Abstract
Solution pH affects numerous biological processes and some biological membranes are exposed to extreme pH environments. We utilized micropipette aspiration of giant unilamellar vesicles composed of 1-stearoyl-2-oleoyl-phosphatidylcholine to characterize the effect of solution pH (2–9) on membrane mechanical properties. The elastic area compressibility modulus was unaffected between pH 3 and 9 but was reduced by ∼30% at pH 2. Fluorescence experiments utilizing the phase-sensitive probe Laurdan confirmed gel-phase characteristics at pH 2, explaining the reduction of membrane elasticity. The membrane bending stiffness, kc, increased by ∼40% at pH 4 and pH 9 over the control value at pH 6.5. Electrophoretic mobility measurements indicate that these changes are qualitatively consistent with theoretical models that predict the effect of membrane surface charge density and Debye length on kc, substantiating a coupling between the mechanical and interfacial electrical properties of the membrane. The effect of pH on intramembrane electrical properties was examined by studying the spectral shifts of the potentiometric probe di-8 ANEPPS. The intramembrane (dipole) potential (Ψd) increased linearly as the solution pH decreased in a manner consistent with the partitioning of hydroxide ions into the membrane. However, changes in Ψd did not correlate with changes in kc. These mechanical and electrical studies lead to the conclusion that the effect of pH on membrane bending stiffness results from alterations in interfacial, as opposed to intramembrane, electrostatics.
INTRODUCTION
The solution pH affects many membrane-mediated biological processes, such as membrane fusion (1), cholesterol domain formation (2,3), drug-liposome interactions (4), membrane phase transitions (5–9), and erythrocyte deformability and spectrin solubility (10). Normally, extracellular fluids have a pH at ∼7.4, and cells regulate their internal pH at ∼7.0. However, in some situations biological membranes are exposed to environments with various pH values. For instance, the inner membranes of endosomes and lysosomes constantly face pH environments as low as 5 (11,12). In the mammalian stomach, apical membranes of gastric surface mucous cells and mucous neck cells, as well as canalicular membranes of parietal cells, constantly face a gastric juice whose pH varies from <1 to 6 (13). The phospholipid layer covering the gastric intestinal tract is also exposed to this corrosive gastric juice (14,15). The mechanism(s) that membranes of these cells and cellular organelles utilize to maintain integrity is still not well understood. Thus, characterizing and understanding the interactions between protons, hydroxide ions, and biomembranes is an important biophysical problem.
Proton-lipid interactions have been studied extensively using a variety of techniques such as EPR and NMR spectroscopy (16), Langmuir film balance (15,17–20), and fluorescence spectroscopy (21). These techniques have documented changes in many membrane properties in response to changes in pH, including liposome stability, lateral phase separation, and the interdigitated gel-to-bilayer gel phase transition (15–21). In particular, calorimetric studies have established that low pH environments (≤2) can increase lecithin membrane phase-transition temperatures (6–9,22,23). From the mechanical standpoint, the effect of pH on membrane interfacial tension has been studied by measuring the curvature change of a phosphatidylcholine (PC) lipid pendant drop exposed to different pH environments (22–26). The interfacial tension of PC lipids was observed to increase near pH 4 (25). However, to the best of our knowledge, the effect of pH on membrane mechanics and electrostatics has not been systematically studied in membrane vesicles. Thus, we were motivated to examine the effect of solution pH (2–9) on the mechanical and electrical properties of liposomal membranes to enable us to better understand the effect of protons and hydroxide ions on membrane stability. An additional outcome of this work is that alterations in solution pH provide a straightforward method to alter membrane electrostatics, and permit us to investigate the relationship between the mechanical and electrical properties of membranes and test theoretical models of these interrelationships.
Biomembranes are stabilized by the opposing forces of surface tension arising from hydrophobic forces and surface pressure resulting from repulsive interactions among lipid headgroups (27,28). Both surface tension and surface pressure are determined by inter- and intramolecular interactions of lipids, which define the cross-sectional area and the acyl-chain length of a given lipid molecule (29,30). For a collection of lipids that have self-assembled into a vesicle, a very small yet finite membrane tension exists that is defined as the difference between surface tension and surface pressure (31). The application of pressure to a vesicular membrane, experimentally realized in the micropipette aspiration method, changes the membrane surface area and can be used to determine the mechanical properties of the membrane. The bending stiffness, kc, can be determined by measuring the tension required to smooth the membrane thermal undulations (32,33). As the membrane is further stretched by applied tension, actual changes in membrane surface area occur and are resisted by the elastic compressibility modulus, KA. The method of micropipette aspiration has been effectively used to study the effect of ions and surfactant molecules on bilayer mechanical properties (32,34–40). For instance, salicylate, an active metabolite of aspirin predominantly anionic at neutral pH, decreases the bending stiffness and the apparent compressibility modulus of the membrane (34). Water-soluble bile acids, which are also mainly in anionic form at neutral pH, also partition into the membrane in a tension-dependent manner and reduce the apparent compressibility modulus (41).
When the membrane is exposed to ions, such as protons and hydroxide ions, inter- and intramolecular interactions, especially electrostatic interactions, can be altered. Zwitterionic PC lipid molecules contain functional groups (phosphate and choline), whose electric charge distribution at the membrane interface is a function of the binding of counterions (27,42–44), including protons and hydroxide ions. These interactions can potentially change interfacial electrical properties such as membrane surface charge density and ζ-potential. The ζ-potential, ζ, which measures the potential slightly above the membrane interface and relates to the membrane surface charge density, is calculated from liposome mobility in an applied electric field (27). The interaction of ions with the membrane can also conceivably affect membrane mechanics, and there is experimental evidence that changes in ionic strength affect lipid packing (44,45). A number of theoretical models have predicted an intricate relationship between the mechanical and electrical properties of membranes (46–50). In particular, Winterhalter and Helfrich (46,47) developed models using Poisson-Boltzmann theory, which predict that changes in interfacial electric properties, such as the Debye length and membrane surface charge density, can affect the membrane bending stiffness. However, testing such models has proven experimentally difficult.
In addition to altering interfacial electrostatics, ions, including protons and hydroxide ions, can also potentially affect intramembrane electrostatics such as the dipole potential (51). The dipole potential, Ψd, arises from intrinsic internal dipole moments carried by both lipid functional groups and interfacial water molecules (27,52,53) and is crucial to many membrane-mediated biological processes (44,52–55). Membranes usually have a large (∼300 mV) dipole potential that is positive inside of the bilayer core, which is believed to be the main reason phospholipid bilayers are more permeable to anions than to cations (51–53). The dipole potential can be determined by measuring spectral shifts of the voltage-sensitive probe di-8 ANEPPS (51,53,56–58). Studies have established that the dipole potential is sensitive to lipid headgroup composition and external ions (51–53,56). The ability of ions to change the dipole potential correlates with the ion's free energy of hydration, following the Hofmeister series (51). It is not known whether there is a correlation between the dipole potential and the mechanical properties of the membrane.
In this study, we utilized micropipette aspiration of giant unilamellar lipid vesicles (GUVs) to investigate the interactions between the electrical and mechanical properties of membranes exposed to different pH environments. We found that the elastic compressibility modulus of SOPC membranes was reduced to 166 mN/m at pH 2, which we attribute to the appearance of gel-phase domains. The bending stiffness increased at pH 4 to 1.2 × 10−19 J and at pH 9 to 1.3 × 10−19 J. To determine whether these effects were related to changes in the interfacial and/or intramembrane electrical properties, we complemented the micromechanical measurements by determining the effect of pH on the ζ-potential, as measured by the electrophoretic mobility of large unilamellar vesicles (LUVs) and on the dipole potential, as measured by spectral shifts in di-8 ANEPPS. The ζ-potential data and measured changes in kc are qualitatively consistent with the theoretical model of Winterhalter and Helfrich (46,47) and support the view that the effect of solution pH on kc arises from contributions of both the membrane surface charge density and the Debye length. The pH effect on the membrane dipole potential is suggested to originate from the preferential interaction of hydroxide ions with the membrane. Thus, our kc, ζ, and Ψd measurements indicate that the pH effect on PC membranes can be twofold. On one hand, protons and hydroxide ions can act as counterions and associate with phosphate and choline groups of PC lipids at the membrane interface. In addition, hydroxide ions affect the internal dipole potential of the membrane. While alterations in interfacial electrostatics correlate with changes in the bending stiffness, there does not appear to be a correlation between the intramembrane potential and the mechanical parameters. Taken together, the data support the view that intermolecular electrostatic interactions at the membrane interface can influence membrane mechanics and consequently affect the bending elasticity of bilayer.
MATERIALS
The phospholipid 1-stearoyl-2-oleoyl-phosphatidyl-choline (SOPC) was purchased from Avanti Polar Lipids (Alabaster, AL). Lipids were dissolved in chloroform and stored at −20°C under nitrogen. Fluorescent probes di-8 ANEPPS and Laurdan were purchased from Molecular Probes (Carlsbad, CA). Bovine serum albumin (BSA) was purchased from Sigma-Aldrich (St. Louis, MO). Glucose, sucrose, sodium hydroxide, and hydrochloric acid were purchased from Fisher (Pittsburgh, PA).
METHODS
Vesicle formation
Vesicles were formed from SOPC using a previously described electroformation procedure (34,59). Briefly, ∼30 μl of 0.5 mg/ml SOPC/chloroform solution was spread on platinum electrodes in a Plexiglass chamber. The lipid film was then dried under vacuum for at least 2 h to completely evaporate chloroform. The chamber was filled with 200 mM sucrose solution and an alternating AC field of 0.5–2V was applied to the electrodes at frequencies varying from 10 Hz to 1 Hz. After ∼2 h, the vesicles were harvested and stored at 4°C under nitrogen. We previously established that this protocol does not oxidize SOPC lipids (34). Thin layer chromatography has been performed to examine the effect of extremely acidic pH (pH 2) and alkaline pH (pH 9) on the chemical composition of PC lipids. No evidence of acid hydrolysis or saponification byproducts were found in SOPC lipids at the tested pH values (data not shown).
Micropipette aspiration
Microaspiration measurements were performed on the stage of a Zeiss Axiovert 200M inverted microscope equipped with differential interference contrast optics and a Zeiss PLAN-NEOFLUAR 40×/0.85 polarized differential interference contrast objective (Carl Zeiss, Thornwood, NY) at 1.6 optovar. Micropipettes were fabricated using a micropipette puller (Sutter P-97, Novato, CA) and then cut cleanly on a custom-made microforge. A 0.02% bovine serum albumin (BSA) solution was used to coat the pipette and coverslips. An extensive washing of the coated pipette and coverslips with both 200 mM glucose solution and distilled water was performed in an effort to eliminate excess BSA on the pipette and coverslips (34,35).
Pressure manipulation was achieved by coupling the micropipette in the chamber to a water-filled reservoir mounted on a motorized mechanical slider (Robocylinder, Torrance, CA) with resolution of 0.01 mm. The pressure level was calibrated and periodically checked using small vesicular debris (∼1–2 μm in diameter) in the chamber. Giant unilamellar vesicles were added in the aspiration chamber filled with an isomolar glucose solution (200 mOsm) that was freshly prepared before each experiment and adjusted to the desired pH. Since this is a study of electrostatic effects of solution pH, specifically effects of protons and hydroxide ions, on membrane mechanics, the solutions were not buffered so that data interpretation would not be complicated by the presence of various ions in a buffer. The pH of the solutions used in experiments was periodically checked after experiments and the change in pH was <0.07 pH units. The insertion of the glass micropipette was also found not to affect solution pH. A negative pressure was applied to create a vesicular projection which was given ∼8–10 s to achieve steady state. Aspiration images were captured using a Zeiss Axiocam MRm camera and analyzed using Zeiss Axiovision 3.0 software. To ensure accuracy of the analysis, measurements were periodically checked using comparative light intensity plots of the corresponding vesicle images (34,60). All measurements were conducted at room temperature.
The membrane tension (τ) and apparent fractional area change (αapp) were obtained using (32,34,39)
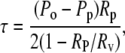 |
(1) |
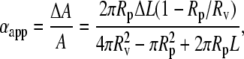 |
(2) |
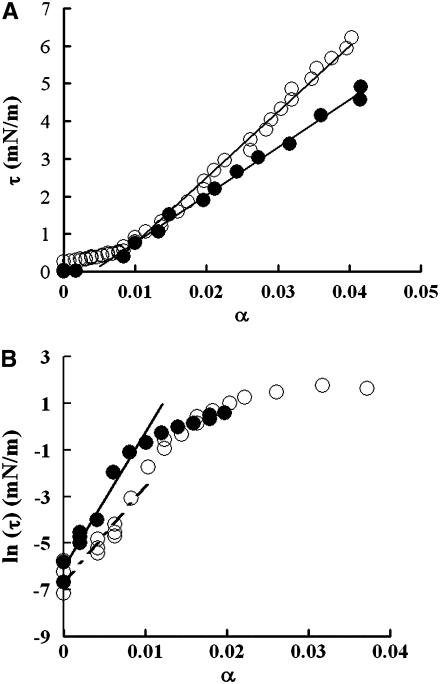
where A is the total membrane surface area; ΔA is the change in area resulting from applied tension; Rv and Rp are radii of the aspirated vesicle and pipette, respectively; L is the vesicle projection length; and ΔL is the change in L resulting from the change in tension. The geometrically measured area expansion is designated as apparent area change. A plot of τ versus αapp reveals an initial exponential domain (τ < 0.5 mN/m) dominated by thermally driven out-of-plane fluctuations of the membrane surface (61) that is followed by a region of linear elastic behavior (τ > 0.5 mN/m) whose slope is the apparent compressibility modulus, Kapp (32,34,39) (Fig. 1 a). The bending modulus, kc, is obtained from the slope of a plot of Ln (τ) versus αapp (Fig. 1 b) in the low-tension region, which is equal to 8π kc/kBT (61).
FIGURE 1.
(a) Plots of tension (τ) versus fractional area change (α) for a representative vesicle in 200 mM glucose at pH 6.5 (○) and for another vesicle in solution at pH 2 (•). The slope of the high tension region (>0.5 mN/m) is the apparent area compressibility modulus (Kapp). (b) Plots of ln (τ) versus α for a control vesicle in 200 mM glucose at pH 6.5 (○) and another vesicle in solution at pH 4 (•), in which α in the low tension region was carefully measured. The slope of the low-tension domain of the plot was used to calculate the bending modulus (kc).
Elastic area compressibility modulus (KA)
In the aspiration experiments, membrane thermal undulations occur throughout the entire tension range. This means that a portion of the measured area change at all tension levels is caused by smoothing of thermal undulations (61). This portion is calculated as
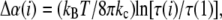 |
(3) |
where τ (1) is the initial tension. To obtain the elastic compressibility modulus (KA), Δα(i) at each tension point i was removed from the measured total area (αapp(i)) using the models developed previously (32,61). Mathematically, the actual elastic area dilation, denoted αA, is calculated at each point as αA(i) = αapp(i) − Δα(i) (32,61). The elastic area compressibility modulus (KA) for each vesicle was calculated from the slope of data replotted as τ versus αA.
Zeta-potential measurements
An SOPC/chloroform solution containing ∼2.5 mg of SOPC lipids was dried under vacuum for at least 2 h to completely evaporate chloroform. The lipid film was rehydrated with ∼4 ml of ultra-pure water at various pH values, and incubated at room temperature under nitrogen gas for at least 30 min and then extruded using the Mini-extruder with 100 nm polycarbonate filters (protocol of Avanti Polar Lipids) to ensure the formation of large unilamellar vesicles (LUVs) ∼100 nm in diameter. The electrophoretic mobility measurements of SOPC LUVs exposed to solutions with different pH values were obtained using the Zeta PALS ζ-potential analyzer (Brookhaven Instrument, Holtsville, NY). The ζ-potential was calculated from the mobility measurements automatically by the instrument utilizing the Smoluchowski equation (62).
Dipole potential measurements
The spectral shifts of the potentiometric styrl probe, di-8 ANEPPS, were utilized to examine changes in dipole potential of membranes exposed to solutions with different pH values. The method follows that of Clarke and Lüpfert (51) and Xu and Loew (56). The LUVs composed of SOPC were synthesized as described above. Approximately 3.5 μl of 1 mg/ml di-8 ANEPPS (in ethanol) was added to the LUV solution to achieve a lipid/probe ratio of ∼400:1. The mixture was incubated under nitrogen gas. Spectral measurements were performed using a SpectraMax M2 spectrophotometer (Molecular Devices, Sunnyvale, CA). The emission wavelength was set at 670 nm and the excitation spectra between 400 nm and 550 nm were recorded (51,57). The ratio (F420/520) of fluorescence intensities at 420 nm and 520 nm of the excitation spectrum was used to calculate the dipole potential using the relationship (51,57):
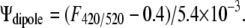 |
(4) |
Laurdan generalized polarization measurements
The bilayer phase behavior was monitored with the fluorescent probe Laurdan, following the techniques of Parassis et al. (63,64) and Bagatolli et al. (65–67). An appropriate amount of SOPC in chloroform was mixed with Laurdan (in ethanol) to achieve a lipid/probe ratio of ∼300:1. The LUVs were formed as described above. The emission spectra between 400 nm and 500 nm were obtained at a fixed excitation wavelength chosen between 320 nm and 410 nm (66). Generalized polarization (GPex) was then obtained by
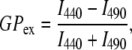 |
(5) |
where I440 and I490 are fluorescent intensities detected at emission wavelength of 440 and 490 nm, respectively. The pattern of the GPex plot as a function of excitation wavelengths was utilized to indicate the bilayer phase behavior. As demonstrated previously, the GP spectra for a PC lipid tilts downward above the Tm, tilts upward near Tm, and is unaffected by the excitation wavelength below the main phase transition temperature (64).
EXPERIMENTAL RESULTS
Effect of pH on apparent area compressibility
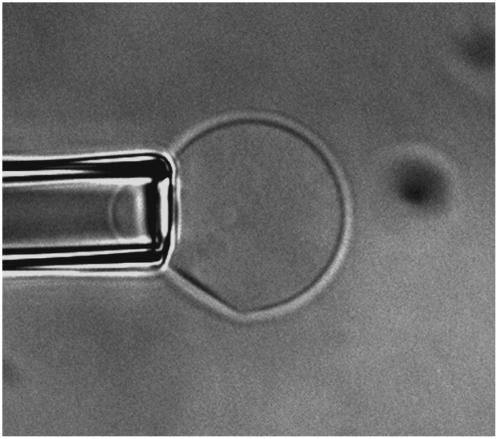
The effect of pH on the apparent compressibility modulus of the membrane was first determined. All the τ versus αapp plots show a linear behavior in the high-tension domain (>0.5 mN/m), illustrating that the membrane behaved as linear elastic material in both acidic and basic environments. Table 1 shows that Kapp of the SOPC membrane was not affected by changes in solution pH until pH 2 (P < 0.05, all data were compared to the measurements conducted at pH 6.5 with no added ions and reported in our previous article (34)). At pH 2, Kapp decreased to ∼135 mN/m, a ∼35% reduction compared to the Kapp value of ∼211 mN/m at pH 6.5. This decrease in Kapp indicates a weakening of the SOPC membrane. The change in lipid packing was also observed optically: many vesicles suspended in solution at pH 2 showed a half-moon shape, in which a portion of the membrane became linear and lost its curvature—an indication of phase separation (Fig. 2). In addition, aspiration on the half-moon-shaped vesicles showed that these vesicles were able to maintain the half-moon shape, indicating that externally applied pressure could not eliminate the phase separation.
TABLE 1.
Effect of pH on membrane mechanical properties
| pH | kc (× 10−19 J) | Kapp (mN/m) | KA (mN/m) | kc-el (× 10−19 J) |
|---|---|---|---|---|
| 2 | 0.87 ± 0.2 (8) | 135 ± 49 (8) | 166 ± 65 (7)* | 0.05 |
| 3 | 0.98 ± 0.34 (10) | 205 ± 27 (10) | 257 ± 48 (10) | 0.19 |
| 4 | 1.22 ± 0.36 (13)* | 205 ± 41 (12) | 246 ± 56 (12) | 0.31 |
| 6.5 | 0.90 ± 0.15 (10)† | 211 ± 23 (17)† | 247 ± 32 (7)† | 0.11 |
| 9 | 1.33 ± 0.33 (12)* | 190 ± 48 (10) | 217 ± 54 (10) | 0.53 |
| 10 mM NaCl at pH 4 | 0.82 ± 0.26 (11) |
Values are shown as mean ± SD. The numbers in parentheses represent the number of vesicles used during experiments.
Indicates values are statistically different from control (pH 6.5), as determined by a Student's t-test results obtained at 95% confidence. The ANOVA test supports the hypothesis that pH has an effect on SOPC bending stiffness (F = 4.8 and P = 0.0024) and on KA at pH 2 (F = 4.18 and P = 0.006).
Data obtained from our previous investigation (34).
FIGURE 2.
Half-moon-shaped vesicle aspirated into a micropipette at pH 2. The vesicle was held at least 50 μm above the bottom surface of the chamber. A portion of the membrane became flat and lost its curvature, an indication of phase separation.
Bending rigidity (kc) and elastic area compressibility modulus (KA)
The effect of pH on bending stiffness is shown in Table 1. The kc of SOPC increased (with the student's t-test P < 0.05) at both pH 4 (∼1.2 × 10−19 J) and pH 9 (∼1.3 × 10−19 J) and remained unchanged at pH 2 (∼0.87 × 10−19 J) when compared to the kc value of ∼0.9 × 10−19 J at pH 6.5 (34), which agrees with earlier measurements for SOPC (32,38). The data was then subjected to an Analysis of Variance (ANOVA) test to assess the statistical significance of the pH effect on kc. The ANOVA test supports the hypothesis that pH has an effect on SOPC bending stiffness (F = 4.8 and P = 0.0024). When kc data for both pH 4 and pH 9 were removed, the ANOVA indicates that the rest of the pH values do not affect kc (F = 0.47 and P = 0.63).
As mentioned above, the observed changes in Kapp could actually reflect alterations in kc. Therefore, the actual elastic area compressibility modulus (KA) is a more appropriate way to indicate changes in elasticity (32,34). When the area dilation caused by smoothing of the thermal undulations was removed, the KA of SOPC at pH 2 was found to decrease to ∼166 mN/m from the control value of ∼246 mN/m previously determined at pH 6.5 (34). Table 1 shows that KA is only affected at pH 2 (Student's t-test P < 0.05). To further assess the statistical significance of pH effect on KA, the ANOVA test was performed. The results support the hypothesis that pH 2 has an effect on KA (F = 4.16 and P = 0.006 with all values of pH; F = 1.15 and P = 0.34 without pH 2).
Effect of pH on electrophoretic mobility and ζ-potential
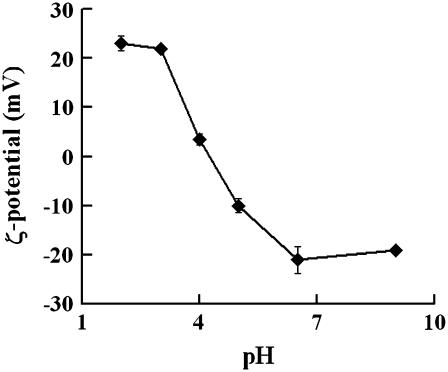
As solution pH varies, different concentrations of protons/ hydroxide act as counterions to neutralize either phosphate or choline groups on PC lipids, thus potentially altering the surface charge (42–44,68,69). Fig. 3 shows that solution pH has significant effects on the surface charge of SOPC vesicles. At pH 2 and 3, SOPC vesicles have a positive ζ-potential, indicating extensive association of protons at the membrane surface. This agrees with other investigations demonstrating that phospholipids carry a positive ζ-potential at low pHs (69). At pH 4, the ζ-potential is nearly zero (∼3.5 mV) and the LUVs showed low mobility, in agreement with other investigations demonstrating that the isoelectric point of PC lipids is ∼pH 4 (24–26,69). At the near neutral pH of 6.5, the ζ-potential is clearly negative (∼−21 mV), in agreement with many investigations which have pointed out that PC is negatively charged between −10 mV and −30 mV at neutral pH (42–44,68,69). Silvander et al. (42) have utilized a surface probe, 4-heptadecyl-7-hydroxycoumarin, to titrate egg PC at low ionic strength and found anionic groups on PC at neutral pH. At pH 9, the ζ-potential is also negative (∼−20 mV), indicating the binding of hydroxide ions with PC headgroups. The observed pattern of change in ζ-potential as a function of solution pH also agrees with the theoretical predictions of Figaszewski and colleagues, who suggested that a form of PC bound with protons mainly exists at extremely low pHs, that pH 4 is the isoelectric point of PC lipids, and that PC lipids associated with hydroxide ions are the predominant form with pH above 4 (24–26).
FIGURE 3.
The ζ-potential, as calculated from electrophoretic mobility data, plotted as a function of solution pH. The values are shown as mean ± SE. As shown, PC vesicles are positively charged at pH 2 and 3, near neutral at pH 4, and negatively charged at both pH 6.5 and 9.
Effect of pH on dipole potential
Ions in the solution can also partition into PC membranes to affect the internal electrical properties. To examine possible changes in dipole potential caused by changing pH, we measured the dipole potential according to the method of Clarke (51,57) and Xu and Lowe (56). The excitation spectra of di-8 ANEPPS incorporated into SOPC LUVs at different solution pHs were obtained. The ratios (F420/520) in Table 2 indicate values between 1.5 and 1.6, in relative agreement with Clarke and Lüpfert (51) who reported the ratio for 18:0/18:1 PC is 1.424. Our results indicate that F420/520 changes significantly as a function of solution pH (Table 2). When compared with data at pH 9, we found that F420/520 increased as pH decreased. We did not test the dipole potential at pH 2 because the pKa of di-8 ANEPPS is ∼1.8 (51), which would complicate data interpretation.
TABLE 2.
Effect of pH on membrane dipole potential
| pH | F (di-8 ANEPPS) | Ψd (mV) |
|---|---|---|
| 2 | N/A | N/A |
| 3 | 1.5801 ± 0.0102* | 221.9 ± 9.4* |
| 4 | 1.6027 ± 0.0330* | 222.7 ± 9.8* |
| 6.5 | 1.5635 ± 0.0215* | 215.5 ± 6.2* |
| 9 | 1.5084 ± 0.0381 | 205.3 ± 11 |
Values are shown as mean ± SD. Experiments were repeated at least three times.
Indicates values are statistically different from pH 9, as determined by a Student's t-test at 95% confidence. The ANOVA test supports the hypothesis that pH has an effect on SOPC dipole potential (F = 11.2 and P = 8.9 × 10−6).
We estimated the dipole potential of SOPC membranes at different pH values (see Table 2). The Ψd values obtained were ∼200–220 mV, within the range of those of PC bilayers obtained by Clarke and colleagues (51,57,58). As shown, the SOPC dipole potential increased as pH decreased from 9 (with the student's t-test P < 0.05 between pH 9 and other pHs, and ANOVA test F = 11.25 and P = 8.9 × 10−6).
Gel-phase characteristics at pH 2
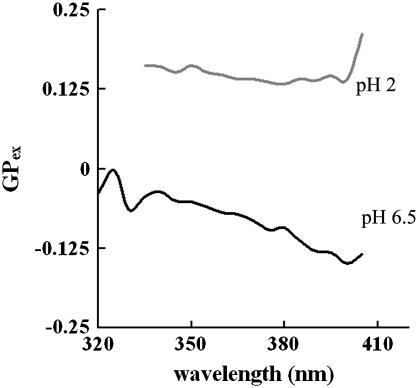
A phase-sensitive fluorescence probe, Laurdan, was utilized to examine possible SOPC gel-phase characteristics at pH 2. The emission generalized polarization (GPex) spectra as a function of excitation wavelength can be utilized to indicate phase behavior below, near and above the main phase transition temperatures (Tm) (66). As indicated by Bagatolli and Gratton (2000), the GP spectra for a PC lipid should tilt downward above the Tm, tilt upward near Tm, and become unaffected by the excitation wavelength below the main phase transition temperature (66). The excitation GP spectra for SOPC at pH 6.5 and pH 2 were examined between 400 nm and 500 nm. In Fig. 4, the downward tilting of the GP spectrum for SOPC at pH 6.5 clearly indicates that SOPC bilayer was in the liquid phase at 23°C. The first portion of the GP spectrum for SOPC at pH 2 is almost independent of the excitation wavelength and the later portion displays an upward tilt, both of which indicate that the LUVs composed of SOPC possess gel phase characteristics at pH 2. Furthermore, the positive GP values at pH 2 suggest that the fluorescence intensities obtained at 440 nm (gel phase maxima) is always higher than that obtained at 490 nm (liquid phase maxima), also indicating that the SOPC membrane has gel phase characteristics at pH 2. This also agrees with findings by Eibl and colleagues who have shown that low acidic pH values (<3) shift the main phase transition temperatures of PC lipids to higher temperatures (6–8).
FIGURE 4.
The excitation GP spectra of SOPC LUVs with Laurdan at pH 6.5 (bottom) and pH 2 (top) at room temperature. The GP spectrum at pH 6.5 shows a downward tilt, indicating a liquid phase. The first portion of the GP spectrum for SOPC at pH 2 is almost independent of the excitation wavelength and the later portion displays an upward tilt, both indicating gel phase characteristics at pH 2. The positive GP values at pH 2 also suggest that the fluorescence intensities obtained at 440 nm (gel phase maxima) is always higher than that obtained at 490 nm (liquid phase maxima), indicating gel phase characteristics at pH 2.
DISCUSSION
Our kc, ζ-potential, and Ψd measurements indicate that solution pH has significant effects on both mechanical and electrical properties of PC membranes. An immediate concern is whether these changes are caused by changes in the titration state of PC functional groups. However, none of the functional groups has a pKa within the range of pH 2–9: the pKa is <2 for phosphate (70), ∼11 for choline (70), and ∼−25 for the ester carbonyl (71). Thus, pH effects observed in this investigation most likely result from other physical interactions, such as counterion binding and partitioning into the membrane.
The ζ-potential measurements indicate that protons and hydroxide ions act as counterions and alter the external electrostatic environment of the membrane. The measured spectral shifts in the response of di-8 ANEPPS point to an effect of pH on the internal electrostatic environment of the membrane. In an attempt to explain the origin of these pH effects on membrane electrostatics and mechanics, we discuss our measurements in light of theoretical models, paying particular attention to the observed correlation between kc and the electrical properties of the membrane.
Effects of pH on membrane interfacial electrostatics and bending stiffness
The membrane bending stiffness contains both electrostatic and mechanical components (46,47). The lack of direct correlation between our measured kc and the change in H+/OH− concentrations suggests that the bending stiffness is unlikely to be influenced by H+/OH− partitioning into the bilayer and directly changing the mechanical packing of the lipids, as expected from the small size of these ions. Thus, we hypothesize that changes in interfacial electrostatics plays the major role in the observed effects of pH on the overall membrane bending stiffness. Electrolytes can not only alter the Debye length, but also potentially change the membrane surface charge and the charge density by acting as counterions and binding to lipids. Many investigators have demonstrated that the electrical properties of even zwitterionic PC headgroups are affected by counterion binding (43–45,62,72), which changes the surface charge and the charge density of membranes. The relationship between membrane surface charge and membrane mechanical properties is an unresolved biophysical problem. The membrane bending stiffness has been theoretically predicted to have an intricate relationship with membrane interfacial electrical properties (46–50). Utilizing Poisson-Boltzmann theory, Winterhalter and Helfrich (47) have constructed a model that predicts that the electrostatic contributions from both solution Debye length and membrane surface charge density can affect bending stiffness (47). In this model (47), the electrostatic portion of the bending stiffness, kc-el, can be expressed as
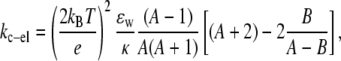 |
(6) |
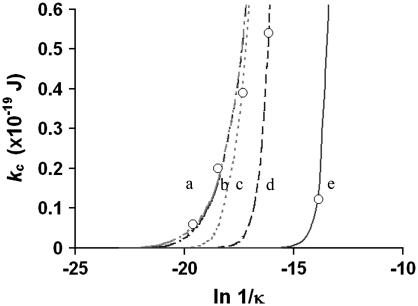
where A = {1+[σe/(2ɛwkBTκ)]2}1/2, B = (ɛi/ɛw)(1/κh), σ is the membrane surface charge density, κ is 1/Debye length, ɛw is the dielectric constant of water, ɛi is the dielectric constant of membranes, e is the elementary charge, and h is the thickness of the membrane. This model predicts that an increase in σ will increase kc-el at a fixed Debye length (47). Likewise, alterations in the Debye length will shift the kc-el versus σ-curve. A particular prediction of this model is that kc-el reaches a plateau when a membrane with a high surface charge density is exposed to a solution with high ionic strength (47). To estimate kc-el, we nominally chose the value of kc we measured at pH 3, where 1/κ = ∼10 nm and surface charge is high (suggested by our ζ-potential measurements). Under these conditions, kc-el should be at a plateau value, which Winterhalter and Helfrich (47) predict to be ∼0.2 × 10−19 J. Subtracting this from our data at pH 3, we estimate that the mechanical component of the bending stiffness, kc-m, is ∼0.78 × 10−19 J. Under the hypothesis that the measured alteration in the overall bending stiffness as a function of pH results from electrostatic effects, we can assume that kc-m remains fixed throughout the tested pH range, and any difference between our measured kc and ∼0.78 × 10−19 J reflect the electrostatic contributions to kc at that specific pH (listed in Table 1). With a view to illustrating trends, we plotted our measured kc-el versus Ln 1/κ (Fig. 5, open circles) and utilized Eq. 6 to estimate the membrane surface charge density, σest, at different pH values (Fig. 5, solid and dashed lines). Fig. 5 demonstrates that, with a fixed surface charge density, an increase in the Debye length will cause an increase in kc-el and changes in σest shift the kc-el versus Ln 1/κ curve, qualitatively in agreement with the prediction of Winterhalter and Helfrich (47).
FIGURE 5.
Plot of measured kc-el against Ln 1/κ (open circles). The membrane surface charge density, σest, was estimated by fitting Eq. 6 (solid and dashed lines) to the experimental data. In the plot, a is pH 2 with σest = 0.1 C/m2, b is pH 3 with σest = 0.03 C/m2, c is pH 4 with σest = 0.003 C/m2, d is pH 9 with σest = 3.3 × 10−4 C/m2, and e is pH 6.5 with σest = 6 × 10−6 C/m2.
The membrane surface charge density can also be estimated from our ζ-potential measurements (73,74). A simple way to relate ζ-potential to the surface potential is by the approximation 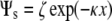 , where Ψs is the surface potential and x is the distance from the shear plane (the boundary between the fixed ion layer and the diffuse ion layer) to the interface, estimated to be ∼0.2 nm (73,74). The surface potential can be utilized to approximate the surface charge density, σcal (69,75)
, where Ψs is the surface potential and x is the distance from the shear plane (the boundary between the fixed ion layer and the diffuse ion layer) to the interface, estimated to be ∼0.2 nm (73,74). The surface potential can be utilized to approximate the surface charge density, σcal (69,75)
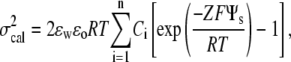 |
(7) |
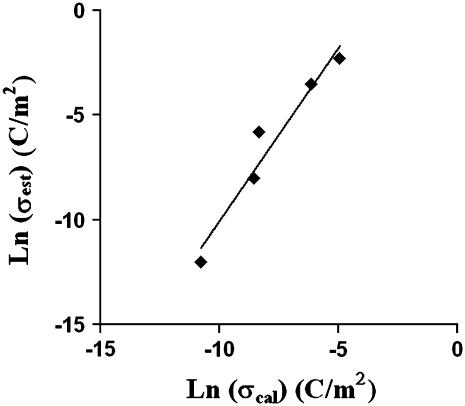
where Ci is the aqueous ion concentration, Z is the valence of the ion, F is the Faraday constant, and R is the gas constant. Using the value of surface charge density estimated from our measured kc and Eq. 6, σest, and the value of surface charge density calculated from our independently measured ζ-potential data, σcal, we plotted Ln (σest) versus Ln (σcal) (Fig. 6). The correlation in Fig. 6 indicates that changes in the membrane surface charge density caused by alterations in solution pH correlate with modifications in the overall bending stiffness. Note that the actual values of σcal do not match those of σest exactly, since both models utilized in calculations are approximations. However, the qualitative trend is important in the comparison. The good correlation in Fig. 6 supports the hypothesis that electrostatics plays a major role in the pH effect on the overall bending stiffness of SOPC membranes.
FIGURE 6.
The correlation between the estimated PC membrane surface charge density, σest, and membrane surface charge density calculated from the ζ-potential data, σcal. The linear correlation (R2 = 0.9466) between σest and σcal indicates that electrostatics play an important role in influencing membrane mechanical properties.
Furthermore, Fig. 5 demonstrates that both the membrane surface charge density and the solution Debye length contribute to the changes in the bending stiffness. At the extreme pH values of 2 and 3, high concentrations of protons (10 mM at pH 2) in the solution result in more ions available to bind with PC lipids when compared to pH 6.5, thus increasing the membrane surface charge (as supported by our ζ-potential measurements in Fig. 3). This is expected to increase the bending stiffness. However, high concentrations of ions also increase κ (the inverse Debye length), which mainly negatively influences the bending stiffness (46,47). Both the model and data support the hypothesis that the sharp decreases in Debye length at pH 2 and 3 out-compete the increase of the surface charge density at the interface, yielding a small kc-el and a negligible change in the overall bending stiffness. At moderate pH values of 4 and 9, the change in surface charge density apparently out-competes the decrease in Debye length, thus increasing the electrical component of the bending stiffness and yielding a larger overall bending stiffness.
To test this hypothesis, we measured kc of SOPC vesicles exposed to a pH 4 solution containing 10 mM NaCl, which gives a shorter Debye length than the original pH 4 solution. As predicted by Winterhalter and Helfrich (47), a decrease in 1/κ to <10 nm would yield a kc-el too small to be detected by experiments. Our measured kc at pH 4 with 10 mM NaCl (∼0.82 × 10−19 J) demonstrates that 10 mM NaCl diminished the effect of the original pH 4 solution on the kc of SOPC (∼1.2 × 10−19 J). The evidence that low concentrations of inorganic monovalent salts do not affect Kapp (35), which is also influenced by kc (32), suggests that 10 mM NaCl does not directly affect membrane-bending stiffness. Thus, we hypothesize that the decrease in overall bending stiffness at pH 4 with the addition of 10 mM NaCl results from the shortening of Debye length when compared to the original pH 4 solution.
Song and Waugh (76) previously investigated the effect of membrane surface charge on membrane bending stiffness using tether-formation experiments. They found no statistically significant difference in the bending stiffness between 100% SOPC and SOPC vesicles containing 14% palmitoyl oleoyl phosphatidylserine (76), which should increase the membrane surface charge density and increase kc-el. The reason for this discrepancy is not clear. One possible explanation could be that it may be energetically unfavorable for the charged PS to enter the highly curved tether. Another possibility is that the packing in mixed PC/PS membranes could reduce kc-m and offset the surface charge density increase.
Effect of pH on membrane dipole potential
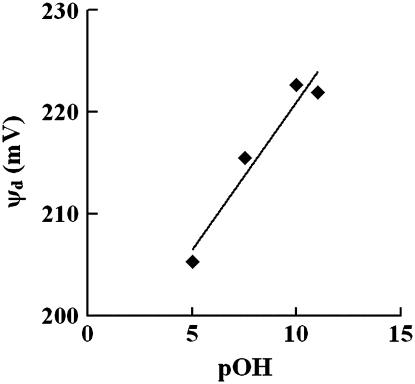
We have demonstrated above that solution pH affects both membrane mechanical and interfacial electrical properties and that alterations in membrane surface charge density and the Debye length can account for the experimentally measured changes in the membrane bending stiffness. This raises the question whether pH also affects the internal electrostatic properties of the membrane and whether there is a correlation between intramembrane electrical properties and mechanics. To investigate this, we measured changes in the intramembrane (dipole) potential as a function of solution pH (Table 2). The data in Table 2 are most clearly displayed by plotting dipole potential versus pOH, which demonstrates a linear correlation (Fig. 7). If the affinity of proton and hydroxide ions for membranes is the same, there should be a symmetric change in dipole potential as a function of pH centered at pH 7. The linear correlation observed in Fig. 7 suggests that either protons or hydroxide ions play a dominant role in affecting Ψd. Clarke and Lüpfert (51) established that the effectiveness of an ion in reducing the membrane dipole potential depends on the free energies of hydration, ΔGhyd, of the ion as well as whether the ion is positively or negatively charged. It was found that hydrophobic anions and hydrophilic cations tend to be more effective at altering the membrane dipole potential (51). Theoretical calculations estimate that ΔGhyd for protons and hydroxide ions is ∼−260 kJ/mol (77). This value indicates that these ions are relatively hydrophobic compared to other ions, such as Mg2+ with ∼−2000 kJ/mol and 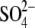 with ∼−1150 kJ/mol (51). Thus, it is likely that the relatively hydrophobic hydroxide ion is more effective at interacting with lipids than an equally hydrophobic proton. Since the ζ-potential is mainly a measure of the surface charge, a negative ζ-potential at pH 6.5 (Fig. 3) also points to the possibility that hydroxide ions associate with PC lipids more favorably than protons. Even with a slight excess of protons in the solution at pH 6.5, more hydroxide ions potentially associate with PC, yielding a negatively charged membrane. This also agrees with the studies of Ninham and colleagues (78,79), who suggest that the affinity of a particular ion for membranes depends upon the polarizability of the ion, or the ionic dispersion coefficient. A much more polarizable hydroxide ion should interact with membranes more favorably than a proton, which is not polarizable (78,79). In addition, both inorganic anions and cations decrease the dipole potential (51). The inability of acidic pHs, even at pH 3, to decrease the SOPC dipole potential also suggests that it is unlikely that protons are the cause of any changes in the dipole potential. Therefore, the partitioning of the anionic hydroxide ions potentially decreases the PC dipole potential, and a decrease in hydroxide concentration as the solution pH decreases is consistent with increases in the dipole potential.
with ∼−1150 kJ/mol (51). Thus, it is likely that the relatively hydrophobic hydroxide ion is more effective at interacting with lipids than an equally hydrophobic proton. Since the ζ-potential is mainly a measure of the surface charge, a negative ζ-potential at pH 6.5 (Fig. 3) also points to the possibility that hydroxide ions associate with PC lipids more favorably than protons. Even with a slight excess of protons in the solution at pH 6.5, more hydroxide ions potentially associate with PC, yielding a negatively charged membrane. This also agrees with the studies of Ninham and colleagues (78,79), who suggest that the affinity of a particular ion for membranes depends upon the polarizability of the ion, or the ionic dispersion coefficient. A much more polarizable hydroxide ion should interact with membranes more favorably than a proton, which is not polarizable (78,79). In addition, both inorganic anions and cations decrease the dipole potential (51). The inability of acidic pHs, even at pH 3, to decrease the SOPC dipole potential also suggests that it is unlikely that protons are the cause of any changes in the dipole potential. Therefore, the partitioning of the anionic hydroxide ions potentially decreases the PC dipole potential, and a decrease in hydroxide concentration as the solution pH decreases is consistent with increases in the dipole potential.
FIGURE 7.
The SOPC dipole potential was plotted against pOH, demonstrating that the dipole potential increases as solution pH decreases (R2 = 0.9403).
The fact that the hydrophobic cations do not affect the dipole potential (51) indicates that the effect of inorganic ions on the membrane dipole potential is potentially dominated by electrostatic interactions. As the membrane dipole potential is positive in the bilayer core, the partitioning of anionic hydroxide ions would diminish the interior positive characteristics and decrease the dipole potential, as supported by our data (Table 2). At acidic pH values, the decreasing hydroxide concentration corresponds to larger dipole potentials. At pH 9, the dipole potential is associated with the highest kc, while the increase in dipole potential at acidic pHs corresponds to a lower kc at pH 3 and pH 6.5 and an unchanged kc at pH 4 when compared with kc at pH 9. Because the di-8 ANEPPS spectral shift is a reflection of the membrane internal properties (56), the lack of direct correlation between bending stiffness and dipole potential further indicates that interfacial electrostatic contributions play a main role in the pH effect on PC bending stiffness.
A final interesting observation on the Ψd measurements is that the concentrations of hydroxide ions needed to affect PC membrane dipole potential are much less than those of Hofmeister ions. The maximal amount of hydroxide ions tested was 10 μM (at pH 9), while concentrations of Hofmeister ions are 500 mM in the study of Clarke and Lüpfert (51). This suggests that hydroxide ions are much more effective in terms of their ability to affect membrane dipole potential. It has been established that proton/hydroxide permeability is as much as nine orders-of-magnitude faster that other monovalent Hofmeister ions, indicating more favorable interactions between protons/hydroxide and membranes (80–82).
Gel-phase characteristics at pH 2
Eibl and colleagues have shown that low acidic pH (pH < 3) increases the main phase transition temperatures (Tm) of C-14/16 lecithin lipids by as much as ∼10°C while higher pH values had no detectable effects on Tm (6–8). This suggests that PC lipid lateral packing is not changed in this pH range (pH > 3), which agrees with our finding that KA was unaffected by changes in pH between 3 and 9. However, our data show a large drop in KA by ∼30% at pH 2, indicating a dramatic disruption in membrane cohesiveness. This observed decrease in KA is not caused by changes in the chemical composition of the SOPC membrane, as shown by thin layer chromatography experiments (data not shown). Theoretical (83,84) and experimental (85–87) studies demonstrated that membrane stability is reduced within the phase transition region. This is attributed to the presence of domains, which disrupt membrane continuity and weaken the entire bilayer structure. In addition to our mechanical data, we visually observed gel-phase characteristics in SOPC membranes at pH 2 (Fig. 2). The uncharacteristically wide spread of our KA data at pH 2 also points to the possibility that multiple phases could exist at pH 2. To further confirm this possibility, we utilized Laurdan to examine the phase behavior of SOPC LUVs at pH 2. We found that the excitation GP spectrum at pH 2 indicates the presence of gel phase characteristics (Fig. 4). Furthermore, although the GUVs we used were single-component system (100% SOPC) which should have a sharp transition, it has been suggested that giant vesicles have a broader transition because of lack of cooperative unit size in the GUVs as compared to LUVs, which are commonly used to determine Tm (67). Thus, an increase in the Tm and a potential broadening of the transition region in GUVs may be the mechanisms that explain the observed gel-phase characteristics in LUVs and GUVs at pH 2 and lower bilayer elasticity.
CONCLUSIONS
The solution pH is a crucial factor in ensuring normal cellular function. We have systematically characterized the effect of pH on the mechanical and electrical properties of SOPC membranes. Mechanical, interfacial electrical, and internal electrical measurements combine to support the view that changes in membrane bending stiffness result from alterations in interfacial electrostatics. Specifically, increases in kc at pH 4 and pH 9 reflect changes in membrane surface charge density and Debye length. Although the membrane dipole potential increases as solution pH decreases, this likely originates from hydroxide ion interactions with membranes and does not correlate with kc. The elastic compressibility of membranes is insensitive to pH, except for a decrease at pH 2, which is attributed to the presence of gel-phase characteristics. Hence, the alteration of solution pH is a straightforward method to study the relationship between mechanical and electrical properties of membranes. These results indicate that membrane electrical properties and mechanical properties are interconnected, supporting the emerging paradigm of the membrane as an electro-mechanical structure.
Acknowledgments
We thank Drs. Huey W. Huang, Jane Grande-Allen, and Michael S. Wong for equipment access. We are grateful to Drs. Nathan Baker and Kevin Mackenzie for insightful discussions and for critiquing a draft of this manuscript.
This study was supported in part by grants from the State of Texas Technology Development and Transfer Program and a National Science Foundation Career Award (BES 0449379).
References
- 1.Chernomordik, L. V., E. Leikina, V. Frolov, P. Bronk, and J. Zimmerberg. 1997. An early stage of membrane fusion mediated by the low pH conformation of influenza hemagglutinin depends upon membrane lipids. J. Cell Biol. 136:81–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redfern, D. A., and A. Gericke. 2004. Domain formation in phosphatidylinositol monophosphate/phosphatidylcholine mixed vesicles. Biophys. J. 86:2980–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redfern, D. A., and A. Gericke. 2005. pH-dependent domain formation in phosphatidylinositol polyphosphate/phosphatidylcholine mixed vesicles. J. Lipid Res. 46:504–515. [DOI] [PubMed] [Google Scholar]
- 4.Carrozzino, J. M., and M. G. Khaledi. 2005. pH effects on drug interactions with lipid bilayers by liposome electrokinetic chromatography. J. Chromatogr. A. 1079:307–316. [DOI] [PubMed] [Google Scholar]
- 5.Cevc, G., A. Watts, and D. Marsh. 1981. Titration of the phase transition of phosphatidylserine bilayer membranes. Effects of pH, surface electrostatics, ion binding, and head-group hydration. Biochemistry. 20:4955–4965. [DOI] [PubMed] [Google Scholar]
- 6.Eibl, H., and A. Blume. 1979. The influence of charge on phosphatidic acid bilayer membranes. Biochim. Biophys. Acta. 553:476–488. [DOI] [PubMed] [Google Scholar]
- 7.Trauble, H., and H. Eibl. 1974. Electrostatic effects on lipid phase transitions: membrane structure and ionic environment. Proc. Natl. Acad. Sci. USA. 71:214–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trauble, H., M. Teubner, P. Woolley, and H. Eibl. 1976. Electrostatic interactions at charged lipid membranes. I. Effects of pH and univalent cations on membrane structure. Biophys. Chem. 4:319–342. [DOI] [PubMed] [Google Scholar]
- 9.Stumpel, J., K. Harlos, and H. Eibl. 1980. Charge-induced pretransition in phosphatidylethanolamine multilayers. The occurrence of ripple structures. Biochim. Biophys. Acta. 599:464–472. [DOI] [PubMed] [Google Scholar]
- 10.Smith, B. D., and P. L. La Celle. 1979. Parallel decrease of erythrocyte membrane deformability and spectrin solubility at low pH. Blood. 53:15–18. [PubMed] [Google Scholar]
- 11.Heuser, J. 1989. Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J. Cell Biol. 108:855–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell, N. A., and J. B. Reece. 2002. Biology. Benjamin/Cummings, San Francisco, CA.
- 13.Barreto, J., and L. M. Lichtenberger. 1992. Vesicle acidification driven by a millionfold proton gradient: a model for acid influx through gastric cell membranes. Am. J. Physiol. 262:G30–G34. [DOI] [PubMed] [Google Scholar]
- 14.Lichtenberger, L. M. 1987. Membranes and barriers: with a focus on the gastric mucosal barrier. Clin. Invest. Med. 10:181–188. [PubMed] [Google Scholar]
- 15.Nylander-Koski, O., H. Mustonen, I. Vikholm, T. Kiviluoto, and E. Kivilaakso. 2001. HCl causes less intracellular acidification in Necturus gastric mucosa surface epithelial cells than other acids. Am. J. Physiol. Gastrointest. Liver Physiol. 281:G675–G680. [DOI] [PubMed] [Google Scholar]
- 16.Sulkowski, W. W., D. Pentak, K. Nowak, and A. Sulkowska. 2005. The influence of temperature, cholesterol content and pH on liposome stability. J. Mol. Struct. 744:737–747. [Google Scholar]
- 17.Smaby, J. M., J. M. Muderhwa, and H. L. Brockman. 1994. Is lateral phase separation required for fatty acid to stimulate lipases in a phosphatidylcholine interface? Biochemistry. 33:1915–1922. [DOI] [PubMed] [Google Scholar]
- 18.Liu, H., R. Z. Lu, J. G. Turcotte, and R. H. Notter. 1994. Dynamic interfacial properties of surface-excess films of phospholipids and phosphonolipids analogs. J. Colloid Interface Sci. 167:378–390. [Google Scholar]
- 19.Petriat, F., and S. Giasson. 2005. Study of pH-sensitive copolymer/phospholipid complexes using the Langmuir balance technique: effect of anchoring sequence and copolymer molecular weight. Langmuir. 21:7326–7334. [DOI] [PubMed] [Google Scholar]
- 20.Leonard, M. R., M. A. Bogle, M. C. Carey, and J. M. Donovan. 2000. Spread monomolecular films of monohydroxy bile acids and their salts: influence of hydroxyl position, bulk pH, and association with phosphatidylcholine. Biochemistry. 39:16064–16074. [DOI] [PubMed] [Google Scholar]
- 21.Furuike, S., V. G. Levadny, S. J. Li, and M. Yamazaki. 1999. Low pH induces an interdigitated gel to bilayer gel phase transition in dihexadecylphosphatidylcholine membrane. Biophys. J. 77:2015–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harlos, K., J. Stumpel, and H. Eibl. 1979. Influence of pH on phosphatidic acid multilayers. A rippled structure at high pH values. Biochim. Biophys. Acta. 555:409–416. [DOI] [PubMed] [Google Scholar]
- 23.Blume, A., and H. Eibl. 1979. The influence of charge on bilayer membranes. Calorimetric investigations of phosphatidic acid bilayers. Biochim. Biophys. Acta. 558:13–21. [DOI] [PubMed] [Google Scholar]
- 24.Petelska, A. D., and Z. A. Figaszewski. 1998. Interfacial tension of two-component bilayer lipid membrane modeling of the cell membrane. Bioelectrochem. Bioenerg. 46:199–204. [Google Scholar]
- 25.Petelska, A. D., and Z. A. Figaszewski. 2000. Effect of pH on the interfacial tension of lipid bilayer membrane. Biophys. J. 78:812–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petelska, A. D., and Z. A. Figaszewski. 2002. Effect of pH on the interfacial tension of bilayer lipid membrane formed from phosphatidylcholine or phosphatidylserine. Biochim. Biophys. Acta. 1561:135–146. [DOI] [PubMed] [Google Scholar]
- 27.Gennis, R. B. 1989. Biomembrane Molecular Structure and Function. C. R. Cantor, editor. Springer-Verlag, New York.
- 28.Cevc, G., and D. Marsh. 1987. Phospholipid Bilayers: Physical Principles and Models. E. E. Bittar, editor. John Wiley & Sons, New York, NY.
- 29.Israelachvili, J. N., D. J. Mitchell, and B. W. Ninham. 1976. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Faraday Trans. II. 72:1525–1568. [Google Scholar]
- 30.Israelachvili, J. N., S. Marcelja, and R. G. Horn. 1980. Physical principles of membrane organization. Q. Rev. Biophys. 13:121–200. [DOI] [PubMed] [Google Scholar]
- 31.Evans, E. A., and R. Skalak. 1980. Mechanics and Thermodynamics of Biomembranes. CRC Press, Boca Raton, FL.
- 32.Rawicz, W., K. C. Olbrich, T. McIntosh, D. Needham, and E. Evans. 2000. Effect of chain length and unsaturation on elasticity of lipid bilayers. Biophys. J. 79:328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Glassinger, E., A. C. Lee, and R. M. Raphael. 2005. Electromechanical effects on tether formation from lipid membranes: a theoretical analysis. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 72:041926. [DOI] [PubMed] [Google Scholar]
- 34.Zhou, Y., and R. M. Raphael. 2005. Effect of salicylate on the elasticity, bending stiffness, and strength of SOPC membranes. Biophys. J. 89:1789–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shoemaker, S. D., and T. K. Vanderlick. 2002. Intramembrane electrostatic interactions destabilize lipid vesicles. Biophys. J. 83:2007–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Needham, D., and R. S. Nunn. 1990. Elastic deformation and failure of lipid bilayer membranes containing cholesterol. Biophys. J. 58:997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Needham, D., and D. V. Zhelev. 1995. Lysolipid exchange with lipid vesicle membranes. Ann. Biomed. Eng. 23:287–298. [DOI] [PubMed] [Google Scholar]
- 38.Ly, H. V., and M. L. Longo. 2004. The influence of short-chain alcohols on interfacial tension, mechanical properties, area/molecule, and permeability of fluid lipid bilayers. Biophys. J. 87:1013–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwok, R., and E. Evans. 1981. Thermoelasticity of large lecithin bilayer vesicles. Biophys. J. 35:637–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hung, V. L., D. E. Block, and M. L. Longo. 2002. Interfacial tension effect of ethanol on lipid bilayer rigidity, stability, and area/molecule: a micropipette aspiration approach. Langmuir. 18:8988–8995. [Google Scholar]
- 41.Evans, E. A., W. Rawicz, and A. Hoffmann. 1994. Lipid Bilayer Expansion and Mechanical Degradation in Solutions of Water-Soluble Bile Acids. A. Hoffmann, G. Baumgartner, and A. Stiehl, editors. San Diego, CA.
- 42.Silvander, M., P. Hansson, and K. Edwards. 2000. Liposomal surface potential and bilayer packing as affected by PEG-lipid inclusion. Langmuir. 16:3696–3702. [Google Scholar]
- 43.Tatulian, S. A. 1983. Effect of lipid phase transition on the binding of anions to dimyristoylphosphatidylcholine liposomes. Biochim. Biophys. Acta. 736:189–195. [DOI] [PubMed] [Google Scholar]
- 44.Petrache, H. I., T. Zemb, L. Belloni, and V. A. Parsegian. 2006. Salt screening and specific ion adsorption determine neutral-lipid membrane interactions. Proc. Natl. Acad. Sci. USA. 103:7982–7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petrache, H. I., S. Tristram-Nagle, D. Harries, N. Kucerka, J. F. Nagle, and V. A. Parsegian. 2006. Swelling of phospholipids by monovalent salt. J. Lipid Res. 47:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Winterhalter, M., and W. Helfrich. 1988. Effect of surface charge on the curvature elasticity membranes. J. Phys. Chem. 92:6865–6867. [Google Scholar]
- 47.Winterhalter, M., and W. Helfrich. 1992. Bending elasticity of electrical charged bilayers: coupled monolayers, neutral surfaces, and balancing stresses. J. Phys. Chem. 96:327–330. [Google Scholar]
- 48.Lekkerkerker, H. N. W. 1989. Contribution of the electric double layer to the curvature elasticity of charged amphiphilic monolayers. Physica A. 159:319–328. [Google Scholar]
- 49.Mitchell, D. J., and B. W. Ninham. 1989. Curvature elasticity of charged membranes. Langmuir. 5:1121–1123. [Google Scholar]
- 50.Duplantier, B., R. E. Goldstein, V. Romero-Rochn, and A. I. Pesci. 1990. Geometrical and topological aspects of electric double layers near curved surfaces. Phys. Rev. Lett. 65:508–511. [DOI] [PubMed] [Google Scholar]
- 51.Clarke, R. J., and C. Lupfert. 1999. Influence of anions and cations on the dipole potential of phosphatidylcholine vesicles: a basis for the Hofmeister effect. Biophys. J. 76:2614–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brockman, H. 1994. Dipole potential of lipid membranes. Chem. Phys. Lipids. 73:57–79. [DOI] [PubMed] [Google Scholar]
- 53.Clarke, R. J. 2001. The dipole potential of phospholipid membranes and methods for its detection. Adv. Colloid Interface Sci. 89–90:263–281. [DOI] [PubMed] [Google Scholar]
- 54.Honig, B. H., W. L. Hubbell, and R. F. Flewelling. 1986. Electrostatic interactions in membranes and proteins. Annu. Rev. Biophys. Biophys. Chem. 15:163–193. [DOI] [PubMed] [Google Scholar]
- 55.Franklin, J. C., and D. S. Cafiso. 1993. Internal electrostatic potentials in bilayers: measuring and controlling dipole potentials in lipid vesicles. Biophys. J. 65:289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu, C., and L. M. Loew. 2003. The effect of asymmetric surface potentials on the intramembrane electric field measured with voltage-sensitive dyes. Biophys. J. 84:2768–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clarke, R. J. 1997. Effect of lipid structure on the dipole potential of phosphatidylcholine bilayers. Biochim. Biophys. Acta. 1327:269–278. [DOI] [PubMed] [Google Scholar]
- 58.Starke-Peterkovic, T., N. Turner, M. F. Vitha, M. P. Waller, D. E. Hibbs, and R. J. Clarke. 2006. Cholesterol effect on the dipole potential of lipid membranes. Biophys. J. 90:4060–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Angelova, M. J., S. Soleau, P. Melaeard, J. F. Faucon, and P. Bothorel. 1992. Preparation of giant vesicles by AC electric fields. kinetic and applications. Prog. Colloid Polym. Sci. 89:127–131. [Google Scholar]
- 60.Evans, E., V. Heinrich, F. Ludwig, and W. Rawicz. 2003. Dynamic tension spectroscopy and strength of biomembranes. Biophys. J. 85:2342–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Evans, E., and W. Rawicz. 1990. Entropy-driven tension and bending elasticity in condensed-fluid membranes. Phys. Rev. Lett. 64:2094–2097. [DOI] [PubMed] [Google Scholar]
- 62.McLaughlin, S. 1989. The electrostatic properties of membranes. Annu. Rev. Biophys. Biophys. Chem. 18:113–136. [DOI] [PubMed] [Google Scholar]
- 63.Parasassi, T., E. de Felip, F. Lepore, and F. Conti. 1986. Calcium-induced phase separation in phospholipid bilayers. A fluorescence anisotropy study. Cell. Mol. Biol. 32:261–266. [PubMed] [Google Scholar]
- 64.Parasassi, T., G. De Stasio, A. d'Ubaldo, and E. Gratton. 1990. Phase fluctuation in phospholipid membranes revealed by Laurdan fluorescence. Biophys. J. 57:1179–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bagatolli, L. A., E. Gratton, and G. D. Fidelio. 1998. Water dynamics in glycosphingolipid aggregates studied by LAURDAN fluorescence. Biophys. J. 75:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bagatolli, L. A., and E. Gratton. 2000. Two photon fluorescence microscopy of coexisting lipid domains in giant unilamellar vesicles of binary phospholipid mixtures. Biophys. J. 78:290–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bagatolli, L. A., and E. Gratton. 1999. Two-photon fluorescence microscopy observation of shape changes at the phase transition in phospholipid giant unilamellar vesicles. Biophys. J. 77:2090–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smejtek, P., A. Blochel, and S. Wang. 1996. Hydrophobicity and adsorption of chlorophenolates to lipid membranes. Chemosphere. 33:177–201. [DOI] [PubMed] [Google Scholar]
- 69.Kinraide, T. B., U. Yermiyahu, and G. Rytwo. 1998. Computation of surface electrical potentials of plant cell membranes. Correspondence to published ζ-potentials from diverse plant sources. Plant Physiol. 118:505–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tocanne, J. F., and J. Teissie. 1990. Ionization of phospholipids and phospholipid-supported interfacial lateral diffusion of protons in membrane model systems. Biochim. Biophys. Acta. 1031:111–142. [DOI] [PubMed] [Google Scholar]
- 71.Loundon, G. M. 1995. Organic Chemistry. A. Scanlan-Rohrer, editor. Benjamin/Cummings, Redwood City, CA.
- 72.Heinz, W. F., and J. H. Hoh. 1999. Relative surface charge density mapping with the atomic force microscope. Biophys. J. 76:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gilbert, D. L., and G. Ehrenstein. 1969. Effect of divalent cations on potassium conductance of squid axons: determination of surface charge. Biophys. J. 9:447–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cole, K. S. 1969. Zeta potential and discrete versus uniform surface charges. Biophys. J. 9:465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lau, A., A. McLaughlin, and S. McLaughlin. 1981. The adsorption of divalent cations to phosphatidylglycerol bilayer membranes. Biochim. Biophys. Acta. 645:279–292. [DOI] [PubMed] [Google Scholar]
- 76.Song, J., and R. Waugh. 1990. Bilayer membrane bending stiffness by tether formation from mixed PC-PS lipid vesicles. J. Biomech. Eng. 112:235–240. [DOI] [PubMed] [Google Scholar]
- 77.Tawa, G. J., I. A. Topol, S. K. Burt, R. A. Calwell, and A. A. Rashin. 1998. Calculation of the aqueous solvation free energy of the proton. J. Chem. Phys. 109:4852–4863. [Google Scholar]
- 78.Bostrom, M., D. R. Williams, and B. W. Ninham. 2001. Specific ion effects: why DLVO theory fails for biology and colloid systems. Phys. Rev. Lett. 87:168103. [DOI] [PubMed] [Google Scholar]
- 79.Bostrom, M., D. R. Williams, P. R. Stewart, and B. W. Ninham. 2003. Hofmeister effects in membrane biology: the role of ionic dispersion potentials. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 68:041902. [DOI] [PubMed] [Google Scholar]
- 80.Nagle, J. F. 1987. Theory of passive proton conductance in lipid bilayers. J. Bioenerg. Biomembr. 19:413–426. [DOI] [PubMed] [Google Scholar]
- 81.Nichols, J. W., and D. W. Deamer. 1980. Net proton-hydroxyl permeability of large unilamellar liposomes measured by an acid-base titration technique. Proc. Natl. Acad. Sci. USA. 77:2038–2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nichols, J. W., M. W. Hill, A. D. Bangham, and D. W. Deamer. 1980. Measurement of net proton-hydroxyl permeability of large unilamellar liposomes with the fluorescent pH probe, 9-aminoacridine. Biochim. Biophys. Acta. 596:393–403. [DOI] [PubMed] [Google Scholar]
- 83.Mouritsen, O. G., and M. J. Zuckermann. 1985. Softening of lipid bilayers. Eur. Biophys. J. 12:75–86. [DOI] [PubMed] [Google Scholar]
- 84.Nagle, J. F., and H. L. Scott, Jr. 1978. Lateral compressibility of lipid mono- and bilayers. Theory of membrane permeability. Biochim. Biophys. Acta. 513:236–243. [DOI] [PubMed] [Google Scholar]
- 85.Evans, E., and R. Kwok. 1982. Mechanical calorimetry of large dimyristoylphosphatidylcholine vesicles in the phase transition region. Biochemistry. 21:4874–4879. [DOI] [PubMed] [Google Scholar]
- 86.Mills, J. K., and D. Needham. 2005. Lysolipid incorporation in dipalmitoylphosphatidylcholine bilayer membranes enhances the ion permeability and drug release rates at the membrane phase transition. Biochim. Biophys. Acta. 1716:77–96. [DOI] [PubMed] [Google Scholar]
- 87.Papahadjopoulos, D., K. Jacobson, S. Nir, and T. Isac. 1973. Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol. Biochim. Biophys. Acta. 311:330–348. [DOI] [PubMed] [Google Scholar]