Abstract
Lactadherin is a secreted extracellular matrix protein expressed in phagocytes and contributes to the removal of apoptotic cells. We examined lactadherin expression in brain sections of patients with or without Alzheimer’s disease and studied its role in the phagocytosis of amyloid β-peptide (Aβ). Cells involved in Alzheimer’s disease, including vascular smooth muscle cells, astrocytes, and microglia, showed a time-related increase in lactadherin production in culture. Quantitative analysis of the level of lactadherin showed a 35% reduction in lactadherin mRNA expression in the brains of patients with Alzheimer’s disease (n = 52) compared with age-matched controls (n = 58; P = 0.003). Interestingly, lactadherin protein was detected in the brains of patients with Alzheimer’s disease and controls, with low expression in areas rich in senile plaques and marked expression in areas without Aβ deposition. Using surface plasmon resonance, we observed a direct protein-protein interaction between recombinant lactadherin and Aβ 1-42 peptide in vitro. Lactadherin deficiency or its neutralization using specific antibodies significantly prevented Aβ 1-42 phagocytosis by murine and human macrophages. In conclusion, lactadherin plays an important role in the phagocytosis of Aβ 1-42 peptide, and its expression is reduced in Alzheimer’s disease. Alterations in lactadherin production/function may contribute to the initiation and/or progression of Alzheimer’s disease.
Alzheimer’s disease (AD) is the leading cause of dementia in the elderly, possibly affecting 20 to 40% of the population older than age 85.1,2,3 The hallmarks of AD are neuronal loss, extracellular accumulation of senile plaques, the major component of which is the amyloid β-peptide (Aβ), and the presence of neurofibrillary tangles.3,4 Excess deposition of Aβ is involved in neur-onal cell death and is thought to result from an imbal-ance between Aβ production, aggregation, and/or clearance.5,6
The Aβ peptides result from the processing of the β-amyloid precursor protein (APP) by the β- and γ-secretases.7,8,9,10 Pathogenic mutations in the APP and presenilin 1 and 2 genes (involved in the γ-secretase activity) have clearly demonstrated the major role of Aβ overproduction in the AD process.3,8,11,12 Recently reinforcing this role, genetic variability in the ubiquilin gene, which regulates endoproteolysis of presenilin and modulates γ-secretase components, has been associated with familial forms of AD.13,14
The most consistent genetic variability associated with the nonfamilial, sporadic form of the disease is that at the apolipoprotein (apo)E locus, which encodes a protein involved in lipid transport.3,15 The molecular mechanisms by which the apoE ε4 allele alters the lifetime risk of AD are currently unknown. ApoE ε4 may modulate Aβ oligomerization into fibrils.16 In addition, recent studies have suggested that apoE may be involved in Aβ clearance, in part through its receptor, the low-density lipoprotein receptor-related protein.17 Understanding how the Aβ peptide is removed from the brain could lead to the identification of critical molecular pathways that could be targeted to reduce Aβ burden.18 However, apart from lipoprotein receptor-related protein- or P-glycoprotein-mediated Aβ efflux,19,20,21 little is known about the mechanisms responsible for Aβ clearance from the brain.18
Lactadherin or Mfge8 (milk fat globule-epidermal growth factor-like-factor VIII) is a glycoprotein originally identified as a component of the membrane of milk fat globules, with an RGD motif that binds to integrins αvβ3 and αvβ5, and a domain involved in binding to phosphatidylserine.22,23,24 As a component of the exosomes of dendritic cells or macrophages,25,26 lactadherin forms a bridge between phosphatidylserine on apoptotic cells and integrins on phagocytes, leading to engulfment and phagocytosis of apoptotic cells.26 This function is critical to tissue homeostasis as total deficiency in lactadherin leads to autoimmune diseases.27,28 Interestingly, apoE has been involved in phagocytosis of both apoptotic cells and Aβ peptide.17,29 We therefore hypothesized that in a similar way, lactadherin may also contribute to clearance of the Aβ peptide.
Materials and Methods
Expression of Lactadherin in Cultured Cells and Supernatants
Primary normal human adult astrocytes were obtained from Clonetics (Cambrex BioScience, Saint-Beauzire, France) and were cultured in Clonetics astrocyte medium (CC-3186). Glial fibrillary acid protein (GFAP) positivity (>80%) was unchanged during the period of the culture experiments (data not shown). Primary cultures of mouse neonatal astrocytes were generated (∼99% positivity for GFAP) after purification of astrocytes as described.30 Primary cultures of mixed glia were prepared from newborn mice as described.31 Pure microglia were magnetically isolated from primary cultures of mixed glia using CD11b microbeads from Miltenyi Biotech (Bergisch Gladbach, Germany), according to the manufacturer’s instructions.
Exosomes were prepared from cell supernatants.32 Protein extracts from cells and exosomes were examined for the presence of lactadherin and other exosomal-specific proteins by Western blotting using specific antibodies.32 We previously described the generation and specificity of the anti-mouse and anti-human lactadherin antibodies.33 Other antibodies were commercially available: goat anti-mouse or anti-human Lamp-2 (Santa Cruz Biotechnology, Santa Cruz, CA), goat or mouse anti-mouse or anti-human tsg101 (Santa Cruz Biotechnology) and rabbit anti-mouse or anti-human MHC class I (Santa Cruz Biotechnology).
Brain Samples
Fifty-two AD brains were obtained at autopsy from patients with sporadic AD accessioned from the Greater Manchester region of United Kingdom during the years 1986 to 2001 (mean age at onset, 65.9 ± 10.3 years; mean age at death, 73.6 ± 9.7 years; 55% of males). All patients were at Braak stages 5 or 6 at time of death. All pathological diagnoses were made in accordance with the Consortum to Establish a Registry to Alzheimer’s Disease neuropathological criteria for AD.34 Fifty-eight control brains presenting Braak stages less than 2 were obtained from routine autopsies performed at the Hospices Civils de Strasbourg (Strasbourg, France) (mean age at death, 79.3 ± 6.5 years, 38% of males).35 Total RNA from frontal areas was extracted from frozen brain tissue using phenol/chloroform protocol (TRIzol reagent; Invitrogen, Cergy, France). The quality of total RNA was assessed using the Agilent 2100 Bioanalyzer (Palo Alto, CA) and the ratio of ribosomal RNA 28S/18S systematically estimated using the Agilent 2100 Bioanalyzer bio-sizing software.36
Real-Time Polymerase Chain Reaction (PCR) on Brain Samples
Real-time quantitative PCR was performed as described.37 The primer sequences were as follows: lactadherin forward, 5′-GACAAGCAGGGCAACTTCAAC-3′; lactadherin reverse, 5′-CAGGATGGGCGTCTCAAAC-AA-3′; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) forward, 5′-GAAGGTGAAGGTCGGAGTC-3′; and GAPDH reverse, 5′-GAAGATGGTGATGGGATTTC-3′.
Immunohistochemical Studies on Brain Samples
Immunohistochemical studies were performed on brain temporal sections using specific anti-lactadherin antibody,33 mouse anti-CD68 antibody (DAKO), mouse anti-GFAP antibody, and/or anti-human Aβ antibody (clone 4G8; Sigma, St. Louis, MO) after pretreatment of the section with 70% formic acid. Sudan Black was used to prevent nonspecific background fluorescence.
Surface Plasmon Resonance
Surface plasmon resonance, a technique for quantitating protein-protein interaction, was measured by a BIAcore X instrument (BIAcore International AB, Uppsala, Sweden) using CM5 sensor chips. Lactadherin was diluted in Hanks’ balanced salt solution buffer. Samples were injected at 25°C at a flow rate of 10 μl/minute over the active CM5 surface on which the Aβ 1-42 peptide had been immobilized to 7000 resonance units. An RGE lactadherin mutant was also used. It contains an Asp69 to glutamic acid point mutation in the RGD motif of lactadherin.33
In Vitro Phagocytosis Studies
Lactadherin-deficient mice and control littermates were obtained as we previously described.33 Macrophages were generated from bone marrow progenitors (from C57BL6 mice, lactadherin-deficient mice, and littermates controls), as described.38 The cells were seeded in uncoated 24-well culture plates with RPMI medium containing 20% fetal calf serum and 20% L929 cell-conditioned medium. Human peripheral blood mononuclear cells were isolated using Ficoll gradient from whole blood (50 ml) of healthy donors (two men, one woman; mean age, 32 ± 5 years) who gave informed consent. The cells were allowed to adhere for 2 hours on uncoated 24-well culture plates, washed, and used for the following experiments.
Cell preparations were incubated with microaggre-gates of 1 μg/ml fluorescein isothiocyanate (FITC)-con-jugated Aβ 1-42 peptide (rPeptide, Athens, GA) as previously described,39 with or without neutralizing anti-mouse or anti-human lactadherin antibody (45 μg/ml), or with an isotype-matched control antibody. The cells were washed, stained with 4,6-diamidino-2-phenylindole, and fixed with 1% paraformaldehyde. Phagocytosed FITC-conjugated Aβ appeared as punctuate fluorescent vesicles under a fluorescence microscope. The percentage of FITC-positive cells, as well as FITC intensity, was quantified using Histolab software (Microvisions, Louisville, KY).
In Vivo Phagocytosis Studies
Lactadherin-deficient mice and control littermates received an intraperitoneal injection of thioglycollate (7%, 2 ml) (Sigma). Three days later, mice were injected intraperitoneally with either microaggregates of FITC-conjugated Aβ 1-42 peptide (500 μl at 5 μg/ml) or saline. Phagocytosis was allowed to proceed for 1 hour in vivo. Cells were collected from the peritoneal cavities, suspended in culture medium, pelleted, extensively washed, counted, and fixed with 1% paraformaldehyde. FITC-labeled macrophages were quantified by flow cytometry (Epics XL; Coulter, Hialeah, FL).
Statistical Analysis
Statistical analyses were performed using SAS statistical software, v.8.2 (SAS Institute Inc., Cary, NC). Values of lactadherin mRNA were log-transformed to normalize their distributions. The level of total RNA degradation (estimated by rRNA 28S/18S ratio) was identified as a confounder for lactadherin/GAPDH ratio using linear regression model (nonparametric Spearman’s test, P = 0.047) and subsequently included (as well as age and gender) in a multivariate analysis of covariance using a general linear model for comparison of mRNA amount between AD and control cases.36 Results are expressed as means ± SD. The Mann-Whitney rank-sum test was used for quantitative data. All reported P values are two-sided.
Results
Age- and Time-Related Increase in the Production of Lactadherin
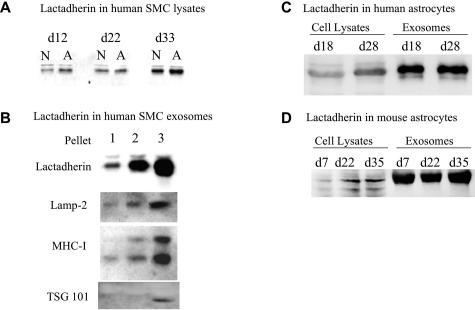
Lactadherin is expressed in vessels of aged persons.40 However, the relation between lactadherin expression and age had not been explored. We examined lactadherin expression in cultured smooth muscle cells obtained from either newborns (<2 years old) or adults (>18 years old). We observed higher lactadherin expression in adult smooth muscle cells compared with those from newborns (Figure 1). Interestingly, lactadherin expression increased in both newborn and adult smooth muscle cells as a function of time (Figure 1) and was secreted in the culture supernatants in exosomes (Figure 1). We obtained similar results in murine smooth muscle cells (data not shown), suggesting a species-independent process. Because AD is a prototype of age-associated disease, we examined lactadherin expression in brain cells. Lactadherin was undetectable in neonatal murine neuronal cells but was readily expressed in neonatal murine astrocytes, as well as in human adult astrocytes (Figure 1, C and D). Lactadherin expression in astrocytes also increased as a function of time in culture and was greatly enriched in astrocyte-derived exosomes (Figure 1, C and D).
Figure 1.
Lactadherin expression in cultured cells. Shown are the results of Western blot analyses. In each panel, equal amounts of proteins were loaded. A: Western blot analysis was performed on protein extracts of confluent human aortic smooth muscle cells (SMCs) obtained at different time points (d indicates day) after the beginning of culture. SMCs were from either newborn (N; <2 years old) or adult (A; >18 years old) humans. Lactadherin expression is higher in adult SMCs and increases as a function of time in culture in both newborn and adult SMCs. B: Lactadherin is enriched in supernatant membrane fractions presenting the characteristics of exosomes, ie, membrane fractions rich in Lamp-2, MHC-I, and tsg101 (pellet 3). Pellets 1, 2, and 3 were obtained after serial centrifugation of cell supernatants at 2,500 × g for 20 minutes (pellet 1; cell debris), then 10,000 × g for 45 minutes (pellet 2, cell microparticles), then 100,000 × g for 90 minutes (pellet 3, exosomes). C: Lactadherin expression in confluent adult human astrocytes. D: Lactadherin expression in confluent murine neonatal astrocytes. Cell-associated lactadherin expression increases with time in culture.
Lactadherin Expression and AD
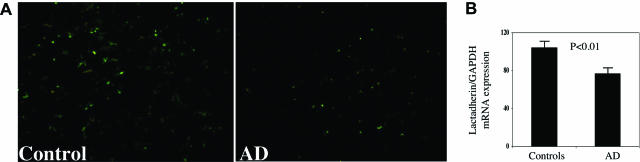
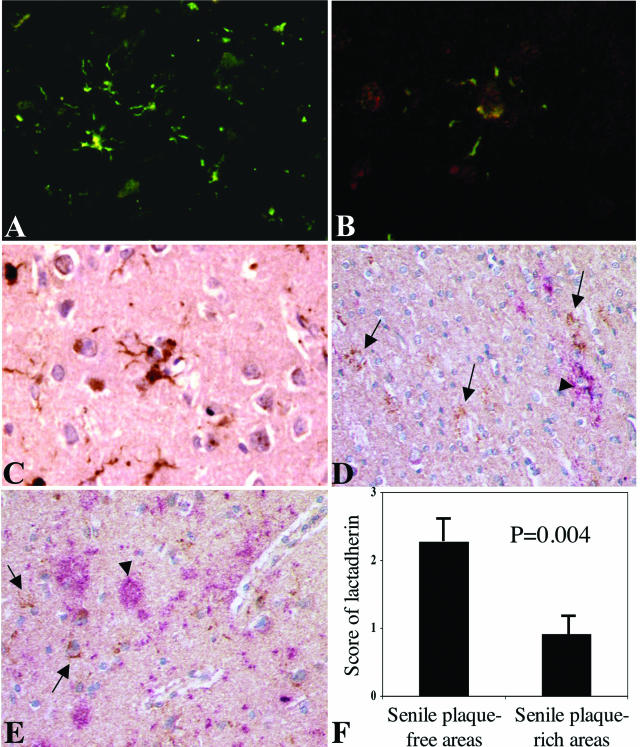
To examine the clinical relevance of these findings, we looked at lactadherin expression in brain specimens of AD patients and controls. Interestingly, we observed a 35% decrease of lactadherin mRNA expression in the brain of AD cases compared with controls (Figure 2). Immunohistochemical analysis of the protein also showed reduced expression in AD sections (Figure 2). Lactadherin expression was mostly detected in astrocytes (Figure 3, A–C). Expression was also detected occasionally in CD68-positive cells (Supplemental Figure 1, see http://ajp.amjpathol.org) and in smooth muscle cells of large arteries (Supplemental Figure 2, see http://ajp.amjpathol.org). Of note, lactadherin expression in AD sections was frequently observed in areas showing little or no Aβ accumulation, whereas it was frequently absent at close vicinity from senile plaques (Figure 3, D–F).
Figure 2.
Lactadherin expression in normal and AD brains. A: Representative immunohistochemical staining of lactadherin protein expression (green) in brains of patients with AD or controls. B: Brain mRNA levels of lactadherin/GAPDH ratio (expressed as percentage of controls), determined using quantitative RT-PCR. Results are expressed as mean ± SD (n = 52 AD brains and 58 control brains).
Figure 3.
Lactadherin expression in brain temporal sections of patients with or without AD. A–E: Representative immunohistochemical staining of lactadherin protein expression in brain specimens of patients with AD (B–E) or controls (A). A: Staining for lactadherin (green) showing expression in cells with astrocyte morphology. B: Lactadherin appears in green and GFAP staining in red. C–E: Lactadherin appears in brown (arrows) and Aβ staining in pink/red (arrowheads). Note that lactadherin-expressing cells are negative for Aβ staining. F: Relation between lactadherin expression and the occurrence of senile plaques. Brain temporal sections of patients with AD were double-stained with lactadherin and Aβ (n = 6 patients). Three Aβ-positive and three Aβ-negative areas were identified in each patient, in which the mean intensity of lactadherin staining was estimated using a semiquantitative immunohistochemical score (0, no staining; 1, weak staining; 2, moderate; 3, strong). The histograms show the mean ± SD scores from six patients. Clearly, lactadherin expression occurred preferentially in Aβ-free areas.
Lactadherin Contributes to Phagocytosis of the Amyloid β-Peptide
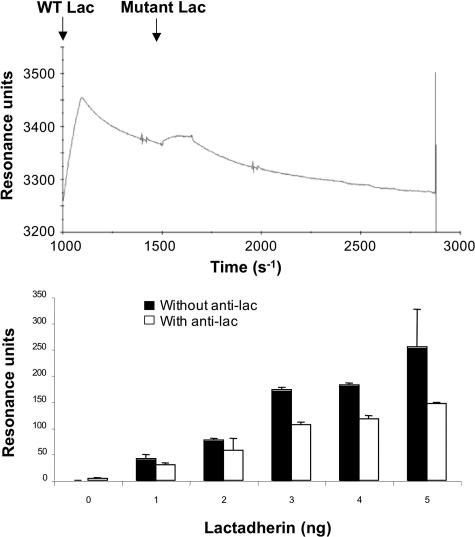
The inverse association between lactadherin expression and Aβ deposits in AD brain suggested a potential role for lactadherin in removal of the Aβ peptide. In addition, using surface plasmon resonance, we observed a dose-dependent increase in direct protein-protein interaction between Aβ 1-42 peptide and recombinant human lactadherin (Figure 4), which was not observed with an RGE mutant of lactadherin. Thus, we examined the role of lactadherin in the phagocytosis of microaggregates of Aβ 1-42.
Figure 4.
Direct protein-protein interaction between recombinant human lactadherin and Aβ 1-42 peptide using surface plasmon resonance. Top: Recombinant human lactadherin or an RGE lactadherin mutant was injected at 25°C at a flow rate of 10 μl/minute over the active CM5 surface on which the Aβ 1-42 peptide had been immobilized. The RGE mutant of lactadherin does not bind Aβ 1-42 peptide. Bottom: Increased lactadherin-Aβ interaction with increasing amounts of lactadherin. The figure also shows partial but significant inhibition of lactadherin-Aβ interaction using anti-human lactadherin antibody (anti-lac, 45 μg/ml) directed against the RGD sequence of human lactadherin.
First, we observed a time-dependent increase in the phagocytosis of FITC-conjugated Aβ 1-42 peptide by bone marrow-derived (Supplemental Figure 3, see http://ajp.amjpathol.org) or peritoneal-derived (not shown) murine macrophages. FITC-conjugated Aβ peptide was seen in vesicular structures within the macrophages (Supplemental Figure 3, see http://ajp.amjpathol.org); its uptake was saturable and was almost totally inhibited by excess of the scavenger receptor ligands, fucoidan and acetylated low-density lipoprotein (not shown), as previously described.39 Interestingly, uptake of FITC-Aβ showed a marked decrease (∼40% reduction, P = 0.03) in cell preparations treated with a neutralizing anti-lactadherin antibody in comparison with preparations treated with a control antibody (Figures 5 and 6). The anti-lactadherin antibody also inhibited the uptake of Aβ microaggregates by cultured murine microglial cells (Figure 5). The results were reproduced in mice deficient for lactadherin, whose macrophage preparations showed a marked reduction in the uptake of Aβ in comparison with control macrophages (Figure 6). Importantly, treatment of blood-derived human macrophages by a neutralizing anti-human lactadherin antibody significantly inhibited the uptake of FITC-Aβ microaggregates in comparison with a control antibody (55% reduction in fluorescence intensity, P = 0.007) (Figure 7). Finally, lactadherin-deficient mice showed significant reduction in the phagocytosis of Aβ 1-42 peptide by peritoneal macrophages in vivo compared with control littermate mice (Figure 8).
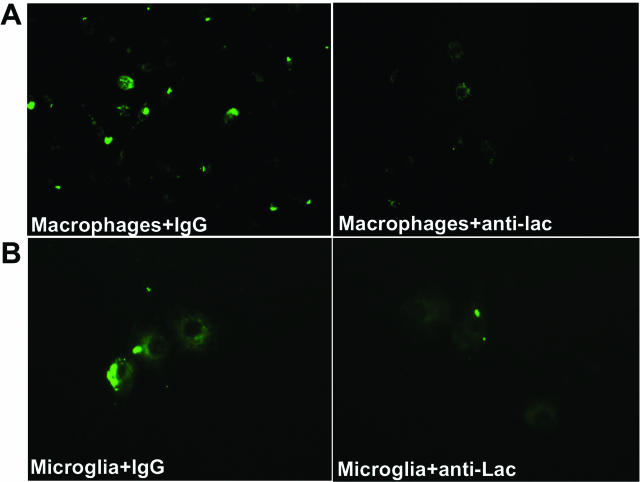
Figure 5.
Lactadherin and phagocytosis of Aβ microaggregates. A: Representative example of uptake of FITC-conjugated Aβ 1-42 peptide by cultured bone marrow-derived murine macrophages obtained from lactadherin wild-type mice with (+anti-lac) or without (+IgG) in vitro pretreatment with a neutralizing anti-mouse lactadherin antibody (n = 3 to 5 per condition). B: Representative example of uptake of FITC-conjugated Aβ 1-42 peptide by primary murine microglial cells from C57BL/6 mice with (+anti-lac) or without (+IgG) in vitro pretreatment with a neutralizing anti-mouse lactadherin antibody (n = 3 per condition).
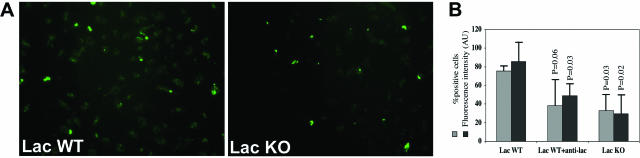
Figure 6.
Lactadherin and phagocytosis of Aβ microaggregates. A: Example of uptake of FITC-conjugated Aβ 1-42 peptide by cultured bone marrow-derived murine macrophages obtained from either lactadherin-deficient mice (lac KO) or wild-type (lac WT) littermate controls. B: Quantitative analysis (Histolab software; Microvision) of the percentage of FITC-positive cells (percent positive cells) and the mean fluorescence intensity (expressed in arbitrary units, AU) in the murine macrophage cultures from lactadherin-deficient mice or from wild-type mice with or without in vitro pretreatment with a neutralizing anti-mouse lactadherin antibody (anti-lac) (n = 3 to 5 per condition). Control IgG antibody was added in lac WT and lac KO conditions.
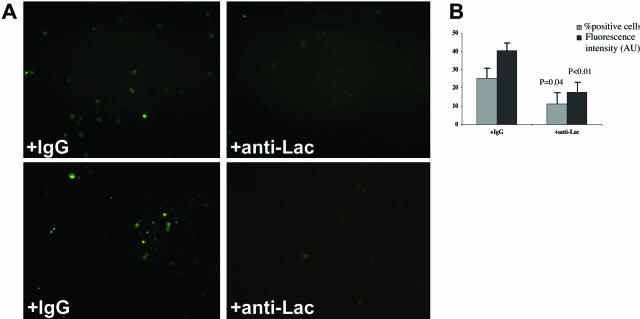
Figure 7.
Lactadherin and phagocytosis of Aβ microaggregates by blood-derived human macrophages in vitro. A: Two examples of uptake of FITC-conjugated Aβ 1-42 peptide by cultured blood-derived human macrophages in the presence (+anti-Lac) or absence (+IgG) of a neutralizing anti-human lactadherin antibody. B: Quantitative analysis (Histolab software; Microvision) of the percentage of FITC-positive cells (percent positive cells) and the mean fluorescence intensity (expressed in arbitrary units, AU) in the above-mentioned cultures (n = 3 per condition). Values are means ± SD. P values are versus IgG.
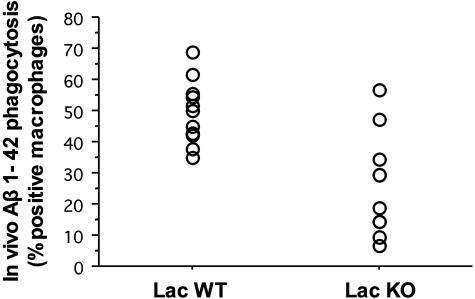
Figure 8.
Lactadherin and phagocytosis of Aβ microaggregates by murine peritoneal macrophages in vivo. Quantification using flow cytometry of the uptake of FITC-conjugated Aβ 1-42 peptide by peritoneal macrophages in vivo. Macrophages of lactadherin-deficient mice (lac KO, n = 10) showed reduced Aβ uptake compared with littermate controls (lac WT, n = 11; P = 0.0009).
Discussion
Aβ accumulation results from an imbalance between production and clearance.3 Although overproduction of Aβ is mostly involved in familial forms of AD, sporadic, late-onset AD is thought to result from impaired clearance, leading to Aβ accumulation and precipitating its conversion into toxic forms. Several mechanisms account for Aβ clearance, including proteolytic degradation,41,42 elimination by passive bulk flow,43 active transport across the blood-brain barrier,20,21,44 and phagocytosis by astrocytes and microglia.45,46
In this study, we have provided a series of data that implicate lactadherin in a major process related to the pathophysiology of AD, namely Aβ-peptide phagocytosis. The age- or time-related expression of lactadherin, its vascular and brain parenchymal expression (astrocytic and microglial), further highlight the potential role of this glycoprotein in the protection against excess Aβ accumulation with aging.
We found a significant reduction in lactadherin mRNA expression in AD brains compared with controls. The difference of lactadherin expression between AD and control brains was independent of RNA degradation and unlikely to be attributable to differences in genetic background between UK and French populations.47,48,49,50 However, at this stage of the analysis, it is not possible to take into account environmental factors such as dietary habits.
The present studies conducted in human brain tissue showing decreased mRNA expression of lactadherin and altered protein expression/distribution in patients with AD are highly suggestive of a role for this glycoprotein in the disease process. The finding that a neutralizing antibody against lactadherin or a complete deficiency of this glycoprotein in mice inhibits Aβ 1-42 phagocytosis by macrophages/microglia, provides an interesting mechanistic insight into the direct role of lactadherin in a critical process contributing to the neuropathology of AD.
A recent study showed that a small fraction of Aβ (<1%) produced by neurons may be secreted in exosomes.51 Thus, it could be argued that association of lactadherin and Aβ on exosomes would rather enhance Aβ secretion and facilitate its extracellular accumulation. However, we were unable to detect significant lactadherin expression in neurons. Thus, exosomal Aβ secreted by neurons would not associate with lactadherin before secretion. In contrast, secreted Aβ could encounter exosomal lactadherin (produced by astrocytes and microglia) in the extracellular milieu, leading to its phagocytosis and clearance. Our observations in vitro and in vivo clearly suggest that when lactadherin is expressed, Aβ phagocytosis is enhanced, impeding its extracellular accumulation. This could explain, at least in part, why Aβ fibrils accumulate in areas with no or low lactadherin expression. In addition to reduced mRNA expression of lactadherin, alterations in vesicular transport, as reported in AD,52 may impair exosome secretion in several cell types, potentially leading to a reduction in lactadherin-mediated Aβ phagocytosis.
It is noteworthy that Aβ peptides, particularly Aβ 1-42, show high affinity for phosphatidylserine,53 the aminophospholipid that interacts with lactadherin for apoptotic cell phagocytosis.26,27 Thus, Aβ peptides may concentrate in phosphatidylserine-rich membranes and become targets for removal by lactadherin (ultimately leading to their degradation). This could be particularly relevant if, as widely reported,3 Aβ peptides induce cell activation or apoptosis, exposing phosphatidylserine at the external membrane leaflet. Our findings that lactadherin contributes to Aβ phagocytosis suggest that Aβ would tend to accumulate and exert its neurotoxic effects in areas with altered lactadherin production. Reduced expression of lactadherin in patients with AD would also lead to accumulation of apoptotic debris. We speculate that increased cell death and defective phagocytosis of apoptotic cells could alter the anti-inflammatory/tolerogenic arm of the immune response and set the stage for exaggerated pathogenic responses,27,54 leading to disease aggravation. Finally, lactadherin deficiency might aggravate tissue response to ischemia,33 magnifying the vascular dysfunction of AD.55
However, it remains to be established that lactadherin deficiency directly leads or contributes to Alzheimer’s neuropathology in experimental models of the disease. Future studies should examine the effect of lactadherin deficiency on the accumulation of senile plaques in animal models of AD. Nevertheless, we believe that the data presented here point to a potentially important and new role for lactadherin in AD and should pave the way for future studies aiming at the comprehension of the precise mechanisms involved in these processes.
Supplementary Material
Acknowledgments
We thank Clotilde Thery and Sebastian Amigorena (Inserm U653) for sharing the polyclonal anti-lactadherin antibodies and the lactadherin-deficient mice and Alain Delcayre (Anosys) for providing the recombinant mouse lactadherin.
Footnotes
Address reprint requests to Ziad Mallat, M.D., Ph.D., INSERM U689, Hôpital Lariboisière, 41, Bd de la Chapelle, 75010 Paris, France. E-mail: mallat@larib.inserm.fr.
Supported by Lille Genopole (for genetic analyses), the Assistance Publique-Hôpitaux de Paris (contrat d’interface to Z.M. and A.T.), the Fondation pour la Recherche Médicale (to K.K.), the Association France Alzheimer (to N.J.), and the European Commission (Network of Excellence contract No. LSHM-CT-2003-503254 to INSERM U689, which is a partner of the European Vascular Genomics Network).
Supplementary material for this article can be found on http://ajp.amjpathol.org.
References
- Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, Finkel SI, Gwyther LP, Khachaturian ZS, Lebowitz BD, McRae TD, Morris JC, Oakley F, Schneider LS, Streim JE, Sunderland T, Teri LA, Tune LE. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA. 1997;278:1363–1371. [PubMed] [Google Scholar]
- Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348:1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease: genes, proteins, and therapy. Physiol Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Annaert W, De Strooper B. A cell biological perspective on Alzheimer’s disease. Annu Rev Cell Dev Biol. 2002;18:25–51. doi: 10.1146/annurev.cellbio.18.020402.142302. [DOI] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Suh YH, Checler F. Amyloid precursor protein, presenilins, and alpha-synuclein: molecular pathogenesis and pharmacological applications in Alzheimer’s disease. Pharmacol Rev. 2002;54:469–525. doi: 10.1124/pr.54.3.469. [DOI] [PubMed] [Google Scholar]
- Esler WP, Wolfe MS. A portrait of Alzheimer secretases—new features and familiar faces. Science. 2001;293:1449–1454. doi: 10.1126/science.1064638. [DOI] [PubMed] [Google Scholar]
- Steiner H, Haass C. Intramembrane proteolysis by presenilins. Nat Rev Mol Cell Biol. 2000;1:217–224. doi: 10.1038/35043065. [DOI] [PubMed] [Google Scholar]
- Wilquet V, De Strooper B. Amyloid-beta precursor protein processing in neurodegeneration. Curr Opin Neurobiol. 2004;14:582–588. doi: 10.1016/j.conb.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M. Genetic dissection of Alzheimer’s disease and related dementias: amyloid and its relationship to tau. Nat Neurosci. 1998;1:355–358. doi: 10.1038/1565. [DOI] [PubMed] [Google Scholar]
- Bertram L, Hiltunen M, Parkinson M, Ingelsson M, Lange C, Ramasamy K, Mullin K, Menon R, Sampson AJ, Hsiao MY, Elliott KJ, Velicelebi G, Moscarillo T, Hyman BT, Wagner SL, Becker KD, Blacker D, Tanzi RE. Family-based association between Alzheimer’s disease and variants in UBQLN1. N Engl J Med. 2005;352:884–894. doi: 10.1056/NEJMoa042765. [DOI] [PubMed] [Google Scholar]
- Bird TD. Genetic factors in Alzheimer’s disease. N Engl J Med. 2005;352:862–864. doi: 10.1056/NEJMp058027. [DOI] [PubMed] [Google Scholar]
- Myers AJ, Goate AM. The genetics of late-onset Alzheimer’s disease. Curr Opin Neurol. 2001;14:433–440. doi: 10.1097/00019052-200108000-00002. [DOI] [PubMed] [Google Scholar]
- Dolev I, Michaelson DM. A nontransgenic mouse model shows inducible amyloid-beta (Abeta) peptide deposition and elucidates the role of apolipoprotein E in the amyloid cascade. Proc Natl Acad Sci USA. 2004;101:13909–13914. doi: 10.1073/pnas.0404458101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koistinaho M, Lin S, Wu X, Esterman M, Koger D, Hanson J, Higgs R, Liu F, Malkani S, Bales KR, Paul SM. Apolipoprotein E promotes astrocyte colocalization and degradation of deposited amyloid-beta peptides. Nat Med. 2004;10:719–726. doi: 10.1038/nm1058. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Moir RD, Wagner SL. Clearance of Alzheimer’s Abeta peptide: the many roads to perdition. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- Deane R, Wu Z, Sagare A, Davis J, Du Yan S, Hamm K, Xu F, Parisi M, LaRue B, Hu HW, Spijkers P, Guo H, Song X, Lenting PJ, Van Nostrand WE, Zlokovic BV. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–344. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, Holtzman DM, Miller CA, Strickland DK, Ghiso J, Zlokovic BV. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–1499. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Deane R, Fagan AM, Spinner ML, Parsadanian M, Finn MB, Jiang H, Prior JL, Sagare A, Bales KR, Paul SM, Zlokovic BV, Piwnica-Worms D, Holtzman DM. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005;115:3285–3290. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs JD, Lekutis C, Singer KL, Bui A, Yuzuki D, Srinivasan U, Parry G. cDNA cloning of a mouse mammary epithelial cell surface protein reveals the existence of epidermal growth factor-like domains linked to factor VIII-like sequences. Proc Natl Acad Sci USA. 1990;87:8417–8421. doi: 10.1073/pnas.87.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MR, Couto JR, Scallan CD, Ceriani RL, Peterson JA. Lactadherin (formerly BA46), a membrane-associated glycoprotein expressed in human milk and breast carcinomas, promotes Arg-Gly-Asp (RGD)-dependent cell adhesion. DNA Cell Biol. 1997;16:861–869. doi: 10.1089/dna.1997.16.861. [DOI] [PubMed] [Google Scholar]
- Andersen MH, Graversen H, Fedosov SN, Petersen TE, Rasmussen JT. Functional analyses of two cellular binding domains of bovine lactadherin. Biochemistry. 2000;39:6200–6206. doi: 10.1021/bi992221r. [DOI] [PubMed] [Google Scholar]
- Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- Asano K, Miwa M, Miwa K, Hanayama R, Nagase H, Nagata S, Tanaka M. Masking of phosphatidylserine inhibits apoptotic cell engulfment and induces autoantibody production in mice. J Exp Med. 2004;200:459–467. doi: 10.1084/jem.20040342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grainger DJ, Reckless J, McKilligin E. Apolipoprotein E modulates clearance of apoptotic bodies in vitro and in vivo, resulting in a systemic proinflammatory state in apolipoprotein E-deficient mice. J Immunol. 2004;173:6366–6375. doi: 10.4049/jimmunol.173.10.6366. [DOI] [PubMed] [Google Scholar]
- Pousset F, Cremona S, Dantzer R, Kelley K, Parnet P. Interleukin-4 and interleukin-10 regulate IL1-beta induced mouse primary astrocyte activation: a comparative study. Glia. 1999;26:12–21. doi: 10.1002/(sici)1098-1136(199903)26:1<12::aid-glia2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Tsuzaki N, Shimojo M, Hamanoue M, Kohsaka S. Microglia isolated from rat brain secrete a urokinase-type plasminogen activator. Brain Res. 1992;577:285–292. doi: 10.1016/0006-8993(92)90285-h. [DOI] [PubMed] [Google Scholar]
- Théry C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol. 2001;166:7309–7318. doi: 10.4049/jimmunol.166.12.7309. [DOI] [PubMed] [Google Scholar]
- Silvestre JS, Thery C, Hamard G, Boddaert J, Aguilar B, Delcayre A, Houbron C, Tamarat R, Blanc-Brude O, Heeneman S, Clergue M, Duriez M, Merval R, Levy B, Tedgui A, Amigorena S, Mallat Z. Lactadherin promotes VEGF-dependent neovascularization. Nat Med. 2005;11:499–506. doi: 10.1038/nm1233. [DOI] [PubMed] [Google Scholar]
- Thaker U, McDonagh AM, Iwatsubo T, Lendon CL, Pickering-Brown SM, Mann DM. Tau load is associated with apolipoprotein E genotype and the amount of amyloid beta protein, Abeta40, in sporadic and familial Alzheimer’s disease. Neuropathol Appl Neurobiol. 2003;29:35–44. doi: 10.1046/j.1365-2990.2003.00425.x. [DOI] [PubMed] [Google Scholar]
- Berr C, Lambert JC, Sazdovitch V, Amouyel P, Chartier-Harlin MC, Mohr M, Heldt N, Kiesmann M, Hauw JJ. Neuropathological epidemiology of cerebral aging: a study of two genetic polymorphisms. Neurobiol Aging. 2001;22:227–235. doi: 10.1016/s0197-4580(00)00227-x. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Mann D, Richard F, Tian J, Shi J, Thaker U, Merrot S, Harris J, Frigard B, Iwatsubo T, Lendon C, Amouyel P. Is there a relation between APOE expression and brain amyloid load in Alzheimer’s disease? J Neurol Neurosurg Psychiatry. 2005;76:928–933. doi: 10.1136/jnnp.2004.048983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutala RV, Reddy PH. The use of real-time PCR analysis in a gene expression study of Alzheimer’s disease post-mortem brains. J Neurosci Methods. 2004;132:101–107. doi: 10.1016/j.jneumeth.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Lake FR, Noble PW, Henson PM, Riches DW. Functional switching of macrophage responses to tumor necrosis factor-alpha (TNF alpha) by interferons. Implications for the pleiotropic activities of TNF alpha. J Clin Invest. 1994;93:1661–1669. doi: 10.1172/JCI117148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- Häggqvist B, Naslund J, Sletten K, Westermark GT, Mucchiano G, Tjernberg LO, Nordstedt C, Engstrom U, Westermark P. Medin: an integral fragment of aortic smooth muscle cell-produced lactadherin forms the most common human amyloid. Proc Natl Acad Sci USA. 1999;96:8669–8674. doi: 10.1073/pnas.96.15.8669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris W, Mansourian S, Chang Y, Lindsley L, Eckman EA, Frosch MP, Eckman CB, Tanzi RE, Selkoe DJ, Guenette S. Insulin-degrading enzyme regulates the levels of insulin, amyloid beta-protein, and the beta-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci USA. 2003;100:4162–4167. doi: 10.1073/pnas.0230450100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N, Mizukami H, Shirotani K, Takaki Y, Muramatsu S, Lu B, Gerard NP, Gerard C, Ozawa K, Saido TC. Presynaptic localization of neprilysin contributes to efficient clearance of amyloid-beta peptide in mouse brain. J Neurosci. 2004;24:991–998. doi: 10.1523/JNEUROSCI.4792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RO, Massey A, Newman TA, Hutchings M, Kuo YM, Roher AE. Cerebral amyloid angiopathy: amyloid beta accumulates in putative interstitial fluid drainage pathways in Alzheimer’s disease. Am J Pathol. 1998;153:725–733. doi: 10.1016/s0002-9440(10)65616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMattos RB, Bales KR, Cummins DJ, Paul SM, Holtzman DM. Brain to plasma amyloid-beta efflux: a measure of brain amyloid burden in a mouse model of Alzheimer’s disease. Science. 2002;295:2264–2267. doi: 10.1126/science.1067568. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Loike JD, Brionne TC, Lu E, Anankov R, Yan F, Silverstein SC, Husemann J. Adult mouse astrocytes degrade amyloid-beta in vitro and in situ. Nat Med. 2003;9:453–457. doi: 10.1038/nm838. [DOI] [PubMed] [Google Scholar]
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S. Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer’s disease. Neuron. 2006;49:489–502. doi: 10.1016/j.neuron.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Bensemain F, Chapuis J, Tian J, Shi J, Thaker U, Lendon C, Iwatsubo T, Amouyel P, Mann D, Lambert JC. Association study of the Ubiquilin gene with Alzheimer’s disease. Neurobiol Dis. 2006;22:691–693. doi: 10.1016/j.nbd.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Chapuis J, Tian J, Shi J, Bensemain F, Cottel D, Lendon C, Amouyel P, Mann D, Lambert JC. Association study of the vascular endothelial growth factor gene with the risk of developing Alzheimer’s disease. Neurobiol Aging. 2006;27:1212–1215. doi: 10.1016/j.neurobiolaging.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Mann D, Harris J, Araria-Goumidi L, Chartier-Harlin MC, Cottel D, Iwatsubo T, Amouyel P, Lendon C. Association study of Notch 4 polymorphisms with Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2004;75:377–381. doi: 10.1136/jnnp.2003.017368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JC, Goumidi L, Vrieze FW, Frigard B, Harris JM, Cummings A, Coates J, Pasquier F, Cottel D, Gaillac M, St Clair D, Mann DM, Hardy J, Lendon CL, Amouyel P, Chartier-Harlin MC. The transcriptional factor LBP-1c/CP2/LSF gene on chromosome 12 is a genetic determinant of Alzheimer’s disease. Hum Mol Genet. 2000;9:2275–2280. doi: 10.1093/oxfordjournals.hmg.a018918. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Honsho M, Zahn TR, Keller P, Geiger KD, Verkade P, Simons K. Alzheimer’s disease beta-amyloid peptides are released in association with exosomes. Proc Natl Acad Sci USA. 2006;103:11172–11177. doi: 10.1073/pnas.0603838103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokin GB, Lillo C, Falzone TL, Brusch RG, Rockenstein E, Mount SL, Raman R, Davies P, Masliah E, Williams DS, Goldstein LS. Axonopathy and transport deficits early in the pathogenesis of Alzheimer’s disease. Science. 2005;307:1282–1288. doi: 10.1126/science.1105681. [DOI] [PubMed] [Google Scholar]
- Kurganov B, Doh M, Arispe N. Aggregation of liposomes induced by the toxic peptides Alzheimer’s Abetas, human amylin and prion (106-126): facilitation by membrane-bound GM1 ganglioside. Peptides. 2004;25:217–232. doi: 10.1016/j.peptides.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci. 2004;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.