and is not reversible. As a consequence, a reconditioning phase of several weeks or longer is required to achieve full-time pacing (3,16,27,51). Implantation of electrodes, which can be placed less invasively, reversibly, and at lower cost, such as intramuscular electrodes through laparoscopic surgery (20, 21), could provide the option of DP rather than mechanical ventilation in acute tetraplegia. In patients in whom there is sufficient improvement in phrenic nerve function to maintain spontaneous ventilation, the electrodes can be subsequently removed. This approach would also significantly reduce or eliminate the need for reconditioning. A clinical trial comparing clinical outcome measures of DP vs mechanical ventilation in acute tetraplegia would be extremely useful in this regard.
Development of a totally implantable system similar to cardiac pacemakers would eliminate the need for attachment of materials to the body surface and connection to an external transmitter. This would further improve patient convenience.
During combined intercostal and unilateral diaphragm contraction, the paralyzed hemidiaphragm most likely moves rostrally and restricts lung expansion. Recent animal studies (67) also suggest that inspired volume by this technique can be further enhanced by plication of the paralyzed hemidiaphragm. Future clinical trials of combined intercostal and unilateral diaphragm pacing therefore may prove more successful with plication of the noncontracting diaphragm.
Finally, many patients have suffered significant injury to both phrenic nerves and therefore cannot be offered DP. Further development of intercostal to phrenic nerve transfer may restore phrenic nerve viability (68), allowing these patients the possibility of DP, as well.
Use of electrical stimulation to activate the expiratory muscles to restore a cough mechanism in patients with SCI is being studied. If this method proves effective, it is possible that this system could be synchronized with DP, because stimulated cough is brief, lasting less than 1 second, and applied intermittently.
Acknowledgments
The authors thank Dana Hromyak for invaluable assistance in the preparation of this review.
Footnotes
This study was supported by the Food and Drug Administration (FD-R-001839), the National Center for Research Resources (M01 RR-00080), and the Department of Veterans Affairs.
REFERENCES
- National Spinal Cord Injury Statistical Center. Annual Statistical Report. Birmingham, AL: University of Alabama at Birmingham; 2005. [Google Scholar]
- Carter RE, Donovan WH, Halstead L, Wilkerson MA. Comparative study of electrophrenic nerve stimulation and mechanical ventilatory support in traumatic spinal cord injury. Paraplegia. 1987;25:86–91. doi: 10.1038/sc.1987.16. [DOI] [PubMed] [Google Scholar]
- Schechter DC. Application of electrotherapy to noncardiac thoracic disorders. Bull N Y Acad Med. 1970;46:932–951. [PMC free article] [PubMed] [Google Scholar]
- Sarnoff SJ, Harenbergh E, Whittenberger JL. Electrophrenic respiration. Am J Physiol. 1948;155:1–9. doi: 10.1152/ajplegacy.1948.155.1.1. [DOI] [PubMed] [Google Scholar]
- Whittenberger JL, Sarnoff SJ, Hardenberg E. Electrophrenic respiration II. Its use in man. J Clin Invest. 1949;28:124–128. [PubMed] [Google Scholar]
- Glenn WWL, Hageman JH, Mauro A, Eisenberg L, Flanigan S, Harvard M. Electrical stimulation of excitable tissue by radiofrequency transmission. Ann Surg. 1964;160:338–350. [PMC free article] [PubMed] [Google Scholar]
- Van Heeckeren DW, Glenn WWL. Electrophrenic respiration by radiofrequency induction. J Thorac Cardiovasc Surg. 1966;52:655–665. [PubMed] [Google Scholar]
- Judson JP, Glenn WWL. Radiofrequency electrophrenic respiration: long-term application to a patient with primary hypoventilation. JAMA. 1968;203:1033–1037. doi: 10.1001/jama.203.12.1033. [DOI] [PubMed] [Google Scholar]
- Glenn WWL, Holcomb WG, Hogan JF, et al. Diaphragm pacing by radiofrequency transmission in the treatment of chronic ventilatory insufficiency: present status. J Thorac Cardiovasc Surg. 1973;66:505–520. [PubMed] [Google Scholar]
- Shaw RK, Glenn WWL, Hogan JF, Phelps ML. Electrophysiological evaluation of phrenic nerve function in candidates for diaphragm pacing. J Neurosurg. 1980;53:345–354. doi: 10.3171/jns.1980.53.3.0345. [DOI] [PubMed] [Google Scholar]
- Glenn WWL, Hogan JF, Phelps ML. Ventilatory support of the quadriplegic patient with respiratory paralysis by diaphragm pacing. Surg Clin North Am. 1980;60:1055–1078. doi: 10.1016/s0039-6109(16)42233-4. [DOI] [PubMed] [Google Scholar]
- Oda T, Glenn WWL, Fukuda Y, Hogan JF, Gorfien J. Evaluation of electrical parameters for diaphragm pacing: an experimental study. J Surg Res. 1981;30:142–153. doi: 10.1016/0022-4804(81)90006-8. [DOI] [PubMed] [Google Scholar]
- Glenn WWL, Hogan JF, Loke JSO, Ciesielski TE, Phelps ML, Rowedder R. Ventilatory support by pacing of the conditioned diaphragm in quadriplegia. N Engl J Med. 1984;310:1150–1155. doi: 10.1056/NEJM198405033101804. [DOI] [PubMed] [Google Scholar]
- Glenn WWL, Phelps ML. Diaphragm pacing by electrical stimulation of the phrenic nerve. Neurosurgery. 1985;17:974–984. doi: 10.1227/00006123-198512000-00021. [DOI] [PubMed] [Google Scholar]
- Glenn WWL, Sairenji H. Diaphragm pacing in the treatment of chronic ventilatory insufficiency. In: Roussos C, Macklem PT, editors. The Thorax: Lung Biology in Health and Disease. Vol 29. New York, NY: Marcel Dekker; 1985. pp. 1407–1440. [Google Scholar]
- Glenn WWL, Phelps ML, Elefteriades JA, Dentz B, Hogan JF. Twenty years experience in phrenic nerve stimulation to pace the diaphragm. Pacing Clin Electrophysiol. 1986;9:780–784. doi: 10.1111/j.1540-8159.1986.tb06627.x. [DOI] [PubMed] [Google Scholar]
- Glenn WWL, Brouillette RT, Dentz B, et al. Fundamental considerations in pacing of the diaphragm for chronic ventilatory insufficiency: a multi-center study. Pacing Clin Electrophysiol. 1988;11:2121–2127. doi: 10.1111/j.1540-8159.1988.tb06360.x. [DOI] [PubMed] [Google Scholar]
- Baer GA, Talonen PP, Shneerson JM, Markkula H, Exner G, Wells FC. Phrenic nerve stimulation for central ventilatory failure with bipolar and four-pole electrode systems. Pacing Clin Electrophysiol. 1990;19:1061–1072. doi: 10.1111/j.1540-8159.1990.tb02153.x. [DOI] [PubMed] [Google Scholar]
- Weese-Mayer DE, Silvestri JM, Kenny AS, et al. Diaphragm pacing with quadripolar phrenic nerve electrode: an international study. Pacing Clin Electrophysiol. 1996;19:1311–1319. doi: 10.1111/j.1540-8159.1996.tb04209.x. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Onders RP, Kowalski KE, Miller ME, Ferek S, Mortimer JT. Phrenic nerve pacing in a tetraplegic patient via intramuscular diaphragm electrodes. Am J Respir Crit Care Med. 2002;166:1604–1606. doi: 10.1164/rccm.200203-175CR. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Onders RP, Ignagni A, Kowalski KE, Mortimer JT. Phrenic nerve pacing via intramuscular diaphragm electrodes in tetraplegic subjects. Chest. 2005;127:671–678. doi: 10.1378/chest.127.2.671. [DOI] [PubMed] [Google Scholar]
- Nochomovitz ML, Hopkins M, Brodkey J, Montenegro H, Mortimer JT, Cherniack NS. Conditioning of the diaphragm with phrenic nerve stimulation after prolonged disuse. Am Rev Respir Dis. 1984;130:685–688. doi: 10.1164/arrd.1984.130.4.685. [DOI] [PubMed] [Google Scholar]
- Hunt CE, Brouillette RT, Weese-Mayer DE, Morrow A, Ilbawi MN. Diaphragm pacing in infants and children. Pacing Clin Electrophysiol. 1988;11:2135–2141. doi: 10.1111/j.1540-8159.1988.tb06362.x. [DOI] [PubMed] [Google Scholar]
- Ilbawi MN, Idriss FS, Hunt CE, Brouillette RT, DeLeon SY. Diaphragmatic pacing in infants: techniques and results. Ann Thorac Surg. 1985;40:323–329. doi: 10.1016/s0003-4975(10)60061-6. [DOI] [PubMed] [Google Scholar]
- Dobelle WH, D'Angelo MS, Goetz BF. 200 cases with a new breathing pacemaker dispel myths about diaphragm pacing. ASAIO J. 1994;40:244–252. doi: 10.1097/00002480-199407000-00003. [DOI] [PubMed] [Google Scholar]
- Elefteriades JA, Quin JA, Hogan JF, et al. Long-term follow-up of pacing of the conditioned diaphragm in quadriplegia. Pacing Clin Electrophysiol. 2002;25:897–906. doi: 10.1046/j.1460-9592.2002.00897.x. [DOI] [PubMed] [Google Scholar]
- Tibballs J. Diaphragmatic pacing: an alternative to long-term mechanical ventilation. Anaesth Intensive Care. 1991;19:597–601. doi: 10.1177/0310057X9101900424. [DOI] [PubMed] [Google Scholar]
- Hackler RH. A 25-year prospective mortality study in the spinal cord injured patient: comparison with the long-term living paraplegic. J Urol. 1977;117:486–488. doi: 10.1016/s0022-5347(17)58506-7. [DOI] [PubMed] [Google Scholar]
- Chen CF, Lien IN. Spinal cord injuries in Taipei, Tiawan, 1978–1981. Paraplegia. 1985;23:364–370. doi: 10.1038/sc.1985.58. [DOI] [PubMed] [Google Scholar]
- Whiteneck GG, Charlifue SW, Frankel HL, et al. Mortality, morbidity, and psychosocial outcomes of persons spinal cord inured more than 20 years ago. Paraplegia. 1992;30:617–630. doi: 10.1038/sc.1992.124. [DOI] [PubMed] [Google Scholar]
- Moxham J, Shneerson JM. Diaphragmatic pacing. Am Rev Respir Dis. 1993;148:533–536. doi: 10.1164/ajrccm/148.2.533. [DOI] [PubMed] [Google Scholar]
- Creasey G, Elefteriades J, DiMarco A, et al. Electrical stimulation to restore respiration. J Rehabil Res Dev. 1996;33:123–132. [PubMed] [Google Scholar]
- Elefteriades JA, Quin JA. Diaphragm pacing. Chest Surg Clin North Am. 1998;8:331–357. [PubMed] [Google Scholar]
- DiMarco AF. Diaphragm pacing in patients with spinal cord injury. Top Spinal Cord Inj Rehabil. 1999;5:6–20. [Google Scholar]
- DiMarco AF. Neural prostheses in the respiratory system. J Rehabil Res Dev. 2001;38:601–607. [PubMed] [Google Scholar]
- Weese-Mayer DE, Hunt CE, Brouillette RT, Silvestri JM. Diaphragm pacing in infants and children. J Pediatr. 1992;120:1–8. doi: 10.1016/s0022-3476(05)80588-8. [DOI] [PubMed] [Google Scholar]
- Vanderlinden RG, Epstein SW, Hyland RH, Smythe HS, Vanderlinden LD. Management of chronic ventilatory insufficiency with electrical diaphragm pacing. Can J Neurol Sci. 1988;15:63–67. doi: 10.1017/s0317167100027219. [DOI] [PubMed] [Google Scholar]
- McLean IC, Mattoni TA. Phrenic nerve conduction studies: a new technique and its application in quadriplegic patients. Arch Phys Med Rehabil. 1981;62:70–73. [PubMed] [Google Scholar]
- McKenzie DK, Gandevia SC. Phrenic nerve conduction times and twitch pressures of the human diaphragm. J Appl Physiol. 1985;58:1496–1504. doi: 10.1152/jappl.1985.58.5.1496. [DOI] [PubMed] [Google Scholar]
- Moosa A. Phrenic nerve conduction in children. Dev Med Child Neurol. 1981;23:434–448. doi: 10.1111/j.1469-8749.1981.tb02016.x. [DOI] [PubMed] [Google Scholar]
- Brouillette RT, Ilbawi MN, Hunt CE. Phrenic nerve pacing in infants and children: a review of experience and report on the usefulness of phrenic nerve stimulation studies. J Pediatr. 1983;102:32–39. doi: 10.1016/s0022-3476(83)80282-0. [DOI] [PubMed] [Google Scholar]
- DiMarco AF. Respiratory muscle stimulation in patients with spinal cord injury. In: Horch KW, Dhillon GS, editors. Neuroprosthetics: Theory and Practice. Hackensack, NJ: Word Scientific; 2004. pp. 951–978. [Google Scholar]
- Similowski T, Straus C, Attali V, Duguet A, Jourdain B, Derenne J. Assessment of the motor pathway to the diaphragm using cortical and cervical magnetic stimulation in the decision-making progress of phrenic pacing. Chest. 1996;110:1551–1557. doi: 10.1378/chest.110.6.1551. [DOI] [PubMed] [Google Scholar]
- Glenn WWL, Holcomb WG, Gee JBL, Rath R. Central hypoventilation; long-term ventilatory assistance by radio-frequency electrophrenic respiration. Ann Surg. 1970;172:755–773. doi: 10.1097/00000658-197010000-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaul DB, Danielson PD, McComb JG, Keens TG. Thoracoscopic placement of phrenic nerve electrodes for diaphragmatic pacing in children. J Pediatr Surg. 2002;37:974–978. doi: 10.1053/jpsu.2002.33821. [DOI] [PubMed] [Google Scholar]
- Shoji T, Oku Y, Ishikawa S, Wada H. Thoracoscopic electrode implantation for diaphragm pacing in dogs. Respiration. 2002;69:69–74. doi: 10.1159/000049373. [DOI] [PubMed] [Google Scholar]
- Morgan JA, Morales DL, John R, et al. Endoscopic, robotically assisted implantation of phrenic pacemakers. J Thorac Cardiovasc Surg. 2003;126:582–583. doi: 10.1016/s0022-5223(03)00721-9. [DOI] [PubMed] [Google Scholar]
- Auerbach AA, Dobelle WH. Transtelephonic monitoring of patients with implanted neurostimulators. Lancet. 1987;1:224–225. doi: 10.1016/s0140-6736(87)90046-8. [DOI] [PubMed] [Google Scholar]
- Mayr W, Bijak M, Girsh W, et al. Multichannel stimulation of phrenic nerves by epineural electrodes. Clinical experience and future developments. ASAIO J. 1993;39:M729–M735. [PubMed] [Google Scholar]
- Thoma H, Gerner H, Holle J, et al. Substitution of paralyzed functions in tetraplegia. Trans Am Soc Artif Intern Organs. 1987;33:472–479. [PubMed] [Google Scholar]
- Nochomovitz ML, DiMarco AF, Mortimer JT, Cherniack NS. Diaphragm activation with intramuscular stimulation in dogs. Am Rev Respir Dis. 1983;127:325–329. doi: 10.1164/arrd.1983.127.3.325. [DOI] [PubMed] [Google Scholar]
- Onders RP, DiMarco AF, Ignagni AR, Aiyar H, Mortimer JT. Mapping the phrenic nerve motor point: the key to a successful laparoscopic diaphragm pacing system in the first human series. Surgery. 2004;136:819–826. doi: 10.1016/j.surg.2004.06.030. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Altose MD, Cropp A, Durand D. Activation of the inspiratory intercostal muscles by electrical stimulation of the spinal cord. Am Rev Respir Dis. 1987;136:1385–1390. doi: 10.1164/ajrccm/136.6.1385. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Budzinska K, Supinski GS. Artificial ventilation by means of electrical activation of the intercostal/accessory muscles alone in anesthetized dogs. Am Rev Respir Dis. 1989;139:961–967. doi: 10.1164/ajrccm/139.4.961. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Supinski GS, Petro JA, Takaoka Y. Evaluation of intercostal pacing to provide artificial ventilation in quadriplegics. Am J Respir Crit Care Med. 1994;150:934–940. doi: 10.1164/ajrccm.150.4.7921466. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Connors AF, Kowalaski KE. Gas exchange during separate diaphragm and intercostal muscle breathing. J Appl Physiol. 2004;96:2120–2124. doi: 10.1152/japplphysiol.00628.2003. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Supinski GS, Petro J, Takaoka Y. Artificial respiration via combined intercostal and diaphragm pacing in a quadriplegic patient [abstract] Am Rev Respir Dis. 1994;149:A135. [Google Scholar]
- DiMarco AF, Kowalski KE, Petro J, et al. Evaluation of intercostal and diaphragm pacing to provide ventilatory support in tetraplegic patients. 2001. ATS International Conference, San Francisco, CA, May 18–23.
- DiMarco AF, Takaoka Y, Kowalski KE. Combined intercostal and diaphragm pacing to provide artificial ventilation in patients with tetraplegia. Arch Phys Med Rehabil. 2005;86:1200–1207. doi: 10.1016/j.apmr.2004.11.027. [DOI] [PubMed] [Google Scholar]
- Salmons S, Henriksson J. The adaptive response of skeletal muscle to increased use. Muscle Nerve. 1981;4:94–105. doi: 10.1002/mus.880040204. [DOI] [PubMed] [Google Scholar]
- Ciesielski TE, Fukuda Y, Glenn WWL, Gorfien J, Jeffery K, Hogan JF. Response of the diaphragm muscle to electrical stimulation of the phrenic nerve: a histochemical and ultrastructural study. J Neurosurg. 1983;58:92–100. doi: 10.3171/jns.1983.58.1.0092. [DOI] [PubMed] [Google Scholar]
- Fodstad H. The Swedish experience in phrenic nerve stimulation. Pacing Clin Electrophysiol. 1987;10:246–251. doi: 10.1111/j.1540-8159.1987.tb05957.x. [DOI] [PubMed] [Google Scholar]
- McMichan JC, Piepgras DG, Gracey DR, Marsh HM, Sittipong R. Electrophrenic respiration. Mayo Clin Proc. 1979;54:662–668. [PubMed] [Google Scholar]
- Weese-Mayer DE, Morrow AS, Brouillette RT, Ilbawi MN, Hunt CE. Diaphragm pacing in infants and children. A life-table analysis of implanted components. Am Rev Respir Dis. 1989;139:974–979. doi: 10.1164/ajrccm/139.4.974. [DOI] [PubMed] [Google Scholar]
- Elefteriades JA, Hogan JF, Handler A, Loke JS. Long-term follow-up of bilateral pacing of the diaphragm in quadriplegia. N Engl J Med. 1992;21:1433–1444. doi: 10.1056/NEJM199205213262113. [DOI] [PubMed] [Google Scholar]
- Oo T, Watt JWH, Soni BM, Sett PK. Delayed diaphragm recovery in 12 patients after high cervical spinal cord injury. A retrospective review of the diaphragm status of 107 patients ventilated after acute spinal cord injury. Spinal Cord. 1999;37:117–122. doi: 10.1038/sj.sc.3100775. [DOI] [PubMed] [Google Scholar]
- Kowalski KE, Cernanec KJ, Romaniuk JR, DiMarco AF. Effects of diaphragm plication during intercostal and unilateral diaphragm stimulation in dogs. 2005. ATS International Conference, San Diego, CA, May 20–25.
- Krieger LM, Krieger AJ. The intercostal to phrenic nerve transfer: an effective means of reanimating the diaphragm in patients with high cervical spine injury. Plast Reconstr Surg. 2000;105:1255–1261. doi: 10.1097/00006534-200004040-00001. [DOI] [PubMed] [Google Scholar]