Abstract
Background/Objective:
We conducted a randomized, double-blind comparison of twice daily bladder irrigation using 1 of 3 different solutions in community-residing persons with neurogenic bladder who used indwelling catheters to evaluate efficacy in treatment of bacteriuria.
Methods:
Eighty-nine persons with bacteriuria were randomized to irrigate their bladders twice daily for 8 weeks with 30 mL of (a) sterile saline, (b) acetic acid, or (c) neomycin-polymyxin solution. Urinalysis, cultures, and antimicrobial susceptibility tests were performed at baseline and weeks 2, 4, and 8 to determine the extent to which each of the solutions affected numbers and types of bacteria, urinary pH, urinary leukocytes, and generation of antimicrobial-resistant organisms.
Results:
Bladder irrigation was well tolerated with the exception of 3 participants who had bladder spasms. None of the 3 irrigants had a detectable effect on the degree of bacteriuria or pyuria in 52 persons who completed the study protocol. A significant increase in urinary pH occurred in all 3 groups. No significant development of resistance to oral antimicrobials beyond what was observed at baseline was detected.
Conclusions:
Bladder irrigation was generally well tolerated for 8 weeks. No advantages were detected for neomycin-polymyxin or acetic acid over saline in terms of reducing the urinary bacterial load and inflammation. We cannot recommend bladder irrigation as a means of treatment for bacteriuria in persons with neurogenic bladder.
Keywords: Neurogenic bladder, Spinal cord injuries, Bacteriuria, Urinary tract infection, Irrigation, Acetic acid, Neomycin-polymyxin
INTRODUCTION
Urinary tract infection (UTI) is the single most frequent secondary medical complications in persons with spinal cord injury (SCI) who develop neurogenic bladder dysfunction, irrespective of the type of bladder management used (1–4). UTIs affect the overall well being in these individuals and those who have neurogenic bladder from various neurologic conditions, contributing substantially to health care costs.
An indwelling urinary catheter is virtually always associated with bacteriuria within a few days after it is inserted as a means to ensure adequate urine drainage and preservation of renal function when normal voiding mechanisms can no longer function as a result of injury or disease. However, use of an indwelling catheter may sometimes be unavoidable. Data from the Model SCI Systems indicate that 8.1% of men and 52.3% of women use indwelling catheters 15 years after injury (5). Approximately 24% of community-residing persons with SCI who receive medical care at our institution use indwelling catheters or suprapubic tubes as the primary means for bladder management.
Untreated asymptomatic bacterial colonization seems to have no long-term serious effects on renal function in most persons in whom low intravesicular pressure can be maintained (6). However, prophylaxis of UTI with systemic antibiotics and sporadic antibiotic treatment of asymptomatic bacteriuria should be avoided, because these interventions have little effect on prevention of symptomatic UTI and may increase colonization with antimicrobial-resistant bacteria (7–12). However, the presence of bacteria in the bladder places the remainder of the urinary tract at risk for further invasion, and uncertainty of this risk remains a major concern. Relapsing asymptomatic bacteriuria can also be a risk factor for development of symptomatic UTI that may be complicated by bacteremia and require hospitalization (13). Persons with SCI may also complain of cloudy, malodorous urine, increased incontinence, autonomic dysreflexia, and overall malaise to exert pressure on their health care providers to give them antibiotics.
Use of systemic antibiotics also places patients at risk for drug toxicities and adverse reactions. Previous reports indicated that 33% of all gram-negative bacteria isolated from the urinary tracts of community-residing persons with SCI were multidrug resistant (MDR) and that the highest rates of antimicrobial resistance occurred in persons with indwelling catheters (14). The frequency of MDR bacteria in the urinary tract of persons with SCI or other neurologic diseases associated with neurogenic bladder limits choices for oral antimicrobials suitable for treatment of symptomatic illness. Problems may also arise when patients are allergic to the medications to which the infecting organisms are susceptible.
Bladder washouts with antiseptics, acidifiers, organic acids, and antibiotic mixtures have been proposed as alternative means to prevent or treat bacteriuria in catheterized persons with SCI on a short-term basis, but very little data are available on the microbiological efficacy, patient tolerability, or potential adverse effects, including selection for drug-resistant organisms. This study addresses the question of whether bladder irrigation can be recommended as a therapeutic alternative in catheterized bacteriuric persons who are not ill enough to require systemic antibiotics. This study was performed with the approval of the institutional review board. Informed consent was obtained from all participants before enrollment.
METHODS
Study Design
This was a randomized, double-blinded study of 3 methods of bladder irrigation performed twice daily for 8 weeks in community-residing persons with neurogenic bladder secondary to SCI or other neurological conditions managed by indwelling Foley catheters. The research question addressed was whether bladder irrigation with one of these agents would provide a significant reduction in bacteriuria.
Study Population and Enrollment
Participants were recruited from community-residing men and women at the time of routine clinic visits for annual evaluations or other reasons. Enrollment was limited to individuals (men or women) who were at least 19 years of age and who were at least 6 months post-SCI or onset of other neurological diseases. All participants had their neurogenic bladders managed by an indwelling Foley catheter or suprapubic tube and had evidence of microscopic bacteriuria and pyuria at time of enrollment. Exclusion criteria were (a) signs or symptoms of serious UTI that required use of systemic antibiotics, such as increased spasticity, abdominal or flank pain, or fever of at least 101°F; (b) use of any urine-acidifying agent, bladder irrigant, or systemic antibiotic within the previous 7 days; (c) prior abnormalities in renal function as documented by a renal scan, radiographs, or other laboratory parameter; (d) pregnancy; and (e) inability or unwillingness to provide informed consent.
Intervention
Participants who consented were randomized, stratified by sex, to receive 30 mL of 1 of 3 bladder irrigant solutions twice daily for 8 weeks in a double-blinded fashion. The first irrigation was shown in the clinic at the time of enrollment, and participants were given a supply of premeasured bladder irrigants in syringes to take home. Additional solutions were provided as needed during the course of the study. Bladder irrigants used were (a) normal saline, (b) 0.25% acetic acid, and (c) neomycin-polymyxin GU irrigant containing 40 mg/mL neomycin sulfate and 200,000 units/mL polymyxin B.
The irrigation procedure involved disconnecting the catheter tubing, attaching a syringe containing 30 mL of irrigant, and pushing the plunger of the syringe to force the solution into the bladder where it remained for 20 minutes. The syringe was disconnected, and the catheter was reconnected to the leg bag. Gloves were worn to minimize contamination of the catheter tubing. The 30-mL irrigant volume was chosen because it represented the maximum amount that could be readily tolerated according to our preliminary pilot study of 5 persons who initially underwent irrigation with 60 mL of irrigant and immediately began to leak the irrigant because of their relatively small bladder capacities, a result of long-term indwelling catheterization. These same persons were able to retain the 30-mL volume without leakage for the 20-minute duration needed.
All participants were contacted by telephone weekly to monitor compliance, assess for tolerability and adverse effects of bladder irrigation, and arrange for collection of follow-up urine specimens. Patients were advised to continue their usual practices for perineal hygiene and catheter care. Quantitative urinalyses and bacterial cultures were performed on urine specimens collected before enrollment and after 2, 4, and 8 weeks, giving a total of 4 data collection time-points for each participant. Participants shipped their urine specimens to the research laboratory by overnight courier, dropped them off at the clinic, or were visited in their homes by the study coordinator for collection.
Urine Collection and Laboratory Studies
The Foley catheter collection port was disinfected with an alcohol swab. Using a needle and syringe, approximately 10 mL was aspirated from the injection port, and then approximately 5 mL was transferred to a prelabeled Vacutainer containing boric acid preservative (Becton Dickinson & Co., Rutherford, NJ). The remaining urine was transferred to a second Vacutainer tube without any additives for urinalysis. For patients whose catheter did not contain a port, the catheter was clamped for 20 to 30 minutes until at least 5 mL of urine collected. The catheter was then unclamped and urine was allowed to flow into a sterile plastic container. Urine was aspirated into the appropriate Vacutainer tubes using the port on the specimen container provided with the urine collection kit. A package containing all of the supplies, detailed written instructions for collecting urine, and an addressed return label was shipped by overnight courier to each participant at the time a urine specimen was due. Urine specimens were transported to the laboratory on ice by overnight courier and processed immediately on receipt. The Vacutainer tube containing preservative maintains bacterial counts at a stable level for 24 hours without refrigeration. We have validated this previously in our laboratory. In several instances, for participants residing locally, the study coordinator visited their homes and collected the urine specimens.
Urine was examined microscopically using a hemo-cytometer after staining with Giemsa to enumerate leukocytes per cubic millimeter. Urine pH was measured by dipstick (Ames Multistix, Miles Laboratories, Elkhart, Indiana). Cultures were performed by immersing a 0.001-mL calibrated platinum loop into the specimen and streaking trypticase soy agar with 5% sheep blood and MacConkey agar plates for quantitative cultures. Agar plates were incubated aerobically at 35°C for up to 48 hours. Colony counts were determined and bacterial isolates were identified using standard biochemical procedures and the API 20 system when necessary (bioMérieux, Durham, NC).
Qualitative antimicrobial susceptibility testing was performed by the agar-disk diffusion (Kirby-Bauer) technique using cation-adjusted Mueller-Hinton agar. Interpretation of results and quality control were done in accordance with criteria published by the National Committee for Clinical Laboratory Standards (15), now the Clinical and Laboratory Standards Institute (CLSI). Antimicrobials tested against gram-negative bacteria included representative oral agents from multiple drug groups: ampicillin, cefazolin (representing oral cephalo-sporins) ciprofloxacin, and trimethoprim/sulfamethoxazole. Only orally administered drugs were assessed for gram-negative bacilli, because the objective of this study was to determine whether bladder irrigation could be an alternative for administration of systemic antibiotics in an outpatient setting. Serious UTIs require intravenous agents, and therefore, bladder irrigation could not be an alternative in that setting. For the purposes of this study, a gram-negative bacillus was designated as MDR if it was resistant in vitro to drugs in at least 2 different antimicrobial classes (ie, β-lactams and fluoroquinolones).
Staphylococci and enterococci constitute the great majority of gram-positive uropathogens. Because development of resistance to oxacillin and vancomycin were the major concerns for staphylococci and entero-cocci, respectively, we assessed resistance to these agents even though they are not administered orally. Staphylococci that are susceptible to oxacillin can be assumed to be susceptible to other β-lactamase stable penicillins and cephalosporins, including some oral agents (15). Ampicillin susceptibility was also assessed for enterococci.
Statistical Analysis
Demographic and injury-related data were recorded for each participant and compared among the 3 groups in aggregate. Variables such as sex, race, type level of neurological lesion, and method of bladder management were compared using χ2 analysis. Other variables such as age and length of time since injury or onset of illness were compared using Student's 2-tailed t test. Nonparametric data including evaluation of urinary leukocytes were compared using the Wilcoxon ranked sign test. A P value of <0.05 was considered statistically significant (16). Bacterial colony counts were recorded as 0 (no growth), 1,000 to 9,000, 10,000 to 90,000, or greater than 100,000 colony-forming units per milliliter (cfu/mL) at each time-point for all participants. Median numbers of urinary leukocytes per cubic millimeter and mean urine pH values were also calculated for each of the 3 irrigant groups at each data collection time-point. The occurrence of individual bacterial species or groups over time was enumerated according to irrigation group and by susceptibility or resistance to individual antimicrobial agents with comparison by χ2 analysis where appropriate.
RESULTS
A total of 89 persons were enrolled into the study. Characteristics of the study population are shown in Table 1. There were 30 persons randomized to irrigate their bladders with acetic acid, 30 with neomycin-polymyxin solution, and 29 with saline. There were 52 (58.4%) persons who completed the entire 8-week protocol. Among those who completed the entire 8-week study, there were no significant differences in any of the demographic and injury-related variables described above across the 3 groups (data not shown).
Table 1.
Characteristics of Study Population (n = 89) *
Bacterial Numbers and Species
As a criterion for entry into the study, each participant had bacteriuria identified by screening microscopic urinalysis. Quantitative culture data obtained before beginning bladder irrigations confirmed bacteriuria with at least 100,000 cfu/mL in all but 2 individuals. Two or more bacterial species were often present simultaneously in individual urine specimens. Cultures collected after 2, 4, and 8 weeks of twice daily irrigations showed this high level of bacteriuria remained in almost every individual in all 3 groups at all time-points, with no distinction among the 3 irrigant solutions. There were only 2 follow-up cultures of participants who initially had at least 100,000 cfu/mL that yielded less than 10,000 cfu/mL.
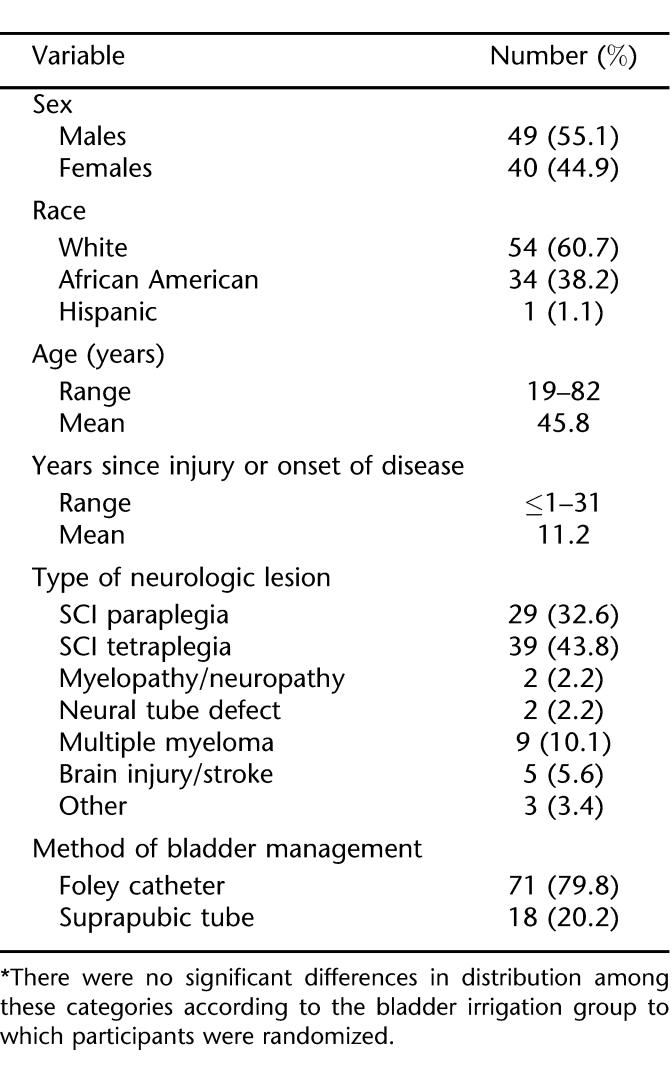
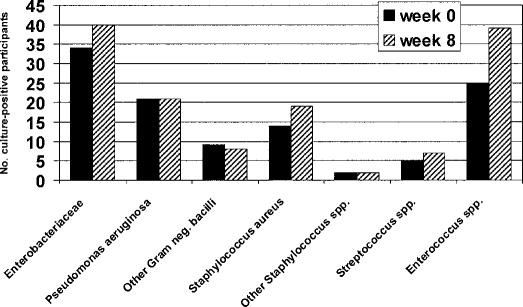
The distribution of bacterial species and groups present in urine before beginning bladder irrigation and at its completion for the 52 participants is shown in Figure 1. The predominant gram-positive bacteria encountered were enterococci and staphylococci. Overall, there were 135 isolates of Enterococcus spp detected in urine specimens over the 8 weeks of bladder irrigation, making them the most common organisms encountered. The diversity of bacterial species in urine specimens was generally similar before and after completing the study, except that the numbers of participants harboring Enterococcus species alone or in conjunction with other types of bacteria rose from 25 (48%) initially to 39 (75%) after 8 weeks, a significant increase over time (P = 0.0006). Nineteen of 22 persons in the neomycin-polymyxin acid group (86%), 7 of 9 (78%) in the acetic acid group, and 13 of 21 (62%) of the saline group had positive cultures for Enterococcus spp after completing the irrigation study (Figure 2). When analyzed according to irrigation group, the increase in occurrence of entero-cocci over time was significant only for the neomycin-polymyxin group (P = 0.02). There were no other apparent differences with respect to the frequency of occurrence of the various bacterial species present in the urine before, during, or after 8 weeks of bladder irrigation among participants across the 3 groups, but numbers of isolates of the other bacterial species were too small for meaningful comparisons.
Figure 1. Distribution of bacterial groups isolated in urine cultures from 52 participants before and after completion of 8 weeks of bladder irrigation. There was no difference over time (P ≥ 0.10) for any bacterial group except Enterococcus spp (P = 0.0006).
Figure 2. Occurrence of Enterococcus spp in each bladder irrigation group over time. There was a significant increase in the total numbers of persons harboring these organisms over time (P = 0.005) and within the neo-mycin-polymyxin group (P = 0.02).
Predominant members of the Enterobacteriaceae that were encountered during the study included, in descending order of frequency, Escherichia coli (43 isolates), Proteus spp (43 isolates), Serratia marcescens (32 isolates), Klebsiella spp (28 isolates), Providencia spp (24 isolates), Citrobacter spp (15 isolates), and Morganella morganii (12 isolates). Pseudomonas aeruginosa was the predominant nonfermentative gram-negative bacillus (78 isolates), occurring alone or in combination with other species in 21 participants before initiation of the study. Interestingly, the same number of participants harbored the organism at the conclusion of the study.
Antimicrobial-resistant Bacteria
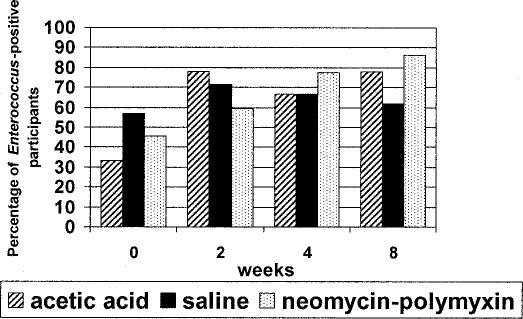
Among the 135 isolates of Enterococcus spp encountered over the course of the study, all of these organisms were susceptible to ampicillin and to vancomycin. Development of resistance to oxacillin or methicillin (MRSA) was the major concern in Staphylococcus aureus. Before initiating bladder irrigation, MRSA occurred in 10 of 52 (19%) study participants. Occurrence of MRSA in study participants at the 8-week time-point was similar (21.2%). No MRSA occurred in the acetic acid group at any time-point. There were 5 persons in the neomycin-polymyxin group who were colonized with MRSA throughout the 8-week period, an additional person who picked up MRSA at 4 weeks, and another at 8 weeks. The occurrence of MRSA in the saline group was less consistent; with 6 of 21 (28.6%) participants harboring this organism, but colonization was intermittent in all but one of them. Occurrence of MRSA in all 3 groups is shown in Figure 3.
Figure 3. Occurrence of MRSA in the 3 bladder irrigation groups stratified across 4 data collection points showing no significant increase over time (P ≥ 0.37).
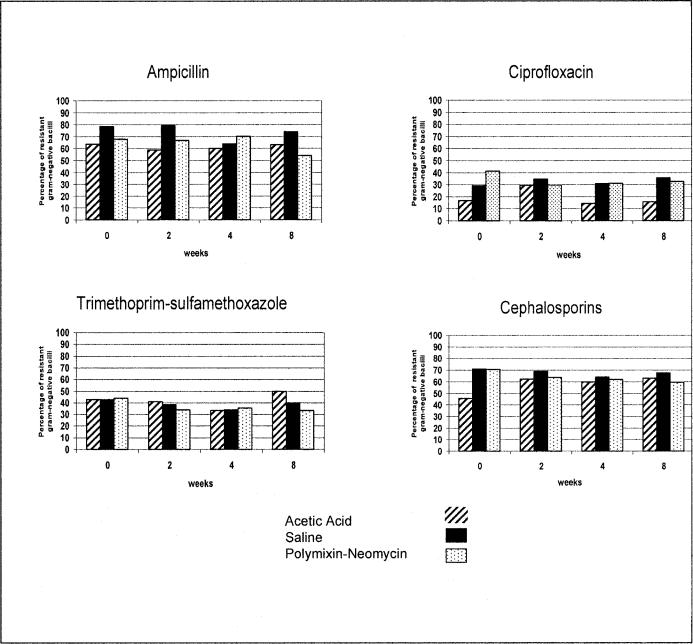
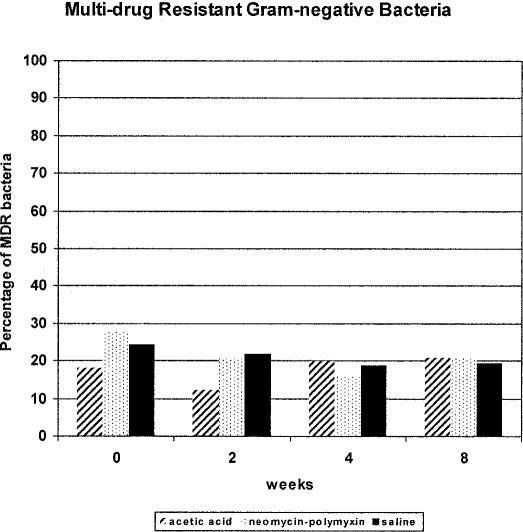
Assessment of the development of antimicrobial resistance among gram-negative bacteria is more complex than for the gram-positive cocci because of the much greater bacterial species diversity encountered in the urinary tract and the greater number of clinically relevant drugs that must be considered. Over the course of the 8-week study protocol, we assessed whether there was a relationship between irrigation with any of the 3 solutions and development of resistance of gram-negative bacilli to common orally administered antimicrobials used for treatment of UTI: ampicillin, cefazolin (representing oral cephalosporins), ciprofloxacin, and trimethoprim-sulfamethoxazole. As shown in Figure 4, there was no significant increase or trend in development of resistance to any of these oral antimicrobial agents individually among the 3 bladder irrigation groups. However, the baseline level of resistance to these agents differed according to the drug class and it was relatively high for most of the agents initially. Moreover, the occurrence of MDR bacteria did not seem to increase to a significant degree over the course of the study in any of the 3 bladder irrigation groups (Figure 5). Ciprofloxacin was the oral agent with the best overall in vitro activity against gram-negative bacilli, but resistance was still evident in many bacterial isolates, as would be expected. Similar to observations with staphylococci and enterococci, individuals tended to be colonized with gram-negative bacterial species initially and harbored the same organisms throughout the irrigation period with minimal effect by any of the 3 bladder irrigants.
Figure 4. Occurrence of gram-negative bacilli resistant to ampicillin, ciprofloxacin, trimethoprim-sulfamethoxazole, or cephalosporins in 3 bladder irrigation groups stratified across 4 data collection time-points showing no significant increases over time (P ≥ 0.11).
Figure 5. Occurrence of MDR gram-negative bacilli in 3 bladder irrigation groups stratified across 4 data collection points showing no significant increases over time (P ≥ 0.41).
Urinary Leukocytes
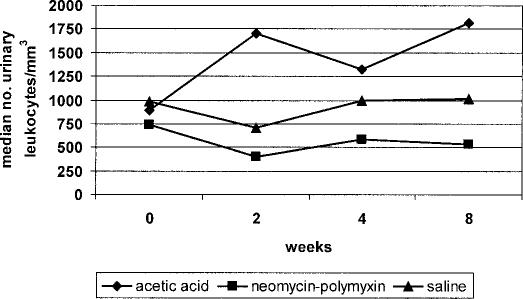
There was no significant difference among the participants in the 3 bladder irrigation groups with respect to percentages of urine specimens in each of the 4 numerical quartiles (<250, 250–749, 750–2,999, >3,000 leukocytes/mm3) over time (P > 0.6) Figure 6 shows the median numbers of urinary leukocytes in each bladder irrigation group stratified by data collection time-point indicating that substantial levels of pyuria occurred among subjects in each group at every time-point. The acetic acid group had the highest median numbers of urinary leukocytes. The neomycin-polymyxin group had the fewest urinary leukocytes overall at each time-point, but the numbers were still consistently high.
Figure 6. Median numbers of urinary leukocytes stratified according to bladder irrigation group and data collection points showing persistence of high level pyuria throughout the study. No significant increases were observed in any group (P ≥ 0.6).
Urinary pH
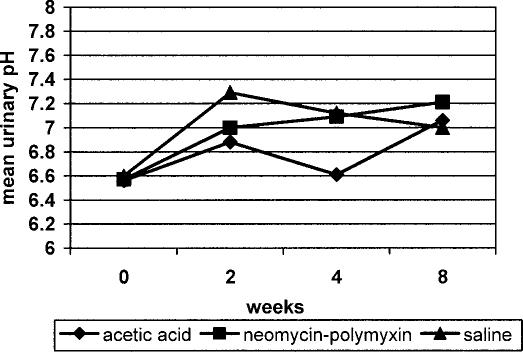
The mean pH values for all 3 irrigation groups were the same at the beginning of the study (6.6) as shown in Figure 7. The acetic acid group had the lowest mean values at the 2- and 4-week time-points, but at 8 weeks, the mean urinary pH had increased for all 3 groups (P = 0.01; range, 7.0–7.2).
Figure 7. Mean urinary pH stratified according to bladder irrigation group and data collection points showing significant changes over the course of the study in all 3 groups (P = 0.01).
Tolerability and Adverse Effects
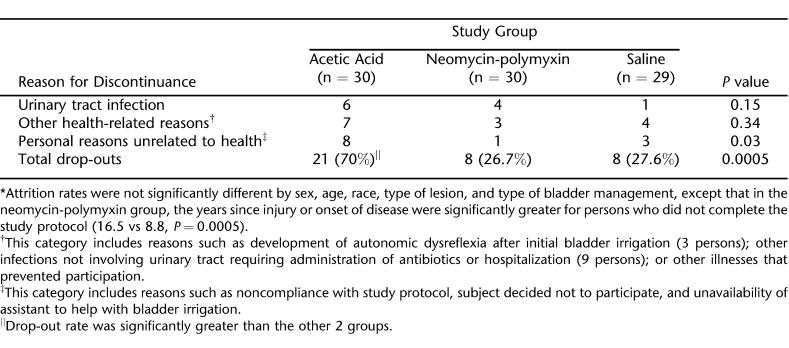
The attrition rates were not significantly different by sex, age, race, type of lesion, and type of bladder management (Foley catheter vs suprapubic tube), except that in the neomycin-polymyxin group, the years since injury or onset of disease were significantly greater for persons who did not complete the study protocol (16.5 vs 8.8, P = 0.04). The overall rate at which subjects in the acetic acid group discontinued the protocol was significantly greater than the other 2 groups (P = 0.0005). Overall, 11 of 89 (12.4%) persons discontinued participation because of development of symptomatic UTIs, but their distribution among the 3 irrigation groups was not significantly different. There were only 3 cases of adverse effects that could be attributed directly to participation in bladder irrigation. These included 3 instances in which participants experienced manifestations of autonomic dysre-flexia shortly after the initial instillation of the irrigant solution. Two of these subjects received neomycin-polymyxin and the other received acetic acid.
A description of the 37 individuals who discontinued the study and reasons for discontinuance are shown in Table 2. Twenty-five persons withdrew because of health-related matters. These included development of symptomatic UTIs and other types of illnesses. Other than the 3 cases of bladder spasms, there were no other instances in which intolerance of the irrigation procedure required discontinuation. However, 12 persons discontinued because of personal reasons, most of which related to perceived difficulty, inconvenience, or unwillingness to perform the twice daily irrigations.
Table 2.
Reasons for Discontinuation of Study Protocol *
DISCUSSION
Instillation of chemical substances into the urinary bladder to combat infection has been practiced for over 40 years in different populations with varied results. Saline irrigant provides a washout effect, but has nothing in it to inhibit bacterial multiplication. Dilute acetic acid is inexpensive and can be bactericidal. It also has the potential to cause bladder irritation, but probably not antimicrobial resistance. Neomycin and polymyxin are the only antimicrobial agents sold commercially in the United States as a urinary bladder irrigant. This combination is bactericidal and active in vitro against several members of the Enterobacteriaceae, P aeruginosa, and S aureus, but it is not active against S marcescens, streptococci, and enterococci. The fact that these antibiotics are poorly absorbed systemically in the absence of compromised renal function makes this solution potentially less likely to generate resistant bacteria or induce toxic side effects than other agents. However, both of these agents are known to produce toxic effects when administered systemically, especially ototoxicity and nephrotoxicity.
We chose an 8-week study duration for twice daily bladder irrigations in hopes of allowing sufficient time for an effect on bacterial growth in urine. Most of the previous bladder irrigation studies in persons with SCI involving antibiotic solutions were conducted for just a few days, with concern over possible neomycin-polymyxin toxicity being a time-limiting factor. Limiting exposure to the irrigation solutions to intermittent instillation twice daily rather than a continuous flow was felt to be a safer mode. A disadvantage of continuous bladder irrigation that has been used in earlier short-term studies is that it may bathe the epithelium of the bladder insufficiently to exert a bactericidal effect, induce autonomic dysreflexia, and cause bladder distention, and it is a much more complex and inconvenient procedure to perform in an outpatient setting (17). Twice daily was the maximum number of times that we expected patients to be able to perform irrigation over such an extended period.
This study showed that, while bladder irrigation was generally well tolerated by the majority of persons for 8 weeks, the procedure was somewhat time-consuming, requiring a personal commitment and motivation on the part of the individual performing the task. Persons whose neurological lesions limited their hand dexterity often required assistance to perform the irrigations. Time requirement and availability of assistance with the irrigation procedure were major factors leading to discontinuation of 12 participants who decided they were unable or unwilling to comply with the protocol. Several other participants had to be withdrawn from the study because they were hospitalized and/or given antimicrobial agents to treat UTIs or unrelated infections. It is impossible to say whether development of symptomatic UTI that occurred in 11 persons was related to the bladder irrigations because the condition is so common in this population. In a nonexperimental setting, many of the events requiring discontinuation of the study protocol would not have required cessation of bladder irrigation.
None of the 3 irrigants had a marked effect on the degree of pyuria. It is logical to expect that acetic acid could induce a greater degree of bladder inflammation because of its acidic properties. However, this effect could not be detected because all 3 irrigant groups had relatively high leukocyte counts at the beginning and high colony counts of bacteria likely to elicit inflammation persisted throughout the study and the numbers of participants in the acetic acid group who completed the study (9 persons) was very small. The presence of the indwelling catheter alone could also incite inflammation. Any effect of acetic acid or the other agents on urine pH would likely be negated by the fact that it was instilled for a short period at intervals several hours apart such any influence on urine pH might be of short duration. We have no explanation for the apparent increase in urine pH that occurred in all 3 irrigant groups over the course of the study.
Bladder irrigation with antibiotics, antiseptics, or other solutions has been promoted primarily for short-term use in catheterized persons to prevent development of bacteriuria. Some studies of bladder irrigation in catheterized persons from non-SCI populations found beneficial effects of short-term intermittent irrigation with neomycin-polymyxin or acetic acid in previously infected bladder drainage systems, but these findings are not universal (18–20).
Although data are limited, some studies have been reported describing the use of bladder irrigation in persons with SCI. Rhame and Perkash (21) reported that more than 50% of hospitalized persons with SCI undergoing intermittent catheterization who also received simultaneous bladder irrigation with neomycin-polymyx-in still developed bacteriuria, which was usually asymptomatic. Haldorson et al (22) found no difference between the incidence of bacteriuria in hospitalized persons with SCI who were irrigated with neomycin-polymyxin in comparison with those who were not irrigated. Haldorson et al (22) detected more enterococci in irrigated patients because these drugs are not effective against these bacteria, but inhibit many of the other competing organisms. This is consistent with our current observations, although we did not encounter an increase in P aeruginosa or yeasts as they did in the earlier studies. Anderson (23) commented on the lack of activity of neomycin against gram-positive bacteria that was reflected in the ability to eliminate gram-negative bacteria but reduce the gram-positive bacteria by only 50% in his study of patients with SCI. Linsenmeyer et al (24) instilled neomycin-polymyxin 3 times a day for 5 days in asymptomatic SCI patients with indwelling catheters and determined that while bacteria were often not eradicated, they sometimes changed in susceptibility such that an oral antimicrobial could be used in a patient with a previously resistant infection. No significant changes occurred in the urinary leukocyte counts before or after irrigations, similar to our observation. Because there was no control group in that study, it is impossible to know whether such an effect on antibiotic susceptibilities could have been achieved with an irrigant that did not contain an antibiotic.
We did not specifically determine whether bacterial isolates recovered from the urine of individuals undergoing bladder irrigation developed resistance to neomycin or polymyxin over the course of the study because these drugs are not routinely tested by our microbiology laboratory. However, we must acknowledge that resistance to neomycin-polymyxin has been reported in association with its use as a bladder irrigant in persons with SCI (21). The lack of effect of bladder irrigation with any of the 3 agents on bacterial numbers in our study was probably not caused by development of antimicrobial resistance in any specific organisms because we saw no appreciable differences in the bacteriology or resistance to other agents among the 3 irrigant groups over the course of the study, except for the significant increase in enterococci in the neomycin-polymyxin group. Even though the enterococci were selectively increased in the neomycin-polymyxin group, they did not become resistant to other agents. Persistence of high numbers of diverse types of bacteria in the saline and acetic acid groups that were largely similar to those in the neomycin-polymyxin groups suggests that none of these agents had any appreciable effect. Even though participants were exposed to the irrigant solutions for 8 weeks, the actual time these solutions were present in the urinary bladder was less than 1 hour each day because of logistics and concern for tolerability and toxicity with the antibiotic solutions. It is possible that this short duration of exposure was inadequate to provide any measurable benefit on reducing the magnitude of bacteriuria and that more frequent irrigations or longer retention times could provide greater microbiological efficacy. Additional limitations include the relatively small sample size and the high attrition rate in the acetic acid group.
CONCLUSIONS
This study showed that twice daily bladder washout with saline, acetic acid, or neomycin-polymyxin solution is well tolerated by most persons with neurogenic bladder managed by an indwelling Foley catheter. The procedure did not cause pain or inflammation beyond what is already present in the urine or lead to the generation of antimicrobial-resistant organisms beyond their baseline occurrence. This conclusion must be tempered with the fact that there was already a high prevalence of MRSA and MDR gram-negative bacilli in the study population. The results were disappointing, although not entirely unexpected, in that that no beneficial effect of any of the 3 irrigant solutions on reducing bacterial colony counts or urinary leukocytes, or improving the quality of urine overall could be shown. In view of these findings, there is no basis on which to recommend the use of bladder irrigation as a routine method for treating asymptomatic bacteriuria in catheterized persons with neurogenic bladder. Future studies incorporating an increased volume of irrigant, frequency of instillations, and/or duration of treatment should be considered.
Acknowledgments
Technical assistance from Al Turnbow, Yvonne Lopez, Kathy Harmon, and Sarah Armstrong is gratefully acknowledged. We also appreciate the advice of Drs. Keith Lloyd and Amie Jackson in planning and development of the study and in identifying patients for enrollment.
Footnotes
This study was supported by Paralyzed Veterans of America Spinal Cord Research Foundation grant RFA-305 and it was presented at the Annual Assembly of the American Academy of Physical Medicine and Rehabilitation, Philadelphia, PA, October 2005.
REFERENCES
- Montgomerie JZ. Infections in patients with spinal cord injuries. Clin Infect Dis. 1997;25:1285–1290. doi: 10.1086/516144. [DOI] [PubMed] [Google Scholar]
- Cardenas DD, Hooton TM. Urinary tract infection in persons with spinal cord injury. Arch Phys Med Rehabil. 1995;76:272–280. doi: 10.1016/s0003-9993(95)80615-6. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Stover SL. Long-term survival and causes of death. In: Stover SL, DeLisa JA, Whiteneck G, editors. Spinal Cord Injury: Clinical Outcomes From the Model Systems. Gaithersburg, MD: Aspen Publisher; 1995. pp. 289–316. [Google Scholar]
- Van Kerrebroeck PE, Koldewijn EL, Scherpenhuizen S, Debruyne FM. The morbidity due to lower urinary tract function in spinal cord injury patients. Paraplegia. 1993;31:320–329. doi: 10.1038/sc.1993.56. [DOI] [PubMed] [Google Scholar]
- Cardenas DD, Farrell-Roberts L, Sipski ML, Rubner D. Management of gastrointestinal, genitourinary, and sexual function. In: Stover SL, DeLisa JA, Whiteneck G, editors. Spinal Cord Injury: Clinical Outcomes From the Model Systems. Gaithersburg, MD: Aspen Publishers; 1995. pp. 120–144. [Google Scholar]
- Sotolongo JR, Jr, Koleilat N. Significance of asymptomatic bacteriuria in spinal cord injury patients on condom catheter. J Urol. 1990;143:979–980. doi: 10.1016/s0022-5347(17)40157-1. [DOI] [PubMed] [Google Scholar]
- Sandock DS, Gothe BG, Bodner DR. Trimethoprim-sulfa-methoxazole prophylaxis against urinary tract infection in the chronic spinal cord injury patient. Paraplegia. 1995;33:156–160. doi: 10.1038/sc.1995.34. [DOI] [PubMed] [Google Scholar]
- Waites KB, Canupp KC, DeVivo MJ. Eradication of urinary tract infection following spinal cord injury. Paraplegia. 1993;31:645–652. doi: 10.1038/sc.1993.104. [DOI] [PubMed] [Google Scholar]
- Waites KB, Canupp KC, Brookings ES, DeVivo MJ. Effect of oral ciprofloxacin on bacterial flora of perineum, urethra, and lower urinary tract in men with spinal cord injury. J Spinal Cord Med. 1999;22:192–198. doi: 10.1080/10790268.1999.11719569. [DOI] [PubMed] [Google Scholar]
- Biering-Sorensen F, Hoiby N, Nordenbo A, Ravnborg M, Bruun B, Rahm V. Ciprofloxacin as prophylaxis for urinary tract infection: prospective, randomized, cross-over, placebo controlled study in patients with spinal cord lesion. J Urol. 1994;151:105–108. doi: 10.1016/s0022-5347(17)34882-6. [DOI] [PubMed] [Google Scholar]
- Pedersen SS, Horbov S, Biering-Sorensen F, Hoiby N. Peroral treatment with ciprofloxacin of patients with spinal cord lesion and bacteriuria caused by multiply resistant bacteria. Paraplegia. 1990;28:41–47. doi: 10.1038/sc.1990.5. [DOI] [PubMed] [Google Scholar]
- Stannard AJ, Sharples SJ, Norman PM, Tillotson GS. Ciprofloxacin therapy of urinary tract infections in paraplegic and tetraplegic patients: a bacteriological assessment. J Antimicrob Chemother. 1990;26(suppl F):13–18. doi: 10.1093/jac/26.suppl_f.13. [DOI] [PubMed] [Google Scholar]
- Elden H, Hizmetli S, Nacitarhan V, Kunt B, Goker I. Relapsing significant bacteriuria: effect on urinary tract infection in patients with spinal cord injury. Arch Phys Med Rehabil. 1997;78:468–470. doi: 10.1016/s0003-9993(97)90158-1. [DOI] [PubMed] [Google Scholar]
- Waites KB, Chen Y, DeVivo MJ, Canupp KC, Moser SA. Antimicrobial resistance in gram-negative bacteria isolated from the urinary tract in community-residing persons with spinal cord injury. Arch Phys Med Rehabil. 2000;81:764–769. doi: 10.1016/s0003-9993(00)90108-4. [DOI] [PubMed] [Google Scholar]
- National Committee for Clinical Laboratory Standards (NCCLS) Performance Standards for Antimicrobial Susceptibility Testing, 11th Informational Supplement. NCCLS; Wayne, PA: 2001. [Google Scholar]
- Diggle PJ, Liang K, Zeger SL. Analysis of Longitudinal Data. New York: Oxford University Press; 1994. [Google Scholar]
- Warren JW, Platt R, Thomas RJ, Rosner B, Kass EH. Antibiotic irrigation and catheter-associated urinary-tract infections. N Engl J Med. 1978;299:570–573. doi: 10.1056/NEJM197809142991103. [DOI] [PubMed] [Google Scholar]
- Kass EH, Sossen HS. Prevention of infection of urinary tract in presence of indwelling catheters; description of electromechanical valve to provide intermittent drainage of the bladder. JAMA. 1959;169:1181–1183. doi: 10.1001/jama.1959.03000280033008. [DOI] [PubMed] [Google Scholar]
- Martin CM, Bookrajjan EN. Bacteriuria prevention after indwelling urinary catheterization. Ann Intern Med. 1962;110:703. [Google Scholar]
- Bruun JN, Digranes A. Bladder irrigation in patients with indwelling catheters. Scand J Infect Dis. 1978;10:71–74. doi: 10.3109/inf.1978.10.issue-1.16. [DOI] [PubMed] [Google Scholar]
- Rhame FS, Perkash I. Urinary tract infections occurring in recent spinal cord injury patients on intermittent catheterization. J Urol. 1979;122:669–673. doi: 10.1016/s0022-5347(17)56552-0. [DOI] [PubMed] [Google Scholar]
- Haldorson AM, Keys TF, Maker MD, Opitz JL. Nonvalue of neomycin instillation after intermittent urinary catheterization. Antimicrob Agents Chemother. 1978;14:368–370. doi: 10.1128/aac.14.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RU. Prophylaxis of bacteriuria during intermittent catheterization of the acute neurogenic bladder. J Urol. 1980;123:364–366. doi: 10.1016/s0022-5347(17)55938-8. [DOI] [PubMed] [Google Scholar]
- Linsenmeyer TA, Jain A, Thompson BW. Effectiveness of neomycin/polymyxin bladder irrigation to treat resistant urinary pathogens in those with spinal cord injury. J Spinal Cord Med. 1999;22:252–257. doi: 10.1080/10790268.1999.11719578. [DOI] [PubMed] [Google Scholar]