Abstract
Natronomonas pharaonis halorhodopsin (pHR) is an archaeal rhodopsin functioning as an inward-directed, light-driven Cl− pump. To characterize the electrophysiological features of the Cl− pump activity of pHR, we expressed pHR in Xenopus laevis oocytes and analyzed its photoinduced Cl− pump activity using the two-electrode voltage-clamp technique. Photoinduced outward currents were observed only in the presence of Cl−, Br−, I−, 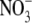 , and SCN−, but not in control oocytes, indicating that photoinduced anion currents were mediated by pHR. The relationship between photoinduced Cl− current via pHR and the light intensity was linear, demonstrating that transport of Cl− is driven by a single-photon reaction and that the steady-state current is proportional to the excited pHR molecule. The current-voltage relationship for pHR-mediated photoinduced currents was also linear between −150 mV and +50 mV. The slope of the line describing the current-voltage relationship increased as the number of the excited pHR molecules was increased by the light intensity. The reversal potential (VR) for Cl− as the substrate for the anion pump activity of pHR was about −400 mV. The value for VR was independent of light intensity, meaning that the VR reflects the intrinsic value of the excited pHR molecule. The value of VR changed significantly for the R123K mutant of pHR. We also show that the Cl− pump activity of pHR can generate a substantial negative membrane potential, indicating that pHR is a very potent Cl− pump. We have also analyzed the kinetics of voltage-dependent Cl− pump activity as well as that of the photocycle. Based on these data, a kinetic model for voltage-dependent Cl− transport via pHR is presented.
, and SCN−, but not in control oocytes, indicating that photoinduced anion currents were mediated by pHR. The relationship between photoinduced Cl− current via pHR and the light intensity was linear, demonstrating that transport of Cl− is driven by a single-photon reaction and that the steady-state current is proportional to the excited pHR molecule. The current-voltage relationship for pHR-mediated photoinduced currents was also linear between −150 mV and +50 mV. The slope of the line describing the current-voltage relationship increased as the number of the excited pHR molecules was increased by the light intensity. The reversal potential (VR) for Cl− as the substrate for the anion pump activity of pHR was about −400 mV. The value for VR was independent of light intensity, meaning that the VR reflects the intrinsic value of the excited pHR molecule. The value of VR changed significantly for the R123K mutant of pHR. We also show that the Cl− pump activity of pHR can generate a substantial negative membrane potential, indicating that pHR is a very potent Cl− pump. We have also analyzed the kinetics of voltage-dependent Cl− pump activity as well as that of the photocycle. Based on these data, a kinetic model for voltage-dependent Cl− transport via pHR is presented.
INTRODUCTION
Halorhodopsin (HR), discovered in the archaebacterium Halobacterium is an inward-directed Cl− pump (1–4). HR, functioning as a Cl− pump, has the ability to transport Cl− against an electrochemical gradient, and can generate an inside-negative membrane potential to support ATP synthesis (1,5,6). Since its original discovery, several HRs from different sources have been reported; but most studies focusing on the functional aspects of HR have used Halobacterium salinarum halorhodopsin (sHR) and Natronomonas pharaonis halorhodospin (pHR) as model systems (4,7). These two proteins show high similarity in amino acid sequences (identity, 66%; homology, 97%). Based on the high sequence homology between the two Cl− pumps and their similar photoinduced intermediates, it has been assumed that these proteins have similar structure (8–11).
Recently, the x-ray crystal structure of sHR was determined at a resolution of 1.8 Å, and it showed striking similarity to that of bacteriorhodopsin (BR) (4,12,13). sHR is composed of seven α-helices, forming a transmembrane channel-like structure. The channel is divided into a cytoplasmic (CP) and an extracellular (EC) half-channels, separated by the chromophore retinal, which is bound through the Schiff base to Lys-242 (12). The crystal structure revealed that Cl− interacts with the proton of the protonated Schiff base and the hydroxyl group of Ser-115, as well as the hydrophobic methyl group of Thr-111. The crystal structure also indicated that Cl− is hydrated by a cluster of three water molecules that form hydrogen bonds with neighboring amino acid residues. It is noteworthy that anion binding is observed in the crystal structure only at this position of the EC channel, implying that Cl− is translocated to the cyotoplasmic space by the photon (12). It is assumed that a Cl−-binding or -interacting site in the cytoplasmic channel is also required for the release of the translocated Cl− into the cytoplasmic space.
Based on the crystal structure, as well as the kinetic analysis, of photoinduced intermediates, the vectorial transport of Cl− via HR is composed of three main processes (6,9,11,12,14): 1), Cl− binding to the vicinity of the protonated Schiff base region of the retinal chromophore (the EC binding site); 2), Cl− translocation; and 3), Cl− release from the CP binding site. Investigation of the electrophysiological features of HR pump activity is very important for a better understanding of the function of the pump at the molecular level. The functional activity of HR has been studied using different experimental approaches (1,5,15–19). The function of HR as an inward-directed Cl− pump has been clarified with cell envelope vesicles from H. salinarum, as well as with intact bacteria (1,5,20). Spectroscopic and flux measurements under conditions of different membrane potential have been done with vesicles or bacteria, but these were difficult experiments since precise analysis of voltage dependence is difficult to study with these systems owing to the small size of the bacteria or membrane vesicles. Direct electrical measurements have been undertaken with HR membrane sheets capacitatively coupled to black lipid membranes (15) or to thin films (16–18), and also with membrane suspensions (19). The latter system has an inherent drawback because the orientation of HR cannot be controlled well. Thus, details of the electrophysiological aspects of the Cl− pump activity of HR are still lacking.
In this study, we used the Xenopus laevis oocyte expression system to elucidate the electrophysiological features of N. pharaonis halorhodopsin (pHR). In this system, the photoinduced currents due to anion transport could be determined precisely to analyze the kinetics of the transport process. Here, we demonstrate that the Cl− pump activity via pHR is dependent on membrane potential. Based on this voltage dependence, we show for the first time that the value of reversal potential (VR) at which the pump current by pHR becomes zero is an intrinsic property of the pump independent of light intensity. These studies also show that the Cl− pump activity by pHR can generate a considerable negative membrane potential (∼−400 mV), indicating that pHR is a highly active Cl− pump.
MATERIALS AND METHODS
Construction of the expression plasmid of the histidine-tagged pHR in the oocyte expression vector pGH19
The pGH19 vector (kindly provided by Dr. Peter S. Aronson, Yale University School of Medicine) contains the 3′- and 5′-untranslated regions of the Xenopus β-globin gene on the downstream and upstream sides, respectively, of the cloning site. The coding region of pHR was amplified by polymerase chain reaction using the primers 5′-GATATATAGCCATGACTGAGACATTGCCACC-3′ (sense) and 5′-TAAGCTTCAGTGGTGGTGGTGGTGGTGCTCCAGGTCGTCAGCGGGAGTGC-3′ (antisense), and pET21c(+)-pHR plasmid (21) as the template. A HindIII site (underlined residues) was added to the 5′-end of the antisense primer for the purpose of subcloning. The polymerase chain reaction product was first subcloned in a pGEM-T vector (Promega, Madison, WI), and the confirmation of its complete sequence was carried out with the Taq DyeDeoxy terminator method, employing an automated Applied Biosystems 377 Prism DNA sequencer (PerkinElmer, Foster City, CA). The insert was then released by EcoRI/HindIII double digestion. The pGH19 vector was linearized with EcoRI/HindIII and then used for the ligation of the pHR cDNA. The resultant product was partially sequenced to confirm the orientation of the insert. The mutant plasmid for the expression of R123K pHR was constructed with a Quickchange site-directed mutagenesis (Stratagene Cloning Systems, San Diego, CA), as described previously (14). The mutation introduced into the plasmid was also confirmed by sequencing.
Functional expression of the histidine-tagged pHR in Xenopus laevis oocytes
The amplified pHR cDNA was expressed heterologously in Xenopus oocytes by cRNA injection. Capped cRNA was synthesized using mMESSAGE mMACHINE kit (Ambion, Austin, TX). Mature oocytes (stage V-VI) from Xenopus were isolated by treatment with collagenase (1.6 mg/ml), manually defolliculated, and maintained at 18°C in modified Barth's medium, supplemented with 3 μM retinal and 50 μg/ml gentamicin, as described previously (22,23). On the following day, oocytes were injected with 50 ng pHR cRNA in a 50 nl volume and incubated for 3–5 days. The oocytes were used for electrophysiological studies 3–5 days after cRNA injection. Electrophysiological studies were performed with the two-electrode voltage-clamp (TEVC) technique, as described previously. The oocyte was superfused with perfusion buffer (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM Hepes, and 6 mM Tris, pH 7.5). After the current stabilized, the oocyte was superfused with the uptake buffer. The oocyte was voltage-clamped at −50 mV. The composition of the uptake buffer was 2 mM Kgluconate, 1 mM Mg(gluconate)2, 1 mM Ca(gluconate)2, 10 mM Hepes, and 100 mM sodium salt (NaCl, NaBr, NaI, NaSCN, NaNO3, or sodium 2-(N-morpholino)ethanesulfonate (NaMes)), pH 7.5. The current-voltage (I-V) relationship was analyzed immediately before and within a few seconds after illumination with green light (530 ± 18 nm) when the current reached the maximum and steady state. The green light was produced by a light-emitting diode, Luxeon V Star (Lumileds Lighting, San Jose, CA). The measurements of currents at different membrane potentials were made using short pulses (100 ms) in the range of −150 mV to +50 mV in 20-mV increments. Each pulse was separated with a pause (250 ms). TEVC experiments were performed with a TEVC amplifier CEZ-1250 (Nihon Kohden Industry, Tokyo, Japan) and a commercially available program (Clampex software, Axon Instruments, Foster City, CA). The photoinduced current at each applied voltage was calculated as the difference between the steady-state currents recorded before and after illumination. Saturation kinetics of photoinduced currents associated with the anion pump activity was analyzed with seven different concentrations of NaX (X = Cl−, Br−, 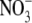 , SCN−, and I−). The light-induced currents are defined by kinetic parameters Imax (the maximal photoinduced current) and K0.5 (the anion concentration necessary for the induction of half-maximal current). Data for the photoinduced current (I) were fitted to the following Michaelis-Menten equation, describing a single saturable component, by an iterative nonlinear least-squares method (Origin, MicroCal, Northampton, MA):
, SCN−, and I−). The light-induced currents are defined by kinetic parameters Imax (the maximal photoinduced current) and K0.5 (the anion concentration necessary for the induction of half-maximal current). Data for the photoinduced current (I) were fitted to the following Michaelis-Menten equation, describing a single saturable component, by an iterative nonlinear least-squares method (Origin, MicroCal, Northampton, MA):
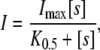 |
(1) |
where [s] is the concentration of the transportable anion in the perfusion buffer.
Protein expression and purification of the histidine-tagged pHR
The experimental details for protein expression and purification employing Escherichia coli BL21(DE3) cells have been described in a previous article (21). Fractions of the proteins using Ni-NTA agarose (Qiagen, Hilden, Germany) were collected by elution (flow rate, 56 mL/h) with buffer E (50 mM Tris-HCl (pH 7.0), 300 mM NaCl, 150 mM imidazole, and 0.1% n-dodecyl β-D-maltopyranoside (dodecyl maltoside (DM)) (Dojindo Lab, Kumamoto, Japan)). The yield of the recombinant pHR was almost the same as that reported previously (21).
Flash photolysis spectroscopy
The photocycle of pHR was analyzed by flash spectroscopy with a computer-controlled flash-photolysis apparatus for measuring transient absorption changes every 0.5 μs in the time range from 10 μs to 220 ms. The computer-controlled flash-photolysis apparatus was constructed as described previously (14). The absorbance of the sample in 50–1000 mM NaCl (containing 10 mM MOPS (pH 7.0) and 0.1% DM) was 0.5 at the absorption maximum, and the temperature was maintained at 20°C.
Data analysis of photocycling
The data collected at all wavelengths from 410 to 710 nm were fitted to a multiexponential equation. Singular value decomposition analysis of the observed data confirmed the existence of four kinetically distinguishable photoinduced intermediates (14). The spectrum of the ith-intermediate, Pi, and its decay time constant, τi, were calculated according to the Chizhov and Engelhard method (11,14).
RESULTS
Induction of photoinduced outward currents by Cl− and other anions in pHR-expressing Xenopus oocytes
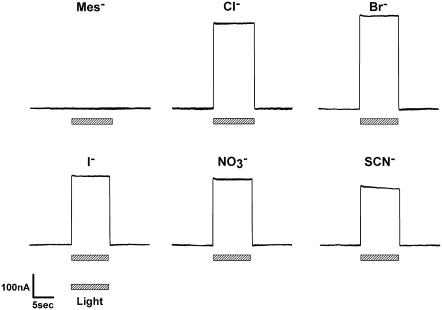
The photoinduced currents were monitored under voltage-clamp conditions in oocytes 3–5 days after microinjection of pHR cRNA. When pHR-expressing oocytes were illuminated with green light (λmax = 530 ± 18 nm) from the light-emitting diode, the presence of Cl− in the perfusion buffer induced marked outward currents (Fig. 1). Similar currents were also observed with other anions, such as Br− and I−, 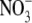 , or SCN−. These currents were, however, specific since the substitution of Cl− with Mes− failed to induce detectable currents. Uninjected oocytes and oocytes injected with water did not show photoinduced currents in the presence of Cl− with or without incubation of the oocytes with retinal (data not shown). These data demonstrate that pHR is able to transport Cl−, Br−, I−,
, or SCN−. These currents were, however, specific since the substitution of Cl− with Mes− failed to induce detectable currents. Uninjected oocytes and oocytes injected with water did not show photoinduced currents in the presence of Cl− with or without incubation of the oocytes with retinal (data not shown). These data demonstrate that pHR is able to transport Cl−, Br−, I−, 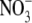 , and SCN−, and that the transport process is associated with the induction of outward currents. Outward currents in oocytes under voltage-clamp conditions indicate the transfer of negative charges into the oocytes, suggesting that pHR-mediated entry of Cl− and other anions into the oocytes is responsible for the outward currents. It is known that pHR transports not only Cl− and other halides (Br− and I−) but also
, and SCN−, and that the transport process is associated with the induction of outward currents. Outward currents in oocytes under voltage-clamp conditions indicate the transfer of negative charges into the oocytes, suggesting that pHR-mediated entry of Cl− and other anions into the oocytes is responsible for the outward currents. It is known that pHR transports not only Cl− and other halides (Br− and I−) but also 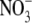 and SCN− (5,24–26). Our electrophysiological data with pHR, expressed heterologously in Xenopus oocytes, confirm these earlier observations.
and SCN− (5,24–26). Our electrophysiological data with pHR, expressed heterologously in Xenopus oocytes, confirm these earlier observations.
FIGURE 1.
Traces of representative currents photoinduced via pHR expressed heterologously in pHR-expressing Xenopus oocytes were superfused with standard buffer containing different anions (as sodium salts) at a concentration of 100 mM. The hatched bars below the current traces indicate the period (10 s) of illumination with green light (530 ± 18 nm). In control oocytes with no pHR expression, there were no detectable currents in response to light pulses (data not shown).
Voltage dependence and VR for pHR-mediated anion pump activity
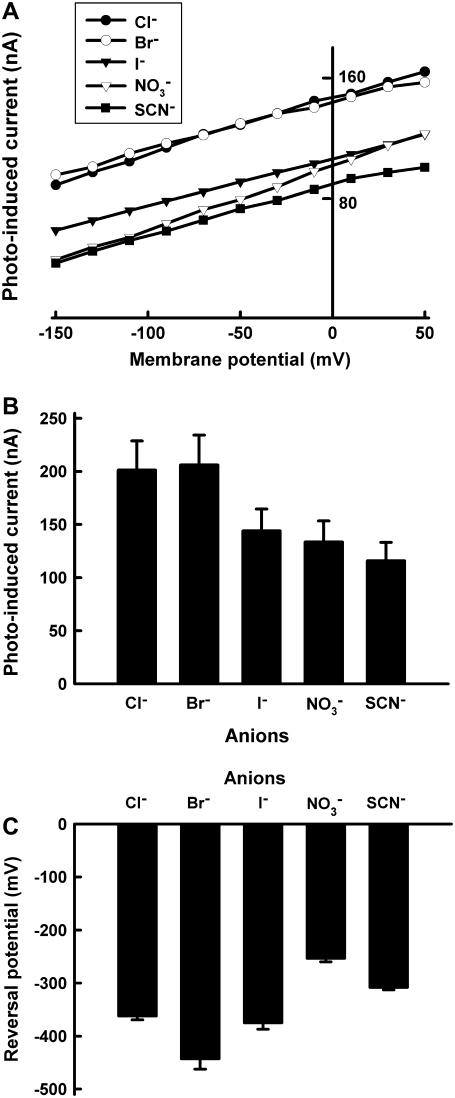
We then characterized the anion pump activity of pHR by using the photoinduced anion-dependent outward currents as the readout of the activity. Fig. 2 summarizes the I-V relationship and anion-dependent photoinduced currents at −50 mV. The I-V curve showed linearity in the measurable range of membrane potential (from −150 mV to +50 mV) (Fig. 2 A). At −50 mV, the order of the anion-dependent current induced by photons is Cl− = Br− > I− > 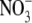 > SCN− (Fig. 2 B). The I-V relationship data were extrapolated to determine the x-intercepts (i.e., zero current), which correspond to the reversal potentials for different anions. The values for VR for different anions ranged from −250 mV to −450 mV (Fig. 2 C): The VR for Br− is the most negative, followed by that for Cl− and I−.
> SCN− (Fig. 2 B). The I-V relationship data were extrapolated to determine the x-intercepts (i.e., zero current), which correspond to the reversal potentials for different anions. The values for VR for different anions ranged from −250 mV to −450 mV (Fig. 2 C): The VR for Br− is the most negative, followed by that for Cl− and I−.
FIGURE 2.
Substrate specificity of pHR. (A) Representative current-voltage relationship (I-V curve) at steady state for pHR-mediated photoinduced currents in the presence of different anions (as sodium salts) (100 mM). (B) Outward photoinduced currents at −50 mV in the presence of various anions. The concentration of anions (as sodium salts) in the perfusion medium was 100 mM. (C) Reversal potentials for the anion pump activity of pHR with different anions. The I-V relationship was linear for all anions tested. Reversal potentials were estimated from the x-intercepts (i.e., zero current) with the extrapolation of the lines describing the I-V relationship. Data represent mean ± SE (n = 4–6).
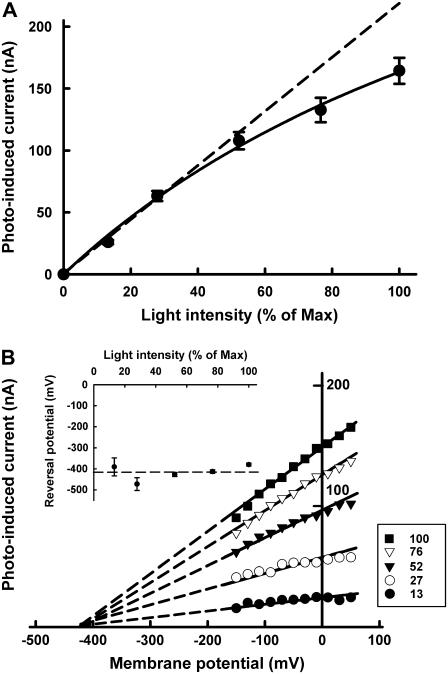
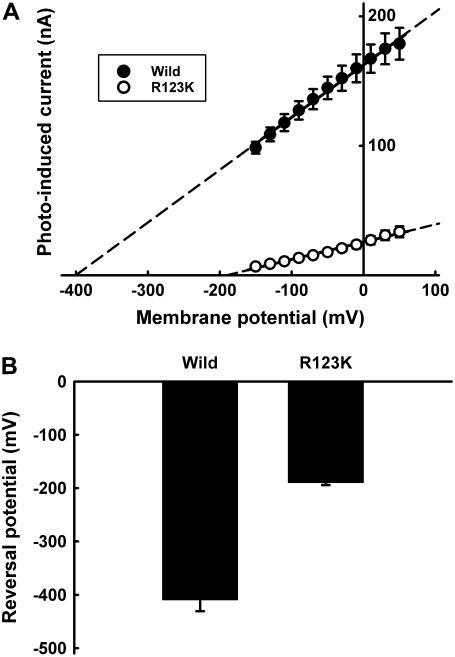
The voltage dependence of the photoinduced current via pHR-mediated Cl− transport was determined at different light intensities (Fig. 3). The magnitude of the photoinduced and Cl−-dependent currents via pHR was linear with light intensity initially but began to plateau subsequently (Fig. 3 A). The profile of the curve indicated that the transport of Cl− is driven by a single-photon reaction and that the steady-state current is proportional to the excited pHR molecule. The slope of the lines describing I-V relationship increased as the light intensity increased (Fig. 3 B). The x-intercept values were similar (∼−400 mV) at all light intensities tested, showing that the VR is independent of light intensity and that this value represents an intrinsic characteristic of the excited pHR molecule. This is further substantiated by the significant change in VR for the Arg-123 (R123K) mutant (Fig. 4). Arg-123 is critical for Cl− recognition and transport, and when this amino acid is mutated, it changes the Cl− pump activity and the VR. Taken collectively, the data show that the Cl− pump activity of pHR is robust and that the pump can theoretically generate a large negative membrane potential (∼−400 mV) in the presence of extracellular Cl−.
FIGURE 3.
Relationship of the Cl− pump activity of pHR to light intensity (A) and membrane potential (B). The photoinduced currents in the presence of 100 mM Cl− were determined at steady state in pHR-expressing oocytes at different light intensities. The light intensity was measured with a photometer and expressed as percentage of maximal light intensity. (A) The dependence of photoinduced current at −50 mV on light intensity. (B) The dependence of photoinduced current on membrane potential at different light intensities. The VR was estimated from the x-intercepts by the extrapolation of the lines describing the I-V relationship. The reversal potentials remained unchanged irrespective of the light intensity. The inset in B represents the dependence of VR on light intensity. Data represent mean ± SE (n = 7–10).
FIGURE 4.
Reversal potentials for wild-type and mutant (R123K) pHRs. (A) Representative I-V curve at steady state for wild-type and mutant (R123K) pHRs. The concentration of Cl− in the perfusion medium (as NaCl) was 100 mM. (B) Reversal potentials for wild-type and mutant (R123K) pHRs. The values were determined from the x-intercepts by the extrapolation of the lines describing the I-V relationship. Data represent mean ± SE (n = 6).
Kinetics of photoinduced currents associated with pHR-mediated Cl− pump activity
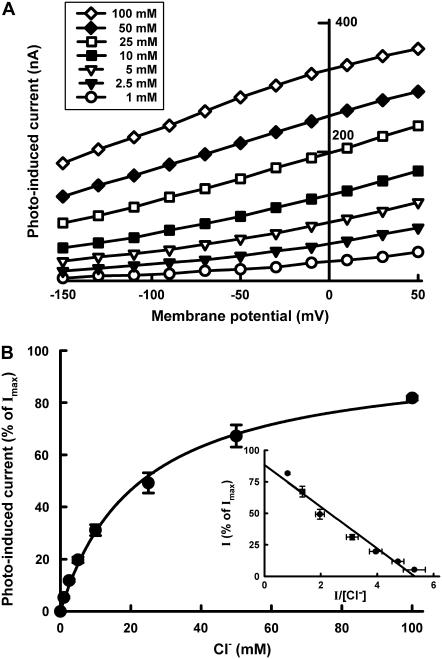
Employing Cl− as the substrate, we analyzed the saturation kinetics of photoinduced currents in pHR-expressing oocytes. The relationship between the photoinduced current and Cl− concentration at different membrane potentials is described in Fig. 5. The photoinduced outward currents were saturable with increasing concentrations of Cl− at all membrane potentials examined. The inset in Fig. 5 shows the Eadie-Hofstee plot at a membrane potential of −50 mV. The kinetic parameters were calculated by fitting data to Eq. 1; estimated values were K0.5 (concentration of Cl− needed for the half-maximal photoinduced current) = 24.0 ± 2.5 mM (mean ± SE) and Imax (maximal photoinduced current) = 324 ± 22 nA (mean ± SE). Under the conditions employed in these studies, the dissociation constant of pHR for Cl− is thus estimated to be ∼25 mM. Similar experiments were conducted with other anions recognized by pHR and kinetic parameters were calculated for each of them (Table 1). The order of the reciprocal of K0.5 value reflecting a binding affinity of anion is: Br− > I− > Cl− > SCN− > 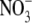 . The K0.5 value seems to be related to the size of the hydrated anions. The order of Imax value is: Cl− > Br− > I− >
. The K0.5 value seems to be related to the size of the hydrated anions. The order of Imax value is: Cl− > Br− > I− > 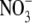 > SCN−.
> SCN−.
FIGURE 5.
Saturation kinetics of the photoinduced Cl− pump activity of pHR. (A) Representative I-V curve at steady state in pHR-expressing oocytes at increasing concentrations of Cl− (as NaCl) (1–100 mM). (B) Photoinduced currents at −50 mV indicative of Cl− entry as a function of Cl− concentration. The experiment was repeated five times with different oocytes. Since the expression levels of pHR varied among the oocytes, data were normalized by the value of Imax, calculated using Eq. 1, in each oocyte. The inset shows the Eadie-Hofstee plot. The K0.5 and Imax values calculated with a Michaelis-Menten-type equation (Eq. 1) were 24.0 ± 2.5 mMand 324 ± 22 nA, respectively. Data represent the mean ± SE (n = 5).
TABLE 1.
Kinetic parameters for anion transport via pHR in the voltage-clamped oocytes at −50 mV
| Kinetic parameters
|
||
|---|---|---|
| Ion | K0.5 (mM) | Imax (nA) |
| Cl− | 24.0 ± 2.5 | 324 ± 22 |
| Br− | 11.2 ± 1.3 | 280 ± 27 |
| I− | 17.2 ± 1.3 | 243 ± 20 |
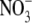 |
45.5 ± 3.8 | 193 ± 21 |
| SCN− | 27.4 ± 2.0 | 160 ± 19 |
The photoinduced currents indicating anion transport rates at −50 mV were determined with increasing concentrations of the anion substrates (1–100 mM). The experiment was repeated with five oocytes. The values for K0.5 and Imax were calculated with a Michaelis-Menten-type equation (Eq. 1). Data represent the mean ± SE (n = 5).
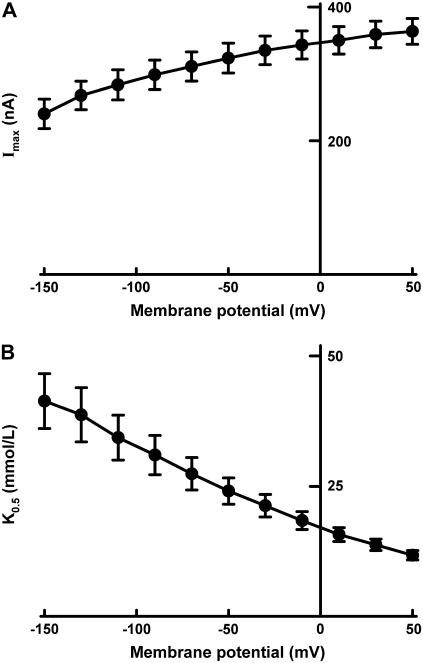
The dependence of the kinetic parameters, K0.5 and Imax, on membrane potential is described in Fig. 6. The Imax decreased as the membrane potential became more negative, indicating that the rate-determining process in pHR-mediated Cl− transport is dependent on membrane potential. The K0.5 also showed marked changes when membrane potential was altered. The value increased markedly as the membrane potential became more negative, implying that the apparent affinity for Cl− decreased at more negative membrane potentials.
FIGURE 6.
Influence of membrane potential on K0.5 (i.e., concentration of Cl− needed for the half-maximal photoinduced current) (A) and Imax (i.e., the maximal photoinduced current at saturating concentrations of Cl−) (B) in pHR-expressing oocytes.
The photocycle of pHR with varying Cl− concentrations
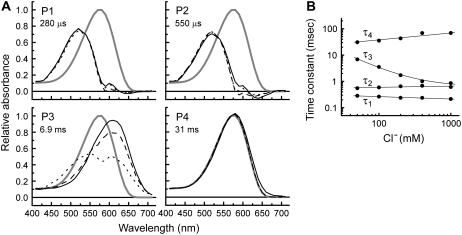
To evaluate the Cl−-dependent and the rate-determining processes during the photocycle reaction, we analyzed the photocycle of pHR at different concentrations of Cl− (0.05–1 M). The absorbance values observed at different wavelengths (410–710 nm) were fitted simultaneously with four exponentials, since singular value decomposition analysis and the calculation of standard deviation demonstrated the existence of four intermediates. The spectra of the kinetically distinguishable photoinduced intermediates were given in Fig. 7 A. The spectra of P1 and P2 were almost the same, and the absorption maximum (λmax) was ∼520 nm, whereas the spectrum of P4 showed the same absorption maximum as the original pigment. The profiles of P1, P2, and P4 intermediates were independent of Cl− concentration. The decay time constants (τ1 and τ2) were also independent of Cl− concentration (Fig. 7 B). The P1 and P2 intermediates are identified as L1 and L2 intermediates, respectively, as indicated by the spectral profile and the decay time constants. Judging from the spectra and the extremely long time constant, the P4 intermediate is identified as pHR′ intermediate. On the other hand, the spectra of P3 intermediate had two absorption maxima, implying that P3 is comprised of at least two physically defined intermediates that attain equilibrium promptly. Only the P3 spectrum revealed large Cl− dependence where considerable shift of the equilibrium occurs from one intermediate with λmax at ∼610 nm to the other with λmax at ∼510 nm. The intermediate with λmax at ∼610 nm can be thought to be Cl−-free, because the λmax value is attributed to a Cl−-free environment of the protonated Schiff base (21,24,26). Váró et al. (9,10) and Chizhov and Engelhard (11) also described the fast equilibrium between anion-bound and anion-free states in the photocycle. The shift of equilibrium in the P3 intermediate with changes in Cl− concentration means that this intermediate is involved in the interaction between Cl− and pHR. The decay time constant (τ3) decreased markedly as Cl− concentration increased. Judging from the photocycle sequence and the acceleration of the transition rate from P3 to P4 (pHR') by the external Cl−, we conclude that the transition is involved in the Cl− binding process. Váró et al. (9,10) also demonstrated that the process of Cl− binding to pHR becomes faster as the external Cl− concentration is increased.
FIGURE 7.
Results of the global fitting of the flash-photolysis data of wild-type pHR. (A) Absorption spectra of unphotolyzed (ground) state (P0,) and four kinetically distinguishable intermediates at three representative Cl− concentrations (solid line, 50mM; dashed line, 400 mM; dotted line, 1000 mM). The P3 state shows the largest dependence on Cl− concentration, both in terms of the time constant (τ3) and the absorption spectrum. The figures represent the time constants corresponding to the states in 50 mM NaCl, pH 7.0, at 20°C. (B) Time constants for the photochemical transitions as a function of Cl− concentration.
DISCUSSION
In this article, we report on the successful expression of functional pHR in Xenopus laevis oocytes for electrophysiological characterization of its anion pump activity. This has allowed us to carry out a detailed analysis of the photoinduced anion-dependent outward currents associated with pHR-mediated entry of anions into the oocyte. As can be seen in Fig. 1, photoinduced outward currents were detectable only in pHR-expressing oocytes when the oocytes were superfused with anions such as Cl−. Substrate specificity studies showed that pHR can recognize and pump a variety of anions, including not only monoatomic (e.g., Cl−, Br−, and I−) but also polyatomic (e.g., 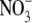 and SCN−) anions. These data are in accordance with those reported previously (5,24,26). The photoinduced currents showed saturation kinetics with all anions that were transported via pHR. Duschl and Lanyi have also determined the Cl− transport activity via pHR, employing envelope vesicles from N. pharaonis (5). The concentration dependence of Cl− transport activity by pHR followed the Michaelis-Menten-type kinetics, with a single saturable component. The K0.5 value for Cl− reported by Duschl and Lanyi was 25 mM, consistent with the data presented here. The K0.5 value, which approximates the dissociation constant for the interaction of the transporter with its substrate, was determined for five different anions (Table 1). Based on these data, the rank order of substrate affinity is as follows: Br− > I− > Cl− > SCN− >
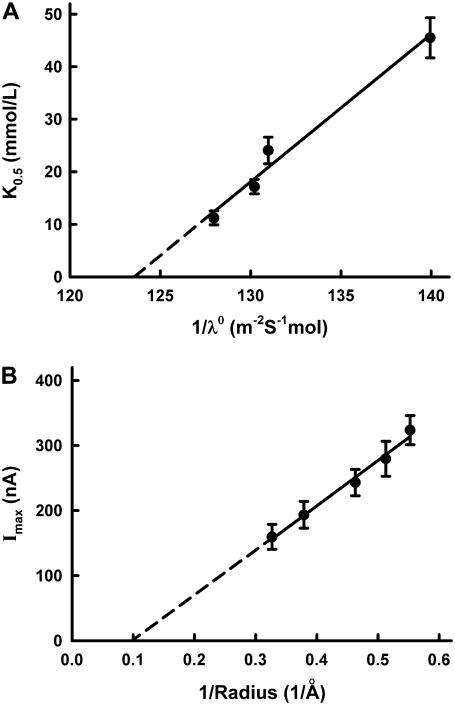
and SCN−) anions. These data are in accordance with those reported previously (5,24,26). The photoinduced currents showed saturation kinetics with all anions that were transported via pHR. Duschl and Lanyi have also determined the Cl− transport activity via pHR, employing envelope vesicles from N. pharaonis (5). The concentration dependence of Cl− transport activity by pHR followed the Michaelis-Menten-type kinetics, with a single saturable component. The K0.5 value for Cl− reported by Duschl and Lanyi was 25 mM, consistent with the data presented here. The K0.5 value, which approximates the dissociation constant for the interaction of the transporter with its substrate, was determined for five different anions (Table 1). Based on these data, the rank order of substrate affinity is as follows: Br− > I− > Cl− > SCN− > 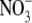 . The order of the affinity seems dependent on the size of hydrated rather than dehydrated anions, because there is good correlation between the K0.5 value and the reciprocal of the limiting equivalent conductivity (λ0) of anions in water (Fig. 8 A). In general, the λ0 value reflects the mobility of ion in water, which is dependent on the Stokes radius of ion. Using the spectroscopic analysis of the binding of different anions to sHR, Schobert and Lanyi demonstrated that the binding affinity is related not to the dehydrated radius of the transportable anion, but to the Stokes (i.e., hydrodynamic) radius, which reflects the radius of the hydration shell around the anion (27). On the basis of the crystal structure of sHR, the binding pocket of Cl− is composed of the protonated Schiff base, Ser-115, Arg-108, Asp-215, and Trp-112. It is noted that the anion Cl− remained partially hydrated by a cluster of three water molecules (12), suggesting that the binding pocket has enough room to accommodate anions with different sizes. This is one of the reasons why the affinity of anions is dictated by the radius of the hydration shell around the anion. In contrast to the K0.5 values, the capacity of the pump activity, Imax, is dependent on the dehydrated sizes of the anions (Fig. 8 B); the rank order of the maximal capacity of the pump activity is as follows:
. The order of the affinity seems dependent on the size of hydrated rather than dehydrated anions, because there is good correlation between the K0.5 value and the reciprocal of the limiting equivalent conductivity (λ0) of anions in water (Fig. 8 A). In general, the λ0 value reflects the mobility of ion in water, which is dependent on the Stokes radius of ion. Using the spectroscopic analysis of the binding of different anions to sHR, Schobert and Lanyi demonstrated that the binding affinity is related not to the dehydrated radius of the transportable anion, but to the Stokes (i.e., hydrodynamic) radius, which reflects the radius of the hydration shell around the anion (27). On the basis of the crystal structure of sHR, the binding pocket of Cl− is composed of the protonated Schiff base, Ser-115, Arg-108, Asp-215, and Trp-112. It is noted that the anion Cl− remained partially hydrated by a cluster of three water molecules (12), suggesting that the binding pocket has enough room to accommodate anions with different sizes. This is one of the reasons why the affinity of anions is dictated by the radius of the hydration shell around the anion. In contrast to the K0.5 values, the capacity of the pump activity, Imax, is dependent on the dehydrated sizes of the anions (Fig. 8 B); the rank order of the maximal capacity of the pump activity is as follows: 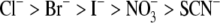 (Table 1). These results suggest that the rate-determining step in the transport cycle shows a dependence on the size of dehydrated ion. Recently, using FTIR spectroscopic analysis data, Shibata et al. demonstrated that the hydrophobicity of the environment in the vicinity of the protonated Schiff base is involved in the translocation of the anion from the EC binding site to the CP binding site (28). The anion might be dehydrated when it is translocated from the EC binding site to the CP binding site (12).
(Table 1). These results suggest that the rate-determining step in the transport cycle shows a dependence on the size of dehydrated ion. Recently, using FTIR spectroscopic analysis data, Shibata et al. demonstrated that the hydrophobicity of the environment in the vicinity of the protonated Schiff base is involved in the translocation of the anion from the EC binding site to the CP binding site (28). The anion might be dehydrated when it is translocated from the EC binding site to the CP binding site (12).
FIGURE 8.
Dependence of K0.5 on the limiting equivalent conductivities (λ0) of anions in water (A) and dependence of Imax on the dehydrated radii (B). The values for the limiting equivalent conductivities (λ0) of anions in water and the dehydrated radii were taken from published reports (33,34).
It is interesting to note that the anion pump activity of pHR shows voltage dependence. The I-V relationship is linear over the range of membrane potential employed (between −150 and +50 mV). The profile of the I-V relationship remains unchanged irrespective of the illumination intensity (Fig. 3). The slope of the I-V curve increased as the number of the excited pHR molecules increased with the light intensity, whereas the VR, obtained by the extrapolations of the lines describing the I-V relationship, did not change with different intensities of light. It seems plausible that the slope of the line describing the I-V relationship is a factor of the number of photoexcited pHR molecules. The linear relationship between photoinduced current and illumination intensity (Fig. 3) demonstrates that the transport of Cl− via pHR is driven by a single photon. On the other hand, the value of VR changed markedly for the mutant of Arg-123 (R123K) (Fig. 4). Since Arg-123 is very critical for the binding and transport of the substrates, the change in VR as a consequence of mutation of this particular residue suggests that VR directly reflects the intrinsic ion motive force of the pHR pump. On the basis of this voltage dependence, we show for the first time that the VR value at which the pump current is reduced to zero reflects the intrinsic motive force of the excited pHR molecule to pump Cl− into the cell. Based on the thermodynamic theory, the Gibbs free energy for the transport of Cl− by the excited pHR molecule (ΔGimf) is given by the following equation, since one chloride anion is transported per one photon absorption:
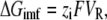 |
(2) |
where zi and F represent the valence of the anion and Faraday's constant, respectively. According to Eq. 2, the ΔGimf value was estimated with VR value −400 mV to be −38.6 (kJ/mol). Employing Planck's constant, the quantum energy for one mole of photon at 580 nm (ΔEphoton) is calculated to be 206 (kJ/mol). The probability that pHR absorbs the light quantum energy, followed by the excitation of pHR, i.e., the isomerization of the retinal from all-trans to 13-cis, is assumed to be 50%. On the basis of a single photon reaction, the conversion efficiency from the energy of the photon stored in the retinal isomerization to the Cl− translocation energy is 18.7%.
It has been well characterized that the light-driven pump BR from H. salinarum can generate electrochemical potential of up to −280 mV inside the cell (29), which corresponds to a proton motive force to be used for ATP synthesis and is coupled to other secondary active transporters in the plasma membrane. Nagel et al. also estimated the VR value based on the extrapolation of the line describing the I-V relationship in Xenopus oocyte expressing BR to be −220 mV, which is in accordance with the electrochemical potential for protons (30). It should be noted that the Cl− pumping activity by pHR can generate a more negative membrane potential, −400 mV, compared with that of BR. The substantial negative VR of pHR implies that it is a much more effective anion pump than BR.
As shown in Figs. 2 and 3, the uniqueness of the I-V relationship resides in its linear nature. This raises an interesting question: What is the mechanism for the voltage-dependent change in the anion pump activity of this transporter? In other words, which particular step(s) in the anion pumping via pHR is (are) regulated by the electric field? The photoinduced current was saturable with respect to Cl− concentration and followed Michaelis-Menten-type kinetics (Fig. 5). Both K0.5 and Imax values showed voltage-dependence; the K0.5 value increased when the membrane potential became more negative, whereas the Imax value decreased. It is of note that the I-V relationship is mainly governed by the voltage-dependent K0.5 and Imax values. To clarify which step in the photocycle is related to these kinetic parameters with regard to Cl− pumping, we performed flash-photolysis analysis with different Cl− concentrations (Fig. 7). In this analysis, we evaluated the rate determining process as well as the Cl−-dependent processes, for two reasons: 1), all intermediates attain steady state under conditions of continuous illumination, and the excited pHR consists almost entirely of the population of intermediate molecules in the rate-determining transition; and 2), the photoinduced currents at steady state show Cl− dependence. It is feasible that a kinetic model describing the photoinduced current at steady state can be simplified, as shown in Fig. 9. Based on these properties of photochemical reaction, we have developed the kinetic model describing the photoinduced current at steady state (See Appendix and Fig. 9). The scheme is comprised of two processes: one is the fast transition of intermediates corresponding to Cl− translocating and releasing processes, and the other is the rate-determining transition corresponding to Cl− binding processes. The photoinduced current is reduced to the following simple equation:
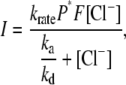 |
(3) |
where ka and kd represent the association and dissociation rate constants, respectively, for Cl− binding to the EC site, krate is the rate constant for the rate-determining transition, i.e., the pHR′→pHR transition, P* is the pHR molecule involved in the photoinduced current, and F is Faraday's constant. Analysis of the data using Eq. 3 shows that the voltage dependence of Imax is attributed to the pHR′→pHR transition, which decreased as the membrane potential became more negative. The K0.5 value increased as the membrane potential became more negative, implying that the transition decreased, i.e., the association rate decreased and/or the dissociation rate increased as the membrane potential became more negative. Taking the negative charge of Cl− into account, we hypothesize that during the vectorial transport of Cl− there exists an electrical field in the EC channel which affects Cl− uptake from the EC bulk space.
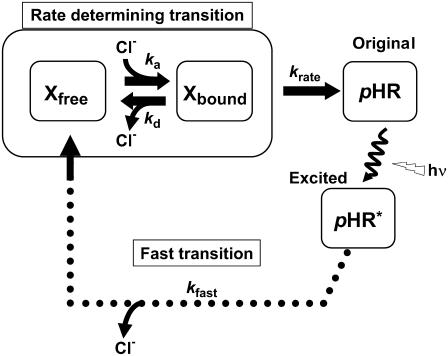
FIGURE 9.
A kinetic model for the photoinduced current at steady state for pHR on the basis of the photocycle. Under conditions of continuous illumination, all intermediates attain steady state, the excited pHR consists almost entirely of the population of intermediate molecules in the rate-determining transition, and the photoinduced current is governed mainly by the rate determining process. Therefore, focusing only on the intermediates involved in the photoinduced current at steady state, we simplify the photocycle scheme of pHR and construct the kinetic model describing the photoinduced current due to Cl− transport via pHR. The kinetic model is comprised of two processes: one is the fast transition of intermediates corresponding to Cl− translocating and releasing processes, and the other is the rate-determining transition corresponding to Cl− binding processes. According to the mass conservation, a mass-balance equation consists of the original pHR, the photoexcited pHR (pHR*) molecules, and Cl− free and bound intermediates (Xfree and Xbound, respectively). Taking into consideration that the krate value is much smaller than any other rate constants (kfast, ka, and kd), the photoinduced current at steady state is reduced to Eq. 3.
The binding of various anions has been studied extensively using detergent-solubilized pHR (21,24,26). Based on the absorption wavelength shift caused by the binding of anion to pHR, it has been concluded that the Cl− binding site has a binding constant of 1–2 mM. Other halides also bind strongly to pHR. On the other hand, the K0.5 values reflecting the binding constants of anions in our studies (Table 1) are at least one order of magnitude larger than the binding constants of anions for detergent-solubilized pHR. There are multiple factors that might explain the difference. The kinetic constants for anion binding to detergent-solubilized pHR were determined from the absorption wavelength shift caused by the binding of the anion to the transporter protein (21,24,26). In contrast, we determined the kinetic constants from the photoinduced outward currents associated with the transport of anions via pHR. The binding of the substrate represents only one of multiple steps involved in the transport process. What we determined in our studies is the kinetic constant for the entire transport process rather than for just the binding of the substrate. In addition, membrane potential might have contributed to the differences between the studies. In our oocyte expression system, measurements of pHR function were made in the presence of membrane potential, which induces electrical field in the EC channel of the transporter. According to stopped-flow experiments on the anion binding to detergent-solubilized pHR, Cl− transport via the transporter occurs mostly by passive diffusion through the EC channel (21). This process is likely to be affected markedly by alterations in the EC channel induced by the electrical field. Furthermore, the binding process of Cl− is coupled to an electrogenic event in the cycle. If the binding site is not available when the occupancy of an intermediate state at this event is lowered by the external potential, the apparent affinity of the binding will be lowered depending on the membrane potential. It is recognized, however, that the membrane potential provides only a partial explanation of the discrepancy in the kinetic constant values. This is because the value for K0.5 is ∼15 mM when the membrane potential is zero (Fig. 6), and this value is still many times higher than the value obtained with detergent-solubilized pHR. The discrepancy between two experimental systems may also be related to the possibility that Cl− binds to different states of pHR depending on the experimental system. The binding constant to pHR expressed in Xenopus oocytes corresponds to the binding to the intermediate (Fig. 9), whereas the binding constant to detergent-solubilized pHR corresponds to binding to the ground state.
Based on the crystal structure of sHR, the Cl− binding site is located in the vicinity of the protonated Schiff base, 18 Å below the extracellular membrane surface, i.e., Cl− is stuck on one-third of its pathway through the membrane (12,13). Supposing that the membrane potential is evenly imposed through the perpendicular vector to the membrane, the membrane potential from the EC bulk space to the EC binding site is one-third of the whole membrane potential. As shown in Fig. 6, the K0.5 value increased almost linearly when the membrane potential was made gradually more negative. Supposing that the increase in K0.5 values is governed by the Nernst equation, the imposed membrane potential through the EC channel can be estimated to be 20% of the whole membrane potential. If the membrane potential is −200 mV, the membrane potential of −40 mV might be imposed at least through the EC channel, which corresponds to 60% of the voltage difference in the EC channel theoretically calculated on the basis of the linear membrane potential gradient. In contrast to the formation of the membrane potential gradient through the EC channel in pHR, a hydrogen-bond network is formed through the EC channel in BR, which facilitates movement of the proton through the EC channel. This hydrogen network is believed to be a proton-wire that can rapidly transfer the proton through the EC channel in BR (31,32). Thus, there is no electrical field through the EC channel in BR.
Under conditions of continuous illumination, the photoinduced current attains a steady state. The photoinduced current is governed by the rate-determining step, which is also regulated by the applied electrical field. According to Michaelis-Menten-type kinetics, the Imax value reflects the rate-determining step. On the basis of flash photolysis analysis, the Imax value is a function of the transition rate constant krate and the photoexcited molecule of pHR. The Imax value reflects the transition of pHR′→pHR, which was estimated to be 10-fold smaller than any other transition in the photocycle. It is important to note that the transition of pHR′→pHR is also regulated by the applied electrical field. On the basis of the binding analysis of Cl− to pHR with stopped-flow experiments (21), the time course of the binding to pHR was composed of two phases, indicating that the uptake process of Cl− through the EC channel is associated with a subtle conformational change or the subtle distortion of the retinal accompanying an intramolecular charge movement. Previously, Manor et al. determined the effect of membrane potential on photochemical reactions of three archaerhodopsins in H. salinarum, sensory rhodopsin I, BR, and sHR (20). Each of these three exhibits a decreased rate of thermal decay of the principal photoinduced intermediate when deenergized cells are energized artificially to generate a more negative membrane potential. The intramolecular charge movements with a vectorial component normal to the plane of the membrane possibly occur in the rate-determining thermal steps of each of the three pigments. In other words, a voltage-dependent conformational change common to their respective photocycles might occur. Especially with regard to BR and HR functioning as ion pumps, these conformational movements might be involved in the electrogenic transport associated with the photocycles. Further analysis of this voltage dependence of the rate-determining step might provide a better insight into the mechanism of the subtle conformational change. The exact mechanism remains yet to be elucidated.
In summary, we have established a pHR expression system in Xenopus laevis oocytes to gain a better insight into the mechanism of electrogenic anion transport via pHR. Using this system, the photoinduced currents due to anion transport could be determined precisely to analyze the kinetics of the transport process. With this approach, we were able to demonstrate that the Cl− pump activity via pHR is dependent on membrane potential. On the basis of this voltage dependence, we show, for the first time that we know of, that the VR value at which the pump current by pHR is reduced to zero represents the intrinsic ion motive force of the excited pHR molecule to pump Cl− into cell. The Cl− pumping activity by pHR can generate a substantial negative membrane potential, −400 mV, i.e., pHR functions as a very potent anion pump.
APPENDIX
It has been demonstrated clearly that Cl− transport into cells via HR is coupled to the cyclic photochemical reaction of HR molecule: all-trans to 13-cis isomerization of the retinal induced by absorption of a light quantum initiates the photochemical reaction, followed by thermal reisomerization to the initial all-trans state. Under conditions of continuous illumination, all intermediates attain steady state and the excited pHR consists almost entirely of the population of the intermediate molecules in the rate-determining transition. The photoinduced current is governed mainly by the rate-determining process. The photoinduced current is also dependent on external Cl− concentration (Fig. 4). Therefore, focusing only on the intermediates involved in the photoinduced current at steady state, we simplify the photocycle scheme of pHR and construct a kinetic model describing the photoinduced current due to Cl− transport via pHR (Fig. 9). The kinetic model is comprised of two processes: 1), the fast transition of intermediates corresponding to Cl− translocating and releasing processes; and 2), the rate determining transition corresponding to Cl− binding processes. According to the mass conservation, a mass-balance equation consists of the original pHR, the photoexcited pHR (pHR*) molecules, and the Cl− free and bound intermediates (Xfree and Xbound, respectively).
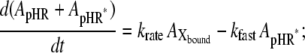 |
(A1) |
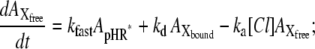 |
(A2) |
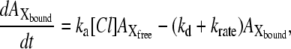 |
(A3) |
where 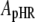 ,
, 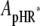 ,
, 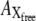 and
and 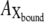 represent the amounts of original pHR, pHR*, Xfree, and Xbound intermediates, respectively; kfast and krate are the rate constants with regard to the fast transition and rate-determining transition processes, respectively; and ka and kd are the association and dissociation rate constants with regard to Cl− binding to the EC site in pHR. Alternatively, the following equation with regard to the total amount of excited pHR molecules involved in the photoinduced current cycle holds as follows:
represent the amounts of original pHR, pHR*, Xfree, and Xbound intermediates, respectively; kfast and krate are the rate constants with regard to the fast transition and rate-determining transition processes, respectively; and ka and kd are the association and dissociation rate constants with regard to Cl− binding to the EC site in pHR. Alternatively, the following equation with regard to the total amount of excited pHR molecules involved in the photoinduced current cycle holds as follows:
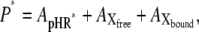 |
(A4) |
where P* is the total amount of pHR molecules involved in the photoinduced current cycle. Under conditions of continuous illumination, all intermediates attain steady state and all mass-balance equations described above are equal to zero. Combining all equations, solving of the term, and substituting the value into Eq. A4 yields the following equation:
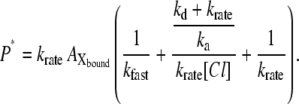 |
(A5) |
All intermediates attain steady state and all transition rates are equal. Thus, the photoinduced current at steady state is expressed as the multiplicity of 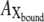 with krate and Faraday's constant, F, as follows:
with krate and Faraday's constant, F, as follows:
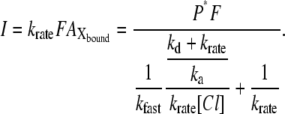 |
(A6) |
Taking into consideration that the krate value is much smaller than any other rate constants (kfast, ka, and kd), Eq. A6 is reduced to
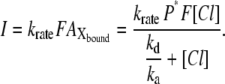 |
(A7) |
Substitutions of krateP*F with Imax and the ratio 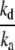 with K0.5 yield the Michaelis-Menten-type equation (Eq. 1),
with K0.5 yield the Michaelis-Menten-type equation (Eq. 1),
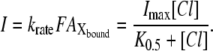 |
(A8) |
The ratio of P* to the unexcited original pHR is designated as α. P* is expressed as
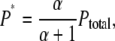 |
(A9) |
where Ptotal is the total amount of the pHR protein expressed in the plasma membrane of Xenopus oocyte. Substitution of Eq. A9 into Eq. A7 explains why the photoinduced current is proportional to the intensity of illumination in the range of low intensity, as shown in Fig. 3.
References
- 1.Schobert, B., and J. K. Lanyi. 1982. Halorhodopsin is a light-driven chloride pump. J. Biol. Chem. 257:10306–10313. [PubMed] [Google Scholar]
- 2.Mukohata, Y., and Y. Kaji. 1981. Light-induced membrane-potential increase, ATP synthesis, and proton uptake in Halobacterium halobium, R1mR catalyzed by halorhodopsin: effects of N,N′-dicyclohexylcarbodiimide, triphenyltin chloride, and 3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile (SF6847). Arch. Biochem. Biophys. 206:72–76. [DOI] [PubMed] [Google Scholar]
- 3.Lanyi, J. K. 1990. Halorhodopsin, a light-driven electrogenic chloride-transport system. Physiol. Rev. 70:319–330. [DOI] [PubMed] [Google Scholar]
- 4.Varo, G. 2000. Analogies between halorhodopsin and bacteriorhodopsin. Biochim. Biophys. Acta. 1460:220–229. [DOI] [PubMed] [Google Scholar]
- 5.Duschl, A., J. K. Lanyi, and L. Zimanyi. 1990. Properties and photochemistry of a halorhodopsin from the haloalkalophile, Natronobacterium pharaonis. J. Biol. Chem. 265:1261–1267. [PubMed] [Google Scholar]
- 6.Rudiger, M., and D. Oesterhelt. 1997. Specific arginine and threonine residues control anion binding and transport in the light-driven chloride pump halorhodopsin. EMBO J. 16:3813–3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lanyi, J. K., A. Duschl, G. W. Hatfield, K. May, and D. Oesterhelt. 1990. The primary structure of a halorhodopsin from Natronobacterium pharaonis. Structural, functional and evolutionary implications for bacterial rhodopsins and halorhodopsins. J. Biol. Chem. 265:1253–1260. [PubMed] [Google Scholar]
- 8.Varo, G., L. Zimanyi, X. Fan, L. Sun, R. Needleman, and J. K. Lanyi. 1995. Photocycle of halorhodopsin from Halobacterium salinarium. Biophys. J. 68:2062–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varo, G., L. S. Brown, J. Sasaki, H. Kandori, A. Maeda, R. Needleman, and J. K. Lanyi. 1995. Light-driven chloride ion transport by halorhodopsin from Natronobacterium pharaonis. 1. The photochemical cycle. Biochemistry. 34:14490–14499. [DOI] [PubMed] [Google Scholar]
- 10.Varo, G., R. Needleman, and J. K. Lanyi. 1995. Light-driven chloride ion transport by halorhodopsin from Natronobacterium pharaonis. 2. Chloride release and uptake, protein conformation change, and thermodynamics. Biochemistry. 34:14500–14507. [DOI] [PubMed] [Google Scholar]
- 11.Chizhov, I., and M. Engelhard. 2001. Temperature and halide dependence of the photocycle of halorhodopsin from Natronobacterium pharaonis. Biophys. J. 81:1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolbe, M., H. Besir, L. O. Essen, and D. Oesterhelt. 2000. Structure of the light-driven chloride pump halorhodopsin at 1.8 Å resolution. Science. 288:1390–1396. [DOI] [PubMed] [Google Scholar]
- 13.Essen, L. O. 2002. Halorhodopsin: light-driven ion pumping made simple? Curr. Opin. Struct. Biol. 12:516–522. [DOI] [PubMed] [Google Scholar]
- 14.Sato, M., M. Kubo, T. Aizawa, N. Kamo, T. Kikukawa, K. Nitta, and M. Demura. 2005. Role of putative anion-binding sites in cytoplasmic and extracellular channels of Natronomonas pharaonis halorhodopsin. Biochemistry. 44:4775–4784. [DOI] [PubMed] [Google Scholar]
- 15.Bamberg, E., P. Hegemann, and D. Oesterhelt. 1984. Reconstitution of halorhodopsin in black lipid membranes. Prog. Clin. Biol. Res. 164:73–79. [PubMed] [Google Scholar]
- 16.Kalaidzidis, I. V., Y. L. Kalaidzidis, and A. D. Kaulen. 1998. Flash-induced voltage changes in halorhodopsin from Natronobacterium pharaonis. FEBS Lett. 427:59–63. [DOI] [PubMed] [Google Scholar]
- 17.Okuno, D., M. Asaumi, and E. Muneyuki. 1999. Chloride concentration dependency of the electrogenic activity of halorhodopsin. Biochemistry. 38:5422–5429. [DOI] [PubMed] [Google Scholar]
- 18.Muneyuki, E., C. Shibazaki, H. Ohtani, D. Okuno, M. Asaumi, and T. Mogi. 1999. Time-resolved measurements of photovoltage generation by bacteriorhodopsin and halorhodopsin adsorbed on a thin polymer film. J. Biochem. (Tokyo). 125:270–276. [DOI] [PubMed] [Google Scholar]
- 19.Ludmann, K., G. Ibron, J. K. Lanyi, and G. Varo. 2000. Charge motions during the photocycle of pharaonis halorhodopsin. Biophys. J. 78:959–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manor, D., C. A. Hasselbacher, and J. L. Spudich. 1988. Membrane potential modulates photocycling rates of bacterial rhodopsins. Biochemistry. 27:5843–5848. [DOI] [PubMed] [Google Scholar]
- 21.Sato, M., T. Kanamori, N. Kamo, M. Demura, and K. Nitta. 2002. Stopped-flow analysis on anion binding to blue-form halorhodopsin from Natronobacterium pharaonis: comparison with the anion-uptake process during the photocycle. Biochemistry. 41:2452–2458. [DOI] [PubMed] [Google Scholar]
- 22.Miyauchi, S., E. Gopal, Y. J. Fei, and V. Ganapathy. 2004. Functional identification of SLC5A8, a tumor suppressor down-regulated in colon cancer, as a Na(+)-coupled transporter for short-chain fatty acids. J. Biol. Chem. 279:13293–13296. [DOI] [PubMed] [Google Scholar]
- 23.Gopal, E., Y. J. Fei, M. Sugawara, S. Miyauchi, L. Zhuang, P. Martin, S. B. Smith, P. D. Prasad, and V. Ganapathy. 2004. Expression of slc5a8 in kidney and its role in Na(+)-coupled transport of lactate. J. Biol. Chem. 279:44522–44532. [DOI] [PubMed] [Google Scholar]
- 24.Scharf, B., and M. Engelhard. 1994. Blue halorhodopsin from Natronobacterium pharaonis: wavelength regulation by anions. Biochemistry. 33:6387–6393. [DOI] [PubMed] [Google Scholar]
- 25.Balint, Z., M. Lakatos, C. Ganea, J. K. Lanyi, and G. Varo. 2004. The nitrate transporting photochemical reaction cycle of the pharaonis halorhodopsin. Biophys. J. 86:1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magyari, K., V. Simon, and G. Varo. 2006. The influence of the halide ions on the photochemical reaction cycle of pharaonis halorhodopsin. J. Photochem. Photobiol. B. 82:16–20. [DOI] [PubMed] [Google Scholar]
- 27.Schobert, B., and J. K. Lanyi. 1986. Electrostatic interaction between anions bound to site I and the retinal Schiff base of halorhodopsin. Biochemistry. 25:4163–4167. [DOI] [PubMed] [Google Scholar]
- 28.Shibata, M., N. Muneda, T. Sasaki, K. Shimono, N. Kamo, M. Demura, and H. Kandori. 2005. Hydrogen-bonding alterations of the protonated Schiff base and water molecule in the chloride pump of Natronobacterium pharaonis. Biochemistry. 44:12279–12286. [DOI] [PubMed] [Google Scholar]
- 29.Michel, H., and D. Oesterhelt. 1976. Light-induced changes of the pH gradient and the membrane potential in H. halobium. FEBS Lett. 65:175–178. [DOI] [PubMed] [Google Scholar]
- 30.Nagel, G., B. Kelety, B. Mockel, G. Buldt, and E. Bamberg. 1998. Voltage dependence of proton pumping by bacteriorhodopsin is regulated by the voltage-sensitive ratio of M1 to M2. Biophys. J. 74:403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luecke, H., B. Schobert, H. T. Richter, J. P. Cartailler, and J. K. Lanyi. 1999. Structural changes in bacteriorhodopsin during ion transport at 2 Å resolution. Science. 286:255–261. [DOI] [PubMed] [Google Scholar]
- 32.Lanyi, J. K., and H. Luecke. 2001. Bacteriorhodopsin. Curr. Opin. Struct. Biol. 11:415–419. [DOI] [PubMed] [Google Scholar]
- 33.Robinson, R. A., and R. H. Stokes. 1959. Electrolyte Solution, 2nd ed. Butterworth, London.
- 34.Israelachvili, J. 1992. Intermolecular and Surface Forces. Academic Press, New York.