Abstract
The transcription factor aryl hydrocarbon receptor (AhR) plays an important role in the response to environmental pollutants. However, its role in normal physiology is unclear. To investigate the role of AhR in acute lung inflammation, control and AhR knockout (KO) mice were exposed to inhaled cigarette smoke or bacterial endotoxin. Smoke-induced lung inflammation was twofold to threefold more severe in AhR KO mice than controls. Intriguingly, levels of tumor necrosis factor-α and interleukin-6 in the bronchoalveolar lavage of air-exposed KO mice were equal to the levels seen in smoke-exposed controls, suggesting that AhR-deficient mice are inflammation prone. AhR KO mice challenged with inhaled endotoxin, which does not contain AhR ligands, also developed greater lung neutrophilia than controls, and bronchoalveolar lavage cells from AhR KO mice produced elevated levels of tumor necrosis factor-α and interleukin-6 when treated with endotoxin in vitro. Nuclear factor-κB DNA-binding activity was elevated in smoke-exposed AhR KO mice compared with controls and was associated with a rapid loss of RelB only in the KO mice. We propose that AhR is a previously unrecognized regulator of inflammation that interacts with nuclear factor-κB so that in the absence of AhR RelB is prematurely degraded, resulting in heightened inflammatory responses to multiple proinflam-matory stimuli.
Cigarette smoking is widely recognized as a cause or leading risk factor for many chronic diseases including cancer, chronic obstructive pulmonary disease, hypertension, and cardiovascular disease. One common process involved in all these conditions is chronic inflammation. For example, recent research has focused on the role of inflammation and inflammatory cell mediators in the pathogenesis of chronic obstructive pulmonary disease.1,2,3,4,5 It is now believed that atherosclerosis and thrombosis are related to inflammatory processes in smokers,6,7 and it has been hypothesized that chronic inflammation creates a predisposition to the development of cancer.8,9 Smoking is also a significant risk factor for a diverse range of diseases with inflammatory components including psoriasis, Graves’ ophthalmopathy, and diabetes.10,11,12 Therefore, to understand the pathogenic role of smoking in chronic disease, it is necessary to understand the molecular and cellular mechanisms by which cigarette smoke induces acute and chronic inflammation.
One signaling pathway that may link cigarette smoke exposure and inflammation involves the aryl hydrocarbon receptor (AhR). The AhR is a ligand-activated, basic helix-loop-helix transcription factor, well known as the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and other polyaromatic hydrocarbons.13,14,15,16 TCDD, a powerful environmental toxicant, was the first AhR ligand identified, and it is believed that a key role of the AhR is to provide protection against environmental toxicants via up-regulation of phase I and phase II genes such as CYP1A1.17,18 Cigarette smoke contains more than 4000 chemicals, including many polyaromatic hydrocarbons that are AhR ligands.19 It has been shown that components of cigarette smoke compete with dioxin for AhR binding20 and that cigarette smoke extract can activate AhR-dependent gene transcription in vitro and in vivo.17,21,22 We have recently reported that cigarette smoke extract induces a proinflammatory response in human lung fibroblasts, with induction of prostaglandin H synthase-2 (cyclooxygenase-2, Cox-2), microsomal prostaglandin E synthase (mPGES)-1, and prostaglandin (PG)E2, which is AhR-dependent.23 Thus, the AhR may be an important regulator of cigarette smoke-induced inflammation, either through direct activation of AhR-dependent gene transcription or via the activation or detoxification of inflammatory components of cigarette smoke via AhR-mediated up-regulation of phase I and phase II genes.
Although the role of the AhR in the response to polyaromatic hydrocarbons has been extensively studied, its role in normal physiology is poorly understood. A potential endogenous AhR ligand has been identified, but its function is unknown.24 Several lines of evidence suggest that the AhR may play a role in regulating immunity and inflammation in the absence of exogenous polyaromatic hydrocarbons. It has been shown that interleukin (IL)-4 induces transcription of the AhR gene in B cells,25 whereas AhR-deficient mice exhibit abnormalities in hematopoietic stem cell development, have significantly higher numbers of pre/pro-B cells, and develop splenomegaly after immunization with ovalbumin.26,27 Furthermore, although it has been shown that cigarette smoke exposure activates the critical proinflammatory transcription factor nuclear factor (NF)-κB and induces transcription of NF-κB-dependent genes,28,29,30,31 recent reports suggest that AhR and NF-κB may regulate each other’s activity through mutual co-repression.32,33 These results suggest that AhR may play a role in regulating in vivo inflammatory responses through interactions with NF-κB both in the presence and the absence of exogenous AhR ligands. This hypothesis was tested by analyzing acute lung inflammation in wild-type and AhR knockout (KO) mice exposed to cigarette smoke or inhaled bacterial lipopolysaccharide (LPS).
Materials and Methods
Mice
AhR KO mice (AhR−/−, strain B6.129-Ahrtm1Bra) were purchased from The Jackson Laboratory (Bar Harbor, ME) and bred at the University of Rochester. This strain carries a targeted deletion of exon 2 of the AhR gene and was backcrossed for 12 generations onto C57BL/6.34 For some experiments, heterozygous colony littermates (AhR+/−) were used as controls; for other experiments, C57BL/6 mice were purchased from The Jackson Laboratory. No differences were observed between C57BL/6 and AhR+/− mice. All animal procedures were performed under the supervision of the University Committee on Animal Research.
Cigarette Smoke Exposure
Age- and gender-matched AhR−/− (KO) mice and C57BL/6 (B6) or heterozygous littermate (AhR+/−) controls were placed in individual compartments of a wire cage, which was placed inside a closed plastic box connected to the smoke source. Research cigarettes (1R3F; University of Kentucky, Lexington, KY) were smoked according to the Federal Trade Commission protocol (1 puff/minute/cigarette of 2 seconds duration and 35-ml volume) in a Baumgartner-Jaeger CSM2072i cigarette smoking machine (CH Technologies, Westwood, NJ). Mainstream cigarette smoke was diluted with filtered air and directed into the exposure chamber. The smoke exposure (total particulate matter per cubic meter of air, TPM) was monitored in real time with a MicroDust Pro aerosol monitor (Casella CEL, Bedford, UK) and verified by gravimetric sampling. The smoke concentration was set at a nominal value of 250 mg/m3 TPM by adjusting the flow rate of the dilution air. The average actual exposure for these experiments was 286 ± 45 mg/m3 TPM. Mice received two 1-hour exposures, 4 hours apart, on days 1 and 2, a single exposure on day 3, and were euthanized 4 or 24 hours after the final exposure. Control mice were exposed to filtered air in an identical chamber according to the same schedule.
LPS Aerosol Exposure
Age- and gender-matched AhR KO and B6 controls were exposed to an aerosol of saline alone (control) or saline containing Pseudomonas aeruginosa LPS (Sigma, St. Louis, MO) as described.35 Total exposure time was 8 minutes, and the estimated deposition in the lungs was 5 endotoxin units (EUs) per animal. Four hours after LPS exposure, the mice were euthanized and the lungs were lavaged as described below.
Tissue Harvest and Bronchoalveolar Lavage (BAL)
Mice were anesthetized with 2,2,2-tribromoethanol (Avertin, 250 mg/kg i.p.; Sigma) and euthanized by exsanguination. The heart and lungs were removed en bloc, and the lungs were lavaged twice with 0.5 ml of phosphate-buffered saline (PBS). The lavage fluid was centrifuged, and the cell-free supernatants were frozen for later analysis. The BAL cell pellet was resuspended in PBS, and the total cell number was determined by counting on a hemocytometer. Differential cell counts (minimum of 300 cells per slide) were performed on Cytospin-prepared slides (Thermo Shandon, Pittsburgh, PA) stained with Diff-Quik (Dade Behring, Newark, DE). In some experiments, the remaining BAL cells were pelleted and frozen for electrophoretic mobility shift assays (EMSAs) (see below). The left and right lungs were frozen separately in liquid nitrogen for later analysis.
Analysis of BAL Fluid
Mouse IL-6, tumor necrosis factor (TNF)-α, MIP-2, and KC were measured in BAL samples by commercial enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, MN). PGE2 was measured by enzyme immunoassay using commercially available reagents (Cayman Chemical, Ann Arbor, MI) as described.36 The limit of detection was 7 pg/ml for IL-6, MIP-2, and KC, 15 pg/ml for TNF-α, and 30 pg/ml for PGE2. To assay β-glucuronidase activity, the liberation of p-nitrophenol from 4-nitrophenol glucuronide (Sigma) was measured in fresh (not frozen) BAL samples as described.37,38 Total protein in lavage fluid was measured by the bicinchoninic (BCA) colorimetric assay (Pierce, Rockford, IL).
Histological Analysis and Immunohistochemistry
Mouse lungs (which had not been lavaged) were fixed by inflation with 10% neutral buffered formalin at a pressure of 15 cm H2O. Tissues were embedded in paraffin, sectioned (5 μm), and stained with hematoxylin and eosin. To visualize tissue neutrophils, sections were stained with a rat monoclonal anti-mouse neutrophil antibody (MCA771GA, 1:25 dilution; Serotec, Oxford, UK), developed with NovaRed (Vector Laboratories, Burlingame, CA), and counterstained with hematoxylin.
Myeloperoxidase Assay
Frozen lung tissue was homogenized in 50 mmol/L potassium phosphate buffer, pH 6.0, containing a protease inhibitor cocktail and 0.5% hexadecyltrimethylammonium bromide (Sigma), sonicated with a probe sonicator (Branson Ultrasonics, Danbury, CT), and then subjected to one freeze-thaw cycle in a dry ice-ethanol bath. The suspension was centrifuged at 16,000 × g, and the resulting supernatant was assayed. Myeloperoxidase activity was determined by monitoring the oxidation of o-dianisidine dihydrochloride (Sigma) spectrophotometrically at 460 nm as described.39
BAL Cell Cultures
BAL cells were obtained from naive control and AhR−/− mice as described, except that the lungs were lavaged 10 times with Dulbecco’s modified PBS (Invitrogen, Carlsbad, CA), containing 1% glucose, for a total volume of 5 ml. BAL cells from four to six mice per strain were pooled, washed twice with PBS, resuspended in RPMI medium containing 10% fetal calf serum and supplemented with l-glutamine (2 mmol/L) and gentamicin (50 μg/ml) (Invitrogen), and counted with a hemocytometer. Quadruplicate cultures were established containing 1 × 105 cells in 0.2 ml of medium per well in 96-well plates and incubated at 37°C. After 2 hours, nonadherent cells were removed by gentle washing with serum-free medium, and the adherent cells were cultured in medium containing 1% fetal calf serum, with or without the addition of LPS (10 EU/ml P. aeruginosa endotoxin; Sigma). In some experiments, the cells were pretreated for 1 hour with the NF-κB inhibitors SN50 (25 μg/ml) or helenalin (5 μmol/L) (Biomol, Plymouth Meeting, PA). After 18 hours, the culture medium was harvested and assayed for inflammatory cytokines by ELISA, as described.
EMSA
Nuclear extracts were prepared from frozen lung tissue or BAL cell pellets as described.40 Nuclear extracts were incubated with radiolabeled oligonucleotide probes for NF-κB and OCT-1 and analyzed by autoradiography of polyacrylamide gels as described.41
Western Blot Analysis
To analyze the immediate effect of smoke and LPS exposure on NF-κB, mice were exposed to mainstream smoke for 1 hour as described and sacrificed 30 minutes and 4 hours later or exposed to LPS as described and sacrificed 30 minutes and 3 hours later. The lungs were lavaged to obtain BAL cells and then homogenized to prepare total and nuclear lung cell protein extracts using a commercially available kit according to the supplier’s instructions (Active Motif, Carlsbad, CA). BAL cells from three mice per group were pooled, and protein extracts were prepared. Lung and BAL proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and analyzed by Western blot using antibodies specific for NF-κB p65 and RelB (Santa Cruz Biotechnology, Santa Cruz, CA) and total and phosphorylated IκBα (Cell Signaling, Danvers, MA).
Statistical Analysis
All results are reported as the mean ± SEM. The differences between air- and smoke-exposed AhR KO and control mice on differential cell counts, lavage cytokines, and biochemical markers were assessed by one-factor analysis of variance and the Student’s t-test. A P value <0.05 was considered significant.
Results
Elevated Inflammatory Cells in BAL of AhR KO Mice Exposed to Cigarette Smoke
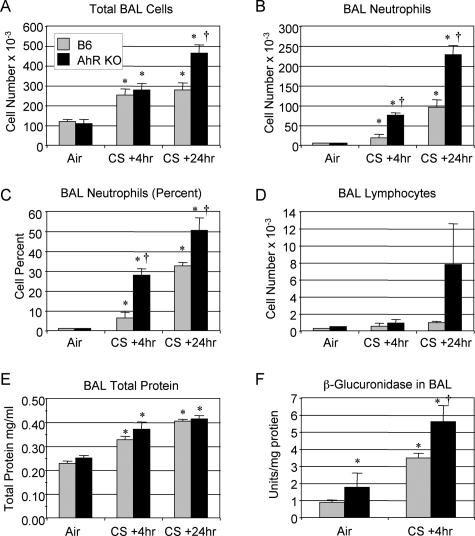
To investigate the potential role of the AhR in regulating the inflammatory response to cigarette smoke, wild-type and AhR KO mice were exposed to cigarette smoke for 1 hour, twice a day, for 3 days and sacrificed 4 or 24 hours after the final smoke exposure. This exposure protocol elicits an acute inflammatory response characterized by a twofold to threefold increase in the total number of cells and a greater than 100-fold increase in the number of neutrophils recovered by BAL (Figure 1). Compared with wild-type B6 mice, AhR KO mice developed significantly greater neutrophilic influx into the lungs, with twofold to threefold more neutrophils recovered in BAL at both 4 and 24 hours after exposure (Figure 1, B and C). The total number of cells recovered by BAL is also significantly elevated in the AhR KO mice 24 hours after exposure (Figure 1A). Cigarette smoke exposure resulted in increased numbers of BAL lymphocytes that were not significant (Figure 1D), but no changes in BAL eosinophils (data not shown). The protein content of BAL fluid, a marker of inflammation, was significantly increased by cigarette smoke exposure, but the differences between wild-type and AhR KO mice were not significant (Figure 1E). β-Glucuronidase, a hydrolytic enzyme found in phagocytic cells and thought to be involved in tissue breakdown in inflammatory lung disease,42 is also elevated in BAL fluid of smoke-exposed animals, with enzyme activity 60% higher in smoke-exposed AhR KO mice compared with controls (Figure 1F). Similar results were observed in experiments comparing AhR heterozygote and KO mice (data not shown).
Figure 1.
AhR KO mice exhibit increased lung inflammation markers in BAL after cigarette smoke exposure. Wild-type (B6, gray bars) and AhR KO (black bars) mice were exposed to air or cigarette smoke (CS) as described and sacrificed 4 or 24 hours after the final exposure. Differential counts were performed on BAL cells, and the total cell number (A), number of neutrophils (B), percentage of neutrophils (C), and number of lymphocytes (D) are reported. E: Total protein in BAL fluid was measured by BCA assay. F: β-Glucuronidase activity was measured in fresh BAL fluid and is expressed as units of activity per mg total protein. Results shown are the means ± SE for n = 6 mice per group from a single experiment and are representative of three independent experiments. *Significant increase over air-exposed wild-type mice; †significant increase over smoke-exposed, wild-type mice (P < 0.05).
AhR KO Mice Exposed to Cigarette Smoke Exhibit Increased Lung Tissue Inflammation
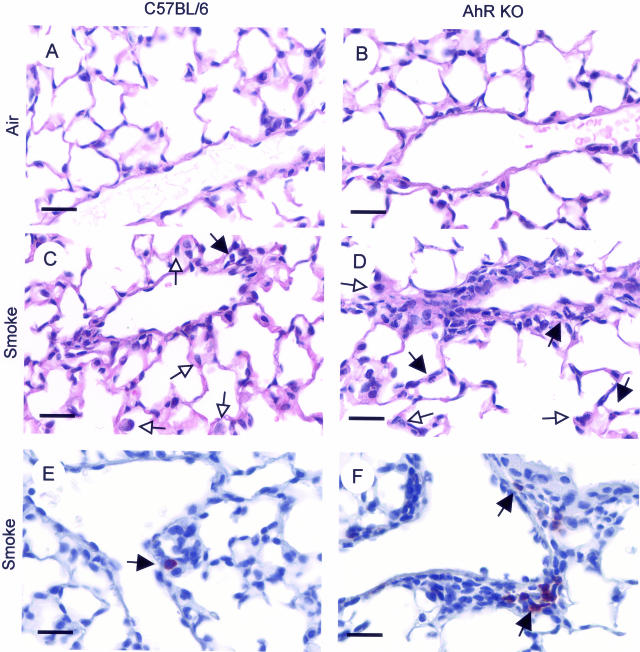
Acute cigarette smoke exposure at the dose used in this study results in a mild inflammation marked by perivascular accumulation of inflammatory cells, including neutrophils, and increased numbers of alveolar macrophages and neutrophils (Figure 2C). Perivascular infiltrates are more pronounced in the AhR KO mice, as is the appearance of neutrophils in alveolar capillaries (Figure 2D). Immunohistochemical staining using an antibody specific for mouse neutrophils confirms these observations, with increased numbers of neutrophils in the airspaces and perivascular infiltrates in AhR KO mice (Figure 2, E and F).
Figure 2.
Lung inflammation is increased in AhR KO mice exposed to cigarette smoke. Mice were exposed to air or smoke as described and sacrificed 24 hours after the final smoke exposure. Lungs were inflated and fixed with formalin, and sections were stained with H&E. A and B: Air-exposed mice have normal alveoli and blood vessels. C: Smoke-exposed mice exhibit perivascular inflammation with extravasating monocytes and neutrophils and monocytes and neutrophils in the alveolar capillaries. D: Perivascular infiltrations and alveolar neutrophils are more prominent in smoke-exposed AhR KO mice. Sections were immunostained with an antibody against mouse neutrophils (red) and counterstained with hematoxylin. Tissue-infiltrating neutrophils are much more prominent in AhR KO mice (F) than wild-type (E). White arrows, monocyte; black arrows, neutrophil. Scale bars = 25 μm.
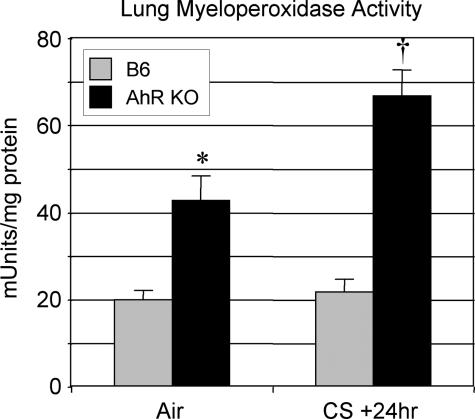
Lung neutrophilia in the AhR KO− mice was confirmed biochemically by assaying whole lung tissue for myeloperoxidase (MPO), an enzyme found in neutrophil granules that can be used to estimate the level of neutrophilic inflammation in tissues.39 MPO activity is significantly higher in naive AhR KO mice than B6 controls and is further increased in these mice by cigarette smoke exposure to levels threefold higher than smoke-exposed B6 controls (Figure 3).
Figure 3.
AhR KO mice exposed to cigarette smoke have elevated levels of myeloperoxidase activity in lung tissue compared with wild-type controls. Wild-type (B6, gray bars) and AhR KO (black bars) mice (n = 4 to 5 per group) were exposed to air or cigarette smoke (CS) and sacrificed 24 hours after the final exposure as described. Myeloperoxidase activity in the left lung was measured as described in Materials and Methods. Results shown are the means ± SE for n = 6 mice per group from a single experiment and are representative of two independent experiments. *Significant increase compared with air-exposed wild-type mice; †significant increase compared with smoke-exposed, wild-type mice (P < 0.05).
AhR KO Mice Produce Elevated Levels of Proinflammatory Cytokines
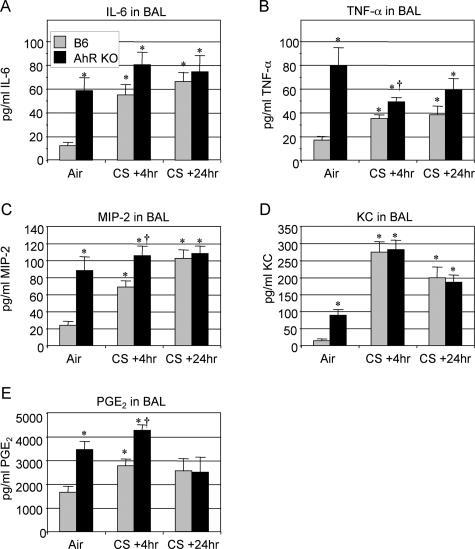
Acute cigarette smoke exposure induces several proinflammatory cytokines in the BAL of wild-type mice, including IL-6, TNF-α, and PGE2 (Figure 4, A, B, and E). The neutrophil chemoattractant CXC chemokines MIP-2 and KC are also significantly elevated by cigarette smoke (Figure 4, C and D). Interestingly, air-exposed AhR KO mice express threefold to fivefold higher levels of proinflammatory cytokines and chemokines than untreated controls. In the case of IL-6, MIP-2, TNF-α, and PGE2, the basal levels in AhR KO mice are as high or higher than in smoke-exposed B6 mice. AhR KO mice exposed to smoke express higher levels of TNF-α, MIP-2, and PGE2 than control mice at 4 hours after exposure but not at 24 hours after exposure (Figure 4, B, C, and E).
Figure 4.
Proinflammatory cytokine levels are elevated in the BAL of smoke-exposed AhR KO mice. Wild-type (B6, gray bars) and AhR KO (black bars) mice (n = 6 per group) were exposed to air or cigarette smoke (CS) and sacrificed 4 or 24 hours after the final exposure. IL-6 (A), TNF-α (B), MIP-2 (C), KC (D), and PGE2 (E) were measured in BAL fluid by commercial ELISA or enzyme immunoassay, as described. Results shown are the means ± SE for n = 6 mice per group from a single experiment and are representative of three independent experiments. *Significant difference from air-exposed wild-type mice; †significant difference from smoke-exposed, wild-type mice (P < 0.05).
Increased BAL Neutrophils in AhR KO Mice Exposed to Inhaled LPS
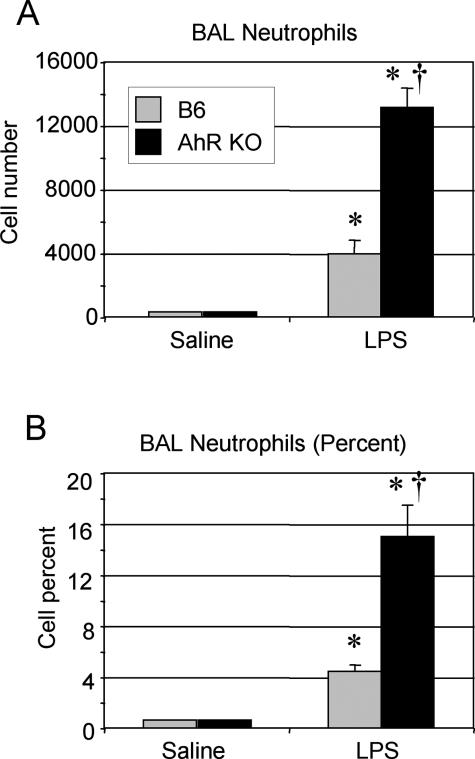
The presence of elevated levels of proinflammatory cytokines in naive AhR KO mice suggested that these mice are more inflammation-prone than wild-type B6 mice. To investigate this possibility, mice were exposed to an aerosol of bacterial LPS, a strong inflammatory stimulus that does not signal through the AhR. Mice exposed to inhaled LPS develop neutrophilic lung inflammation within 4 hours.35 BAL from AhR KO mice contained two to three times more neutrophils than B6 controls (Figure 5), indicating that the AhR mice develop a heightened inflammatory response to LPS, as well as cigarette smoke.
Figure 5.
AhR KO mice exhibit increased neutrophils in BAL after exposure to bacterial endotoxin. Wild-type (B6, gray bars) and AhR KO (black bars) mice were exposed to an aerosolized suspension of bacterial endotoxin (LPS) in saline, or saline aerosol alone, and sacrificed 4 hours later. Differential cell counts were performed on BAL cells, and the number (A) and percentage (B) of neutrophils in the BAL is reported. Values shown are the mean ± SE of n = 4 mice per group and are representative of two independent experiments. *Significant increase compared with air-exposed wild-type mice; †significant increase compared with smoke-exposed, wild-type mice (P < 0.05).
Production of Inflammatory Cytokines in Vitro Is Elevated in Cells from AhR KO Mice
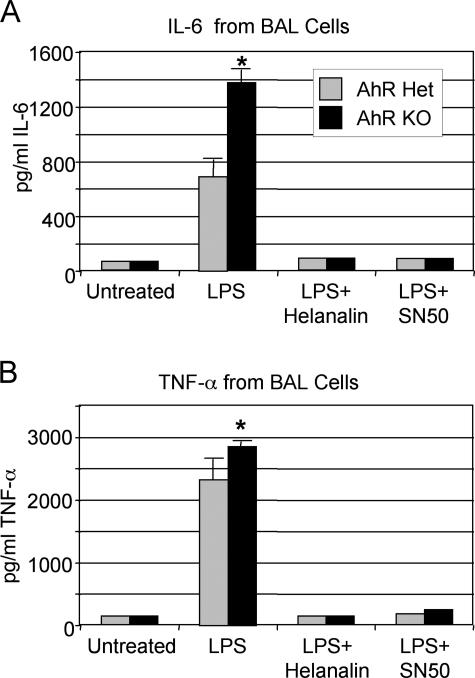
The heightened response to LPS seen in the AhR KO mice was further investigated in vitro. Cells recovered from the lungs of naive AhR KO and control mice (either B6 or heterozygous littermates) by BAL were cultured with LPS for 18 hours. These BAL cells from untreated mice were >99% macrophages when checked by differential counting (data not shown). LPS stimulated the release of proinflammatory cytokines including IL-6 and TNF-α from BAL cells in vitro (Figure 6). BAL cells from AhR KO mice produced significantly higher levels of IL-6 and TNF-α than cells from AhR heterozygous controls.
Figure 6.
BAL cells from AhR KO mice treated in vitro with bacterial endotoxin produce elevated levels of proinflammatory cytokines. Lung cells were obtained by lavage from six naive AhR heterozygous (Het) and KO mice, pooled by strain, and cultured in vitro with or without 3.3 ng/ml bacterial endotoxin (LPS), 5 μmol/L helenalin, or 25 μg/ml SN50. After 18 hours, culture supernatants were harvested and assayed for IL-6 (A) and TNF-α (B) by commercial ELISA. The results shown are the mean ± SEM of quadruplicate cultures and are representative of three independent experiments performed using both AhR Het littermates and B6 mice as controls. *Significant increase compared with control cells (P < 0.05).
Mouse TNF-α, IL-6, and MIP-2 are regulated by NF-κB,43,44,45 and recent reports have suggested that AhR and NF-κB may regulate each other’s activity.32,33 To investigate the NF-κB dependence on cytokine production in the AhR KO mice, BAL cells from naive mice were incubated with LPS and the NF-κB inhibitors helenalin and SN50. Both helenalin and SN50, which block NF-κB activity by different mechanisms,46,47 reduced the production of IL-6 and TNF-α to background levels, confirming that IL-6 and TNF-α production is NF-κB-dependent in the AhR KO mice (Figure 6, A and B).
AhR KO Mice Exhibit Enhanced NF-κB DNA Binding Activity after Exposure to Cigarette Smoke
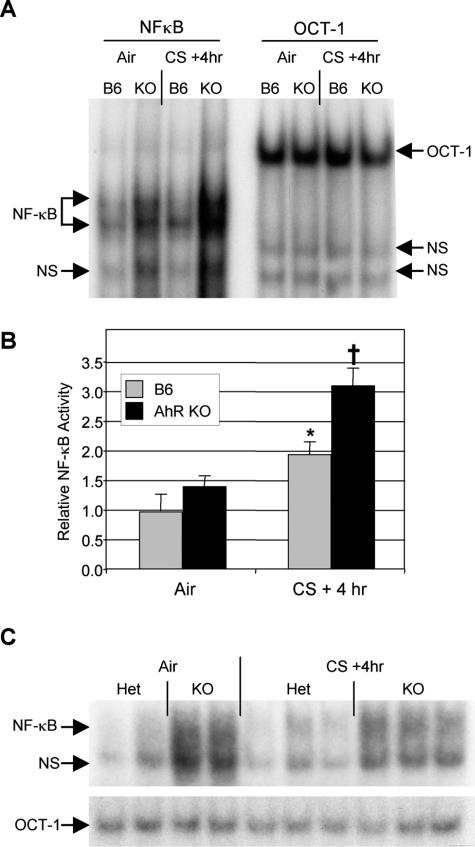
To determine whether NF-κB activity was altered in AhR-deficient mice, EMSAs were performed on nuclear extracts prepared from lung tissue of mice exposed to air or cigarette smoke. Cigarette smoke induced a twofold increase in NF-κB-specific DNA binding in the lungs of B6 mice (Figure 7, A and B). Interestingly, air-exposed AhR KO mice had a 40% higher basal level of NF-κB activity, although this was not significant (P = 0.13). Smoke exposure induced a twofold increase in NF-κB activity in the AhR KO mice, resulting in significantly higher activity than smoke-exposed B6 mice. NF-κB activity was also higher in BAL cells harvested from air and smoke-exposed AhR KO mice compared with controls (Figure 7C).
Figure 7.
AhR KO mice exhibit elevated levels of NF-κB DNA-binding activity in lung tissue with and without cigarette smoke exposure. Nuclear extracts were made from lung tissue of wild-type (B6) and AhR KO mice exposed to air or cigarette smoke as described. DNA-binding activity to radiolabeled oligonucleotide probes containing NF-κB (left) or OCT-1 (right) consensus binding sequences was analyzed by EMSA. Arrows indicate nonspecific (NS), NF-κB-specific, and OCT-1-specific DNA binding activity. A total of 20 mice were analyzed (n = 4 per air-exposed group and 6 per smoke-exposed group). A: One representative animal per group; B: NF-κB activity relative to air-exposed B6 mice, determined by densitometric analysis of all 20 mice, normalized to OCT-1. *Significant increase over air-exposed B6 mice; †significant exposure over both air-exposed KO and smoke-exposed B6, P < 0.05. C: BAL cells harvested from air- and smoke-exposed AhR heterozygous (Het) and KO mice were pooled in groups of two to obtain sufficient nuclear extract for EMSA (a total of four Het and six KO mice were exposed in this experiment). NF-κB and OCT-1 binding activity was determined as described.
AhR KO Mice Exhibit a Rapid Loss of Nuclear RelB after Smoke and LPS Exposure
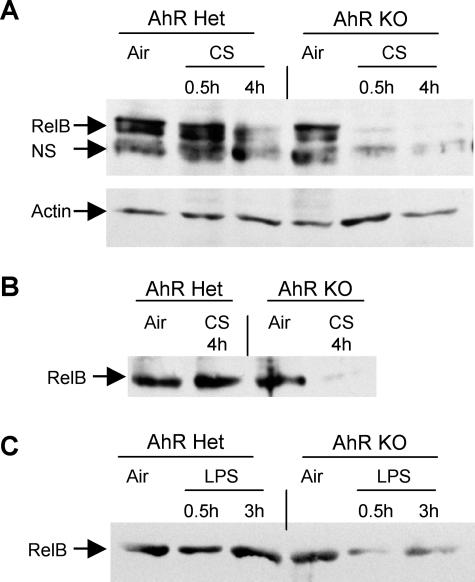
To investigate the mechanism of increased NF-κB DNA binding, whole cell and nuclear extracts from lung were analyzed by Western blot. Cigarette smoke induced nuclear accumulation of p65 (RelA) and phosphorylation of IκBα in both control and AhR KO mice, but there were no differences between the groups (data not shown). Control mice also exhibited a loss of RelB from lung cell nuclei 4 hours after smoke exposure. Intriguingly, AhR KO mice exhibited a profound, rapid loss of nuclear RelB 30 minutes after smoke exposure (Figure 8A). The inflammatory cells extracted by BAL were also analyzed by Western blot. Although no differences were observed between KO and control mice with respect to p65 and IκBα, cigarette smoke exposure induced a loss of nuclear RelB from the BAL cells of AhR KO mice but not control mice (Figure 8B). Because the AhR KO mice exhibited enhanced inflammation to LPS, as well as to cigarette smoke, we determined the effect of LPS exposure on RelB. Similar to the response seen to cigarette smoke, LPS exposure induced rapid loss of RelB in BAL cells from KO but not control mice (Figure 8C).
Figure 8.
AhR KO mice exhibit rapid loss of RelB after smoke and LPS exposure. Control (Het) and AhR KO mice were exposed to cigarette smoke for 1 hour, and BAL cells and lung tissue were harvested 30 minutes and 4 hours later. Control mice were exposed to air only. A: Nuclear extracts were prepared from lung tissue and analyzed by Western blot. One representative mouse per group is shown. Equal amounts of protein were loaded in each lane. The blots were probed with antibodies to RelB and actin (a loading control). NS, a nonspecific band sometimes observed with this antibody. Data are representative of two independent experiments with three mice per group in each experiment. B: The BAL cells retrieved from all three mice in each group were pooled and total protein extracts were prepared and analyzed by Western blot for RelB. Equal amounts of protein were loaded in each lane. The results are representative of two independent experiments. C: Control (Het) and AhR KO mice were exposed to LPS as described, and BAL cells were harvested after 30 minutes and 3 hours. Control mice were exposed to saline only. BAL cell protein extracts were analyzed by Western blot for RelB.
Discussion
Chronic inflammation associated with cigarette smoking is a major contributing factor to the pathogenesis of smoking-related diseases, including chronic obstructive pulmonary disease and cardiovascular disease, and may also explain why smoking is a significant risk factor for other diseases including diabetes and Graves’ ophthalmopathy.1,2,5,6,7,10,11,12 Cigarette smoke and smoke extracts have been shown to induce lung inflammation in vivo and the production of inflammatory mediators in vitro.4,28,38,48,49 A number of molecular pathways have been implicated including the MAP kinases, chromatin remodeling, oxidative stress responses, and NF-κB.5,50 Recently, it has been shown that cigarette smoking activates AhR-dependent gene transcription,17,21,22 and we have recently demonstrated that smoke extract stimulates a proinflammatory response in primary human lung fibroblasts that is AhR-dependent.23 These results suggest that the AhR is important in regulating cigarette smoke-induced inflammation in vivo.
To test the hypothesis that AhR is a regulator of smoked-induced inflammation, mice lacking AhR were exposed to cigarette smoke using an exposure protocol that results in mild acute inflammation in B6 mice.38 AhR KO mice developed more severe inflammation, with increased BAL neutrophils and β-glucuronidase activity (Figure 1), increased tissue neutrophils (Figures 2 and 3), and elevated levels of proinflammatory cytokines (Figure 4). Of great interest was the fact that naive AhR KO mice expressed elevated levels of the proinflammatory cytokines TNF-α and IL-6, neutrophilic chemokines MIP-2 and KC, and the inflammatory prostaglandin PGE2 (Figure 4). This suggested that AhR KO mice were not merely more prone to cigarette smoke-induced inflammation but were generally prone to inflammatory stimuli. To investigate this possibility, we used bacterial endotoxin, an inflammatory stimulus that does not signal through the AhR. AhR KO mice challenged with inhaled LPS developed more acute lung inflammation and neutrophilia than wild-type controls (Figure 5). Because alveolar macrophages are one of the key cell types involved in the inflammatory response in the lung,3,51 we tested the response of BAL cells (containing >99% alveolar macrophages) isolated from naive mice to LPS in vitro. Although BAL cells from control strains produced significant amounts of TNF-α and IL-6, BAL cells from AhR KO mice expressed twofold to threefold higher levels of these inflammatory cytokines (Figure 6). These results confirm that AhR KO mice exhibit a phenotype of heightened responsiveness to multiple inflammatory stimuli.
TNF-α and IL-6 are both regulated by NF-κB. Given the recent data suggesting that NF-κB and AhR engage in mutual repression,52 we hypothesized that the loss of AhR leads to derepression of NF-κB. We tested this hypothesis by performing EMSAs using lung tissue from mice that had been exposed to air or cigarette smoke. Wild-type mice exhibited a twofold increase in NF-κB activity after exposure to mainstream cigarette smoke (Figure 7, A and B). In contrast, NF-κB activity was significantly elevated in smoke-exposed AhR KO mice compared with both air-exposed KOs and smoke-exposed controls (Figure 7, A and B). NF-κB activity is also elevated in BAL cells from AhR KO mice (Figure 7C). Thus, AhR and NF-κB seem to coordinately regulate cigarette smoke-induced inflammation so that the loss of the AhR leads to increased NF-κB activity and increased smoke-induced inflammation. To further explore the regulation of NF-κB in AhR KO mice, levels of p65 (RelA), RelB, and total and phosphorylated IκBα were analyzed by Western blot. Although there were no differences in the levels of p65 or IκBα between AhR KO and control mice, lung cell nuclei and BAL cells from AhR KO mice exhibited a rapid loss of RelB after exposure to both cigarette smoke and LPS that was not seen in control mice (Figure 8).
These results demonstrate that AhR plays a critical role in modulating innate immune responses in the lung. The current literature on AhR suggests two mechanisms by which the AhR might regulate cigarette smoke-induced lung inflammation; a direct mechanism in which AhR ligands in cigarette smoke induce transcription of AhR-dependent genes, some of which may be involved in immune responses, and an indirect mechanism involving the activation or detoxification of inflammatory chemicals in cigarette smoke via AhR-dependent up-regulation of phase I and phase II enzymes.17,18 Significantly, our results indicate that AhR modulates inflammation through a novel mechanism involving interaction with RelB. Although RelB can activate NF-κB-dependent gene transcription via the alternative pathway,53 recent evidence indicates that RelB plays a critical role as a negative regulator of immune responses. For example, morphine-induced suppression of cytokine production is RelB-dependent,54 whereas RelB−/− fibroblasts exhibit prolonged induction of inflammatory cytokines when stimulated with LPS.55 RelB−/− mice housed under standard conditions develop severe multiorgan inflammation.56 Of particular interest is a recent report that endotoxin tolerance in monocytes is RelB-dependent. Endotoxin-tolerant monocytes respond to LPS treatment with degradation of IκBα and nuclear translocation of p65 without concomitant activation of cytokine genes. However, small inhibitory RNAs that decreased RelB production restored the LPS response, whereas overexpression of RelB in nontolerant cells inhibited the endotoxin response.57 This is comparable with our finding that NF-κB DNA-binding activity is increased in the AhR KO mice but that IκBα phosphorylation and p65 nuclear translocation are not affected. We suggest that, as in endotoxin tolerance, the loss of RelB leads to increased p65 activity, possibly through loss of inhibitory p65/RelB heterodimers.57,58
Our results demonstrate for the first time that the AhR is crucial for the regulation of inflammation. Our new findings suggest that the mechanism involves interaction with RelB such that loss of the AhR leads to a rapid loss of RelB on stimulation with either cigarette smoke or LPS. The rapidity of the decline, from both lung nuclear fractions and BAL cells, combined with previous reports that RelB synthesis is a late effect of activation of the alternative NF-κB pathway,53 suggests the mechanism involves active degradation of RelB. The fact that loss of RelB occurs in response to both LPS and cigarette smoke suggests that the mechanism does not involve signaling by exogenous AhR ligands, including cigarette smoke. It is possible that the effect is ligand-independent, or that it involves endogenous AhR ligands. The role of the AhR in normal homeostasis is poorly understood. A putative endogenous AhR ligand has been identified in porcine lung, but its function is unknown.24 AhR KO mice exhibit abnormalities in liver development, hematopoiesis, and reproduction,26,34,59 implying that endogenous ligands are involved in regulating these developmental processes. It should be noted that different AhR ligands can have very different activities in vivo and in vitro. For example, mice carrying the AhRd allele are only weakly responsive to TCDD but exhibit enhanced responses to benzo[a]pyrene, a component of tobacco smoke.60,61 The putative endogenous ligand ITE activates an AhR reporter gene in the same tissues of developing embryos as TCDD, but without causing the same developmental abnormalities.62 It will be interesting to determine whether AhR regulation of RelB levels is dependent or independent of endogenous ligands and to determine whether the regulation of RelB is altered in B6.AhRd congenic mice, which carry a low-affinity AhR allele.
Footnotes
Address reprint requests to Dr. Patricia J. Sime, University of Rochester, Division of Pulmonary and Critical Care Medicine, 601 Elmwood Ave., Box 692, Rochester, NY 14642. E-mail: patricia_sime@urmc.rochester.edu.
Supported in part by the National Institutes of Health [grants K08HL04492, HL075432, ES09430, DE011390, HL078603, EY017123, National Institute of Environmental Health Sciences center grant P30ES01247, National Institute of Environmental Health Sciences training grant ES-07026 (to H.F.L.), NS054578 (to S.B.M.), and Environmental Protection Agency grant R827354], Philip Morris USA, and the American Lung Association (fellowship to C.J.B.).
References
- Di Stefano A, Caramori G, Ricciardolo FL, Capelli A, Adcock IM, Donner CF. Cellular and molecular mechanisms in chronic obstructive pulmonary disease: an overview. Clin Exp Allergy. 2004;34:1156–1167. doi: 10.1111/j.1365-2222.2004.02030.x. [DOI] [PubMed] [Google Scholar]
- Wright JG, Christman JW. The role of nuclear factor kappa B in the pathogenesis of pulmonary diseases: implications for therapy. Am J Respir Med. 2003;2:211–219. doi: 10.1007/BF03256650. [DOI] [PubMed] [Google Scholar]
- Churg A, Zay K, Shay S, Xie C, Shapiro SD, Hendricks R, Wright JL. Acute cigarette smoke-induced connective tissue breakdown requires both neutrophils and macrophage metalloelastase in mice. Am J Respir Cell Mol Biol. 2002;27:368–374. doi: 10.1165/rcmb.4791. [DOI] [PubMed] [Google Scholar]
- Miller LM, Foster WM, Dambach DM, Doebler D, McKinnon M, Killar L, Longphre M. A murine model of cigarette smoke-induced pulmonary inflammation using intranasally administered smoke-conditioned medium. Exp Lung Res. 2002;28:435–455. doi: 10.1080/01902140290096728. [DOI] [PubMed] [Google Scholar]
- Spurzem JR, Rennard SI. Pathogenesis of COPD. Semin Respir Crit Care Med. 2005;26:142–153. doi: 10.1055/s-2005-869535. [DOI] [PubMed] [Google Scholar]
- MacCallum PK. Markers of hemostasis and systemic inflammation in heart disease and atherosclerosis in smokers. Proc Am Thorac Soc. 2005;2:34–43. doi: 10.1513/pats.200406-036MS. [DOI] [PubMed] [Google Scholar]
- Hunninghake DB. Cardiovascular disease in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2:44–49. doi: 10.1513/pats.200410-050SF. [DOI] [PubMed] [Google Scholar]
- Ohshima H, Tazawa H, Sylla BS, Sawa T. Prevention of human cancer by modulation of chronic inflammatory processes. Mutat Res. 2005;591:110–122. doi: 10.1016/j.mrfmmm.2005.03.030. [DOI] [PubMed] [Google Scholar]
- Steele VE, Hawk ET, Viner JL, Lubet RA. Mechanisms and applications of non-steroidal anti-inflammatory drugs in the chemoprevention of cancer. Mutat Res. 2003;523–524:137–144. doi: 10.1016/s0027-5107(02)00329-9. [DOI] [PubMed] [Google Scholar]
- Eliasson B. Cigarette smoking and diabetes. Prog Cardiovasc Dis. 2003;45:405–413. doi: 10.1053/pcad.2003.00103. [DOI] [PubMed] [Google Scholar]
- Chodorowska G, Kwiatek J. Psoriasis and cigarette smoking. Ann Univ Mariae Curie Sklodowska [Med] 2004;59:535–538. [PubMed] [Google Scholar]
- Holm IA, Manson JE, Michels KB, Alexander EK, Willett WC, Utiger RD. Smoking and other lifestyle factors and the risk of Graves’ hyperthyroidism. Arch Intern Med. 2005;165:1606–1611. doi: 10.1001/archinte.165.14.1606. [DOI] [PubMed] [Google Scholar]
- Okino ST, Whitlock JP., Jr The aromatic hydrocarbon receptor, transcription, and endocrine aspects of dioxin action. Vitam Horm. 2000;59:241–264. doi: 10.1016/s0083-6729(00)59009-8. [DOI] [PubMed] [Google Scholar]
- Shimada T, Inoue K, Suzuki Y, Kawai T, Azuma E, Nakajima T, Shindo M, Kurose K, Sugie A, Yamagishi Y, Fujii-Kuriyama Y, Hashimoto M. Arylhydrocarbon receptor-dependent induction of liver and lung cytochromes P450 1A1, 1A2, and 1B1 by polycyclic aromatic hydrocarbons and polychlorinated biphenyls in genetically engineered C57BL/6J mice. Carcinogenesis. 2002;23:1199–1207. doi: 10.1093/carcin/23.7.1199. [DOI] [PubMed] [Google Scholar]
- Whitlock JP., Jr Mechanistic aspects of dioxin action. Chem Res Toxicol. 1993;6:754–763. doi: 10.1021/tx00036a003. [DOI] [PubMed] [Google Scholar]
- Swanson HI. DNA binding and protein interactions of the AHR/ARNT heterodimer that facilitate gene activation. Chem Biol Interact. 2002;141:63–76. doi: 10.1016/s0009-2797(02)00066-2. [DOI] [PubMed] [Google Scholar]
- Dertinger SD, Nazarenko DA, Silverstone AE, Gasiewicz TA. Aryl hydrocarbon receptor signaling plays a significant role in mediating benzo[a]pyrene- and cigarette smoke condensate-induced cytogenetic damage in vivo. Carcinogenesis. 2001;22:171–177. doi: 10.1093/carcin/22.1.171. [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem. 2004;279:23847–23850. doi: 10.1074/jbc.R400004200. [DOI] [PubMed] [Google Scholar]
- Lodovici M, Akpan V, Evangelisti C, Dolara P. Sidestream tobacco smoke as the main predictor of exposure to polycyclic aromatic hydrocarbons. J Appl Toxicol. 2004;24:277–281. doi: 10.1002/jat.992. [DOI] [PubMed] [Google Scholar]
- Löfroth G, Rannug A. Ah receptor ligands in tobacco smoke. Toxicol Lett. 1988;42:131–136. doi: 10.1016/0378-4274(88)90070-7. [DOI] [PubMed] [Google Scholar]
- Gebremichael A, Tullis K, Denison MS, Cheek JM, Pinkerton KE. Ah-receptor-dependent modulation of gene expression by aged and diluted sidestream cigarette smoke. Toxicol Appl Pharmacol. 1996;141:76–83. doi: 10.1006/taap.1996.0262. [DOI] [PubMed] [Google Scholar]
- Meek MD, Finch GL. Diluted mainstream cigarette smoke condensates activate estrogen receptor and aryl hydrocarbon receptor-mediated gene transcription. Environ Res. 1999;80:9–17. doi: 10.1006/enrs.1998.3872. [DOI] [PubMed] [Google Scholar]
- Martey CA, Baglole CJ, Gasiewicz TA, Sime PJ, Phipps RP. The aryl hydrocarbon receptor is a regulator of cigarette smoke induction of the cyclooxygenase and prostaglandin pathways in human lung fibroblasts. Am J Physiol. 2005;289:L391–L399. doi: 10.1152/ajplung.00062.2005. [DOI] [PubMed] [Google Scholar]
- Song J, Clagett-Dame M, Peterson RE, Hahn ME, Westler WM, Sicinski RR, DeLuca HF. A ligand for the aryl hydrocarbon receptor isolated from lung. Proc Natl Acad Sci USA. 2002;99:14694–14699. doi: 10.1073/pnas.232562899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka G, Kanaji S, Hirano A, Arima K, Shinagawa A, Goda C, Yasunaga S, Ikizawa K, Yanagihara Y, Kubo M, Kuriyama-Fujii Y, Sugita Y, Inokuchi A, Izuhara K. Induction and activation of the aryl hydrocarbon receptor by IL-4 in B cells. Int Immunol. 2005;17:797–805. doi: 10.1093/intimm/dxh260. [DOI] [PubMed] [Google Scholar]
- Thurmond TS, Staples JE, Silverstone AE, Gasiewicz TA. The aryl hydrocarbon receptor has a role in the in vivo maturation of murine bone marrow B lymphocytes and their response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2000;165:227–236. doi: 10.1006/taap.2000.8942. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Sosa M, Elizondo G, Lopez-Duran RM, Rivera I, Gonzalez FJ, Vega L. Over-production of IFN-gamma and IL-12 in AhR-null mice. FEBS Lett. 2005;579:6403–6410. doi: 10.1016/j.febslet.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Martey CA, Pollock SJ, Turner CK, O’Reilly KM, Baglole CJ, Phipps RP, Sime PJ. Cigarette smoke induces cyclooxygenase-2 and microsomal prostaglandin E2 synthase in human lung fibroblasts: implications for lung inflammation and cancer. Am J Physiol. 2004;287:L981–L991. doi: 10.1152/ajplung.00239.2003. [DOI] [PubMed] [Google Scholar]
- Nishikawa M, Kakemizu N, Ito T, Kudo M, Kaneko T, Suzuki M, Udaka N, Ikeda H, Okubo T. Superoxide mediates cigarette smoke-induced infiltration of neutrophils into the airways through nuclear factor-kappaB activation and IL-8 mRNA expression in guinea pigs in vivo. Am J Respir Cell Mol Biol. 1999;20:189–198. doi: 10.1165/ajrcmb.20.2.3305. [DOI] [PubMed] [Google Scholar]
- Mochida-Nishimura K, Surewicz K, Cross JV, Hejal R, Templeton D, Rich EA, Toossi Z. Differential activation of MAP kinase signaling pathways and nuclear factor-kappaB in bronchoalveolar cells of smokers and nonsmokers. Mol Med. 2001;7:177–185. [PMC free article] [PubMed] [Google Scholar]
- Di Stefano A, Caramori G, Oates T, Capelli A, Lusuardi M, Gnemmi I, Ioli F, Chung KF, Donner CF, Barnes PJ, Adcock IM. Increased expression of nuclear factor-kappaB in bronchial biopsies from smokers and patients with COPD. Eur Respir J. 2002;20:556–563. doi: 10.1183/09031936.02.00272002. [DOI] [PubMed] [Google Scholar]
- Kim DW, Gazourian L, Quadri SA, Romieu-Mourez R, Sherr DH, Sonenshein GE. The RelA NF-kappaB subunit and the aryl hydrocarbon receptor (AhR) cooperate to transactivate the c-myc promoter in mammary cells. Oncogene. 2000;19:5498–5506. doi: 10.1038/sj.onc.1203945. [DOI] [PubMed] [Google Scholar]
- Ke S, Rabson AB, Germino JF, Gallo MA, Tian Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-alpha and lipopolysaccharide. J Biol Chem. 2001;276:39638–39644. doi: 10.1074/jbc.M106286200. [DOI] [PubMed] [Google Scholar]
- Lahvis GP, Bradfield CA. AhR null alleles: distinctive or different? Biochem Pharmacol. 1998;56:781–787. doi: 10.1016/s0006-2952(98)00134-8. [DOI] [PubMed] [Google Scholar]
- Johnston CJ, Finkelstein JN, Gelein R, Oberdorster G. Pulmonary cytokine and chemokine mRNA levels after inhalation of lipopolysaccharide in C57BL/6 mice. Toxicol Sci. 1998;46:300–307. doi: 10.1006/toxs.1998.2557. [DOI] [PubMed] [Google Scholar]
- Koumas L, King AE, Critchley HO, Kelly RW, Phipps RP. Fibroblast heterogeneity: existence of functionally distinct Thy 1(+) and Thy 1(−) human female reproductive tract fibroblasts. Am J Pathol. 2001;159:925–935. doi: 10.1016/S0002-9440(10)61768-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl PD, Fishman WH. Measurement of beta-glucuronidase activity. Bergmeyer HU, editor. Weinheim: Deerfield Beach, FL,; Methods of Enzymatic Analysis, Enzymes 2. 1984:pp 246–256. [Google Scholar]
- Thatcher TH, McHugh NA, Egan RW, Chapman RW, Hey JA, Turner CK, Redonnet MR, Seweryniak KE, Sime PJ, Phipps RP. Role of CXCR2 in cigarette smoke-induced lung inflammation. Am J Physiol. 2005;289:L322–L328. doi: 10.1152/ajplung.00039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with ‘mini-extracts’, prepared from a small number of cells. Nucleic Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez SH, Sanchez JF, Dimitri CA, Gelbard HA, Dewhurst S, Maggirwar SB. Neurotrophins prevent HIV Tat-induced neuronal apoptosis via a nuclear factor-kappaB (NF-kappaB)-dependent mechanism. J Neurochem. 2001;78:874–889. doi: 10.1046/j.1471-4159.2001.00467.x. [DOI] [PubMed] [Google Scholar]
- Pérez-Arellano JL, Barrios MN, Martin T, Sanchez ML, Jimenez A, Gonzalez-Buitrago JM. Hydrolytic enzyme of the alveolar macrophage in diffuse pulmonary interstitial disease. Respir Med. 1996;90:159–166. doi: 10.1016/s0954-6111(96)90158-4. [DOI] [PubMed] [Google Scholar]
- Kim DS, Han JH, Kwon HJ. NF-kappaB and c-Jun-dependent regulation of macrophage inflammatory protein-2 gene expression in response to lipopolysaccharide in RAW 264.7 cells. Mol Immunol. 2003;40:633–643. doi: 10.1016/j.molimm.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Collart MA, Baeuerle P, Vassalli P. Regulation of tumor necrosis factor alpha transcription in macrophages: involvement of four kappa B-like motifs and of constitutive and inducible forms of NF-kappa B. Mol Cell Biol. 1990;10:1498–1506. doi: 10.1128/mcb.10.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal PB. Regulation of IL6 gene expression. Res Immunol. 1992;143:724–734. doi: 10.1016/0923-2494(92)80011-9. [DOI] [PubMed] [Google Scholar]
- Torgerson TR, Colosia AD, Donahue JP, Lin YZ, Hawiger J. Regulation of NF-kappa B, AP-1, NFAT, and STAT1 nuclear import in T lymphocytes by noninvasive delivery of peptide carrying the nuclear localization sequence of NF-kappa B p50. J Immunol. 1998;161:6084–6092. [PubMed] [Google Scholar]
- Lyss G, Knorre A, Schmidt TJ, Pahl HL, Merfort I. The anti-inflammatory sesquiterpene lactone helenalin inhibits the transcription factor NF-kappaB by directly targeting p65. J Biol Chem. 1998;273:33508–33516. doi: 10.1074/jbc.273.50.33508. [DOI] [PubMed] [Google Scholar]
- Masubuchi T, Koyama S, Sato E, Takamizawa A, Kubo K, Sekiguchi M, Nagai S, Izumi T. Smoke extract stimulates lung epithelial cells to release neutrophil and monocyte chemotactic activity. Am J Pathol. 1998;153:1903–1912. doi: 10.1016/S0002-9440(10)65704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beisswenger C, Platz J, Seifart C, Vogelmeier C, Bals R. Exposure of differentiated airway epithelial cells to volatile smoke in vitro. Respiration. 2004;71:402–409. doi: 10.1159/000079647. [DOI] [PubMed] [Google Scholar]
- Moodie FM, Marwick JA, Anderson CS, Szulakowski P, Biswas SK, Bauter MR, Kilty I, Rahman I. Oxidative stress and cigarette smoke alter chromatin remodeling but differentially regulate NF-kappaB activation and proinflammatory cytokine release in alveolar epithelial cells. FASEB J. 2004;18:1897–1899. doi: 10.1096/fj.04-1506fje. [DOI] [PubMed] [Google Scholar]
- Chen ZT, Li SL, Cai EQ, Wu WL, Jin JS, Zhu B. LPS induces pulmonary intravascular macrophages producing inflammatory mediators via activating NF-kappaB. J Cell Biochem. 2003;89:1206–1214. doi: 10.1002/jcb.10590. [DOI] [PubMed] [Google Scholar]
- Tian Y, Ke S, Denison MS, Rabson AB, Gallo MA. Ah receptor and NF-kappaB interactions, a potential mechanism for dioxin toxicity. J Biol Chem. 1999;274:510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Martucci C, Franchi S, Lattuada D, Panerai AE, Sacerdote P. Differential involvement of RelB in morphine-induced modulation of chemotaxis NO, and cytokine production in murine macrophages and lymphocytes. J Leukoc Biol. 2007;81:344–354. doi: 10.1189/jlb.0406237. [DOI] [PubMed] [Google Scholar]
- Xia Y, Pauza ME, Feng L, Lo D. RelB regulation of chemokine expression modulates local inflammation. Am J Pathol. 1997;151:375–387. [PMC free article] [PubMed] [Google Scholar]
- Weih F, Carrasco D, Durham SK, Barton DS, Rizzo CA, Ryseck RP, Lira SA, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-kappa B/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- Yoza BK, Hu JY, Cousart SL, Forrest LM, McCall CE. Induction of RelB participates in endotoxin tolerance. J Immunol. 2006;177:4080–4085. doi: 10.4049/jimmunol.177.6.4080. [DOI] [PubMed] [Google Scholar]
- Marienfeld R, May MJ, Berberich I, Serfling E, Ghosh S, Neumann M. RelB forms transcriptionally inactive complexes with RelA/p65. J Biol Chem. 2003;278:19852–19860. doi: 10.1074/jbc.M301945200. [DOI] [PubMed] [Google Scholar]
- Gonzalez FJ, Fernandez-Salguero P, Lee SS, Pineau T, Ward JM. Xenobiotic receptor knockout mice. Toxicol Lett. 1995;82–83:117–121. doi: 10.1016/0378-4274(95)03548-6. [DOI] [PubMed] [Google Scholar]
- Galván N, Teske DE, Zhou G, Moorthy B, MacWilliams PS, Czuprynski CJ, Jefcoate CR. Induction of CYP1A1 and CYP1B1 in liver and lung by benzo(a)pyrene and 7,12-dimethylbenz(a)anthracene do not affect distribution of polycyclic hydrocarbons to target tissue: role of AhR and CYP1B1 in bone marrow cytotoxicity. Toxicol Appl Pharmacol. 2005;202:244–257. doi: 10.1016/j.taap.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Galván N, Jaskula-Sztul R, MacWilliams PS, Czuprynski CJ, Jefcoate CR. Bone marrow cytotoxicity of benzo[a]pyrene is dependent on CYP1B1 but is diminished by Ah receptor-mediated induction of CYP1A1 in liver. Toxicol Appl Pharmacol. 2003;193:84–96. doi: 10.1016/s0041-008x(03)00338-7. [DOI] [PubMed] [Google Scholar]
- Henry EC, Bemis JC, Henry O, Kende AS, Gasiewicz TA. A potential endogenous ligand for the aryl hydrocarbon receptor has potent agonist activity in vitro and in vivo. Arch Biochem Biophys. 2006;450:67–77. doi: 10.1016/j.abb.2006.02.008. [DOI] [PubMed] [Google Scholar]