Abstract
Natural killer T (NKT) cells have recently been implicated in atherogenesis, primarily for their ability to recognize and respond to lipid antigens. Because the atherosclerotic lesion is characterized by the retention and modification of lipids in the vascular wall, NKT cells may be involved in promoting the local vascular inflammatory response. Here, we investigate the proatherogenic role of NKT cells in an adoptive transfer model of atherosclerosis, using as recipients immune-deficient, atherosclerosis-susceptible RAG1−/−LDLR−/− mice. The adoptive transfer of an NKT cell-enriched splenocyte population from Vα14Jα18 T-cell receptor transgenic mice resulted in a 73% increase in aortic root lesion area compared with recipients of NKT cell-deficient splenocytes derived from CD1d−/− mice after 12 weeks of Western-type diet feeding. The total serum from hypercholesterolemic mice leads to a small but significant activation of Vα14Jα18 T-cell receptor-expressing hybridoma line by dendritic cells that is CD1d-dependent. Therefore, these studies demonstrate that NKT cells are proatherogenic in the absence of exogenous stimulation, and this activity is likely associated with endogenous lipid antigens carried by lipoproteins in the circulation and perhaps also in the atherosclerotic plaque.
Atherosclerosis is a complex chronic inflammation within the vessel wall responding to retained and modified lipids and lipoproteins. Both the innate and adaptive immune responses are involved.1,2,3 Our group and others have previously demonstrated that adaptive immunity is not absolutely required for atherogenesis because robust lesions still develop in the arterial vasculatures of hypercholesterolemic mice that completely lack functional T and B cells.4,5,6 Nevertheless, it is widely held that the adaptive immune response plays an important regulatory role in the disease process. Therefore, any subtle modulation of the inflammatory response may lead to significant changes in lesion size and morphology and ultimately clinically relevant endpoints.
Natural killer T (NKT) cells are a distinct subset of T lymphocytes unique in their ability to respond to glycolipid antigens presented by the major histocompatibility complex class I-like CD1d molecule when recognized by their semi-invariant T-cell receptor (TCR), predominantly Vα14Jα18/Vβ8 in mice (Vα24Jα18/Vβ11 in humans).7 After activation, NKT cells are able to rapidly and robustly secrete large amounts of both proinflammatory and anti-inflammatory cytokines [including interferon (IFN)-γ and interleukin (IL)-4, respectively], thereby playing an important regulatory role in a number of pathological states.8
Recently, NKT cells have been implicated in atherosclerosis. In humans, immunohistochemical techniques have localized NKT cells to the shoulder regions of carotid artery plaques9 as well as in atherosclerotic tissue derived from abdominal aortic aneurysms.10 In experimental mouse models, two basic strategies have implicated NKT cells as proatherogenic. In the absence of NKT cells attributable to CD1d deficiency, a reduction in atherosclerosis in both the aortic root and throughout the rest of the aorta has been noted.11,12,13,14 On the other hand, the exogenous administration of the nonphysiological but strongly activating glycolipid α-galactosylceramide (α-GalCer) results in a 50 to 100% increase in aortic atherosclerosis in apoE−/− mice.11,12,13 Therefore, it seems as if NKT cell activation has the potential to exacerbate the atherogenic process.
In this study, an alternate approach to investigate the participation of NKT cells in atherogenesis has been taken. We have previously shown that robust atherosclerosis can develop in the absence of an adop-tive immune system.4,15 Using the immune-deficient RAG1−/−LDLR−/− mouse as recipients, we show here the selective reconstitution of the adaptive immune system in these mice through the adoptive transfer of mature peripheral lymphocyte populations from the spleens of either C57BL/6 (wild-type), CD1d−/− (NKT cell-deficient), or Vα14Jα18 TCR transgenic (NKT cell-enriched) mice and the resultant effects on atherosclerosis. No nonphysiological exogenous antigen administration is involved, implying that the differences noted relate to the presentation of endogenous lipid antigens to the transferred NKT cells. In addition to addressing the role of different levels of NKT cells in atherosclerosis, we have asked whether the lipoproteins derived from atherosclerosis-susceptible mouse models contain a measurable CD1d-restricted stimulating lipid antigen recognized by NKT cells in a very sensitive in vitro assay.
Materials and Methods
Mice
All mice were housed in specific pathogen-free barrier facilities at the University of Chicago and experimental procedures performed in accordance with National Institutes of Health guidelines under protocols approved by the Institutional Animal Care and Use Committee. The donor Vα14Jα18 TCRα chain transgenic (Vα14tg) mice on the C57BL/6 (B6) background were kindly provided by Dr. Albert Bendelac from the University of Chicago and have been described previously;16 the donor B6 mice were transgene-negative littermates of the Vα14tg mice, and the donor CD1d−/− mice on the B6 background have been described previously.17 Recipient mice were 8- to 10-week-old female recombination activating gene 1 (RAG1), LDL receptor (LDLR) double-knockout mice, backcrossed 10 times onto the B6 background as previously characterized.15
Adoptive Transfer
Lymphocytes were isolated from the spleens of donor mice using a Lympholyte-M (Cedarlane Laboratories, Burlington, ON, Canada) density gradient, and 20 × 106 cells from either B6, CD1d−/−, or Vα14tg donors were intravenously injected in 0.2-ml PBS volume into the retro-orbital plexus of the recipient female RAG1−/−LDLR−/− mice. After the adoptive transfer, the mice were immediately switched from a standard laboratory chow diet (TD7913, 6.25% fat; Harlan Teklad, Indianapolis, IN) to the high-fat Western-type diet (WTD) (TD88137, 21% saturated fat, 0.15% cholesterol; Harlan Teklad) and bled monthly to determine fasting plasma cholesterol and triglyceride levels using kits from Roche Diagnostics, Indianapolis, IN. Lipoproteins in the plasma were fractionated via tandem Superose 6 fast-performance liquid chromatography (FPLC) columns from Amersham Biosciences, Piscataway, NJ, and the levels of each of the lipoproteins in the plasma determined as described previously.4 Total IgG in plasma was examined by immunoblotting, and levels of IgM and IgG subtypes in the plasma were determined by enzyme-linked immunosorbent assay (Southern Biotechnology, Birmingham, AL).
Tissue Preparation and Histomorphology
After 12 weeks on the WTD, the mice were anesthetized with ketamine/xylazine and exsanguinated, vasculatures were perfused with paraformaldehyde under physiological pressures, and the upper aortic vasculature tissue was mounted in OCT as described previously.4 For orientation purposes, a piece of liver was embedded adjacent to the heart on the side of the greater curvature of the aortic arch. Serial 10-μm cross-sections were collected from the innominate artery through the aortic root. Lesions in the ascending thoracic aorta were assessed from three oil red O-stained sections, each separated by 100 μm and located between 100 and 300 μm below the apex of the lesser curvature of the aortic arch. Aortic root lesions were evaluated from three sections, each separated by 100 μm, beginning at the site of appearance of the coronary artery and valve leaflets. Atherosclerosis was quantified from digitally captured images and OpenLab Software, version 3.1.5 (Improvision Inc., Lexington, MA). For qualitative histological analysis, serial sections were also stained with either Mayer’s hematoxylin and alcoholic eosin or Gomori’s trichrome acid fuchsin.
Flow Cytometry
At sacrifice, single cell splenocyte suspensions were prepared from the recipient mice as before. Aliquots containing 106 cells were first FcγR blocked (2.4G2) and then stained with fluorescently labeled anti-CD3 (17A2), NK1.1 (PK136), IgM (II/41) (all from eBioscience, San Diego, CA), and α-GalCer-loaded CD1d-tetramers (mCD1d/PBS-57; National Institutes of Health MHC Tetramer Core Facility, Bethesda, MD). Flow cytometry was performed using a FACSCalibur (BD Biosciences, San Jose, CA) with subsequent data analysis using WinMDI 2.9 software (J. Trotter, Scripps Research Institute, La Jolla, CA).
Semiquantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Liver or spleen samples were obtained from the mice before vascular perfusion and were flash-frozen in liquid N2. RNA was isolated using RNeasy mini spin column kit (Qiagen, Valencia, CA). Reverse transcription was performed using oligo dT primers (Invitrogen, Carlsbad, CA) and the Transcriptor RT system (Roche). For each gene screened, serial dilutions of template cDNA were assayed to define the linear range of amplification for subsequent polymerase chain reaction using FastStart Taq High-Fidelity PCR system (Roche). The PCR primers used can be found in Table 1. The PCR products were run on ethidium bromide-stained agarose gels in TBE. Gel images were acquired with a FluorChem 8800 image detection system (Alpha Innotech, San Leandro, CA) and target brands quantified using AlphaEaseFC analysis software. Expression of each gene was normalized to the expression of the pan T-cell marker CD3ε.
Table 1.
Primer Sequences and Target Band Size Used for RT-PCR
| Gene | Amplicon | Primers |
|---|---|---|
| Vα14Jα18 iTCR | 305 bp | Forward: 5′-GTTGTCCGTCAGGGAGAGAA-3′ |
| Reverse: 5′-CAATCAGCTGAGTCCCAGCT-3′ | ||
| GAPDH | 438 bp | Forward: 5′-TGCCATTTGCAGTGGCAAAGTGG-3′ |
| Reverse: 5′-TTGTCATGGATGACCTTGGCCAGG-3′ | ||
| IFN-γ | 245 bp | Forward: 5′-AGCGGCTGACTGAACTCAGATTG-3′ |
| Reverse: 5′-GTCACAGTTTTCAGCTGTATAGG-3′ | ||
| IL-4 | 285 bp | Forward: 5′-AGCCATATCCACGGATGAGACAAA-3′ |
| Reverse: 5′-GCAGCTTATCGATGAATCCAGGCA-3′ | ||
| IL-10 | 485 bp | Forward: 5′-ACTGCTATGCTGCCTGCTCTTACT-3′ |
| Reverse: 5′-TGGCCTTGTAGACACCTTGGTCTT-3′ | ||
| TNF-α | 603 bp | Forward: 5′-CTCAGCCTCTTCTCATTCCTGCTT-3′ |
| Reverse: 5′-AATGACTCCAAAGTAGACCTGCCC-3′ | ||
| TGF-β1 | 695 bp | Forward: 5′-TAAAGAGGTCACCCGCGTGCTAAT-3′ |
| Reverse: 5′-TGTACTGTGTGTCCAGGCTCCAAA-3′ | ||
| CD3ε | 350 bp | Forward: 5′-CATCTGTATCACTCTGGGCTTGCT-3′ |
| Reverse: 5′-AAGGTTCTAGGACACGTGTTCACC-3′ |
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; TGF, transforming growth factor.
Lipid Stimulation Assay
Eight-week-old LDLR−/−, apoE−/− mice were fed the high-fat WTD for 4 weeks to induce hypercholesterolemia with age-matched chow-fed B6 mice used as controls. Mice were anesthetized and bled via the retro-orbital sinus to obtain plasma for subsequent stimulation assay experiments. Either 10 μl of unfractionated total serum or low-density lipoprotein (LDL) isolated by FPLC from WTD-fed LDLR−/− mice was used in the NKT cell hybridoma stimulation assay with bone marrow-derived dendritic cells (BMDCs) done in triplicate. LDL was oxidized by incubation with 10 μmol/L CuSO4 for 16 hours. In brief, BMDCs were generated from either B6 or CD1d−/− bone marrow cells cultured for 6 days with IL-4 and granulocyte-monocyte colony-stimulating factor. BMDCs were then incubated in 96-well flat-microwell plates (4 × 104 cells in 100 μl of media per well) in the presence of each lipid fraction as indicated. After 4 hours, BMDCs were activated with 10 ng/ml tumor necrosis factor (TNF)-α overnight, and washed before incubation with 5 × 104 Vα14Jα18TCR expressing DN32.D3 hybridoma cells for 24 hours. IL-2 released in cultured supernatants was measured using CTLL-2 indicator cells as described.18
Statistical Analyses
All results are expressed as mean ± SEM. Statistical analyses were performed using StatView 5.0.1 (SAS Institute Inc., Cary, NC) using analysis of variance and Student’s t-tests with nonparametric Mann-Whitney tests for analysis of atherosclerotic lesions. The significance level was set at P < 0.05.
Results
Splenocyte Adoptive Transfer Reconstitutes the Immune System of Recipient RAGI−/−LDLR−/− Mice
Significant B-cell and T-cell populations were identified in the spleens of all recipient mice via fluorescence-activated cell sorting staining with anti-IgM and CD3, respectively (Table 2). No quantitative differences in B- or T-lymphocyte populations were observed between groups. Grossly, the spleen weights of all three groups of recipient mice (Table 3) fell between that of immune-competent LDLR−/− mice (0.105 ± 0.005 g) and immune incompetent RAG1−/−LDLR−/− mice (0.033 ± 0.002 g) fed a WTD for 12 weeks and both age and gender-matched; further evidence supporting reconstitution of peripheral lymphoid organs. Finally, the functionality of B cells was assessed by probing the plasma of the recipient mice for immunoglobulins. All three groups had IgG immunoglobulin levels in their plasma comparable with those of immune competent mice with no differences observed between experimental groups. The analysis of immunoglobulin subtypes revealed a similarity of IgG2a, IgG2b, and IgG3 among all three groups; however, total plasma IgM was significantly elevated in the CD1d−/− recipients compared with the Vα14 splenocyte recipients (data not shown).
Table 2.
FACS Analysis of Splenocyte Populations 12 Weeks after Adoptive Transfer and WTD Feeding Demonstrate the Reconstitution of the Peripheral Immune Compartments of Recipient Adaptive Immune-Deficient Mice
| Donor | CD3Pos/tetramerPos | CD3Pos/tetramerNeg | IgMPos | NK1.1Pos/CD3Neg |
|---|---|---|---|---|
| B6 | 0.10 ± 0.01 | 35.7 ± 3.7 | 19.5 ± 1.7 | 12.1 ± 1.2 |
| Vα14tg | 0.98 ± 0.19* | 27.7 ± 3.0 | 24.5 ± 2.7 | 10.2 ± 1.1 |
| CD1d−/− | 0.02 ± 0.01† | 26.0 ± 6.2 | 20.1 ± 4.1 | 8.1 ± 1.5 |
Data are expressed as mean percentages of gated lymphocytes ± SEM, with n = 4 to 5 mice per group.
P < 0.002 for Vα14tg versus B6.
P < 0.004 for CD1d−/− versus B6 or Vα14tg.
Table 3.
Vital Parameters for Adoptive Transfer Recipient Mice
| Donor | Chol | TG | VLDL | LDL | HDL | WT | Spleen WT |
|---|---|---|---|---|---|---|---|
| B6 | 34.4 ± 2.0 | 3.45 ± 0.25 | 20.6 ± 2.3 | 18.7 ± 1.2 | 2.02 ± 0.13 | 21.9 ± 0.8 g | 0.06 ± 0.007 g |
| Vα14tg | 39.4 ± 2.2 | 4.74 ± 0.41 | 19.6 ± 3.1 | 16.3 ± 0.9 | 1.86 ± 0.28 | 23.7 ± 0.6 g | 0.06 ± 0.004 g |
| CD1d−/− | 45.0 ± 2.4* | 5.77 ± 1.01* | 25.5 ± 3.1 | 17.3 ± 0.5 | 2.22 ± 0.18 | 24.0 ± 0.7 g | 0.09 ± 0.014 g |
Fasting plasma total cholesterol and triglyceride levels measured at sacrifice after 12 weeks of WTD feeding. No differences were observed in plasma total cholesterol or triglyceride levels between groups after 4 or 8 weeks on WTD. Plasma lipoproteins from a subset of mice in each group were separated via FPLC and the amount of VLDL, LDL, and HDL cholesterol was calculated based on the percentage of total cholesterol carried in each peak. Data are mean ± SEM, lipid values reported in micromolar plasma.
P < 0.05 CD1d−/− versus B6.
To assess the effectiveness of the adoptive transfer in reconstituting the adaptive immune system of the recipient RAG1−/−LDLR−/− mice with NKT cells, FACS analysis of the resulting splenocyte populations was performed at sacrifice 12 weeks after adoptive transfer and WTD feeding. As shown in Figure 1A, NKT cells were found in the recipients of Vα14tg and B6 splenocytes but not CD1d−/− splenocytes at the time of sacrifice. For the recipients of Vα14tg and B6 splenocytes, the levels of NKT cells in their spleens were ∼10% of the levels present in the spleens of donors. In addition, transcripts for the invariant T-cell receptor (iTCR) α chain (Vα14tgJα18) used by NKT cells were robustly expressed in the livers and spleens of the Vα14tg recipients, only faintly in the livers of B6 recipients, and not at all in the livers or spleens of the CD1d−/− recipients (Figure 1B). Together, these results demonstrate that the adoptively transferred lymphocyte populations effectively reconstituted the adaptive immune system of the recipient RAG1−/−LDLR−/− immunodeficient mice, and some of these cells persisted to the time of sacrifice at 12 weeks after the transfer and initiation of WTD.
Figure 1.
Adoptive transfer of total splenocyte populations effectively reconstitutes the NKT cell pools of recipient immune-deficient mice. A: FACS analysis of splenic lymphocytes from donor mice (top) or corresponding recipient RAG1−/−LDLR−/− mice (bottom) performed at sacrifice, 12 weeks after adoptive transfer and WTD feeding. Each FACS plot is representative of three to four independent experiments. B: Expression of the iTCR by NKT cells in the livers and spleens of recipient RAG1−/−LDLR−/− mice 12 weeks after adoptive transfer and WTD feeding reflects the different levels of NKT cells repopulating these mice as assessed by RT-PCR.
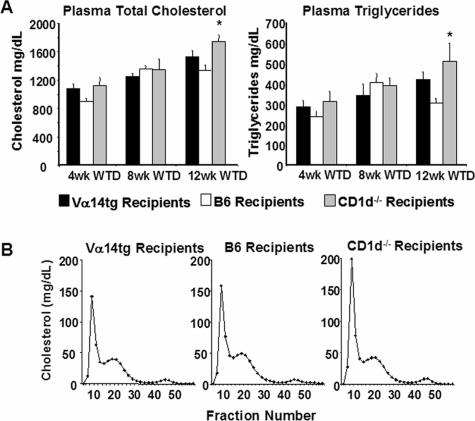
Plasma Lipid Levels and Lipoprotein Distribution in Recipient Mice
As shown in Figure 2A, the feeding of the high-fat WTD elicited a robust hyperlipidemia in all three groups, although significantly higher plasma total cholesterol and triglyceride levels only at the terminal 12-week time point were observed in the recipients of CD1d−/− splenocytes compared with the recipients of B6 splenocytes. Subsequent plasma lipoprotein fractionation via FPLC revealed that this elevation was localized primarily to the very low-density lipoprotein (VLDL) fraction with LDL and high-density lipoprotein (HDL) unchanged (Figure 2B). The data on lipids and lipoproteins, as well as body and spleen weights, are summarized in Table 3.
Figure 2.
A: Plasma cholesterol and triglyceride levels in recipients of splenocytes from Vα14tg, B6, and CD1d−/− mice at 4, 8, and 12 weeks after initiation of WTD. B: Typical FPLC lipoprotein profiles of recipients of splenocytes from Vα14tg, B6, and CD1d−/− mice 12 weeks after splenocyte adoptive transfer and WTD feeding. FPLC fractions were monitored for cholesterol content. *P < 0.03 B6 versus CD1d−/−.
NKT Cells Exacerbate Atherosclerosis
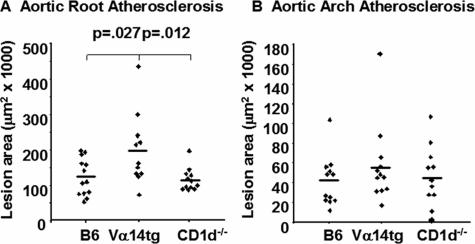
The atherosclerotic lesion burden was assessed in the arterial vasculatures of the mice after 12 weeks of WTD feeding. As illustrated in Figure 3A, the aortic root lesions were 73% larger in the recipients of the enriched NKT cell (Vα14tg) splenocytes as compared with recipients of the NKT cell-deficient (CD1d−/−) splenocytes (198,795 ± 30,428 μm2 versus 115,127 ± 9154 μm2, P = 0.012), and 62% larger than the control B6 splenocyte recipients as well (122,937 ± 14,482 μm2, P = 0.027). On the other hand, there was no difference in atherosclerotic burden between recipients of B6 and CD1d−/− splenocytes. In contrast to the aortic root, there was no difference in the extent of atherosclerosis in a second vascular site, the ascending thoracic aorta, between the three experimental groups (Figure 3B). Although the enriched NKT cell recipients demonstrated a 23% increase in lesion size as compared with the NKT cell-deficient cell population (56,930 ± 11,568 μm2 versus 46,442 ± 9264 μm2) and a 38% increase relative to control B6 splenocyte recipients (41,128 ± 7305 μm2), none of these differences were statistically significant. Interestingly, no lesions were found in the cross-section taken from the innominate arteries of any experimental group. Furthermore, there were no macroscopic lesions observed in the thoracic aorta, abdominal aorta, or iliac arteries in any of these groups.
Figure 3.
Quantification of the atherosclerotic lesional area in the aortic root (A) and the ascending thoracic aortic arch (B) 12 weeks after splenocyte adoptive transfer and WTD feeding. n = 11 to 13 mice per group.
Histological Evaluation of Atherosclerotic Lesions
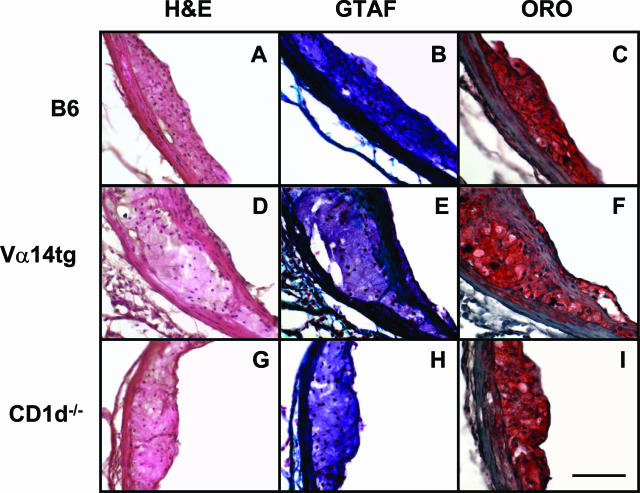
To gain a qualitative assessment of the lesions that developed in these mice, serial sections were stained with oil red O for neutral lipids, Mayer’s hematoxylin and alcoholic eosin (H&E) for overall cellular architecture, and Gomori’s trichrome acid fuchsin to identify collagen and extracellular matrix components. Shown in Figure 4 are images of the stained lesions in the sinus adjacent to the left coronary artery, which had the largest amount of lesion of the three sinuses in the aortic root. As expected, the larger aortic root lesions from the recipients of the enriched NKT cell splenocytes were more mature and complex than those from the recipients of B6 or CD1d−/− splenocytes. The aortic root lesions in the recipients of the NKT cell-enriched splenocytes demonstrated far more smooth muscle cell involvement in the form of collagen and fibrous cap formation, in contrast to the predominantly foam cell-rich intimal xanthomatas of the B6 and CD1d−/− splenocyte recipients. Furthermore, this effect of NKT cell sufficiency on the differential involvement of vascular smooth cells held even after correcting for the different average size of the atherosclerotic lesions between groups. None of the aortic root lesions under these adoptive transfer conditions and at this time-point demonstrated any significant necrotic cores, extracellular cholesterol pools, or calcification. The lesions in the other two sinuses in the aortic root were primarily foam cell-enriched immature lesions in all three experimental groups. Finally, the lesions forming along the lesser curvature of the ascending aortic arch across all groups were predominantly foam cell-rich intimal xanthomatas with little fibrous cap formation evident for lesions smaller than ∼60,000 μm2 (data not shown). Almost all lesions in this site were smaller than 60,000 μm2 (Figure 3B).
Figure 4.
Histomorphology of representative atherosclerotic lesions found in the left coronary sinus of the aortic root in the recipients of B6 (A–C), Vα14tg (D–F), or CD1d−/− (G–I) splenocyte populations 12 weeks after splenocyte adoptive transfer and WTD feeding. Ten-μm serial sections were stained with H&E (A, D, G), Gomori’s trichrome acid fuschin (B, E, H), and oil red O (C, F, I). Original magnifications, ×40. Scale bar = 100 μm.
Hepatic Cytokine Production
Among the lymphocytes of the liver, a large proportion are NKT cells.19 As shown in Figure 1B, the liver exhibits the differential expression of the iTCR, which is expected based on the genotype of the donors of the splenocytes, ie, large amount of iTCR message in recipients of Vα14 transgenic splenocytes and the absence of this message in recipients of CD1d−/− splenocytes. To assess the activity of the transplanted lymphocyte populations, the transcript levels for a variety of Th1- and Th2-type cytokines in the liver was examined by RT-PCR (see Table 1 for primer sequences). Surprisingly, no differences in either Th1 or Th2 cytokine transcript levels were observed between groups despite clear differences in NKT cell levels as measured by iTCR message (data not shown). There was substantial variability between individual mice of each group. This negative result should be interpreted carefully because this represents the transcriptional activity of the whole liver and not isolated NKT cells. This is obvious from the fact that there is at least as much IFN-γ and IL-4 message in the livers of recipients of CD1d−/− splenocytes, which contain no NKT cells, as in the recipients of splenocytes from Vα14tg mice. In addition, message levels are not necessarily equivalent to protein production. Furthermore, the pattern of cytokine production in the artery wall may not mimic the pattern displayed in the livers.
NKT Cell Stimulation Assay
To determine whether NKT cells can be activated by endogenous lipid antigens present in the plasma of hypercholesterolemic mice, serum samples from control chow-fed B6 mice, WTD-fed LDLR−/− mice, WTD-fed apoE−/− mice, or WTD-fed LDLR−/− apoE−/− mice were incubated with cultured dendritic cells derived either from B6 or CD1d−/− mice and subsequently assayed for their ability to stimulate Vα14Jα18TCR-expressing DN32.D3 hybridoma cells to secrete IL-2, a surrogate for CD1d-restricted activation of NKT cells. As indicated in Figure 5A, all serum samples contained a CD1d-dependent factor (ie, antigen) that promoted IL-2 secretion from the reporter hybridoma. When compared with the serum from chow-fed B6 mice, only serum from LDLR−/− mice showed a statistically significant increase in stimulatory activity. In all cases, this stimulatory activity was modest relative to the large stimulation elicited by the glycolipid superantigen α-GalCer. To test if this stimulatory activity may be present in a lipoprotein fraction, we isolated LDL from the plasma of LDLR−/− mice fed a WTD and demonstrated a CD1d-dependent stimulatory activity of the LDL that was abrogated by a 16-hour incubation of the LDL with copper, which generated an extensively oxidized LDL (Figure 5B).
Figure 5.
NKT cell lipid stimulation assay. A: IL-2 release from DN32.D3 Vα14Jα18 iTCR-expressing hybridoma cells after incubation with cultured B6 (light bars) or CD1d−/− (dark bars) BMDCs that had been incubated with unfractionated total serum from different hypercholesterolemic mouse models. As a positive control, the strongly activating glycolipid antigen α-GalCer (100 ng/ml) was used. B: LDL isolated from WTD-fed LDLR−/− mice was assayed, as in A above, either directly or after copper-mediated oxidation (16 hours) using dendritic cells from B6 (light bars) or CD1d−/− (dark bars) mice. *P < 0.01 LDLR−/− versus B6 and apoE−/− serum.
Discussion
In this study, we have shown that the adoptive transfer of splenocytes enriched in the CD1d-restricted population of NKT cells (ie, from Vα14tg mice) to RAG1−/− LDLR−/− recipients enhances atherosclerosis in the aortic root compared with those lacking NKT cells (ie, splenocytes from CD1d−/− donors). In contrast, the atherosclerosis in the ascending thoracic aorta of these recipients did not differ significantly. No known exogenous antigen was used in these experiments, so it is probable that the activation of NKT cells relies on endogenous antigen.
We also found that the serum of mice, particularly LDLR−/− mice fed the WTD, accumulated modestly increased levels of CD1d-dependent antigen. LDL isolated from these animals also contained the putative antigen that was apparently destroyed by extensive oxidation. The increase of this antigen in the serum of LDLR−/− mice is consistent with its presence in LDL, which is taken up by cells via an LDL receptor-dependent endocytic pathway. Most endogenous antigens that are loaded onto CD1d on antigen-presenting cells require endocytosis such as that mediated by the LDL receptor.20 Unlike native and minimally modified LDL, extensively oxidized LDL is not recognized by the LDL receptor. It remains to be seen whether more modest oxidation of LDL would also destroy the antigenic activity for NKT cells.
The precise nature of the lipid antigen is unknown at this time, but the availability of this high-sensitivity assay presents the possibility of further fractionation and purification of the component of LDL responsible for the antigenic activity. Glycosphingolipids are potential endogenous candidates. Various NKT cell hybridomas have been described that recognize glycerophospholipids, such as phosphatidylinositol or phosphatidylethanolamine presented in the context of CD1d.21,22 However, these glycerophospholipids are not recognized by the NKT cell hybridoma used for our assay.21 Much further work is required to identify the putative antigen present in LDL from hypercholesterolemic LDLR−/− mice.
The evidence that NKT cells mediate an increase in murine atherosclerosis has been evaluated in several previous studies.11,12,13,14 Two approaches have been used. An absence of CD1d, and hence NKT cells, in atherosclerosis-susceptible mouse models (LDLR−/− or apoE−/− mice) results in a reduction in atherosclerosis, from which it is implied that NKT cells are proatherogenic. The contrary approach involved the repeated administration of α-GalCer or a related glycolipid antigen, OCH, resulting in both cases in an increase in atherosclerosis. Atherosclerosis decreased or increased, respectively, approximately twofold, not very different from the 73% increase observed in this present study when the number of NKT cells in the mice was increased by adoptive transfer. In only two of these previous studies were LDLR−/− mice used. In one case,14 CD1d−/− mice were crossed with LDLR−/− mice and fed a high-fat diet containing 1.25% cholesterol. In this study, an effect of CD1d deficiency on atherosclerosis was noted only after 4 weeks of high-fat feeding and not at 8 or 12 weeks of diet. In the second case,12 wild-type or CD1d−/− bone marrow was transferred into LDLR−/− recipients and the mice fed an atherogenic diet containing 1.25% cholesterol and 0.5% cholic acid, the latter being a proinflammatory agent. In each of these cases, CD1d was either totally absent or lacking from bone marrow-derived cells. We used a cholic acid-free diet with lower cholesterol levels (0.15% cholesterol) in the studies presented here.
This present study uses a different strategy, namely the complementation of immune-deficient LDLR−/− mice with splenocytes from wild-type mice, mice transgenic for the invariant NKT cell receptor, or mice lacking CD1d. In this model, we demonstrate an increase in lesion extent in the aortic root of RAG1−/−LDLR−/− recipients of splenocytes enriched in NKT cells, which is consistent with the proposed proatherogenic role of these cells. In these studies, each group of recipients was CD1d+ because irradiation was not used to ablate rapidly turning over cells. Even when CD1d−/− splenocytes were transferred, the host expressed CD1d on bone marrow-derived cells, hepatocytes, and gastrointestinal epithelial cells.
That the splenocyte transfer complemented all of the immune-deficient recipients is evidenced by several observations. There was an increase in spleen weight. CD3-positive T cells as well as IgM-positive cells were present in the spleens of the recipient mice. Plasma immunoglobulins of each subtype were also present at levels similar to those found in the plasma of untreated LDLR−/− mice. Surprisingly, the plasma IgM was more elevated in the recipients of CD1d−/−-deficient splenocytes. High levels of iTCR mRNA found in NKT cells were observed in the livers and spleens of recipients of transgenic splenocytes. In addition, the spleen of recipients given transgenic NKT and wild-type splenocytes contained CD1d-tetramer-positive NKT cells; however, after 12 weeks of WTD, these cells were present at approximately one tenth of the level found in the spleens of the corresponding Vα14 transgenic donors (Figure 1A). This level is comparable with that in the wild-type donor mice, suggesting that even without exogenous stimulation, NKT cells are proatherogenic at wild-type levels of these cells.
The transferred total splenocytes contain other immune cells, such as NK cells, B cells, and other T cells, which could interact with NKT cells and influence the outcome of the transfer. Cytokines produced by activated NKT cells communicate with these other immune cells and could account for their role in atherogenesis. NKT cells may be polarized either in a Th1 or Th2 direction.7 Among the lymphocytes of the liver, NKT cells comprise ∼50% of the hepatic αβ T-cell population.23 Yet we found no increase in either IFN-γ or IL-4 transcripts in the livers from the recipients of NKT cells.
Although we observed a statistically significant increase in aortic root atherosclerosis between recipients of Vα14 transgenic splenocytes and those of wild-type or CD1d−/− splenocytes, there was no difference between the latter two groups. This is in contrast to the differences that have been reported for LDLR−/− mice and LDLR−/−CD1d−/− mice.14 There are several possible explanations for this apparent discrepancy. We may have examined our animals too late after the initiation of WTD, ie, 12 weeks, a time point when no differences were observed in this previous study. The fact that recipients of CD1d−/− splenocytes exhibited higher terminal plasma lipids (Figure 2) than the recipients of B6 splenocytes could have countered the vascular effects of CD1−/− splenocyte transfer. The Vα14 transgenic recipients are enriched in invariant NKT cells expressing the iTCR, whereas the CD1d-deficient mice lack other CD1d-restricted immune cells.24 Furthermore, it is possible that the host expression of CD1d influences the phenotype of the recipients of CD1d−/− splenocytes. Finally, it is possible that there is a threshold level of NKT cells required for an effect on atherosclerosis, and the recipients of B6 and CD1d−/− splenocytes did not achieve this threshold (Table 2).
In conclusion, these adoptive transfer studies demonstrate the possibility and utility of the selective complementation of immune-deficient atherosclerosis-sensitive mouse models that lack all T and B cells. This approach complements traditional knockout studies and bone marrow transplantation but without the confounding effects of irradiation in the latter case. This may offer a tool for dissecting the contributions of individual subpopulations of particular immune cells to the process of atherogenesis. Other groups have complemented similar models with CD4+ T cells in atherogenesis studies.25 Here, we have applied this approach to investigate the role specifically played by invariant NKT cells. Furthermore, we have also demonstrated that a putative antigen for NKT cells exists in the plasma and LDL of LDLR−/− mice. Studies are ongoing to identify this antigen.
Acknowledgments
We thank Dr. Albert Bendelac for providing breeding pairs of the Vα14tg mice.
Footnotes
Address reprint requests to Godfrey S. Getz, M.D., Ph.D., Department of Pathology, The University of Chicago, Box MC1089, Chicago, IL 60637. E-mail: g-getz@uchicago.edu.
Supported by the National Institutes of Health (grants HL056827 and HL068661 to G.S.G., and cardiovascular pathophysiology and biochemistry training grant HL007237 and medical scientist training program grant GM007281 to P.V.) and the Cancer Research Institute (to Y.S.).
References
- Getz GS. Thematic review series: the immune system and atherogenesis. Immune function in atherogenesis. J Lipid Res. 2005;46:1–10. doi: 10.1194/jlr.R400013-JLR200. [DOI] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Vanderlaan PA, Reardon CA. The unusual suspects: an overview of the minor leukocyte populations in atherosclerosis. J Lipid Res. 2005;46:829–838. doi: 10.1194/jlr.R500003-JLR200. [DOI] [PubMed] [Google Scholar]
- Reardon CA, Blachowicz L, White T, Cabana V, Wang Y, Lukens J, Bluestone J, Getz GS. Effect of immune deficiency on lipoproteins and atherosclerosis in male apoprotein E deficient mice. Arterioscler Thromb Vasc Biol. 2001;21:1011–1016. doi: 10.1161/01.atv.21.6.1011. [DOI] [PubMed] [Google Scholar]
- Dansky HM, Charlton SA, Harper MM, Smith JD. T and B lymphocytes play a minor role in atherosclerotic plaque formation in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci USA. 1997;94:4642–4646. doi: 10.1073/pnas.94.9.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Leung C, Schindler C. Lymphocytes are important in early atherosclerosis. J Clin Invest. 2001;108:251–259. doi: 10.1172/JCI11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;26:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- Van Kaer L. Natural killer T cells as targets for immunotherapy of autoimmune diseases. Immunol Cell Biol. 2004;82:315–322. doi: 10.1111/j.0818-9641.2004.01252.x. [DOI] [PubMed] [Google Scholar]
- Bobryshev YV, Lord RSA. Co-accumulation of dendritic cells and natural killer T cells within rupture-prone regions in human atherosclerotic plaques. J Histochem Cytochem. 2005;53:781–785. doi: 10.1369/jhc.4B6570.2005. [DOI] [PubMed] [Google Scholar]
- Chan WL, Pejnovic N, Liew TV, Hami1ton H. Predominance of Th2 response in human abdominal aortic aneurysm: mistaken identity for IL-4-producing NK and NKT cells? Cell Immunol. 2005;233:109–114. doi: 10.1016/j.cellimm.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, Berne GP. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med. 2004;199:417–422. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y, Iwabuchi K, Fujii S, Ishimori N, Dashtsoodol N, Watano K, Mishima T, Iwabuchi C, Tanaka S, Bezbradica JS, Nakayama T, Taniguchi M, Miyake S, Yamamura T, Kitabatake A, Joyce S, Van Kaer L, Onoe K. Natural killer T cells accelerate atherogenesis in mice. Blood. 2004;104:2051–2059. doi: 10.1182/blood-2003-10-3485. [DOI] [PubMed] [Google Scholar]
- Major AS, Wilson MT, McCaleb JL, Su YR, Stanic AK, Joyce S, Van Kaer L, Fazio S, Linton MF. Quantitative and qualitative differences in proatherogenic NKT cells in apo1ipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:2351–2357. doi: 10.1161/01.ATV.0000147112.84168.87. [DOI] [PubMed] [Google Scholar]
- Aslanian AM, Chapman HA, Charo IF. Transient role for CDld-restricted natural killer T cells in the formation of atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2005;25:628–632. doi: 10.1161/01.ATV.0000153046.59370.13. [DOI] [PubMed] [Google Scholar]
- Reardon CA, Blachowicz L, Lukens J, Nissenbaum M, Getz GS. Genetic background selectively influences innominate artery atherosclerosis: immune system deficiency as a probe. Arterioscler Thromb Vasc Biol. 2003;23:1449–1454. doi: 10.1161/01.ATV.0000079793.58054.2E. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Hunziker RD, Lantz O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J Exp Med. 1996;184:1285–1293. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–467. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD 1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- Godfrey DI, Kronenberg M. Going both ways: immune regulation via CD1d-dependent NKT cells. J Clin Invest. 2004;114:1379–1388. doi: 10.1172/JCI23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Elzen P, Garg S, León L, Brigl M, Leadbetter EA, Gumperz JE, Dascher CC, Cheng Y-Y, Sacks FM, Illarionov PA, Besra GS, Kent SC, Moody DB, Brenner MB. Apolipoprotein-mediated pathways of lipid antigen presentation. Nature. 2005;437:906–910. doi: 10.1038/nature04001. [DOI] [PubMed] [Google Scholar]
- Gumperz JE, Roy C, Makowska A, Lum D, Sugita M, Podrebarac T, Koezuka Y, Porcelli SA, Cardell S, Brenner MB, Behar SM. Murine CD1d-restricted T cell recognition of cellular lipids. Immunity. 2000;12:211–221. doi: 10.1016/s1074-7613(00)80174-0. [DOI] [PubMed] [Google Scholar]
- Rauch J, Gumperz J, Robinson C, Sköld, Roy C, Young DC, Lafleur M, Moody DB, Brenner MB, Costello CE, Behar SM. Structural features of the acyl chain determine self-phospholipid antigen recognition by a CD1d-restricted invariant NKT (iNKT) cell. J Biol Chem. 2003;278:47508–47515. doi: 10.1074/jbc.M308089200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohteki T, MacDonald HR. Major histocompatibility complex class I related molecules control the development of CD4+8- and CD4−8-subsets of natural killer 1.1+ T cell receptor-alpha/beta+ cells in the liver of mice. J Exp Med. 1994;180:699–704. doi: 10.1084/jem.180.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar SM, Podrebarac TA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J Immunol. 1999;162:161–167. [PubMed] [Google Scholar]
- Zhou X, Nicoletti A, Elhage R, Hansson GK. Transfer of CD4(+) T cells aggravates atherosclerosis in immunodeficient apolipoprotein E knockout mice. Circulation. 2000;102:2919–2922. doi: 10.1161/01.cir.102.24.2919. [DOI] [PubMed] [Google Scholar]