Abstract
Damage of the placenta resulting from ischemia-reperfusion is important to the pathophysiology of preeclampsia. Here we investigated whether low concentrations of glyceryl trinitrate (GTN), a nitric oxide mimetic with anti-apoptotic properties, inhibit hypoxia/reoxygenation-induced apoptosis in the syncytiotrophoblast of chorionic villous explants from human placentas. Compared with villi analyzed immediately after delivery or maintained under normoxic conditions, villi exposed to a 6-hour cycle of hypoxia/reoxygenation exhibited greater numbers of syncytiotrophoblasts with terminal dUTP nick-end labeling (TUNEL)-positive nuclei in the syncytiotrophoblast. This increased number of TUNEL-positive nuclei was paralleled by higher levels of 4-hydroxynonenal (marker of lipid peroxidation), nitrotyrosine residues, and active caspase-3 and polyADP-ribose polymerase expression. Morphological analysis of explants exposed to hypoxia/reoxygenation revealed apoptotic and aponecrotic features similar to those of chorionic villi from preeclamptic pregnancies. Treatment with GTN during the hy-poxia/reoxygenation cycle blocked the increases in the number of TUNEL-positive nuclei and in the levels of 4-hydroxynonenal, nitrotyrosine, and active caspase-3. Incubation with GTN also attenuated the hypoxia/reoxygenation-induced polyADP-ribose polymerase expression and the apoptotic and aponecrotic morphological alterations. These results suggest that small concentrations of nitric oxide protect chorionic villi from hypoxia/reoxygenation-induced damage and provide a rationale for the use of low doses of nitric oxide mimetics in the treatment and/or prevention of preeclampsia.
Preeclampsia is a disease of human pregnancy characterized by a systemic maternal inflammatory response associated with endothelial dysfunction, hypertension, and proteinuria. This condition affects 5 to 7% of all pregnancies and is the main cause of perinatal mortality and morbidity in developed countries. There is also evidence that the risk of subsequent cardiovascular disease is significantly increased in women affected by preeclamptic pregnancies.1,2
Although the pathophysiology of preeclampsia has not been fully defined, there is evidence that placental oxidative stress attributable to abnormal uteroplacental blood circulation plays a critical role. In preeclampsia, the transformation that normally leads to spiral arterioles with large diameters is defective, and consequently, placental perfusion is compromised.3,4 Furthermore, it has been suggested that uteroplacental blood flow in preeclampsia is intermittent or pulsatile, likely attributable to the persistent sensitivity of the maladapted spiral arterioles to maternal vasopressor molecules.5,6 It has been postulated that the abnormally decreased and intermittent perfusion of the intervillous space of the placenta results in oxidative damage and the release of apoptotic and aponecrotic placental tissue into the maternal circulation.7 The presence of large amounts of syncytiotrophoblast microfragments in the maternal circulation is thought to promote the maternal systemic inflammatory response and endothelial dysfunction characteristic of preeclampsia.8 Indeed, an increased prevalence of apoptotic nuclei has been reported in the syncytiotro-phoblast of placentas from pregnancies complicated by preeclampsia.9 Using an in vitro model of hypoxia/reoxygenation (H/R) that replicates the oxidative stress that placental tissues undergo during preeclampsia, Hung and colleagues6 demonstrated that the syncytiotrophoblast of normal chorionic villi exposed to H/R undergoes apoptotic and aponecrotic changes similar to those observed in the syncytiotrophoblast of chorionic villi from preeclamptic pregnancies.
In a recent study we demonstrated that carbon monoxide (CO) is able to inhibit the H/R-induced apoptosis of the syncytiotrophoblast in chorionic villi from term human placentas.10 Nitric oxide (NO), like CO, is a small polyvalent molecule that plays a role in regulating multiple biological functions. It induces vasodilation, regulates platelet adhesion, is involved in various aspects of vascular remodeling, acts as a neurotransmitter, and is a mediator of cell growth and apoptosis. Many cell types, including trophoblast cells, produce NO.11,12,13 Recent studies have shown that NO protects cultured extravillous trophoblast cells from apoptosis through a mechanism involving the activation of soluble guanylyl cyclase (sGC).14 Thus, in the present study we used a well established explant model6 to determine whether low concentrations of the NO mimetic glyceryl trinitrate (GTN; nanomolar to micromolar range) are able to attenuate the changes associated with the apoptotic and aponecrotic effects of H/R in the syncytiotrophoblast of term chorionic villi. These changes were assessed by a variety of approaches including the terminal dUTP nick-end labeling (TUNEL) assay and immunodetection of 4-hydroxynonenal (4-HNE, a marker of lipid peroxidation), nitrotyrosine residues, caspase-3, and polyADP-ribose polymerase (PARP). Morphological alterations were assessed by light and electron microscopy.
Materials and Methods
Collection and Culture of Chorionic Villous Explants
Human term placentas (n = 13) were obtained from nonlaboring normal pregnancies immediately after cesarean deliveries at Kingston General Hospital. Collection of placentas was done with the approval of the Queen’s University Research Ethics Board. After the removal of the basal plate from placental lobules, tissue cubes of ∼2 cm3 were dissected from at least seven randomly selected sites free of calcification across the placenta. The tissue was transferred to the laboratory in a sterile sealed container in ice-cold phosphate-buffered saline (PBS).
Chorionic villi (5 to 10 mg) from the collected tissue were dissected on ice, rinsed once with ice-cold PBS, and twice with CMRL-1066 culture medium (Invitrogen, Burlington, ON, Canada). Five explants were cultured in individual Costar Netwell supports (15-mm diameter, 74-μm mesh; Cole-Parmer, Anjou, QC, Canada) in 1.2-ml culture medium supplemented with 5% heat-inactivated fetal bovine serum, 2.2 mg/ml NaHCO3, 100 μg/ml streptomycin sulfate, 100 IU/ml penicillin G, 1 μg/ml insulin, and 2 μg/ml l-glutamine (Sigma-Aldrich Canada Ltd., Oakville, ON, Canada). For H/R exposures, explants (total of 65) were treated with or without GTN (1 μmol/L or 1 nmol/L; Sabex, Boucherville, QC, Canada) and incubated for 3 hours in an atmosphere of 0.5% O2 (3.8 mm Hg), 5% CO2, and 94.5% N2 maintained by means of a ProOx O2 regulator (Biospherix Inc., Redfield, NY) in a humidified Plexiglas chamber at 37°C. Tissues were then transferred to medium continuously flushed with 21% O2/5% CO2/balance N2 and were incubated in a standard incubator at 5% CO2 and 21% O2 (159 mm Hg) for 3 additional hours. These reoxygenation conditions were in accordance to the method of Hung and colleagues6 and were chosen to maximize oxidative stress. Additional controls consisted of explants (n = 5 placentas, for a total of 25 explants) kept in normoxic conditions (5% O2, 90% N2, and 5% CO2) for 6 hours. These oxygen concentrations (38 mm Hg) are similar to those measured within the intervillous space of the term placenta.15,16 At times 0, 3, and 6 hours, explants were collected from all experimental groups and either flash-frozen for molecular analysis or fixed in 4% paraformaldehyde or 2% paraformaldehyde/0.5% glutaraldehyde for histological analysis.
TUNEL Assay and Apoptotic Index
Paraformaldehyde-fixed explants were embedded in paraffin and cut into serial sections. To assess apoptosis of the syncytiotrophoblast layer, a fluorescence TUNEL assay was performed according to the manufacturer’s instructions (In Situ Cell Death Detection kit; Roche Molecular Biochemicals, Laval, QC, Canada). In brief, deparaffinized, dewaxed, and rehydrated sections were pretreated with 20 μg/ml proteinase K (Sigma-Aldrich) in 10 mmol/L Tris-HCl for 15 minutes, blocked with 10% normal goat serum, and then stained for TUNEL using a reaction mixture containing fluorescein-dUTP. Negative controls consisted of sections incubated without terminal deoxynucleotidyl transferase (TdT). All sections were blinded by a third party and observed with a Leica inverted microscope (Leica Microsystems, Heidelberg, Germany) using a ×20 objective lens. Three fields containing a minimum of 900 syncytiotrophoblast nuclei were randomly selected in each section, and digital images of the TUNEL-stained (green) and 4,6-diaminodino-2-phenylindole (DAPI)-stained (blue) sections were captured and deconvoluted using Slidebook 4.1 software (Intelligent Imaging Innovations Inc., Denver, CO). TUNEL-positive (apoptotic) and DAPI-stained (total) nuclei were counted in the syncytiotrophoblast layer using Image-Pro Plus 5.1 software (Media Cybernetics Inc., Silver Spring, MD). The apoptotic index in each section was calculated as the percentage of TUNEL-positive syncytiotrophoblast nuclei divided by the total number of DAPI-stained syncytiotrophoblast nuclei.
Localization of TUNEL-Positive Nuclei and 4-HNE
Double-fluorescence labeling was used to determine the localization of TUNEL-positive nuclei and 4-HNE within chorionic villous explants. In brief, deparaffinized, de-waxed, and rehydrated sections were pretreated in 20 μg/ml proteinase K (Sigma) in 10 mmol/L Tris-HCl for 15 minutes, blocked with 10% normal goat serum, and stained for TUNEL. Sections were then sequentially incubated with a mouse monoclonal antibody against 4-HNE (1:300; Japan Institute for the Control of Ageing, Shizuka, Japan) for 2 hours and after several washes, the sections were incubated with a fluorescent goat anti-mouse secondary antibody (1:600; 555-nm excitation wavelength, 565-nm emission wavelength; Alexa, Invitrogen). The sections were then subjected to TUNEL staining as per the manufacturer’s instructions. All sections were mounted using Vectashield (Vector Laboratories, Burlington, ON, Canada) and observed with a Leica inverted confocal microscope (Leica Microsystems) at a magnification of ×60 with simultaneous excitation and detection of both dyes.
Immunohistochemical Staining for Nitrotyrosine Residues and the p85 Fragment of PARP
Paraffin-embedded placental sections were randomly selected and deparaffinized by heating at 40°C for 20 minutes followed by sequential incubations in Hemo-D and decreasing concentrations of ethanol (each for 2 to 3 minutes). Endogenous peroxidase activity was quenched in 3% hydrogen peroxide in methanol, and nonspecific binding was blocked by incubating the sections in 5% normal goat serum. After extensive washes with PBS, the sections were reacted with a rabbit polyclonal anti-nitrotyrosine antibody (1:500; Upstate Biotechnology Inc., Lake Placid, NY) for 1 hour or with a rabbit polyclonal anti-PARP p85 fragment antibody (1:500; Promega, Nepean, ON, Canada) for 4 hours at room temperature followed by the addition of biotinylated anti-rabbit secondary antibody (1:200; Vector Laboratories). Further processing of the sections for the detection of nitrotyrosine residues or the p85 fragment of PARP was performed according to the instructions provided with the Vectastain Elite ABC kit (Vector Laboratories). Colorimetric detection was achieved using diaminobenzidine as chromogen and hydrogen peroxide as substrate for horseradish peroxidase. Quantification of the p85-fragmented PARP staining was performed using Image-Pro Plus 5.1 software.
Western Blot Analysis of 4-HNE, Nitrotyrosine Residues, Caspase-3, and PARP
To further assess oxidative stress and activation of apoptosis, the levels of 4-HNE, nitrotyrosine residues, active caspase-3, and PARP were determined by Western blot analysis of extracts of flash-frozen villous explants (n = 5 to 7 placentas). In brief, explants were cut and lysed using a buffer containing 2% sodium dodecyl sulfate, 10 mmol/L Tris (pH 7.5), and 0.15 mmol/L NaCl. Lysates were homogenized and subjected to DNA shearing (10 times with a 25-gauge needle) and centrifugation. The culture supernatants were collected and stored at −80°C until use. Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and the resolved proteins were transferred onto an Immobilon-P membrane (Millipore Corp., Bedford, MA). After a 1-hour incubation in blocking solution consisting of 5% dry milk and 0.05% Tween 20 in PBS (PBS-T), the membranes were incubated with mouse monoclonal antibody against 4-HNE (1:500; Japan Institute for the Control of Ageing), rabbit polyclonal anti-nitrotyrosine antibody (1:1000; Upstate Biotechnology), goat polyclonal antibody against caspase-3 (1:1000; R&D Research, Minneapolis, MN), or rabbit polyclonal anti-PARP (1:500; Promega) for 1 to 2 hours at room temperature followed by three 5-minute washes in PBS-T. The membranes were then incubated with secondary goat anti-mouse (1:4000; Vector Laboratories Inc.) or rabbit anti-goat IgG (1:2000; Vector Laboratories Inc.) labeled with horseradish peroxidase. After three additional 5-minute washes with PBS-T, the blots were developed using a chemiluminescence Western blotting plus kit (Perkin Elmer, Burlington, ON, Canada), and subsequently exposed onto X-OMAT Kodak film (Eastman-Kodak, Rochester, NY). Uniformity of loading of protein extracts was determined by probing with a monoclonal anti-β-actin antibody (clone AC-15, 1:8000; Bio-Rad, Mississauga, ON, Canada). Relative intensities of bands were determined by densitometry using AlphaErase software (Alpha Innotech Corp., San Leandro, CA).
Light and Electron Microscopy
Explants fixed in 2% paraformaldehyde/0.5% glutaraldehyde and embedded in araldite resin were used for light and electron microscopic analysis. Semithin sections (1 μm) were prepared for light microscopy and for the selection of areas to conduct further ultrastructural analysis at the electron microscopic level. Morphological assessments at the light microscopic level were performed using toluidine blue-stained sections. For electron microscopic analysis, ultrathin sections (70 nm) were cut and counterstained with uranyl acetate and lead citrate. An additional control for the ultrastructural analysis consisted of chorionic villi obtained from placentas of preeclamptic women. Preeclampsia was defined by the development of elevated maternal blood pressure (>140/90 mm Hg) and proteinuria (≥300 mg/24 hours). Morphological analysis of tissues was performed using a Hitachi 7000 transmission electron microscope at magnifications of ×3500 to ×15,000.
Statistical Analysis
Statistical significance was determined using one-way analysis of variance, when more than two groups were compared, followed by a post hoc Tukey-Kramer test. Nonpaired and paired Student’s t-tests were used when only two groups were compared. All statistical tests were two-sided. Data are presented as the mean ± SE, and were considered significant at P < 0.05.
Results
GTN Decreases the Level of H/R-Induced Apoptosis in the Syncytiotrophoblast
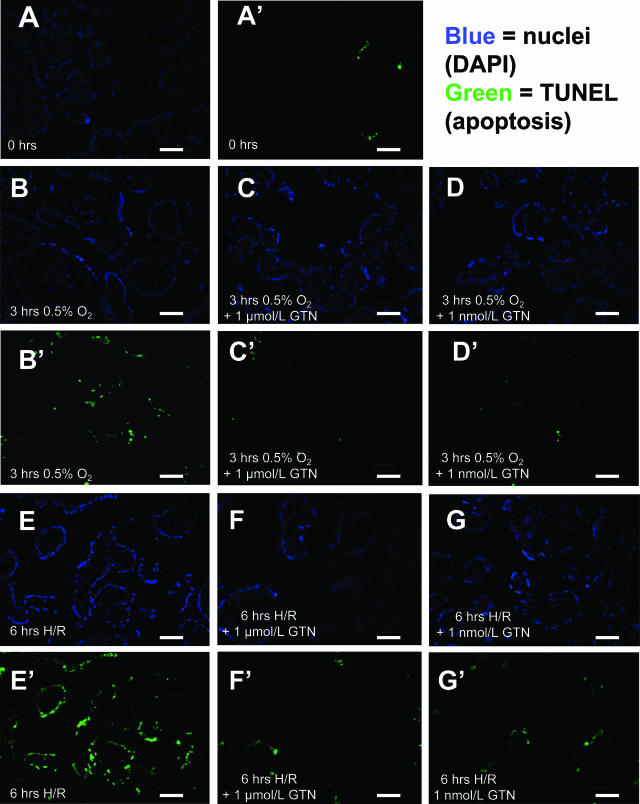
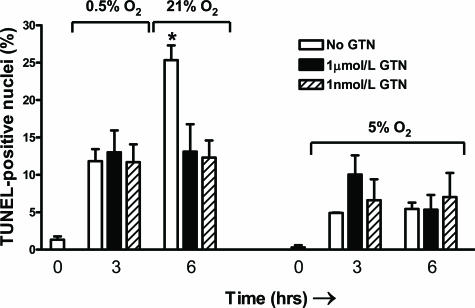
Explants fixed immediately after delivery and dissection had very few TUNEL-positive nuclei in the syncytiotrophoblast, with a mean apoptotic index of 1.1 ± 0.4% (Figure 1, A and A′). After 3 hours of incubation in 0.5% O2 without subsequent reoxygenation, the apoptotic index increased to 11.8 ± 1.6% in the untreated control villi (P < 0.001; Figure 1, B and B′) and to 13.0 ± 1.5% and 11.7 ± 2.4% (P < 0.01 for both), respectively, in the explants treated with 1 μmol/L or 1 nmol/L GTN (Figure 1, C, C′, D, and D′). Apoptotic indices were not significantly different in control and GTN-treated explants after the 3-hour incubation in hypoxia alone (Figure 2).
Figure 1.
Nuclear labeling (DAPI, A–G) and TUNEL assay (A′–G′) of villous explants fixed immediately after delivery (A and A′), untreated explants after 3 hours of hypoxia alone (B and B′), GTN-treated explants after 3 hours of hypoxia alone (C and D and C′ and D′), untreated explants after 3 hours of hypoxia and 3 hours of reoxygenation (H/R, E and E′), and GTN-treated explants after H/R (F and G and F′ and G′). GTN-treated explants exhibited fewer TUNEL-positive syncytiotrophoblast nuclei compared with untreated explants after a 6-hour H/R insult. Scale bars = 50 μm.
Figure 2.
Percentage of TUNEL-positive nuclei in untreated versus GTN-treated villous explants after a 6-hour H/R insult or after a 6-hour incubation in 5% O2. Treatment of explants with GTN significantly inhibited syncytiotrophoblast apoptosis after a 6-hour exposure to H/R. Relatively low levels of apoptosis were observed in control explants incubated under normoxic conditions (5% O2) with or without GTN. Asterisk indicates a significant difference (P < 0.001) between untreated explants after the 6-hour H/R exposure versus untreated explants after 3 hours of hypoxia alone. The H/R-exposed group consisted of explants isolated from 13 placentas whereas the group exposed to 5% O2 consisted of explants isolated from five placentas. Data are presented as means ± SE.
Compared with the apoptotic index in the syncytiotrophoblast of untreated control explants at the end of the 3-hour incubation in hypoxia, a subsequent 3-hour incubation in 21% oxygen resulted in a further 2.1-fold increase in the apoptotic index (P < 0.001; Figure 1, E, G, E′, and G′; and Figure 2). In contrast, this increase in TUNEL staining after the 3-hour reoxygenation period did not occur in the explants incubated with either 1 μmol/L or 1 nmol/L GTN (Figure 1, E, G, E′, and G′; and Figure 2). Compared with explants processed immediately after delivery, the number of TUNEL-positive nuclei in the syncytiotrophoblast of explants incubated under normoxic conditions (5% O2) for 3 hours did not exhibit a statistically significant increase (Figure 2). However, after 6 hours in normoxia, the apoptotic index in untreated explants increased significantly from 0.3 to 5.4% (P < 0.05). Both untreated control and GTN-treated explants maintained at 5% O2 throughout the incubation period exhibited similar levels of apoptosis at 6 hours (5.4 ± 0.8%, 5.3 ± 1.0%, and 6.7 ± 3.4%, respectively; Figure 2).
Immunodetection of 4-HNE and TUNEL Assay
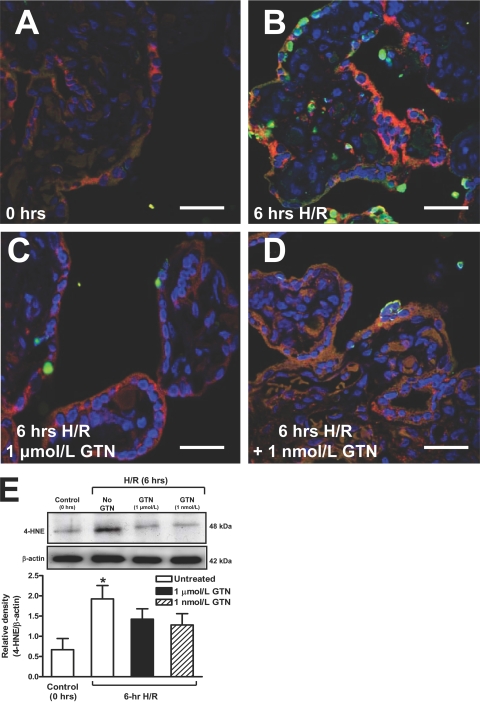
The localization of 4-HNE- and TUNEL-positive nuclei within chorionic villi was determined by immunofluorescence labeling (Figure 3). In placental explants processed immediately after delivery, the immunofluorescence staining intensity for 4-HNE was very low and present only in the syncytiotrophoblast (Figure 3A). In contrast, staining for 4-HNE was much greater in untreated explants subjected to a cycle of H/R (Figure 3B). This increase in 4-HNE expression in the untreated explants was paralleled by an increase in the number of TUNEL-positive nuclei in these samples. However, regions of the syncytiotrophoblast that showed intense 4-HNE staining did not always contain TUNEL-positive nuclei. This may be attributable to the fact that generation of reactive oxygen species and the subsequent peroxidation of lipids occur early during the H/R stress, whereas DNA fragmentation is a later event in the apoptotic process. Staining for 4-HNE and TUNEL was markedly attenuated in explants exposed to H/R in the presence of 1 μmol/L or 1 nmol/L GTN (Figure 3, C and D). Explants incubated with preimmune IgG exhibited virtually no detectable levels of fluorescence (data not shown).
Figure 3.
Double labeling for TUNEL (green) and 4-HNE (red) in villous tissues fixed immediately after delivery (A), in control untreated explants after a 6-hour exposure to H/R (B), and in GTN-treated explants after a 6-hour exposure to H/R (1 μmol/L and 1 nmol/L GTN; C and D, respectively). E: Western blot and densitometric analysis performed using explants from three to six placentas confirmed the above results. The asterisk indicates a significant difference (P < 0.05) between the levels of 4-HNE in untreated villi after a 6-hour H/R insult compared with the levels of 4-HNE in control villi processed immediately after delivery. Scale bars = 30 μm.
Results of the immunofluorescence staining for 4-HNE were confirmed by Western blot analysis. As shown in Figure 3E, compared with the levels of 4-HNE protein adduct in extracts of explants processed immediately after delivery, 4-HNE levels in chorionic villi increased by almost fourfold after H/R (P < 0.05). However, compared with explants processed immediately after delivery, the levels of 4-HNE in the GTN-treated explants did not increase significantly.
Immunodetection of Nitrotyrosine Residues and Cleaved PARP
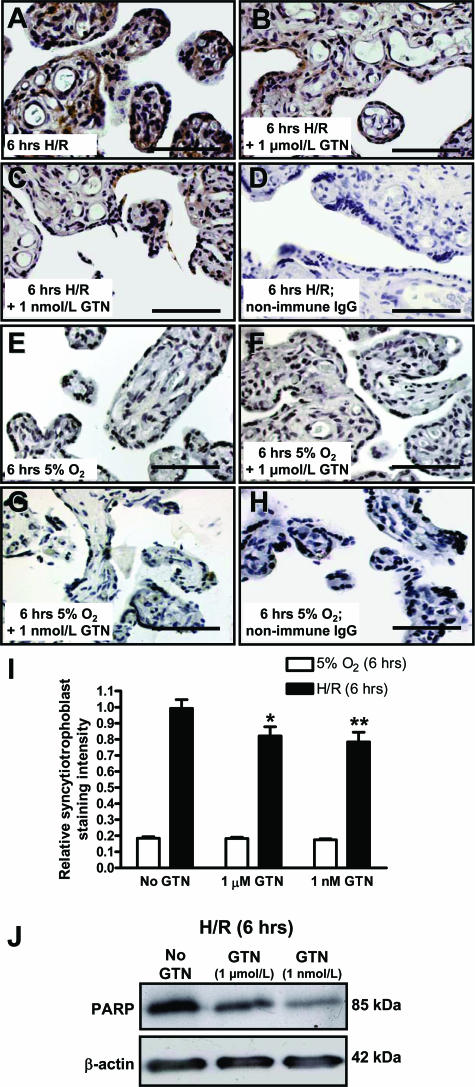
Explants processed immediately after delivery exhibited barely detectable levels of nitrotyrosine staining (Figure 4A) or cleaved PARP immunostaining (not shown). However, after H/R explants exhibited intense immunostaining for both nitrotyrosine (Figure 4B) and cleaved PARP (Figure 5A). Most of the staining for nitrotyrosine and PARP was localized to the syncytiotrophoblast, with some PARP staining also localizing to the stroma. However, immunostaining for both nitrotyrosine and cleaved PARP was markedly attenuated in explants exposed to H/R in the presence of GTN (Figure 4, C and D; and Figure 5, B and C). Exposure of explants to 5% O2 for 6 hours with or without GTN resulted in relatively low levels of PARP immunostaining (Figure 5, E–G). Replacement of the primary antibody with nonimmune rabbit IgG resulted in the absence of staining in explants exposed to H/R or maintained in 5% O2 (Figure 5, D and H).
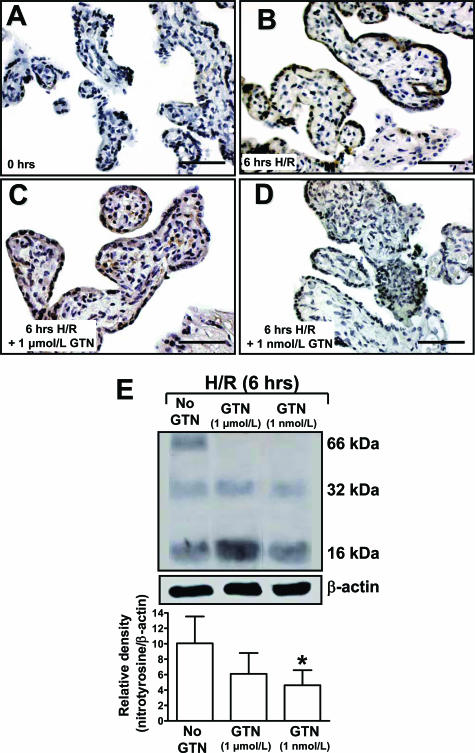
Figure 4.
Nitrotyrosine immunohistochemical staining of explants processed immediately after delivery (A); after 6 hours of H/R (B); after 6 hours of H/R in the presence of 1 μmol/L GTN (C); and after 6 hours of H/R in the presence of 1 nmol/L GTN (D). Densitometric analysis of Western blots of protein extracts (E) showed a significant difference between the levels of nitrotyrosine in explants exposed to H/R without GTN compared with explants exposed to H/R in the presence of 1 nmol/L GTN (asterisk indicates P < 0.05). The presence of 1 μmol/L GTN during H/R did not result in a statistically significant decrease in the level of nitrotyrosine residues. Data are presented as the mean relative densities (±SE) of Western blot analyses performed on explants from four different placentas. Scale bars = 50 μm.
Figure 5.
Immunohistochemical and Western blot analysis of the p85 fragment of PARP. Compared with untreated explants exposed to H/R (A), treatment of H/R-exposed explants with GTN resulted in decreased syncytiotrophoblast immunoreactivity for PARP (B and C). Lower levels of PARP immunostaining were observed in explants exposed to 5% O2 in the absence or presence of GTN throughout the 6-hour incubation period (E–G). Negative controls consisted of sections incubated with nonimmune rabbit IgG (D and H). Combined immunohistochemical staining intensities from five different placentas, as determined by image analysis using ImagePro, revealed significantly lower PARP staining in the syncytiotrophoblast exposed to H/R in the presence of GTN as compared with similar explants exposed to H/R in the absence of GTN (I; single and double asterisks = P < 0.05 and P < 0.01, respectively; one-way analysis of variance followed by Tukey-Kramer test). I: Similar analysis revealed much lower PARP staining intensity in the syncytiotrophoblast of villi maintained under normoxic conditions (5% O2) but not significant differences in the staining intensities in control versus GTN-treated explants. Data are presented as means ± SE. J: PARP immunohistochemistry results from explants exposed to H/R were further confirmed by Western blot analysis. Scale bars = 50 μm.
Quantitative analysis of PARP staining of the syncytiotrophoblast layer was confirmed by image analysis using ImagePro software. Results revealed that, compared with untreated explants after H/R, treatment with GTN (1 μmol/L and 1 nmol/L) led to decreased levels of syncytiotrophoblast PARP staining after H/R (P < 0.05; Figure 5I). In explants maintained under normoxic conditions (5% O2) for 6 hours, the levels of cleaved PARP immunostaining in the syncytiotrophoblast were low and were not affected by GTN treatment.
Western Blot Analysis of Nitrotyrosine, PARP, and Caspase-3
Levels of nitrotyrosine residues were assessed to determine whether endogenous NO leads to peroxynitrite formation and protein nitration after H/R. Nitrotyrosine immunoblotting revealed the presence of three nitrated fragments of 66, 32, and 16 kd in tissue samples exposed to H/R without GTN (Figure 4E). In contrast, the GTN-treated samples revealed only two bands at 32 and 16 kd. Significantly higher levels of nitrotyrosine residues were present in tissues exposed to H/R without GTN than in tissues exposed to H/R in the presence of 1 nmol/L GTN (P < 0.05) as determined by densitometric analysis (Figure 4E).
Cleaved PARP immunostaining results were also confirmed by Western blot analysis (Figure 5J). Densitometric analysis revealed that the expression of active fragment 85 for PARP was significantly higher in tissue samples exposed to H/R alone than in samples exposed to H/R in the presence of 1 nmol/L GTN (P < 0.05). PARP levels in control tissues exposed to 5% O2 for 6 hours were low and unaffected by GTN treatment (not shown).
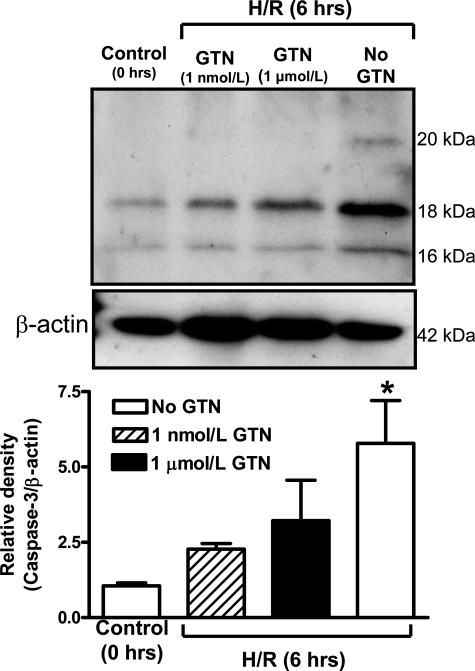
Active caspase-3 levels were assessed because this enzyme is a downstream mediator of apoptosis. Expression of cleaved caspase-3 revealed two polypeptides of 16 and 18 kd (Figure 6). An additional active caspase-3 polypeptide fragment of 20 kd was present only in lysates from untreated explants exposed to H/R. Densitometric analysis revealed that the combined levels of these active caspase-3 polypeptide fragments were significantly increased in explants exposed to H/R without GTN than in explants processed immediately after delivery (P < 0.05; Figure 6). In contrast, this increase in the levels of active caspase-3 fragments did not occur in explants exposed to H/R in the presence of 1 μmol/L or 1 nmol/L GTN (Figure 6).
Figure 6.
Effects of H/R and GTN on the levels of caspase-3 polypeptide fragments as determined by Western blot analysis. Densitometric data are presented as means ± SE from five placentas and show significantly increased levels of caspase-3 fragments in explants exposed to H/R alone as compared with the levels of caspase-3 in control explants processed immediately after delivery (0 hours). β-Actin was used to normalize for loading variability. The asterisk indicates a significant difference (P < 0.05) between the levels of active caspase-3 in untreated villi after a 6-hour H/R insult compared with the levels of active caspase-3 in control villi processed immediately after delivery. Compared with control explants (0 hours), explants exposed to H/R in the presence of GTN did not exhibit significantly increased levels of caspase-3 fragments.
Light and Electron Microscopic Analysis
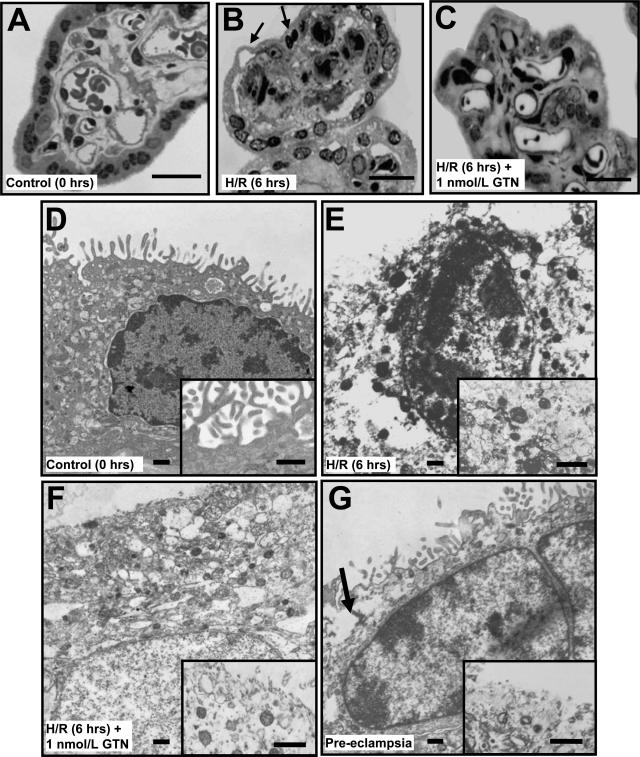
Light microscopic examination of semithin sections of chorionic villi fixed immediately after delivery revealed syncytial nuclei with irregular shapes and normal distribution of chromatin and intact syncytial membranes (Figure 7A). However, after the H/R insult, the untreated explants exhibited several morphological features characteristic of apoptosis and/or aponecrosis (Figure 7B).17 These included nuclei with extensive chromatin condensation and separation of the syncytium from the underlying stroma. In contrast, explants treated with GTN (1 nmol/L) displayed decreased amounts of condensed chromatin and little evidence of syncytial separation from the underlying stroma (Figure 7C). These changes were confirmed by electron microscopy (Figure 7, D–F). Explants processed immediately after delivery had an intact membrane with abundant microvilli and preserved nuclei (Figure 7D, inset). After H/R, there was evidence of severe tissue degeneration, with the syncytiotrophoblast displaying increased vacuolization (Figure 7E), extensive chromatin condensation, interruption of the membrane and loss of microvilli (Figure 7E, inset). In explants treated with GTN throughout the H/R insult, the syncytial membrane was mostly intact and exhibited less chromatin condensation (Figure 7F); however, compared with villi fixed immediately after delivery, GTN-treated explants had reduced numbers of microvilli (Figure 6F, inset). Some of the morphological features present in chorionic villi exposed to H/R without GTN, such as disruption of the syncytial membrane, were also present in chorionic villi from placentas of preeclamptic women (Figure 7G, inset).
Figure 7.
Morphological analysis of chorionic villi at the light (A–C) and electron (D–G) microscopic levels. Villi were processed immediately after delivery (A, D); after 6 hours of H/R (B, E); and after 6 hours of H/R in the presence of GTN (1 nmol/L; C, F). G: An additional control consisted of villi from a preeclamptic pregnancy. Apoptotic changes such as the lifting of the syncytiotrophoblast layer and the presence of picnotic nuclei (B, arrows) were more evident in untreated explants after H/R than in explants treated with GTN (C, F) or fresh placental tissues (A, D). D: Ultrastructural analysis revealed an intact syncytial membrane with abundant microvilli in villi from uncomplicated pregnancies fixed immediately after delivery (inset). E: After H/R the syncytial nuclei often appeared small and with extensive chromatin condensation. E, inset: The presence of cytoplasmic vacuolization and loss of syncytial membrane integrity were also evident in explants after H/R. F: Treatment with GTN during the exposure to H/R resulted in a higher number of well-preserved syncytial nuclei and greater continuity of the cytoplasmic membrane (inset). Compared with explants fixed immediately after delivery, the number of microvilli in tissues treated with GTN during exposure to H/R was reduced (inset). Villi from a preeclamptic pregnancy exhibited some of the morphological features as villi exposed to H/R, such as disruption of the syncytiotrophoblast membrane (G, arrow; inset). Scale bars: 25 μm (A–C); 0.5 μm (D–G).
Discussion
The present study demonstrates that low concentrations of the NO mimetic GTN can effectively protect the syncytiotrophoblast of third trimester chorionic villi from H/R-induced apoptosis. Previous studies have shown that NO can prevent H/R damage in other tissues such as the heart,18 the kidney,19 and the brain.20 Our results extend our previous study in which we showed that physiological concentrations of CO also inhibit H/R-induced damage in the syncytiotrophoblast of chorionic villi.10 Given the availability of various NO mimetic agents with known toxicology and pharmacokinetics, administration of low doses of such agents may be useful in the treatment and/or prevention of the systemic inflammation and endothelial activation associated with preeclampsia.
The H/R model used in our study was an adaptation of the method developed by Hung and colleagues,6 who showed that exposure of chorionic villous explants to a 7-hour cycle of H/R results in apoptotic and aponecrotic changes similar to those described here. In our study, the number of TUNEL-positive syncytiotrophoblast nuclei was greater in explants exposed to reoxygenation after hypoxia than in explants maintained under normoxic conditions throughout the incubation period. Hung and colleagues6 demonstrated that H/R is a more potent inducer of syncytiotrophoblast apoptosis than hypoxia alone. Furthermore, blood flow to the intervillous spaces of the placenta may be intermittent in preeclamptic pregnancies, with periods of ischemia followed by periods of reperfusion.5,6 As confirmed in our present study, others have shown that the syncytiotrophoblast of placentas from women with preeclampsia exhibits apoptotic and aponecrotic alterations similar to those observed in chorionic villi exposed to H/R in vitro.9 Together, these findings suggest that the quality of the uteroplacental circulation is a critical aspect of the pathophysiology of preeclampsia.
The precise role of endogenous NO in normal pregnancy and in the pathophysiology of preeclampsia is still unclear, and studies in which the circulating levels of NO metabolites have been measured in women with preeclampsia have yielded confusing results. Regardless of the levels of placental NO production, it has been postulated that NO availability for normal physiological signaling is decreased in preeclampsia.21 In support of this hypothesis is the fact that the superoxide anion (O2−) generated during cycles of H/R reacts rapidly with NO to form peroxynitrite, thereby decreasing the amount of free NO available.22,23 Our immunohistochemistry findings revealed that nitrotyrosine residues resulting from protein nitration by peroxynitrite were increased in explants exposed to H/R, thereby suggesting decreased NO availability for normal signaling. In addition, there is evidence that hypoxia increases arginase activity and thus diverts l-arginine metabolism away from the NO-generating pathway toward the ornithine pathway.24 In the absence of l-arginine, NOS itself generates O2−, which further leads to increased formation of peroxynitrite instead of the NO required for normal physiological signaling via activation of sGC.21 Indeed, it has been reported that in preeclampsia a lower than normal l-arginine concentration caused by arginase overexpression redirects NOS-3 toward increased peroxynitrite formation.21,23
Generation of NO is also dependent on the availability of co-factors and co-substrates including NADPH, FAD, tetrahydrobiopterin (BH4), and O2.25,26,27,28 Consequently, endogenous NO production is a complex process. However, there is evidence that when O2 availability is limited, as is likely the case during the ischemic periods associated with the placenta in preeclampsia, production of NO is decreased.27,28 Therefore, decreased NO availability in the placenta may occur in preeclampsia as a result of hypoxia, ie, during the periods of ischemia, and also because of increased O2− and peroxynitrite levels. Peroxynitrite is a potentially toxic molecule with biological effects that are often opposite to those of NO.21 For example, peroxynitrite induces cellular apoptosis by activating caspase-3 and the subsequent proteolytic cleavage of PARP.29 Once PARP is cleaved it no longer supports the enzymatic DNA repair function, and there is some evidence that cleaved PARP may inhibit access to DNA by other repair enzymes.30 Although PARP is not absolutely required for apoptosis to proceed, the cleavage of PARP may contribute to the commitment to apoptosis.30,31 In contrast, it has been reported that NO can inhibit apoptosis by inactivating caspase-3 activity through S-nitrosylation.32 In our study we found that small concentrations of the NO mimetic GTN were able to inhibit the H/R-mediated stimulation of caspase-3 and PARP. Whether this effect of GTN is attributable to S-nitrosylation of sulfhydryl groups requires additional investigation. However, a previous study provided evidence that NO is able to inhibit tumor necrosis factor-induced apoptosis of trophoblast cells through a mechanism involving cGMP and independently of caspase-3 inactivation.14
In the present study, evidence of oxidative stress in explants exposed to H/R was provided by the increased levels of 4-HNE and nitrotyrosine in the syncytiotrophoblast layer. Interaction of reactive oxygen species with polyunsaturated fatty acids of membrane lipids results in the production of aldehydes and alkenals such as malonaldehyde and 4-HNE.33 These secondary products react with protein and DNA and are known to be cytotoxic.34 Lipid peroxidation increases during the progression of degenerative conditions, such as H/R-induced tissue damage35 and the formation of atherosclerotic plaques.36 In our study, treatment of explants with GTN resulted in decreased levels of 4-HNE and nitrotyrosine residues. Although elucidation of the precise mechanism by which GTN decreases the levels of these molecules in chorionic villi requires further investigation, there is evidence that small concentrations of NO can attenuate the cellular injury caused by H2O2, O2−, and alkyl peroxides.37 This protective effect of NO appears to be attributable to inhibition of lipid peroxidation.38,39 Moreover, activation of cGMP-dependent protein kinase by NO has been shown to attenuate oxidative stress-induced lipid peroxidation and apoptosis in neuroblastoma cells.40 Thus, we propose that maintenance of low levels of NO activity via administration of small concentrations of NO mimetic agents protects the syncytiotrophoblast from H/R-induced apoptosis at least in part through a mechanism involving inhibition of lipid peroxidation. We emphasize the use of low concentrations (<1 μmol/L) of NO mimetics because at higher concentrations NO can lead to phenotypes different from those induced by the low-concentration cGMP-dependent pathway.41
Caspase-3 plays a crucial role as a final execution enzyme in both the intrinsic and extrinsic pathways of apoptosis. It is synthesized as a proenzyme that contains an N-terminal prodomain followed by a larger subunit (p17) and a smaller subunit (p12).42 During activation, cleavage between the large and the small subunits occurs. It was suggested that all forms of caspase-3 containing different versions of the large subunit are active.43 In our study, treatment with GTN prevented the appearance of the 20-kd active fragment of caspase-3 in explants exposed to H/R. Therefore, because caspase-3 is responsible for the cleavage of PARP, it was not surprising that the levels of cleaved PARP in GTN-treated explants exposed to H/R were also decreased.
Morphological signs of apoptosis and aponecrosis were present in explants exposed to H/R. These included syncytial degeneration, loss of microvilli, and an increased number of nuclei displaying condensed chromatin. These alterations were not evenly distributed throughout the syncytial layer, suggesting that either the level of oxidative stress was not uniform or that different regions of the villous tree exhibit heterogeneous susceptibility to oxidative stress. Overall, these morphological alterations induced by H/R were attenuated in explants treated with GTN.
Although the precise role of NO in the regulation of cellular responses to oxidative stress is not well understood, there is evidence that at physiological concentrations NO can act as a redox buffer quenching the oxidation reactions mediated by reactive species such as O2− and peroxides.37 We propose that oxidative damage to the syncytiotrophoblast is attributable in part to H/R-mediated fluctuations in the level of endogenous NO signaling. Thus, maintenance of physiological NO activity through administration of low doses of NO mimetics may be useful in the treatment and/or prevention of preeclampsia.
Acknowledgments
We thank Mr. John Dacosta, Mrs. Judy Pang, Mr. Jason Caldwell, and Mr. Richard Casselman for their technical help.
Footnotes
Address reprint requests to Charles H. Graham, Department of Anatomy and Cell Biology, Botterell Hall, 9th Floor, Queen’s University, Kingston, ON, Canada K7L 3N6. E-mail: grahamc@post.queensu.ca.
Supported by the Heart and Stroke Foundation of Ontario (grant number T 5722), the Canadian Institutes of Health Research (Pre-Eclampsia New Emerging Team grant), and the Heart and Stroke Foundation of Canada (grant number PG-030-0175-PE-NET).
References
- Marín R, Gorostidi M, Portal CG, Sanchez M, Sanchez E, Alvarez J. Long-term prognosis of hypertension in pregnancy. Hypertens Pregnancy. 2000;19:199–209. doi: 10.1081/prg-100100136. [DOI] [PubMed] [Google Scholar]
- Nisell H, Lintu H, Lunell NO, Mollerstrom G, Pettersson E. Blood pressure and renal function seven years after pregnancy complicated by hypertension. Br J Obstet Gynaecol. 1995;102:876–881. doi: 10.1111/j.1471-0528.1995.tb10874.x. [DOI] [PubMed] [Google Scholar]
- Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–191. [PubMed] [Google Scholar]
- Roberts JM, Lain KY. Recent insights into the pathogenesis of pre-eclampsia. Placenta. 2002;23:359–372. doi: 10.1053/plac.2002.0819. [DOI] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol. 2001;159:1031–1043. doi: 10.1016/S0002-9440(10)61778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circ Res. 2002;90:1274–1281. doi: 10.1161/01.res.0000024411.22110.aa. [DOI] [PubMed] [Google Scholar]
- Sargent IL, Germain SJ, Sacks GP, Kumar S, Redman CW. Trophoblast deportation and the maternal inflammatory response in pre-eclampsia. J Reprod Immunol. 2003;59:153–160. doi: 10.1016/s0165-0378(03)00044-5. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597–602. doi: 10.1053/plac.2000.0560. [DOI] [PubMed] [Google Scholar]
- Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstet Gynecol. 2000;96:271–276. doi: 10.1016/s0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- Bainbridge SA, Belkacemi L, Dickinson M, Graham CH, Smith GN. Carbon monoxide inhibits hypoxia/reoxygenation-induced apoptosis and secondary necrosis in syncytiotrophoblast. Am J Pathol. 2006;169:774–783. doi: 10.2353/ajpath.2006.060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt L, Eis AL, Brockman DE, Kossenjans W, Greer I, Lyall F. Inducible (type II) nitric oxide synthase in human placental villous tissue of normotensive, pre-eclamptic and intrauterine growth-restricted pregnancies. Placenta. 1997;18:261–268. doi: 10.1016/s0143-4004(97)80060-4. [DOI] [PubMed] [Google Scholar]
- Thomsen LL, Miles DW, Happerfield L, Bobrow LG, Knowles RG, Moncada S. Nitric oxide synthase activity in human breast cancer. Br J Cancer. 1995;72:41–44. doi: 10.1038/bjc.1995.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Dash PR, Cartwright JE, Baker PN, Johnstone AP, Whitley GS. Nitric oxide protects human extravillous trophoblast cells from apoptosis by a cyclic GMP-dependent mechanism and independently of caspase 3 nitrosylation. Exp Cell Res. 2003;287:314–324. doi: 10.1016/s0014-4827(03)00156-3. [DOI] [PubMed] [Google Scholar]
- Fujikura T, Yoshida J. Blood gas analysis of placental and uterine blood during cesarean delivery. Obstet Gynecol. 1996;87:133–136. doi: 10.1016/0029-7844(95)00300-2. [DOI] [PubMed] [Google Scholar]
- Soothill PW, Nicolaides KH, Rodeck CH, Campbell S. Effect of gestational age on fetal and intervillous blood gas and acid-base values in human pregnancy. Fetal Ther. 1986;1:168–175. doi: 10.1159/000262264. [DOI] [PubMed] [Google Scholar]
- Formigli L, Papucci L, Tani A, Schiavone N, Tempestini A, Orlandini GE, Capaccioli S, Orlandini SZ. Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J Cell Physiol. 2000;182:41–49. doi: 10.1002/(SICI)1097-4652(200001)182:1<41::AID-JCP5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Maulik N, Engelman DT, Watanabe M, Engelman RM, Rousou JA, Flack JE, III, Deaton DW, Gorbunov NV, Elsayed NM, Kagan VE, Das DK. Nitric oxide/carbon monoxide. A molecular switch for myocardial preservation during ischemia. Circulation. 1996;94:II398–II406. [PubMed] [Google Scholar]
- Chander V, Chopra K. Protective effect of nitric oxide pathway in resveratrol renal ischemia-reperfusion injury in rats. Arch Med Res. 2006;37:19–26. doi: 10.1016/j.arcmed.2005.05.018. [DOI] [PubMed] [Google Scholar]
- Chiueh CC. Neuroprotective properties of nitric oxide. Ann NY Acad Sci. 1999;890:301–311. doi: 10.1111/j.1749-6632.1999.tb08007.x. [DOI] [PubMed] [Google Scholar]
- Lowe DT. Nitric oxide dysfunction in the pathophysiology of preeclampsia. Nitric Oxide. 2000;4:441–458. doi: 10.1006/niox.2000.0296. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Noris M, Todeschini M, Cassis P, Pasta F, Cappellini A, Bonazzola S, Macconi D, Maucci R, Porrati F, Benigni A, Picciolo C, Remuzzi G. L-arginine depletion in preeclampsia orients nitric oxide synthase toward oxidant species. Hypertension. 2004;43:614–622. doi: 10.1161/01.HYP.0000116220.39793.c9. [DOI] [PubMed] [Google Scholar]
- Albina JE, Henry WL, Jr, Mastrofrancesco B, Martin BA, Reichner JS. Macrophage activation by culture in an anoxic environment. J Immunol. 1995;155:4391–4396. [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr DJ. Mammalian nitric oxide synthases. Biochim Biophys Acta. 1999;1411:217–230. doi: 10.1016/s0005-2728(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Whorton AR, Simonds DB, Piantadosi CA. Regulation of nitric oxide synthesis by oxygen in vascular endothelial cells. Am J Physiol. 1997;272:L1161–L1166. doi: 10.1152/ajplung.1997.272.6.L1161. [DOI] [PubMed] [Google Scholar]
- McCormick CC, Li WP, Calero M. Oxygen tension limits nitric oxide synthesis by activated macrophages. Biochem J. 2000;350:709–716. [PMC free article] [PubMed] [Google Scholar]
- Lin KT, Xue JY, Lin MC, Spokas EG, Sun FF, Wong PY. Peroxynitrite induces apoptosis of HL-60 cells by activation of a caspase-3 family protease. Am J Physiol. 1998;274:C855–C860. doi: 10.1152/ajpcell.1998.274.4.C855. [DOI] [PubMed] [Google Scholar]
- Smulson ME, Pang D, Jung M, Dimtchev A, Chasovskikh S, Spoonde A, Simbulan-Rosenthal C, Rosenthal D, Yakovlev A, Dritschilo A. Irreversible binding of poly(ADP)ribose polymerase cleavage product to DNA ends revealed by atomic force microscopy: possible role in apoptosis. Cancer Res. 1998;58:3495–3498. [PubMed] [Google Scholar]
- Trucco C, Oliver FJ, de Murcia G, Menissier-de Murcia J. DNA repair defect in poly(ADP-ribose) polymerase-deficient cell lines. Nucleic Acids Res. 1998;26:2644–2649. doi: 10.1093/nar/26.11.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rössig L, Fichtlscherer B, Breitschopf K, Haendeler J, Zeiher AM, Mulsch A, Dimmeler S. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J Biol Chem. 1999;274:6823–6826. doi: 10.1074/jbc.274.11.6823. [DOI] [PubMed] [Google Scholar]
- Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim Biophys Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Braughler JM, Hall ED. Central nervous system trauma and stroke. I. Biochemical considerations for oxygen radical formation and lipid peroxidation. Free Radic Biol Med. 1989;6:289–301. doi: 10.1016/0891-5849(89)90056-7. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME, Palinski W, Yla-Herttuala S, Butler S, Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990;10:336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Wink DA, Miranda KM, Espey MG, Pluta RM, Hewett SJ, Colton C, Vitek M, Feelisch M, Grisham MB. Mechanisms of the antioxidant effects of nitric oxide. Antioxid Redox Signal. 2001;3:203–213. doi: 10.1089/152308601300185179. [DOI] [PubMed] [Google Scholar]
- Rubbo H, Parthasarathy S, Barnes S, Kirk M, Kalyanaraman B, Freeman BA. Nitric oxide inhibition of lipoxygenase-dependent liposome and low-density lipoprotein oxidation: termination of radical chain propagation reactions and formation of nitrogen-containing oxidized lipid derivatives. Arch Biochem Biophys. 1995;324:15–25. doi: 10.1006/abbi.1995.9935. [DOI] [PubMed] [Google Scholar]
- Struck AT, Hogg N, Thomas JP, Kalyanaraman B. Nitric oxide donor compounds inhibit the toxicity of oxidized low-density lipoprotein to endothelial cells. FEBS Lett. 1995;361:291–294. doi: 10.1016/0014-5793(95)00178-c. [DOI] [PubMed] [Google Scholar]
- Andoh T, Chiueh CC, Chock PB. Cyclic GMP-dependent protein kinase regulates the expression of thioredoxin and thioredoxin peroxidase-1 during hormesis in response to oxidative stress-induced apoptosis. J Biol Chem. 2003;278:885–890. doi: 10.1074/jbc.M209914200. [DOI] [PubMed] [Google Scholar]
- Hanafy KA, Krumenacker JS, Murad F. NO, nitrotyrosine, and cyclic GMP in signal transduction. Med Sci Monit. 2001;7:801–819. [PubMed] [Google Scholar]
- Nicholson DW, Ali A, Thornberry NA, Vaillancourt JP, Ding CK, Gallant M, Gareau Y, Griffin PR, Labelle M, Lazebnik YA, Munday NA, Raju SM, Smulson ME, Yamin TT, Yu VL, Miller DK. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Salvesen GS. Properties of the caspases. Biochim Biophys Acta. 1998;1387:17–31. doi: 10.1016/s0167-4838(98)00133-2. [DOI] [PubMed] [Google Scholar]