Abstract
We find that in contrast to strongly adherent, slow moving cells such as fibroblasts, neutrophils exert contractile stresses largely in the rear of the cell (uropod) relative to the direction of motion. Rather than the leading edge pulling the cell, the rear is both anchoring the cell and the area in which the contractile forces are concentrated. These tractions rapidly reorient themselves during a turn, on a timescale of seconds to minutes, and their repositioning precedes and sets the direction of motion during a turn. We find the total average root mean-squared traction force to be 28 ± 10 nN during chemokinesis, and 67 ± 10 nN during chemotaxis. We hypothesize that the contraction forces in the back of the neutrophil not only break uropodial adhesive contacts but also create a rearward squeezing contractility, as seen in amoeboid or amoeboidlike cells and the formation of blebs in cells, causing a flow of intracellular material to the fluidlike lamellipod. Our findings suggest an entirely new model of neutrophil locomotion.
An intense effort has been made to understand and quantify the mechanism by which the neutrophil translates outside-in signaling into directional cell motion (1). Actin polymerization is concentrated in the lamellipodia (2), while actin-myosin complexes and Rho-A activity exist mostly in the uropod (3). To turn, the neutrophil has to redistribute its cytoskeletal and intracellular components to alter its direction. However, the spatial and temporal distribution of traction stresses, or their modulation during turning and persistence, has never been measured in neutrophils. Here we show the location and magnitude of traction stresses created by neutrophils while undergoing migration in a uniform concentration of chemoattractant (chemokinesis) and in the presence of a chemoattractant gradient (chemotaxis).
Neutrophils are key players in the cellular immune response and capable of migrating quickly at speeds up to 20 μm/min (4). The rapid motion and a neutrophil's ability to turn rapidly imply that neutrophils have unique methods for developing contractile stress and orientation. The expected inverse correlation between speed and force suggests that the forces generated by migrating neutrophils would be small and difficult to detect, but we find carefully developed traction force microscopy (5,6) measurements are adequate to resolve neutrophil motile forces.
To measure neutrophil traction stresses we utilized a surface composed of a polyacrylamide gel (Young's modulus = 9000 Pa (7)) prepared as previously reported (5,6). The substrate was coated with a combination of E-selectin/Fc chimera (41 ± 3/μm2) and ICAM-1/Fc chimera (36 ± 5/μm2) after crosslinking the gel with protein G and coincubating the chimeras at 5 μg/mL each over the gel (see Supplementary Material).
For chemokinesis measurements, the gel was mounted in a flow chamber. Flow was introduced into the chamber, and the neutrophils rolled along the surface until we introduced a concentration of 2nM fMLP, which immediately resulted in firm adhesion, a period of spreading, and chemokinesis (8) (see Supplementary Material). During chemokinesis, neutrophils displayed random motion, with an average migration velocity of 3.5 ± 0.2 μm/min (n = 4 days, 23 cells). This migration velocity is less than that measured for neutrophils migrating on protein-coated polystyrene surfaces (∼12 μm/min) (8). The difference could stem from differences in surface compliance or cell adhesiveness between polystyrene and gel surfaces. The random motility coefficient was 4.4 ± 0.8 μm2/min, and the index of migration, which is a measure of the fraction of the trajectory that occurs in the direction of flow relative to the entire trajectory length, was 0.01 ± 0.05, indicating neutrophils were moving chemokinetically.
During chemokinesis, the neutrophil undergoes persistent motion for short times and random motion over long times, during which changes in direction are routine (4,9). A typical image of the neutrophil, a vector representation of the traction stress, and a pseudocolor image emphasizing the spatial location of stresses illustrate that while the neutrophil is moving persistently, the traction stresses are consistently located either toward the back or along the back edge of the neutrophil, consistent with the location of the actin-myosin bundles and Rho-A (3,10) (see Supplementary Material Fig. S1). Traction stresses are only occasionally, but not consistently, located anywhere in the leading edge of the cell. The total average root mean-square (RMS) force (5) of neutrophils undergoing chemokinesis was found to be 28 ± 10 nN (n = 4 cells), in remarkable agreement with what was previously reported for neutrophil migration in tissue (11) or forces created during neutrophil phagocytosis using micropipette counterpressure (12,13).
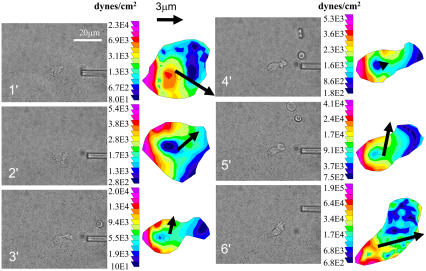
In Fig. 1, we show a set of spatiotemporal traction maps of a neutrophil undergoing a turn during chemokinesis (5,6,14). The time interval between images is ∼1–2 min, and the vector arrow indicates the direction of cell motion in the 1–2 min after the traction stress was imaged; the correlation between traction orientation and motion suggests how the two are coupled. Tractions are located in the uropod, counteropposed to the direction in which the cell will move in the next 1–2 min. In this sequence, the neutrophil is moving persistently to the upper left (in images i–iii) until it initiates a turn (in image iv) through regeneration of a new force center, which initiates its motion downward. Thus, the physical motion of turning is preceded by the regeneration of a new locus of force, which dictates the ultimate direction of motion.
FIGURE 1.
Traction maps of a neutrophil undergoing a turn during chemokinesis. Neutrophils were infused into a parallel-plate flow chamber, in which the bottom surface was composed of a polyacrylamide gel cross-linked to E-selectin and ICAM-1. The neutrophils were perfused at 180 s−1 resulting in rolling until 2 nM fMLP was introduced into the system (see Supplementary Material). The traction map is provided in pseudocolor, indicating regions of low and high force (dark blue to light purple). The black vectors overlaid on the color maps represent the magnitude and direction of the movement of the cell centroid between the current and the next images (∼1–2 min apart). The scale vector represents 2 μm and the color bar represents dynes/cm2.
We performed similar measurements of neutrophils undergoing chemotaxis. We used a micropipette to release a point source of 100 nM fMLP, thus creating a chemoattractant concentration gradient. The neutrophils formed strong traction stresses in the back of the cell relative to the location of the point source, while force generation in the lamellipodia was rare, as is illustrated in Fig. 2. From the traction images, we calculated an average RMS force of 67 ± 10 nN (n = 8 cells). These forces formed and their direction was maintained as the neutrophil crawled toward the point source. The micropipette was placed to the right of the chamber for the first five images, and was moved to the upper right of the cell for the sixth image. The corresponding traction maps indicate that the forces in the cell are low as the micropipette is initially introduced and continue to grow by the fifth image, as the cell responds chemotactically and larger forces are created in the uropod. The direction of motion is toward the pipette, even as the pipette is moved, and the cell responds by maintaining the concentration of stress in the uropod. When the pipette is moved suddenly, requiring a slight change in direction, the orientation of traction force generation in the uropod is altered preceding the change of direction. During chemotaxis, unlike chemokinesis, no force generation is seen in the lamellipod, even during a turn. Total RMS forces generated by the neutrophils in Figs. 1 and 2 are plotted in Supplementary Material Fig. S2.
FIGURE 2.
Force traction maps of human neutrophils undergoing chemotaxis. A micropipette introduces a point source of 100 nM fMLP under static conditions. (First and third columns) Phase images of a neutrophil undergoing chemotaxis toward a micropipette. The micropipette is placed to the right of the cell for the first 5 min and is then moved to the upper-right corner for the sixth minute. Scale bar indicates 20 μm. (Second and fourth columns) Corresponding color traction maps of the cell to its left with the black vector representing the magnitude and direction of movement of the cell centroid between subsequent images (∼1 min apart). The scale vector represents 3 μm and the pseudocolor bar representing traction stresses is given in dynes/cm2.
Forces generated during chemotaxis, 67 ± 10 nN, are generally higher than those created and maintained during chemokinesis. A histogram of forces created during chemokinesis and chemotaxis indicates while forces are on average higher during chemotaxis, extremely large forces as ≥90 nN occasionally occur during chemotaxis (Supplementary Material Fig. S3).
Our measurements show a concentration of the traction stresses in the neutrophil's uropod. Separately, it has been shown by others that important signaling molecules in the Rho-GTPase family, such as RhoA (3,10) as well as actin-myosin bundles (3), are concentrated in the uropod. Further, Rho GTPases have been directly implicated in force generation of breast epithelial cells (15). Based on our results and these other published reports, we hypothesize that RhoA is responsible for the uropodial stresses and thereby sets the direction of neutrophil migration. However, further direct testing of this hypothesis—in which knockdown or knockout experiments are coupled to traction measurements—is needed.
Furthermore, our data indicates that neutrophils migrate in a sequence that is largely the reverse of what has been seen in strongly adherent, slow moving cells such as fibroblasts (14). The accepted model for cell motility in fibroblasts is that cells move through adhesion, lamellipodia or filipodia extension, contraction along the leading edge, and rear de-adhesion (14,16,17).
In contrast, neutrophil motility seems to be organized and initiated in the uropod, leading to forced lamellipodial polymerization and then the adhesion of the lamellipod. Rather than the leading edge pulling the cell, the rear is anchoring the cell and serving as a locus of force generation. Thus, neutrophil motility follows the reverse of the commonly accepted sequence. Significant traction stresses in the front seem to appear only when the neutrophil needs to “decide” on the direction of the next step (see Fig. 1), which has been hypothesized to be the result of stochastic noise in perceived concentration gradients while in the absence of a gradient (9). We can theorize that lateral traction stresses might contribute to the motility of neutrophils by squeezing and pressurizing the neutrophil interior, thus rushing material to the leading edge (18), causing a fluidlike lamellipod similar to that seen in amoeboid and amoeboid-like cells (19,20). In chemotaxis, it is possible that a persistently perceived chemoattractant gradient (10), where the stochastic noise is now centered on the mean of the gradient, allows for a more efficient spatially organized signaling cascade (3). This would cause a maximal accumulation of molecular motors and key enzymes to the uropod, leading to higher force generation and establishing directional persistence. It now remains to be seen how such directionality can be altered intelligently through molecular manipulation.
SUPPLEMENTARY MATERIAL
An online supplement to this article can be found by visiting BJ Online at http://www.biophysj.org.
Acknowledgments
The authors thank Eric Johnston and Andrew Trister for technical support and Sally Zigmond for useful discussions.
We acknowledge support from National Institutes of Health grant No. HL18208.
References
- 1.Li, S., J. L. Guan, and S. Chien. 2005. Biochemistry and biomechanics of cell motility. Annu. Rev. Biomed. Eng. 7:105–150. [DOI] [PubMed] [Google Scholar]
- 2.Pollard, T. D., and G. G. Borisy. 2003. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 112:453–465. [DOI] [PubMed] [Google Scholar]
- 3.Xu, J., F. Wang, A. Van Keymeulen, P. Herzmark, A. Straight, K. Kelly, Y. Takuwa, N. Sugimoto, T. Mitchison, and H. R. Bourne. 2003. Divergent signals and cytoskeletal assemblies regulate self-organizing polarity in neutrophils. Cell. 114:201–214. [DOI] [PubMed] [Google Scholar]
- 4.Lauffenburger, D. A., and J. J. Linderman. 1993. Receptors: Models for Binding, Trafficking, and Signaling. Oxford University Press, New York.
- 5.Reinhart-King, C. A., M. Dembo, and D. A. Hammer. 2005. The dynamics and mechanics of endothelial cell spreading. Biophys. J. 89:676–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dembo, M., and Y. L. Wang. 1999. Stresses at the cell-to-substrate interface during locomotion of fibroblasts. Biophys. J. 76:2307–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeung, T., P. C. Georges, L. A. Flanagan, B. Marg, M. Ortiz, M. Funaki, N. Zahir, W. Ming, V. Weaver, and P. A. Janmey. 2005. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil. Cytoskeleton. 60:24–34. [DOI] [PubMed] [Google Scholar]
- 8.Smith, L. A., J. Haun, H. Aranda-Espinoza, and D. A. Hammer. 2006. Interplay between shear stress and adhesion on neutrophil locomotion. Biophys. J. 92:632–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tranquillo, R. T., D. A. Lauffenburger, and S. H. Zigmond. 1988. A stochastic model for leukocyte random motility and chemotaxis based on receptor binding fluctuations. J. Cell Biol. 106:303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van Keymeulen, A., K. Wong, Z. A. Knight, C. Govaerts, K. M. Hahn, K. M. Shokat, and H. R. Bourne. 2006. To stabilize neutrophil polarity, PIP3 and Cdc42 augment RhoA activity at the back as well as signals at the front. J. Cell Biol. 174:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guilford, W. H., R. C. Lantz, and R. W. Gore. 1995. Locomotive forces produced by single leukocytes in vivo and in vitro. Am. J. Physiol. 268:C1308–C1312. [DOI] [PubMed] [Google Scholar]
- 12.Evans, E., A. Leung, and D. Zhelev. 1993. Synchrony of cell spreading and contraction force as phagocytes engulf large pathogens. J. Cell Biol. 122:1295–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herant, M., V. Heinrich, and M. Dembo. 2006. Mechanics of neutrophil phagocytosis: experiments and quantitative models. J. Cell Sci. 119:1903–1913. [DOI] [PubMed] [Google Scholar]
- 14.Munevar, S., Y. Wang, and M. Dembo. 2001. Traction force microscopy of migrating normal and H-Ras transformed 3T3 fibroblasts. Biophys. J. 80:1744–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paszek, M. J., N. Zahir, K. R. Johnson, J. N. Lakins, G. I. Rozenberg, A. Gefen, C. A. Reinhart-King, S. S. Margulies, M. Dembo, D. Boettiger, Daniel A. Hammer, and Valerie M. Weaver. 2005. Tensional homeostasis and the malignant phenotype. Cancer Cell. 8:241–254. [DOI] [PubMed] [Google Scholar]
- 16.Alberts, B. 2002. Molecular Biology of the Cell. Garland Science, New York.
- 17.Lauffenburger, D. A., and A. F. Horwitz. 1996. Cell migration: a physically integrated molecular process. Cell. 84:359–369. [DOI] [PubMed] [Google Scholar]
- 18.Condeelis, J. 1993. Life at the leading edge: the formation of cell protrusions. Annu. Rev. Cell Biol. 9:411–444. [DOI] [PubMed] [Google Scholar]
- 19.Yanai, M., C. M. Kenyon, J. P. Butler, P. T. Macklem, and S. M. Kelly. 1996. Intracellular pressure is a motive force for cell motion in Amoeba proteus. Cell Motil. Cytoskeleton. 33:22–29. [DOI] [PubMed] [Google Scholar]
- 20.Yanai, M., J. P. Butler, T. Suzuki, H. Sasaki, and H. Higuchi. 2004. Regional rheological differences in locomoting neutrophils. Am. J. Physiol. Cell Physiol. 287:C603–C611. [DOI] [PubMed] [Google Scholar]