Abstract
Background/Objective:
To assess the effects of theophylline on pulmonary function in patients with chronic traumatic tetraplegia, we conducted a double-blind placebo-controlled crossover study in 10 patients.
Methods:
The patients (age: 41 ± 3 years; time from injury: 16 ± 3 years; neurological levels: C3 to C7-T1) were randomized to receive oral theophylline or placebo for 6 weeks. After 2 months of washout, the patients received the medication not taken in the first trial for an additional 6 weeks. We measured lung volumes, expiratory flow rates, maximal inspiratory pressure (MIP), and maximal expiratory pressure (MEP) at both baseline and at the end of each treatment arm. Theophylline blood serum assays were measured during the first week of the treatment and on the day of respiratory measurements.
Results:
Mean theophylline level on the day of treatment completion was 12.6 ± 1.4 μg/mL. In analyzing the data from the group of 10 patients, the percent changes from baseline in total lung capacity, forced vital capacity, forced expiratory volume at 1 second, MIP, and MEP did not differ significantly between the two treatment arms (P > 0.05 in all).
Conclusions:
These data show that in this small group of 10 subjects with chronic tetraplegia, administration of oral theophylline did not improve pulmonary function.
Keywords: Spinal cord injuries, Tetraplegia, Pulmonary function, Respiration, Theophylline, Aminophylline, Motor recovery, Diaphragm
INTRODUCTION
Patients with cervical tetraplegia have weak respiratory muscles, which may predispose them to retention of bronchial secretions, atelectasis, pneumonia, respiratory muscle fatigue, and respiratory failure. Theophylline has been traditionally used in patients with airway disease, primarily for its bronchodilating and central nervous-stimulating effects. Over the last 2 decades, studies involving healthy volunteers have shown that theophylline can also increase the contractility of the diaphragm and protect against respiratory muscle fatigue (1,2). A similar effect has also been reported in patients with chronic obstructive airway disease (COPD) and in postoperative patients with respiratory muscle dysfunction (3–7). In a controlled study in COPD patients, Murciano and colleagues (5) reported that administration of theophylline at therapeutic dosages increased maximal transdiaphragmatic pressure by 16% and suppressed diaphragmatic fatigue in all patients. In another study, also in COPD patients (4), the same investigators found that the long-term administration of theophylline was associated with improvements in dyspnea scores and arterial blood gas levels and was related to improvements in respiratory muscle function.
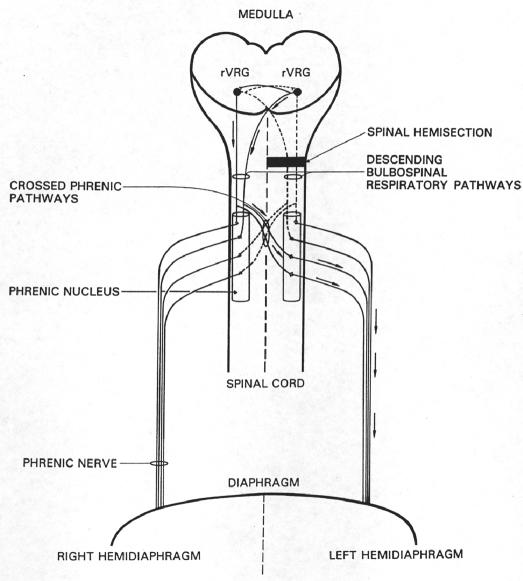
Theophylline has been shown to have beneficial effects on phrenic nerve and diaphragm activation after experimental cervical spinal cord injury in animal models (8–10). Specifically, systemic theophylline administration restores phrenic nerve activity and hemidiaphragm function in rats that have been subjected to an acute ipsilateral C2 spinal cord hemisection that completely disrupts descending respiratory drive to the phrenic motor nucleus. In the hemisection model, theophylline is believed to restore phrenic nerve activity by its action as an adenosine A1-receptor antagonist (9,10). Acting at the A1 receptor, theophylline facilitates synaptic function by activating an uninjured, previously quiescent respiratory tract. This tract crosses the midline of the cord from the contralateral intact cord below the level of the injury to innervate phrenic motoneurons ipsilateral and caudal to the hemisection (Figure 1). The latent pathway is referred to as the crossed phrenic pathway (11). Restored phrenic nerve activity correlates with restored function of the otherwise paralyzed ipsilateral hemi-diaphragm (12). The crossed phrenic pathway has been demonstrated in several mammalian species, but its correlate in man is uncertain. However, a case report citing the use of theophylline in a patient with non–ventilator-dependent tetraplegia noted improvement in measures of pulmonary muscle strength surface diaphragm electromyography (sEMG, maximal inspiratory pressure [MIP]) and central respiratory drive (P100 during MIP) after 3 weeks of oral administration (13). Additionally, a case report in which a patient with ventilator-dependent tetraplegia received aminophylline therapy describes subsequent increased activation of the patient's diaphragm, as evidenced by sEMG and the ability of the patient to be liberated from ventilatory support (14).
Figure 1. Inspiratory drive to phrenic motoneurons is mediated by medullary neurons in the rostral division of the ventral respiratory group (rVRG). These neurons project bilaterally to the phrenic nuclei. Both the crossed and uncrossed rVRG pathways have latent spinal decussating collaterals that also innervate the phrenic nuclei (ie, the crossed phrenic pathways). Hemisection rostral to the phrenic nucleus interrupts (dotted lines) the major bulbospinal drive to the ipsilateral phrenic nucleus, which results in paralysis of the left hemidiaphragm. Systemic administration of theophylline enhances descending inspiratory drive and activates the previously latent crossed phrenic pathways, which can now depolarize phrenic motoneurons ipsilateral and caudal to the hemisection. Arrows indicate the pathway followed by respiratory impulses to restore function to the hemi-diaphragm paralyzed by the spinal cord injury. (Reprinted from reference 29 with permission from Academic Press.).
In spite of the numerous animal studies (8–10,15) and the 2 clinical case reports (13,14) that indicate that theophylline improves respiratory muscle function after cervical spinal cord injury, a detailed clinical study assessing the effects of theophylline on improving measures of respiratory function in a group of patients with chronic tetraplegia has never been conducted. In the present study, using a double-blind placebo-controlled crossover design, we administered sustained-release oral theophylline to a group of 10 patients with chronic tetraplegia and assessed changes in maximum inspiratory and expiratory pressure and pulmonary function indices after 6 weeks of treatment.
METHODS
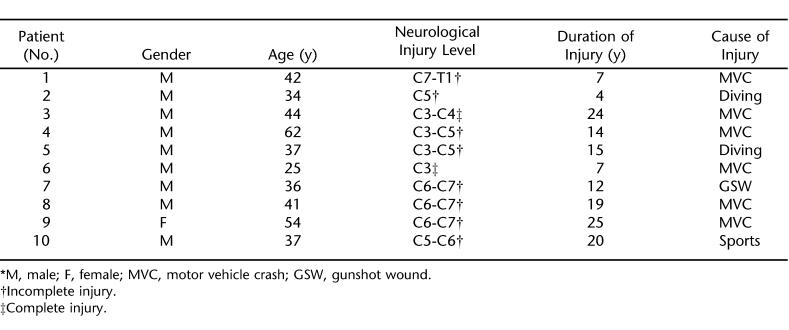
Following a protocol approved by our institutional Human Investigation Committee and after obtaining informed consent, patients with chronic tetraplegia who were not receiving mechanical ventilatory assistance were recruited for the study (neurological levels ranged from C3 to C7-T1) (Table 1). Patients with a history of obstructive airway disease or cardiac, liver, or renal disease were excluded from the study.
Table 1.
Patient Characteristics *
Baseline (pretreatment) pulmonary function testing and measurements of static inspiratory and expiratory pressures were completed in all patients. Spirometry (forced vital capacity (FVC), forced expiratory volume at 1 second (FEV1), and lung volumes (Collins, Braintree, MA) were acquired by methods adhering to American Thoracic Society (ATS) standards for acceptability and reproducibility (16); the helium dilution method was used to measure lung volumes. All patients in the study fulfilled the acceptability and reproducibility requirements set forth by ATS at each study time point. MIP was measured during maximal voluntary efforts at residual volume (RV) and maximal expiratory pressure (MEP) during maximal expiratory effort at total lung capacity (TLC).
To measure respiratory pressures, patients breathed on a flanged-style mouthpiece connected to a small tube and a differential pressure transducer (Validyne, North-ridge, CA). The tube connecting to the mouth piece included a 1-mm id orifice to prevent closure of the glottis. Respiratory flow was measured with a pneumo-tachograph (Hans Rudolph, Kansas City, MO) connected to the distal end of the tube, and volume was measured by digitally integrating the flow signal. Lung volume, mouth pressure, and flow signals were digitized (Power-Lab, AD Instruments, Colorado Springs, CO) and recorded on a computer hard disk for analysis. Measurements of respiratory muscle strength using a flanged-style mouthpiece typically yield lower MEP values when compared with those obtained with a tube-style mouthpiece. This is true for both normal volunteers (17) and for patients with tetraplegia (18) and is related to the inability of the buccal muscles to maintain an adequate mouth seal with the flanged-style mouthpiece. We paid special attention during measurements of MEP to ensure that there was no air leak around the mouthpiece during forceful expiration. Patients were made aware of this, and the operator held the mouthpiece in the occasional patient who could not assure a proper seal.
For MIP, the patients wore a nose clip and breathed quietly on the mouthpiece for about 1 minute before exhaling to RV. A 3-way valve was then occluded, and the patient performed a maximal inspiratory (Mueller) maneuver. For MEP, the patient inhaled to TLC and, after airway occlusion, performed a maximal expiratory effort. Patients were asked to maintain the pressure for at least 1 second. The best of 4 reproducible mouth pressures was chosen for analysis. During measurements, patients were encouraged to give the maximal effort. A visual feedback of attained pressures was displayed on a computer monitor to all patients. All respiratory pressure measurements were obtained by the same individual. Both the person taking the measurements and the patient were blind with regard to whether theophylline or placebo had been taken for the previous 6 weeks.
Protocol
Following baseline measurements of pulmonary function testing, patients were randomly assigned (by means of sealed envelopes) to receive either oral sustained-release theophylline or placebo for 6 weeks. A serum theophylline level was obtained on all patients after 7 to 8 days and adjustments of the prescribed dosage were made, if needed, to achieve a therapeutic level (10–20 μg/mL). All dosage adjustments were made by a physician who did not participate in the measurements, the randomization process, or data analysis. After 6 weeks of treatment, the patients returned to the laboratory for repeat pulmonary function testing and respiratory muscle strength measurements. At the time of testing, a serum theophylline level was drawn. Blood samples for theophylline assay were drawn within the same time frame (within 3–4 hours) after the last dose of medication in all patients, regardless of the study arm. After a washout period of 2 months, the 2 patient groups were crossed over. Thus, the patients who received theophylline in the first arm received the placebo for the next 6 weeks, and the patients who were initially administered the placebo received theophylline for the same length of time. During the second arm, pulmonary function and respiratory muscle measurements were performed as described above.
Data are reported as mean ± SEM. Respiratory pressures are reported as the percent change from baseline. The 2 treatments for each response (MIP, MEP, FEV1, FVC, and TLC) were compared using a general linear model (GLM). The model included the following effects: sequence, sequence within subjects (random effect), period, treatment, and an error. The 95% confidence interval for the difference between drug (Th) and placebo (P) was calculated based on the error mean square of the GLM. The analysis was performed using a commercially available software program (SAS, version 6.04; SAS Inc, Cary, NC). Significance was set at P < 0.05.
RESULTS
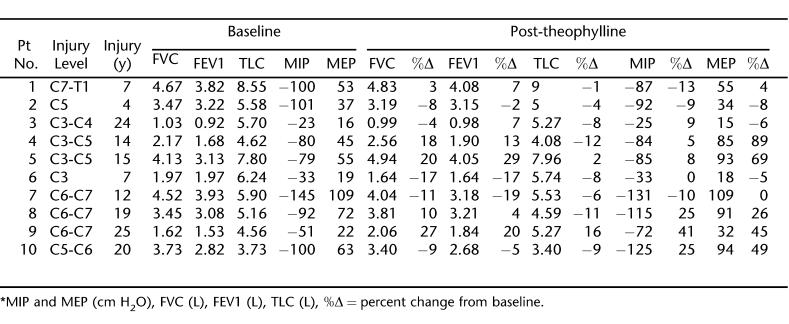
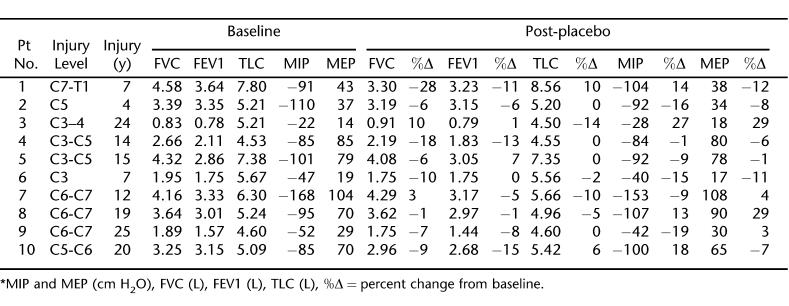
Twenty-four patients with chronic tetraplegia were initially recruited for the study. Of these, 10 patients completed both arms of the study. The remaining 14 patients withdrew from the study for various reasons, including noncompliance with the study protocol and adverse effects of the theophylline (nausea and stomach upset). The characteristics of the 10 patients who completed the study are shown in Table 1. The mean serum theophylline level at the end of the first treatment week was 12.2 ± 1.2 μg/mL, and on the day of treatment completion, this level measured 12.6 ± 1.4 μg/mL. While receiving placebo, all patients had theophylline levels below detectable values. The baseline respiratory values and the effects of 6 weeks of theophylline and placebo treatment on these values for each patient are shown in Tables 2 and 3, respectively.
Table 2.
Theophylline's Effect *
Table 3.
The Placebo Effect *
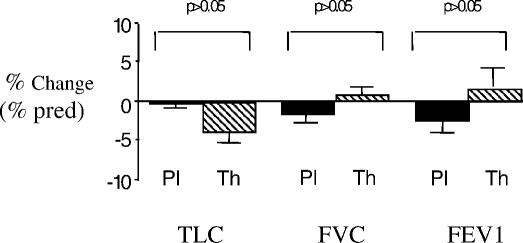
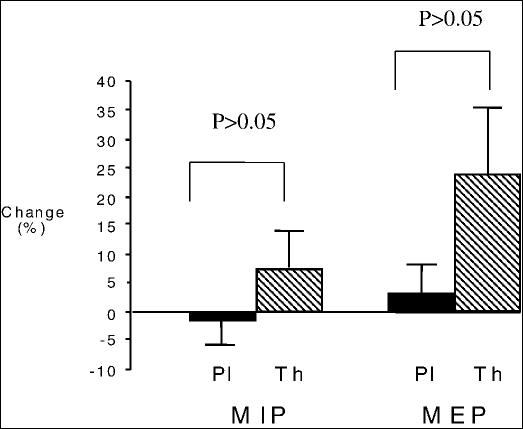
As can be seen from Tables 2 and 3, there was a high level of variability in measured respiratory parameters among the 10 patients before and after each arm of treatment. When group means were determined, the percent change from baseline values in TLC, FVC, or FEV1 did not differ significantly between the two treatment arms (Figure 2; P > 0.05 in all). In addition, the mean percent change in MIP or MEP with theophylline treatment did not differ from the changes while the participants were on placebo (7.5 ± 5.4% vs −1.4 ± 4.4%; P > 0.05 for MIP and 24.0 ± 11.8% vs −3.6 ± 4.5%; P > 0.05 for MEP) (Figure 3).
Figure 2. Percent changes from baseline measurements in percent predicted (% pred) values for total lung capacity (TLC), forced vital capacity (FVC), and forced expiratory volume at 1 second (FEV1) during theophylline (Th) (cross-hatched bars) or placebo (Pl) (solid bars) treatment. No statistically significant changes were found within the group of 10 patients with tetraplegia (P > 0.05).
Figure 3. Percent changes from baseline measurements in maximal inspiratory (MIP) and maximal expiratory pressure (MEP) in the group of 10 patients after taking theophylline (Th) or placebo (Pl). There is a trend for an increase in both pressures, which did not reach statistical significance.
DISCUSSION
Respiratory complications are a considerable source of morbidity and mortality in patients with chronic tetraplegia, not only during the period immediately following spinal cord injury, but also years after the acute injury. Months to years following the initial injury, the increased risk for pulmonary complications, especially atelectasis and pneumonia, persists (19). The risk for ventilatory failure is particularly high in patients with complete injury and borderline respiratory muscle function (20). Pharmacological treatments that may prevent ventilatory failure or delay its onset have not been tested in these patients.
The question as to whether theophylline increases respiratory muscle strength in patients with abnormal muscle function as well as in normal volunteers has been highly debated over the last 2 decades. In agreement with the data of Murciano et al (5), Nietrzeba et al (3) found that intravenous aminophylline improved maximal inspiratory pressure, peak inspiratory flow rate, and exercise performance in patients with COPD. In contrast, Cooper et al (7) showed that acute administration of aminophylline had no effect on respiratory muscle strength and exercise tolerance in patients with COPD. The results from studies in healthy volunteers are equally controversial, with improvements in respiratory muscle strength seen in some (1,2,21) but not all studies (22–25). To help explain these disparate data, other studies have indicated that theophylline may increase diaphragmatic contractility at relatively higher concentrations that are poorly tolerated by patients or healthy individuals (26).
Theophylline may improve expiratory muscle strength by enhancing conduction in descending bulbospinal respiratory motor pathways that innervate the intercostals and abdominal muscles. Alternately, it has been suggested (27) that theophylline may work by enhancing contraction of muscle fibers via inotropic effects on skeletal muscle. It is also possible that bronchodilation resulting from theophylline administration may result in increased FVC and thus increased MEP. Our study was not specifically designed to determine the mechanism by which theophylline might improve MEP or MIP.
The present study is the first controlled study to examine the effects of theophylline on pulmonary function in a group of patients with tetraplegia. Our data show that theophylline had no effect on respiratory muscle indices or on pulmonary function testing (Figure 2). Animal studies have clearly shown that theophylline is effective in improving respiratory muscle function after experimental cervical spinal cord injury when latent respiratory pathways in the spinal cord are spared and they are available to be activated by the drug (8–10). As we try to relate the animal studies using theophylline (8–10,15) to human cases, we would expect that if the patient's injury is extensive (complete disruption of spinal pathways) and involves these latent spinal pathways, then the efficacy of theophylline would not be as great. Therefore, those patients with an incomplete injury might exhibit sparing of latent respiratory pathways that theophylline could target. The level of injury varied among patients and included patients with low (C7-T1), intermediate (C4–C5), or high (C3) cervical lesions. Therefore, respiratory muscle function varied among our patients, as shown by the indices of pulmonary function testing and the respiratory muscle strength (Table 2). Measurements of MIP and MEP showed that 4 out of 10 patients had weak inspiratory muscles (MIP lower than −85 cmH2O), whereas all patients had weak expiratory muscles (mean MEP: 49 cmH2O) (28). Additionally, the aforementioned animal studies were performed in acutely injured animals. It may be the case that in chronically injured humans, the window of opportunity for plasticity in the system has closed, or that plasticity has already occurred to its full extent. In this case, theophylline may be of little use. The variability within our patient group in levels of prestudy respiratory dysfunction, levels of injury, completeness of injury, and time since injury coupled with our small number of patients (n = 10) likely led to our lack of significant findings with theophylline treatment. Furthermore, to the best of our knowledge, a comprehensive study of the effects of theophylline on acutely injured patients has not been conducted.
In this study, the average theophylline level was similar to levels reported by previous studies examining the effects of theophylline in normal volunteers (1,2) or in patients with respiratory muscle weakness (4,5). On further analysis, increases in MIP or MEP observed in some patients after theophylline administration (Table 2) were not associated with high therapeutic range levels (26). It is noteworthy that the withdrawal rate from the study was very high. Patients withdrew from the study mainly because of their inability to comply with the study protocol or because of side effects, such as nausea or stomach upset, related to theophylline usage.
CONCLUSION
Based on the data collected in this study, we conclude that the long-term administration of theophylline does not have a significant effect on pulmonary function in patients with chronic traumatic tetraplegia. However, studies in traumatic tetraplegia involving groups with a larger number of patients who are more homogeneous in terms of spinal injury level, injury extent, and time from injury should be conducted to further investigate theophylline's possible efficacy in treating respiratory muscle dysfunction in these patients.
Acknowledgments
We wish to thank Elias Zintzaras, MSc, PhD (Department of Biomathematics University of Thessaly School of Medicine, Larisa, Greece), for his help with the statistical analysis. His expertise was invaluable to the study.
Footnotes
This study was supported by National Institutes of Health grant HD31550 (H.G.G.).
REFERENCES
- Gauthier AP, Yan S, Sliwinski P, Macklem PT. Effects of fatigue, fiber length, and aminophylline on human diaphragm contractility. Am J Respir Crit Care Med. 1995;152:204–210. doi: 10.1164/ajrccm.152.1.7599825. [DOI] [PubMed] [Google Scholar]
- Wanke T, Merkle M, Zifko U, et al. The effect of aminophylline on the force-length characteristics of the diaphragm. Am J Respir Crit Care Med. 1994;149:1545–1549. doi: 10.1164/ajrccm.149.6.8004311. [DOI] [PubMed] [Google Scholar]
- Nietrzeba RM, Elliott CG, Adams TD, Yeh MP, Yanowitz FG. Effects of aminophylline upon the exercise performance of patients with stable chronic airflow obstruction. Bull Eur Physiopathol Respir. 1984;20:361–367. [PubMed] [Google Scholar]
- Murciano D, Aubier M, Lecocguic Y, Pariente R. Effects of theophylline on diaphragmatic strength and fatigue in patients with chronic obstructive pulmonary disease. N Engl J Med. 1984;311:349–353. doi: 10.1056/NEJM198408093110601. [DOI] [PubMed] [Google Scholar]
- Murciano D, Auclair MH, Pariente R, Aubier M. A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. N Engl J Med. 1989;320:1521–1525. doi: 10.1056/NEJM198906083202304. [DOI] [PubMed] [Google Scholar]
- Siafakas NM, Stoubou A, Stathopoulou M, Haviaras V, Tzanakis N, Bouros D. Effect of aminophylline on respiratory muscle strength after upper abdominal surgery: a double blind study. Thorax. 1993;48:693–697. doi: 10.1136/thx.48.7.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CB, Davidson AC, Cameron IR. Aminophylline, respiratory muscle strength and exercise tolerance in chronic obstructive airway disease. Bull Eur Physiopathol Respir. 1987;23:15–22. [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Alkylxanthine-induced recovery of respiratory function following cervical spinal cord injury in adult rats. Exp Neurol. 2001;168:123–134. doi: 10.1006/exnr.2000.7581. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Theophylline-induced recovery in a hemidiaphragm paralyzed by hemisection in rats: contribution of adenosine receptors. Neuropharmacology. 1998;37:113–121. doi: 10.1016/s0028-3908(97)00190-1. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Actions of specific adenosine receptor A1 and A2 agonists and antagonists in recovery of phrenic motor output following upper cervical spinal cord injury in adult rats. Clin Exp Pharmacol Physiol. 2002;29:915–923. doi: 10.1046/j.1440-1681.2002.03750.x. [DOI] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemi-section in the adult rat. Exp Neurol. 1992;116:219–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy A, Schrimsher G, Reier P, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemi-diaphragm following upper cervical spinal cord injury in adult rats. Neurorehabil Neural Repair. 1999;13:225–234. [Google Scholar]
- Ferguson G, Khanchandani N, Lattin C, Goshgarian HG. Clinical effects of theophylline on inspiratory muscle drive in tetraplegia. Neurorehabil Neural Repair. 1999;13:191–197. [Google Scholar]
- Bascom AT, Lattin CD, Aboussouan LS, Goshgarian HG. Effect of acute aminophylline administration on diaphragm function in high cervical tetraplegia: a case report. Chest. 2005;127:658–661. doi: 10.1378/chest.127.2.658. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Ellenberger JJ, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei in adult rats: a possible substrate for the crossed phrenic phenomenon. Exp Neurol. 1991;111:135–139. doi: 10.1016/0014-4886(91)90061-g. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society Standardization of Spirometry. 1994 Update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- Koulouris N, Mulvey DA, Laroche CM, et al. Comparison of two different mouthpieces for the measurement of Pimax and Pemax in normal and weak subjects. Eur Respir J. 1988;1:863–867. [PubMed] [Google Scholar]
- Tully K, Koke K, Garshick E, Lieberman SL, Tun CG, Brown R. Maximal expiratory pressures in spinal cord injury patients using two mouthpieces. Chest. 1997;112:113–116. doi: 10.1378/chest.112.1.113. [DOI] [PubMed] [Google Scholar]
- DeVivo MJ, Krause S, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- McKinley WO, Jackson AB, Cardenas DD, et al. Long-term medical complications after traumatic spinal cord injury: a regional model systems analysis. Arch Phys Med Rehabil. 1999;80:1402–1410. doi: 10.1016/s0003-9993(99)90251-4. [DOI] [PubMed] [Google Scholar]
- Aubier M, De Troyer A, Sampson M, Macklem PT, Roussos C. Aminophylline improves diaphragmatic contractility. N Engl J Med. 1981;305:279–252. doi: 10.1056/NEJM198107303050503. [DOI] [PubMed] [Google Scholar]
- Moxham J, Miller J, Wiles CM, Morris AJR, Green M. Effect of aminophylline on the human diaphragm. Thorax. 1985;40:288–292. doi: 10.1136/thx.40.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foxworth JW, Reisz GR, Knudson PG, Cuddy DR, Pyszczynski DR, Emory CE. Theophylline and diaphragmatic contractility: investigation of a dose-response relationship. Am Rev Respir Dis. 1988;138:1532–1534. doi: 10.1164/ajrccm/138.6.1532. [DOI] [PubMed] [Google Scholar]
- Levy RD, Nava S, Gibbons L, Bellmare F. Aminophylline and human diaphragm strength in vivo. J Appl Physiol. 1990;68:2591–2596. doi: 10.1152/jappl.1990.68.6.2591. [DOI] [PubMed] [Google Scholar]
- DeGarmo C, Cerny D, Conboy K, Ellis EF. In vivo effects of theophylline on diaphragm, bicep, and quadriceps strength and fatigability. J Allergy Clin Immunol. 1988;82:1041–1045. doi: 10.1016/0091-6749(88)90142-x. [DOI] [PubMed] [Google Scholar]
- Brophy C, Mier A, Moxham J, Green M. The effect of aminophylline on respiratory and limb muscle contractility in man. Eur Resp J. 1989;2:652–655. [PubMed] [Google Scholar]
- Aubier M. Effect of theophylline on diaphragmatic and other skeletal muscle function. J Allergy Clin Immunol. 1986;78:787–792. doi: 10.1016/0091-6749(86)90062-x. [DOI] [PubMed] [Google Scholar]
- Black LF, Hyatt RE. Maximal respiratory pressures: normal values and relationship to age and sex. Am Rev Respir Dis. 1969;99:696–702. doi: 10.1164/arrd.1969.99.5.696. [DOI] [PubMed] [Google Scholar]
- Castro-Moure F, Goshgarian HG. Morphological plasticity induced in the phrenic nucleus following cervical cold block of descending respiratory drive. Exp Neurol. 1997;147:299–310. doi: 10.1006/exnr.1997.6615. [DOI] [PubMed] [Google Scholar]