Abstract
Background:
Patients with spinal cord injury are at risk for knee effusion, most likely as a result of repetitive microtrauma. Patients with paralysis are susceptible to effusions of the hip similar to those seen in documented cases regarding the knee. The etiology is likely similar and is related to repetitive microtrauma, such as that experienced when aggressive range of motion exercises are applied.
Design:
Case report.
Setting:
Acute rehabilitation department of a spinal cord injury center.
Findings:
A 19-year-old man with a complete cervical spinal cord injury presented to acute rehabilitation on postinjury day 25 with a C6 American Spinal Injury Association classification A injury, complete. He was found to have bilateral hip effusions. Joint aspiration yielded a right sterile hydroarthrosis and a left sterile hemarthrosis. During his rehabilitation stay, the patient developed one mildly elevated alkaline phosphatase level, but he showed no radiographic evidence of heterotopic ossification and maintained full passive range of motion of the hips.
Conclusion:
This case indicates that hip effusion may be a similar, less-common occurrence than knee effusion in patients with spinal cord injury. In this case, bilateral aseptic hip effusion was not associated with heterotopic ossification. More research is needed to determine the etiology and sequelae of this condition.
Keywords: Spinal cord injuries, Rehabilitation, Hip joint, Effusion, Physical therapy, Tetraplegia, Hemarthrosis, Hydrarthrosis, Heterotopic ossification
INTRODUCTION
Spinal cord injury (SCI) results in a multitude of physiologic changes and pathologic sequelae. Described in this report is the finding of hemarthrosis and hydroarthrosis in the hip joints of a patient with a complete cervical SCI. While effusions of the knee have been frequently reported in patients with SCI, effusions of the hip have not. This case indicates that hip joints may be subject to factors similar to those associated with effusions of the knee. Comparison is drawn to similar reported knee joint findings in acute SCI (1–4).
History and Physical Examination
J.P. was a 19-year-old white man who was an unrestrained driver involved in a motor vehicle collision. Computerized tomography (CT) imaging on presentation revealed right C6-C7 and left C7-T1 facet joint dislocation, with anterolisthesis of C6 on C7 and acute bleeding around the spinal cord. Also noted was fracture of C7. Magnetic resonance imaging (MRI) evaluation on day of admission confirmed the prior CT findings and demonstrated abnormal signal and blood in the cord from C5 to T1-T2, with ventral epidural hemorrhage from the dens to C6-C7 and soft tissue swelling in the prevertebral areas and posterior cervical areas. The patient was also found to have bilateral subdural hematomas, right temporal contusion, right pneumothorax, right humeral fracture, and fracture of the right fourth metacarpal head. Radiographic examination of the pelvis on the day of injury showed no fracture or dislocation. CT of the pelvis revealed no hip pathology. On postinjury day 2, the patient underwent open reduction of the facet dislocation and instrumented posterior arthrodesis of the C5-T1 vertebrae with right iliac crest bone graft. Postoperatively he was placed in a halo vest.
J.P.'s acute-care stay was complicated by ventilator-dependent respiratory failure due to pulmonary effusions and pneumonia, Clostridium difficile colitis, sinusitis, and stage IV sacral pressure ulceration. He also developed a pulmonary embolus, for which a vena cava filter was placed on postinjury day 4. He had a percutaneous endoscopic gastrostomy (PEG) placed, and a tracheotomy was performed on postinjury day 6.
Previous medical history was significant only for depression. The patient had no known drug allergies. He received Lovenox 40 mg subcutaneously once a day for deep vein thrombosis prophylaxis. J.P. was living with his parents and admitted to using alcohol, cocaine, and marijuana. He had been employed as a longshoreman. Family history was noncontributory. Review of systems was unobtainable at the time of admission.
On physical examination, neurological level was C6 American Spinal Injury Association A; the lower extremities were flaccid. Passive range of motion of the lower extremities was full at rehabilitation admission and discharge.
Hospital Course
J.P. was admitted to the inpatient rehabilitation service on postinjury day 25. During the acute rehabilitation stay, he developed recurrent fevers. At about 7 weeks postinjury, MRI of the pelvis was obtained to exclude osteomyelitis of the sacrum secondary to the sacral pressure ulcer. MRI revealed a midline pressure ulcer extending to the coccyx. Coincidentally, MRI also revealed bilateral hip joint effusions, with evidence of synovial thickening, an abnormal degree of enhancement, and bilateral extension of effusion to the obturator internus muscle as well as along the ischial tuberosity (Figure 1). Also noted was a large left posterior extension of joint fluid, which appeared to communicate with the trochanteric bursa. There was no evidence of heterotopic ossification on the MRI or on a CT of the pelvis performed 2 days later. There was no evidence of osteoarthropathy or fracture of the hips on CT or MRI. Physical examination revealed no edema or loss of range of motion of either hip.
Figure 1. T2 weighted MRI of the hips showing bilateral areas of periarticular contrast enhancement representing hip effusions.
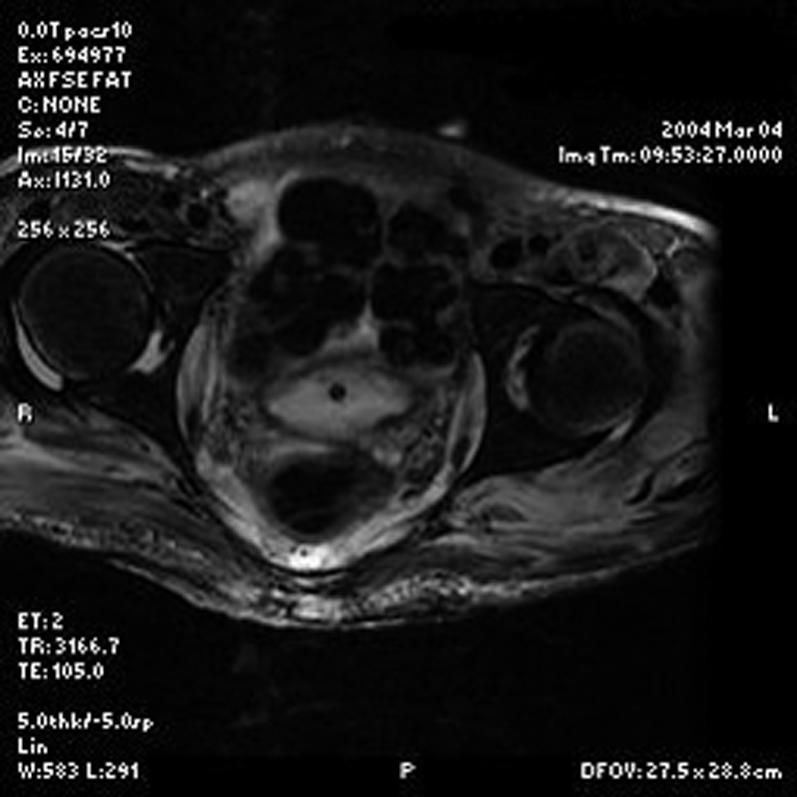
Aspiration of the hip joints yielded a straw-colored fluid from the right side and a bloody fluid from the left side. Both collections of fluid were cultured and found to be sterile. Gram stain of the right hip joint aspirate showed no red cells or white cells. Gram stain of the left hip joint aspirate showed many red blood cells and scant white blood cells. Alkaline phosphatase level at that time was normal. Alkaline phosphatase levels were checked approximately every month thereafter, with the only abnormal result at approximately 20 weeks postinjury (96 IU/L; reference, 29–92 IU/L). Plain radiographs of the pelvis obtained during hospitalization—the last study being 35 weeks postinjury—showed no heterotopic ossification of either hip joint. In addition, at 35 weeks postinjury, the patient still had full passive range of motion of both hips.
DISCUSSION
Spinal cord injury may result in several skeletal/joint complications including osteoporosis, neuropathic joint, and heterotopic calcification. Knee effusions have been previously described in patients with paralysis. Hip effusions in individuals with flaccid paralysis have been found subsequent to electrically stimulated quadriceps training and cycle ergometry (8). Mukand et al (1) notes that paralysis and sensory deficits predispose patients with SCI to traumatic knee effusions. Varghese and Chung (2) have postulated that microtrauma due to physical therapies or position of the joint or joint capsule distension due to decreased muscular support may result in knee effusion. Harvey et al (9) found that physical therapists may use as much as 6 times the force tolerated by neurologically intact individuals when performing hamstring stretches on flaccid patients. Buschbacher et al (3) offer several hypotheses with regard to the finding of noninflammatory effusions in the knees of paretic patients. They suggest that altered biomechanics of the joint may cause irritation of tissues or lead to diminished nutrition to the joint cartilage. Either or both mechanisms may lead to effusion.
We propose that similar mechanisms may be responsible for effusions of the hip. As in the study of Mukand et al (1), the preponderance of red cells vs white cells in the joint aspirate was interpreted to indicate that the etiology was most likely due to the microtrauma sustained during range of motion exercises. Furthermore, the study by Varghese and Chung (2) found evidence of knee effusion in 8 patients with SCI, 6 of which had flaccid paraplegia. They surmised that range of motion of the knee joints during physical therapy may have caused joint trauma, thereby leading to effusion. In their study, 1 of 4 joints aspirated displayed hemarthrosis.
Causes of hip effusion in nonparalyzed patients include rheumatoid arthritis, trauma, crystal disease, fracture, ligamentous injury, joint infection, degenerative joint disease, and benign transient synovitis. With limb paralysis, decreased afferent pain input predisposes the patient to development of neuropathic hip injury or Charcot joint. Johnson (5) notes that neuropathic hip disease is relatively uncommon, as the hip is subject to less strain and is better protected than the knee or foot.
The etiology of joint effusion in the patient with paralysis is multifactorial and often difficult to ascertain without imaging and laboratory support, as described by Mukand et al (1). Interestingly, in their series of 8 patients, 2 individuals (aged 24 and 28 years) were found to have pseudogout, a diagnosis generally associated with an older population. Unfortunately, in this case, the aspirates were not tested for crystalline disease. However, the lack of white cells in the right hip aspirate indicated that crystalline disease was an unlikely etiology (6,7).
The association of joint effusion with the development of heterotopic ossification in patients with SCI has been suggested (7,10), but not confirmed. In this case, no evidence of heterotopic ossification was found. The occurrence of nonbloody joint effusions in association with ligamentous injury is well known. In this case, hemarthrosis was found in one hip, which is consistent with findings in similar reports of effusions of the knee.
CONCLUSION
The finding of aseptic hip joint effusion in this patient with tetraplegia demonstrates that joint effusion in SCI is not limited to the knee. The decreased muscular support, lack of sensation, and laxity of periarticular ligaments of the hip joint found in patients with flaccid paralysis may predispose them to traumatic hip effusions. Range of motion exercises performed in physical therapy may further increase the likelihood of traumatic hip effusion in this population of patients. Imaging and laboratory work-up, including joint aspiration, are warranted to diagnose any treatable causes. The long-term sequelae of hip joint effusion are not well understood.
Acknowledgments
The authors acknowledge the assistance of Tejas Shinde, MD, and Diane Deely, MD (Department of Radiology, Thomas Jefferson University Hospital), in the preparation of this report.
REFERENCES
- Mukand J, Sniger W, Kaufman J, Biener-Bergman S. Common causes of knee effusions in spinal cord injury: a random study. Am J Phys Med Rehabil. 1998;77:113–117. [PubMed] [Google Scholar]
- Varghese G, Chung TS. Benign hydroarthrosis of knee in patients with spinal cord injury. Arch Phys Med Rehabil. 1976;57:468–469. [PubMed] [Google Scholar]
- Buschbacher R, Coplin B, Buschbacher L, McKinley W. Noninflammatory knee joint effusions in spinal cord–injured and other paralyzed patients. Am J Phys Med Rehabil. 1991;70:309–312. [PubMed] [Google Scholar]
- Furman R, Nicholas JJ, Jivoff L. Elevation of serum alkaline phosphatase coincident with ectopic bone formation in paraplegic patients. J Bone Joint Surg. 1970;52:1131–1137. (A) [PubMed] [Google Scholar]
- Johnson JT. Neuropathic injuries of the hip. Clin Orthop. 1973;90:29–32. [PubMed] [Google Scholar]
- Fauci A, Fauci AS, Reginato AJ, Hoffman GS. Arthritis due to deposition of calcium crystals. In: Fauci AS, Braunwald E, Isselbacher KJ, editors. Harrison's Principles of Internal Medicine. 14th ed. Vol 2. New York, NY: McGraw Hill; 1998. pp. 1942–1943. In: [Google Scholar]
- Rott KT, Agudelo CA. Gout. JAMA. 2003;289:2857–2860. doi: 10.1001/jama.289.21.2857. [DOI] [PubMed] [Google Scholar]
- Nicholas JJ. Hydroarthrosis [letter to the editor] Arch Phys Med Rehabil. 1977;58:183. [PubMed] [Google Scholar]
- Nash MS, Tehranzadeh J, Green BA, Rountree MT, Shea JD. Magnetic resonance imaging of osteonecrosis and osteoarthrosis in exercising quadriplegics and paraplegics. Am J Phys Med Rehabil. 1994;73:184–192. doi: 10.1097/00002060-199406000-00007. [DOI] [PubMed] [Google Scholar]
- Harvey LA, McQuade L, Hawthorne S, Byak A. Quantifying the magnitude of torque physiotherapists apply when stretching the hamstring muscles of people with spinal cord injur. Arch Phys Med Rehabil. 2003;84:1072–1075. doi: 10.1016/s0003-9993(03)00131-x. [DOI] [PubMed] [Google Scholar]


