Abstract
Neutrophil activation to release granules containing proteases and other enzymes is a primary cause of tissue damage during ischemia/reperfusion injury. Because the contribution of specific granule enzymes to this injury remains poorly defined, the role of cathepsin G in renal ischemia/reperfusion injury was tested. Bilateral renal ischemia led to the expiration of 64% of wild-type mice within 4 days of reperfusion, whereas all cathepsin G-deficient mice survived. Serum creatinine increased to similar levels at 24 hours after reperfusion and then decreased to background in both groups of mice. Ischemic kidneys from both groups had similar levels of neutrophil infiltration and of CXCL1, CXCL2, and myeloperoxidase protein 9 hours after reperfusion, but at 24 hours, these acute inflammatory response components were decreased more than 50% in kidneys from cathepsin G-deficient versus wild-type mice. Ischemic kidneys from surviving wild-type mice had severe tubular necrosis and tubular cell apoptosis 24 hours after reperfusion with subsequent development of fibrosis 30 days later. In contrast, ischemic kidneys from cathepsin G-deficient mice had a 70% decrease in tubular cell apoptosis with little detectable collagen deposition. These data identify cathepsin G as a critical component sustaining neutrophil-mediated acute tissue pathology and subsequent fibrosis after renal ischemia/reperfusion injury.
The imposition of tissue ischemia and reperfusion injury is an inherent component of solid organ transplantation that has a critical impact on the eventual outcome of the graft. Prolonged ischemic times are associated with increased incidence of delayed renal allograft function as well as with increased incidence of acute rejection and development of graft fibrosis and arteriopathy.1,2,3,4 Reperfusion of ischemic tissues induces an intense inflammatory response that includes cytokine and chemokine production and high-level expression of adhesion and MHC molecules.5,6,7,8,9 These factors promote the rapid infiltration of neutrophils, which are activated to mediate tissue damage during ischemia/reperfusion (I/R) injury of solid organs. Strategies either depleting neutrophils before reperfusion or inhibiting their infiltration into ischemic tissues have been extremely effective in attenuating injury of ischemic organs in animal models.6,8,9,10,11,12
Neutrophil infiltration into inflammatory sites including ischemic tissue is directed by chemoattractants including complement cleavage products and several CXC and CC chemokines.13,14,15,16 In addition to directing neutrophil recruitment, ligand binding to CXCR1 and CXCR2 stimulates neutrophils to release granules containing proteases, cytokines, chemokines, and other chemoattractants that amplify the intensity and extend the duration of tissue inflammation.17,18 Cathepsin G is a serine protease that makes up approximately 20% of the azurophilic granule proteins of neutrophils.19 Cathepsin G has many important roles in neutrophil function during inflammatory processes, including degradation of extracellular matrix components and cytokines, modulation of integrin clustering on neutrophils, and direct chemoattraction of T cells and other leukocytes.20,21,22,23,24,25,26 Cathepsin G−/− mice have normal development of neutrophils but abnormal wound-healing responses and increased susceptibility to fungal and bacterial infections.27,28,29 The role of specific neutrophil granule components including cathepsin G in the injury elicited during reperfusion of ischemic tissue injury remains poorly understood. The identification of key neutrophil products mediating this injury may be particularly useful in providing specific targets for the design of strategies to inhibit tissue damage in ischemic tissues. The goal of the current study was to test the role of cathepsin G during neutrophil infiltration and the development of tissue pathology after reperfusion of ischemic kidneys. The results identify cathepsin G as a major factor sustaining neutrophil infiltration and activation in the pathology of this injury.
Materials and Methods
Experimental Animals
129/SvJ wild-type mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Cathepsin G−/− mice on the 129/SvJ background27 were obtained from Dr. Timothy J. Ley at Washington University School of Medicine (St. Louis, MO) and maintained in the Biological Resources Unit of the Cleveland Clinic Foundation. Mice were housed under pathogen-free conditions according to National Institutes of Health guidelines. Adult males, 8 to 12 weeks old, were used throughout the study. The use of these animals in this study was approved by the Cleveland Clinic Institutional Animal Care and Use Committee.
Renal Ischemia and Reperfusion
Renal I/R injury was imposed on wild-type and cathepsin G−/− mice as previously reported.8 Mice were given 20 U of sodium heparin intraperitoneally 30 minutes before surgery. The mice were anesthetized with phenobarbital and kept warm under a 60-W light bulb until surgery. Under aseptic conditions, the abdominal cavity was opened with a midline incision, and the unilateral or bilateral renal pedicle was occluded nontraumatically with a microvascular clamp. The abdominal cavity was flushed with warm (37°C) Ringer’s solution, and the wound was temporarily closed with a 4-0 silk suture. Mice were placed on a heat pad under a 60-W light bulb to maintain intraperitoneal temperature at 32°C. Kidneys were subjected to ischemia for 60 minutes with varying times of reperfusion. Immediate and complete renal reperfusion was confirmed visually, and the peritoneal cavity was closed. After recovery from anesthesia, mice were given access to food and water. Except for the occlusion of renal pedicles, sham-operated mice were treated in an identical manner.
Histology and Terminal Deoxynucleotide Transferase dUTP Nick-End Labeling (TUNEL) Analysis
For immunohistology, kidney halves were embedded in OCT compound (Sakura Finetek U.S.A., Torrance, CA) and immediately frozen in liquid nitrogen 24 hours after I/R injury. Coronal sections were cut (8-μm thickness), mounted onto slides, dried for 1 hour, and then fixed in acetone for 10 minutes. Slides were immersed in phosphate-buffered saline (PBS) for 10 minutes and in 0.03% H2O2 in PBS for 5 minutes at room temperature to eliminate endogenous peroxidase activity. Endogenous biotin activity was blocked with Biotin Blocking System (DAKO, Carpinteria, CA). Slides were stained for 1 hour at room temperature with rat anti-Gr1 monoclonal antibody RB6-8C5 diluted at 10 μg/ml in PBS with 1% bovine serum albumin to detect neutrophils. Control slides were incubated with rat IgG as the primary antibody. After three washes in PBS for 5 minutes each, slides were incubated for 20 minutes with biotinylated rabbit anti-rat IgG, diluted 1:300 in the same buffer. After three washes in PBS, slides were incubated with streptavidin-horseradish peroxidase (DAKO) for 20 minutes. The substrate-chromagen solution was prepared by dissolving a 10-mg 3,3′-diaminobenzidine tablet (Sigma Chemical Co., St. Louis, MO) in 15 ml of PBS, and 12 μl of 30% H2O2 was added just before use. After three washes in PBS for 5 minutes each, the 3,3′-diaminobenzidine solution was applied to the slides and incubated for 2 to 3 minutes. After a wash in dH2O, slides were counterstained with hematoxylin, rinsed with dH2O, coverslipped, and viewed with a light microscope. Images were captured using Image Pro Plus (Media Cybernetics, Silver Spring, MD). The number of neutrophils was counted in 10 random fields per slide and four slides per kidney for four different kidneys at ×200 magnification in a blinded manner.
For morphology experiments, the kidneys were fixed with 10% buffered formalin. Paraffin-embedded sections were prepared and stained with hematoxylin and eosin (H&E) or with Masson trichrome staining. Apoptotic cells were visualized with the terminal deoxynucleotide transferase FragEL DNA fragmentation kit (Oncogene, Boston, MA), analogous to terminal deoxynucleotide transferase-mediated nick end-labeling, according to the manufacturer’s protocol. The number of apoptotic cells was counted in a blinded manner in 10 random fields per slide and four slides per kidney for four different kidneys at ×200 magnification.
Measurement of Renal Function
Mice were anesthetized with isofluorane and bled from the postorbital plexus using a heparin-coated microcapillary tube every 24 hours. The serum was stored at −80°C until measurement. Serum creatinine was measured using the Creatinine kit (Sigma Diagnostics, Inc., St. Louis, MO).
Preparation of Tissue Homogenates for Enzyme-Linked Immunosorbent Assay (ELISA)
Kidneys were harvested at 9 and 24 hours after I/R injury, cut into halves, and frozen in liquid nitrogen. The frozen tissue was dissolved in 500 μl of PBS with 0.01 mol/L ethylenediaminetetraacetic acid and a proteinase inhibitor cocktail (10 μg/ml phenylmethyl sulfonyl fluoride, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 100 μg/ml Pefabloc SC, and 100 μg/ml chymostatin), and then 1 ml of 1.5% Triton X-100 in PBS was added. After incubation with agitation for 1 hour at 4°C, samples were centrifuged, and the total protein concentration was determined using the DC Protein kit (Bio-Rad, Richmond, CA). KC/CXCL1 and MIP-2/CXCL2 concentrations were quantitated by sandwich ELISA using Quantikine M kits (R&D Systems, Minneapolis, MN). To assess the extent of neutrophil accumulation in kidneys, the concentration of myeloperoxidase was determined using the Mouse MPO ELISA test kit (Cell Sciences, Canton, MA).
Statistical Analysis
Data are expressed as mean ± SD. Differences in levels of cytokine production and neutrophil infiltration in ischemic kidneys between groups were analyzed by a one-way analysis of variance or unpaired Students’ t-test using StatView (Abacus Concepts, Inc., Berkeley, CA), and P < 0.05 was considered a significant difference. Differences in viability between groups were analyzed by log-rank test, and P < 0.01 was considered a significant difference.
Results
Ischemia/Reperfusion Injury in the Absence of Cathepsin G
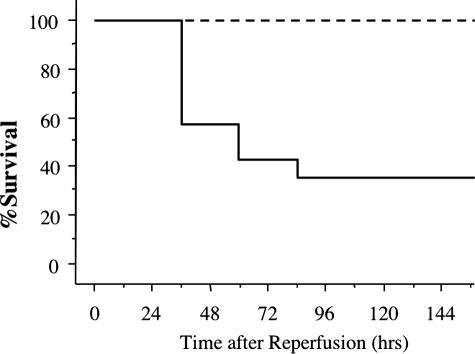
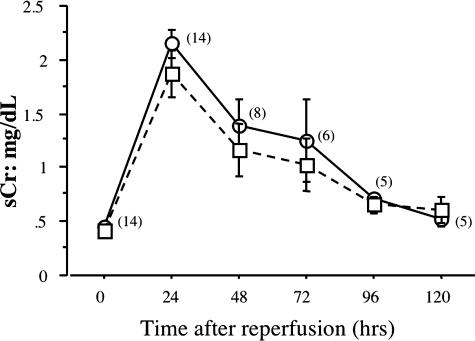
The potential role of cathepsin G in renal I/R injury was investigated by first testing the survival of wild-type 129/SvJ and cathepsin G−/− mice subjected to bilateral ischemia for 60 minutes and then reperfusion. Renal ischemia of wild-type mice led to 64% of the animals expiring within 4 days after reperfusion (Figure 1). In contrast, all cathepsin G−/− mice survived the injury. At several time points after reperfusion, peripheral blood was drawn from surviving mice in each group, and levels of serum creatinine were determined as an indicator of renal dysfunction. Despite the differences in viability after renal I/R injury, serum creatinine increased and reached similar peak levels 24 hours after reperfusion and returned to near normal levels by 96 hours after reperfusion in both wild-type and cathepsin G−/− animals (Figure 2).
Figure 1.
Absence of renal I/R induced mortality in cathepsin G−/− mice. Groups of 14 wild-type 129/SvJ (solid line) and eight cathepsin G−/− (dashed line) mice were subjected to 60 minutes of warm bilateral renal ischemia followed by reperfusion.
Figure 2.
Absence of cathepsin G does not attenuate renal dysfunction after I/R injury. Kidneys from groups of 14 wild-type 129/SvJ (circles) and eight cathepsin G−/− (squares) mice were subjected to bilateral ischemia for 60 minutes, and every 24 hours after reperfusion, peripheral blood was drawn to assay levels of serum creatinine. The number of surviving wild-type mice for each test point is indicated in parentheses.
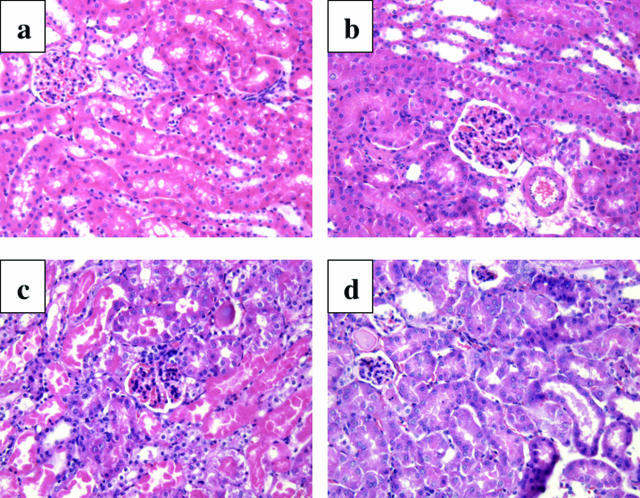
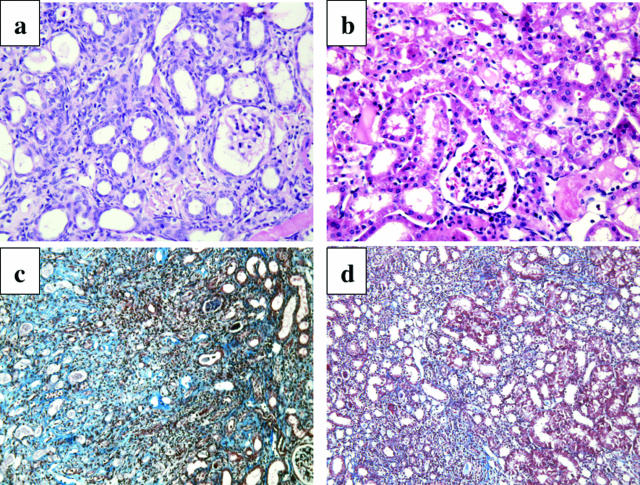
Wild-type and cathepsin G−/− mice were then subjected to unilateral renal ischemia, and the tissue damage in the ischemic kidneys and nonischemic/contralateral kidneys was assessed 24 hours after reperfusion. Tubular necrosis with intense vacuolization in proximal tubules as well as cast formation was clearly evident in ischemic kidneys from the wild-type mice (Figure 3c) when compared with the paired nonischemic kidneys (Figure 3b). In ischemic kidneys from cathepsin G−/− mice, cast formation was less evident, and there were clear decreases in necrosis and tubular vacuolization (Figure 3d).
Figure 3.
Reduced renal histopathology after renal I/R in the absence of cathepsin G. Kidneys from 129/SvJ wild-type and cathepsin G−/− mice were subjected to unilateral ischemia for 60 minutes. Kidneys were retrieved 24 hours after reperfusion, and formalin-fixed sections of a kidney from a normal wild-type mouse (a), contra-lateral (b) and ischemic (c) kidneys from wild-type mice, and an ischemic kidney from cathepsin G−/− mouse (d) were prepared and stained with H&E. Magnification, ×200.
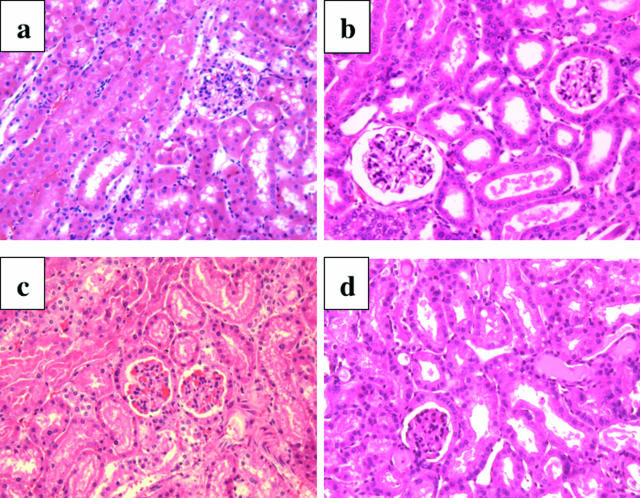
The histopathology of ischemic kidneys retrieved from wild-type mice that expired after reperfusion was compared with kidneys from wild-type mice that survived the injury. Because all mice expiring from renal I/R injury had done so by day 4 after reperfusion, this time point was used to retrieve ischemic kidneys from surviving mice to make this comparison. Kidneys retrieved within 30 minutes of expiration on day 2 (Figure 4c) or day 4 (Figure 4d) after reperfusion exhibited the severe tubular necrosis with vacuolization in proximal tubules and caste formation observed in the previous experiment. In contrast, ischemic kidneys retrieved from surviving wild-type mice on day 4 after reperfusion did not exhibit tubular necrosis and had clear decreases in vacuolization of proximal tubules and caste formation (Figure 4b) similar to the histology of the ischemic kidneys retrieved from cathepsin G−/− mice 24 hours after reperfusion (Figure 3c).
Figure 4.
Reduced renal histopathology after renal I/R in wild-type mice surviving the injury. Kidneys from 129/SvJ wild-type mice were subjected to bilateral ischemia for 60 minutes. Ischemic kidneys were retrieved within 30 minutes after animal expiration at various times after reperfusion or from sham-operated mice on day 4 after reperfusion, and formalin-fixed sections were prepared and stained with H&E. Kidneys were from a sham-operated mouse (a), a surviving mouse on day 4 after reperfusion (b), an expired mouse on day 2 (c), and an expired mouse on day 4 (d). Magnification, ×200.
Neutrophil Infiltration into Ischemic Kidneys during Reperfusion
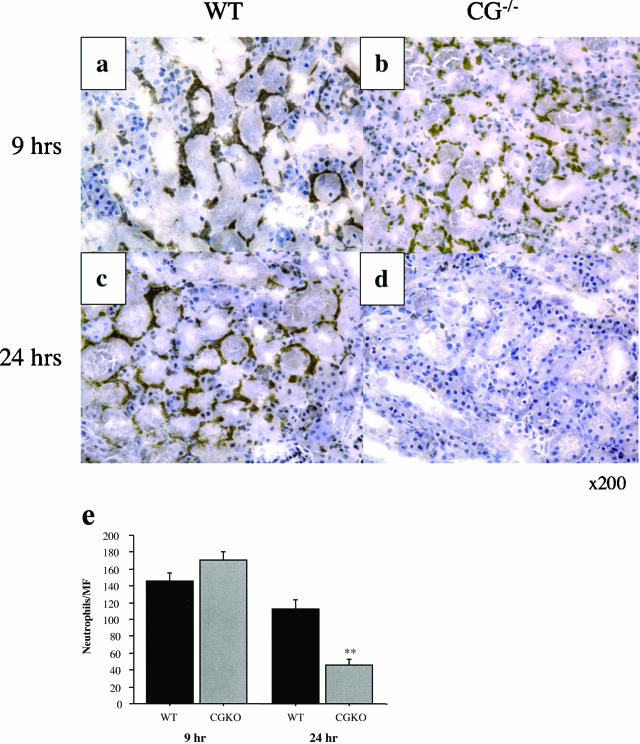
Neutrophil infiltration into kidneys in wild-type versus cathepsin G−/− mice after I/R injury was assessed by immunohistochemistry. Kidneys were harvested at 9 and 24 hours after reperfusion, and frozen sections were prepared and stained with anti-Gr1 monoclonal antibody to detect neutrophil infiltration. At 9 hours after reperfusion, neutrophil infiltration into kidneys of both groups was prominent, primarily localized within the corticomedullary junction (Figure 5, a and b). Neutrophil infiltration into ischemic kidneys from wild-type animals remained clearly evident at 24 hours after reperfusion (Figure 5c) but was clearly decreased in ischemic kidneys from cathepsin G−/− animals (Figure 5d).
Figure 5.
Lack of sustained neutrophil infiltration after reperfusion of ischemic kidneys in the absence of cathepsin G. Wild-type 129/SvJ and cathepsin G−/− mice were subjected to 60 minutes of warm ischemia. Kidneys were retrieved 9 or 24 hours after reperfusion, and frozen sections were prepared and stained to detect neutrophils. Renal sections are from wild-type 129/SvJ mice at 9 hours after reperfusion (a), cathepsin G−/− mice at 9 hours after reperfusion (b), wild-type 129/SvJ mice at 24 hours after reperfusion (c), and cathepsin G−/− mice at 24 hours after reperfusion (d). Magnification, ×200. e: The numbers of neutrophils were counted in 10 microscopic (magnification, ×200) fields/slide for four slides/kidney and four kidneys per group. The mean number of neutrophils per field ± SD is shown for each group. **P < 0.001.
Random microscopic fields of sections from both groups at each reperfusion time point were examined, and the number of infiltrating neutrophils was compared in ischemic kidneys from wild-type and cathepsin G-deficient mice, respectively. The number of kidney-infiltrating neutrophils was similar in both wild-type and cathepsin G−/− groups at 9 hours after reperfusion (Figure 5e). By 24 hours after reperfusion, neutrophil infiltration into ischemic kidneys from cathepsin G−/− mice was decreased more than 50% compared with wild-type mice.
Production of KC/CXCL1 and MIP-2/CXCL2 in Ischemic Kidneys
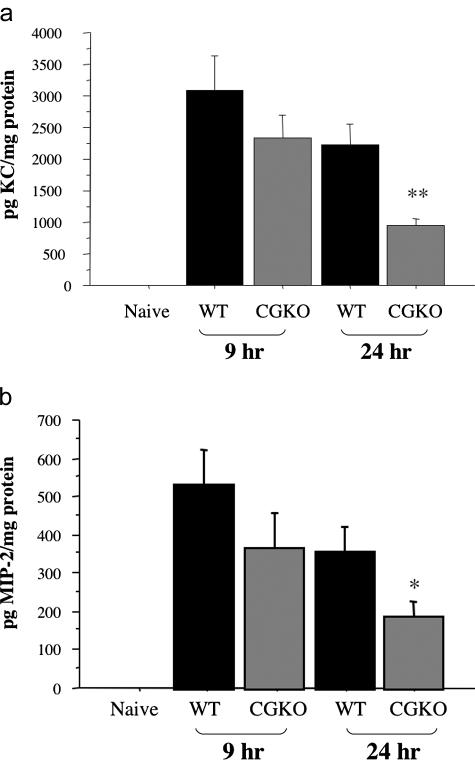
To investigate potential mechanisms underlying the changes in neutrophil infiltration during reperfusion of ischemic kidneys from wild-type versus cathepsin G-deficient mice, the production of KC/CXCL1 and MIP-2/CXCL2 in the kidneys was assessed. Ischemic kidneys were harvested at 9 and 24 hours after reperfusion, and prepared tissue lysates were tested for levels of KC/CXCL1 and MIP-2/CXCL2 protein. In ischemic kidneys from wild-type animals, production of KC/CXCL1 and MIP-2/CXCL2 protein peaked at 9 hours after reperfusion and decreased slightly by 24 hours after reperfusion (Figure 6, a and b). Reperfusion of ischemic kidneys from cathepsin G−/− mice also induced production of both chemokines at levels near those observed in wild-type animals at 9 hours, but at 24 hours, these levels were less than one-half those observed in the wild type.
Figure 6.
Production of CXCL1 and CXCL2 is not sustained during renal I/R injury in the absence of cathepsin G. Kidneys from groups of six wild-type 129/SvJ and cathepsin G−/− mice were subjected to 60 minutes of warm ischemia and harvested 9 and 24 hours after reperfusion. Tissue lysates of ischemic kidneys and naïve wild-type kidneys were prepared and tested by ELISA for quantitation of KC/CXCL1 (a) and MIP-2/CXCL2 (b) protein levels. The chemokine levels in renal lysates from naïve cathepsin G−/− mice were at similar low levels as naïve wild-type mice (data not shown). The mean concentration of chemokine protein per milligram of protein in the tissue lysate ± SD is shown. *P < 0.05; **P < 0.001.
Release of Myeloperoxidase in Ischemic Kidneys during Reperfusion
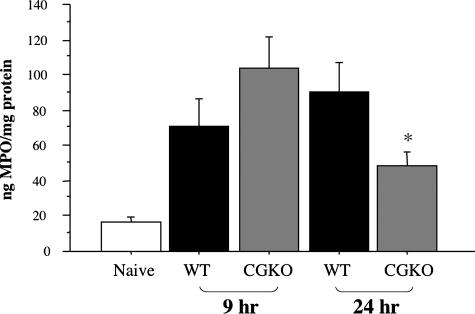
Determination of myeloperoxidase levels in the prepared tissue homogenates was used to assess the activation of infiltrating neutrophils during reperfusion of ischemic kidneys from wild-type and cathepsin G−/− mice. At 9 hours after reperfusion, myeloperoxidase was at high levels in ischemic kidneys from wild-type mice and was slightly increased in cathepsin G−/− mice (Figure 7). Myeloperoxidase levels increased in ischemic kidneys from wild-type mice at 24 hours but were significantly decreased in cathepsin G−/− mice.
Figure 7.
Temporal production of myeloperoxidase (MPO) is not sustained during renal I/R injury in the absence of cathepsin G. Tissue lysates of kidneys from wild-type 129/SvJ and cathepsin G−/− mice subjected to 60 minutes of warm ischemia and harvested 9 and 24 hours after reperfusion as well as from naïve wild-type mice were tested by ELISA for quantitation of MPO. MPO levels in renal lysates from naïve cathepsin G−/− mice were at similar low levels as naïve wild-type mice (data not shown). *P < 0.05.
Decreased Tubular Cell Apoptosis and Fibrosis in Ischemic Kidneys from Cathepsin G-Deficient Mice
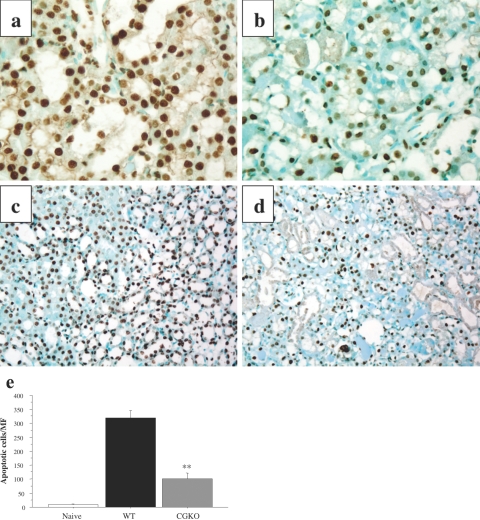
To further compare the extent of tissue damage in ischemic kidneys from wild-type and cathepsin G−/− mice, TUNEL analyses were performed to identify apoptotic cells in the kidneys 24 hours after reperfusion. TUNEL labeling of ischemic kidneys from wild-type mice indicated many dark-brown apoptotic cells with pyknotic nuclei in the proximal and distal convoluted tubules (Figure 8, a and c). Ischemic kidneys from cathepsin G−/− mice had a clear decrease in TUNEL-positive cells after reperfusion (Figure 8, b and d). When random sections were viewed and numbers of apoptotic cells were counted, there was a 70% decrease in the number of TUNEL-positive cells in ischemic kidneys from cathepsin G−/− when compared with wild-type mice (Figure 8e).
Figure 8.
Decreased apoptosis in kidneys subjected to I/R injury in the absence of cathepsin G. Wild-type 129/SvJ (a and c) and cathepsin G−/− (b and d) mice were subjected to 60 minutes of warm renal ischemia. After 24 hours of reperfusion, the ischemic kidneys were removed, and formalin-fixed sections were prepared and stained for TUNEL labeling to detect apoptotic cells. Magnification: ×400 (a and b); ×100 (c and d). e: The numbers of TUNEL-positive cells were counted in 10 microscopic (magnification, ×200) fields/slide for four slides/kidney and four kidneys per group. The mean positive number per field ± SD is shown for each group. **P < 0.001.
Finally, the kidneys were retrieved 30 days after I/R from surviving mice in the two groups and stained to assess the deposition of collagen as an indicator of fibrosis. In kidneys from wild-type mice, there was a clear decrease in the proximal and distal tubules and renal interstitial tissue was replaced by collagen deposition (Figure 9, a and c). In contrast, kidney tubular structure was maintained in ischemic kidneys from cathepsin G−/− mice with little evidence of collagen deposition (Figure 9, b and d).
Figure 9.
Decreased collagen deposition in kidneys subjected to I/R injury in the absence of cathepsin G. Wild-type 129/SvJ (a and c) and cathepsin G−/− (b and d) mice were subjected to 60 minutes of warm, unilateral renal ischemia. Ischemic kidneys were harvested 30 days after reperfusion. Formalin-fixed sections were prepared and stained with H&E (a and b) and Masson trichrome (c and d). Magnification: ×200 (a and b); ×100 (c and d).
Discussion
In most instances of inflammation, neutrophils are the first leukocytes recruited into the tissue site.30 This early recruitment is directed by neutrophil chemoattractants rapidly produced by the vascular endothelium and tissue parenchymal cells after the delivery of the initial signals of inflammation. Using renal I/R and vascularized cardiac allograft models in mice, studies from this laboratory have documented the production of CXCL1 and CXCL2 and neutrophil tissue infiltration within 3 hours of reperfusion.8,31,32 Furthermore, interleukin (IL)-8 mRNA is expressed at high levels in clinical renal grafts 30 minutes after reperfusion, and the length of ischemic times correlates with the levels of IL-8 expression induced during reperfusion of clinical renal allografts emphasizing the relationship between ischemic time and inflammatory cytokine production.33 In animal models, neutrophil infiltration into ischemic tissues is inhibited by antibodies to these chemokines resulting in attenuation of early neutrophil-mediated tissue damage.8,10,34,35,36 These studies highlight the prominent role of neutrophil activation in mediating the tissue injury induced after reperfusion of ischemic tissues.
A large amount of the early tissue damage that occurs during inflammation is mediated through activation of the neutrophils to produce reactive oxygen radicals and to release granules containing proteolytic enzymes and chemoattractants for other leukocyte populations. Cathepsin G is a primary granule component with potent proinflammatory properties.37 Cathepsin G is a serine protease that digests extracellular matrix proteins and enhances the activity of chemokines and matrix metalloproteinases by digesting the proteins into more potent cleavage products.20,21,22,24,25,26 In addition to increased susceptibility to microbial infections, cathepsin G−/− mice have reduced susceptibility to immune complex-mediated pathology characterized by decreased neutrophil accumulation in the immune complex deposition site.23,27,28 Based on these documented roles of cathepsin G in neutrophil function during immune responses and tissue pathology, we initiated experiments to test neutrophil infiltration and the extent of tissue injury after reperfusion of ischemic kidneys in the absence of the neutrophil-derived protease.
The intensity of early renal inflammation and injury was similar in kidneys from wild-type and cathepsin G−/− mice subjected to ischemia and reperfusion. There was no difference in production of the neutrophil chemoattractants CXCL1 and CXCL2 in the ischemic kidneys at 9 hours after reperfusion, the time we have previously shown these chemokines reach peak levels in the ischemic kidneys during this injury.8 The intensity of neutrophil infiltration and activation as indicated by myeloperoxidase release was also similar in kidneys from wild-type and cathepsin G-deficient mice at this time point. These results suggest that early signals initiating the inflammatory response after reperfusion of ischemic kidneys occur independently of cathepsin G. In addition, serum creatinine rose to similar levels and with similar kinetics in wild-type and cathepsin G−/− mice subjected to bilateral renal ischemia and reperfusion. This increase is indicative of early renal injury in both groups of mice, and then the serum creatinine falls to normal levels as the kidneys recover from the injury. However, 40 to 60% of the wild-type mice typically expire within 3 to 4 days after renal I/R, whereas cathepsin G−/− mice completely tolerated the injury, and there was no loss of these mice.
The major defect underlying the absence of severe tissue injury after renal I/R in cathepsin G−/− mice is the inability to sustain the presence of neutrophils in the kidney after reperfusion. The decreased neutrophil infiltration observed at 24 hours after reperfusion in ischemic kidneys from cathepsin G−/− mice was accompanied by decreases in the production of CXCL1 and CXCL2. Whether this decreased chemokine production underlies the low neutrophil infiltration at this time or is a consequence of the decreased neutrophil infiltration observed is unclear at this time. CXCL1 and CXCL2 are produced by tubular epithelial cells 9 hours after reperfusion, but other sources, including the infiltrating neutrophils, may produce these chemokines as the ischemic injury progresses with time.38 The production of these chemokines by neutrophils is likely to amplify and sustain the intensity of neutrophil infiltration into tissue sites of inflammation. Recent studies by Raptis et al23 have indicated decreased neutrophil infiltration and production of CXCL1 and CXCL2 in response to immune complexes and to zymosan inserted into air pouches of cathepsin G-deficient mice when compared with wild-type mice. The short-term infiltration of neutrophils into kidneys of cathepsin G−/− mice after I/R injury also resulted in limited tissue exposure to myeloperoxidase. Although myeloperoxidase is a major mediator of tissue damage through generation of hypochlorous acid and reactive oxygen species, the short term of myeloperoxidase observed in the kidneys from cathepsin G-deficient animals argues that the initial burst of myeloperoxidase does not induce or sustain substantial injury in the absence of cathepsin G.
The limited exposure of the ischemic kidney to these neutrophil-mediated inflammatory events during reperfusion in the absence of cathepsin G resulted in clear reductions in tissue injury. One important consequence was a substantial decrease in tubular cell apoptosis in the ischemic kidneys. Several studies have demonstrated tubular cell apoptosis after reperfusion of ischemic kidneys and the correlation between the number of apoptotic cells and the severity of renal dysfunction.39,40 Cathepsin G has been shown to mediate the detachment and apoptosis of cultured cardiomyocytes, but its role in cell detachment and apoptosis in vivo is untested.41 Myeloperoxidase-generated hypochlorous acid and oxygen radicals are also principal mediators of apoptosis during tissue injury.42,43 Whether the absence of cathepsin G or the resultant limited exposure to myeloperoxidase or other neutrophil-derived enzymes underlies this decrease in apoptosis cannot be defined from these studies. Importantly, the induction of apoptosis during renal I/R injury has been proposed to be a principal factor leading to the tissue fibrosis that develops after ischemic injury.44,45 Consistent with the lack of sustained neutrophil activity and decreased apoptosis of tubular epithelial cells, there was a clear decrease in collagen deposition observed in kidneys from cathepsin G−/− mice after I/R injury when compared with wild-type mice.
The results of this study are the first to identify cathepsin G as a major mediator of tissue injury in ischemic kidneys during reperfusion. The sustained neutrophil-mediated pathological events during ischemic reperfusion of kidneys in wild-type mice leads to a significant mortality, whereas the abbreviated term of these early events after reperfusion was not sufficient to lead to any loss in viability in the cathepsin G-deficient animals. Other than the rise in serum creatinine and a brief period of neutrophil infiltration, there is little other evidence of ongoing injury in the kidneys in the absence of cathepsin G. This suggests the ability of the kidneys to recover from short-term inflammation and neutrophil activation but that a longer period of neutrophil-mediated inflammation in the kidney results in tubular apoptosis and the initiation of inflammatory events leading to the development of tissue fibrosis. The results suggest that specifically targeting cathepsin G may be an effective strategy to attenuate tissue injury after ischemia without the systemic immunosuppression that would result from neutrophil depletion.
Acknowledgments
We thank Dr. Timothy Ley for generously providing breeding pairs of cathepsin G−/− mice and the Biological Resources Unit of the Lerner Research Institute for excellent care of the animals used in these studies.
Footnotes
Address reprint requests to Robert L. Fairchild, NB3-59, Department of Immunology, Cleveland Clinic Foundation, 9500 Euclid Ave., Cleveland, OH 44195-0001. E-mail: fairchr@ccf.org.
This work was funded by grants from the National Institute of Allergy and Infectious Diseases (RO1 AI40459 and RO1 AI51620 to R.L.F.) and from the Roche Organ Transplant Research Foundation (60495086 to R.L.F.).
References
- Dragun D, Hoff U, Park JK, Qun Y, Schneider W, Luft FC, Haller H. Prolonged cold preservation augments vascular injury independent of renal transplant immunogenicity and function. Kidney Int. 2001;60:1173–1181. doi: 10.1046/j.1523-1755.2001.0600031173.x. [DOI] [PubMed] [Google Scholar]
- Kouwenhoven EA, de Bruin RW, Bajema IM, Marquet RL, IJzermans JN. Prolonged ischemia enhances acute rejection in rat kidney grafts. Transplant Proc. 2001;33:361–362. doi: 10.1016/s0041-1345(00)02797-4. [DOI] [PubMed] [Google Scholar]
- Terasaki PI, Cecka JM, Gjertson DW, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333:333–336. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- Land W, Messmer K. The impact of ischemia/reperfusion injury on specific and non-specific, early and late chronic events after organ transplantation. Transplant Rev. 1996;10:236–253. [Google Scholar]
- Bergese SD, Huang EH, Pelletier RP, Widmer MB, Ferguson RM, Orosz CG. Regulation of endothelial VCAM-1 expression in murine cardiac grafts: expression of allograft endothelial VCAM-1 can be manipulated with antagonist of IFN-alpha or IL-4 and is not required for allograft rejection. Am J Pathol. 1995;147:166–175. [PMC free article] [PubMed] [Google Scholar]
- Colletti LM, Kunkel SL, Walz A, Burdick MD, Kunkel RG, Wilke CA, Strieter RM. Chemokine expression during hepatic ischemia/reperfusion-induced lung injury in the rat: the role of epithelial neutrophil activating protein. J Clin Invest. 1995;95:134–141. doi: 10.1172/JCI117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski I, Pratschke J, Wilhelm MJ, Gasser M, Tilney NL. Molecular and cellular events associated with ischemia/reperfusion injury. Ann Transplant. 2000;5:29–35. [PubMed] [Google Scholar]
- Miura M, Fu X, Zhang Q-W, Remick DG, Fairchild RL. Neutralization of Groα and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am J Pathol. 2001;159:2137–2145. doi: 10.1016/s0002-9440(10)63065-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. J Clin Invest. 1997;99:2682–2690. doi: 10.1172/JCI119457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugini D, Azzollini N, Gagliardini E, Cassis P, Bertini R, Colotta F, Norris M, Remuzzi G, Benigni A. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion injury. Kidney Int. 2005;67:1753–1761. doi: 10.1111/j.1523-1755.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- Seekamp A, Mulligan MS, Till GO, Ward PA. Requirements for neutrophil products and L-arginine in ischemia-reperfusion injury. Am J Pathol. 1993;142:1217–1226. [PMC free article] [PubMed] [Google Scholar]
- Klausner JM, Paterson IS, Goldman G, Kobzik L, Rodzen C, Lawrence R, Valeri CR, Shepro D, Hechtman HB. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol. 1989;256:F794–F802. doi: 10.1152/ajprenal.1989.256.5.F794. [DOI] [PubMed] [Google Scholar]
- Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines-CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Leigh LEA, Ghebrehiwet B, Perera TS, Bird LN, Strong P, Kishore U, Reid KBM, Eggleton P. C1q-mediated chemotaxis by human neutrophil involvement of gC1qR and G-protein signaling. Biochem J. 1998;330:247–254. doi: 10.1042/bj3300247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- Ward PA, Newman LI. A neutrophil chemotactic factor from human C5. J Immunol. 1969;102:93–99. [PubMed] [Google Scholar]
- Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/ interleukin-8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Smith CW. Mechanisms of neutrophil-induced parenchymal cell injury. J Leukoc Biol. 1997;61:647–653. doi: 10.1002/jlb.61.6.647. [DOI] [PubMed] [Google Scholar]
- Bank U, Ansorge S. More than destructive: neutrophil derived serine proteases in cytokine bioactivity control. J Leukoc Biol. 2001;69:197–206. [PubMed] [Google Scholar]
- Berahovich RD, Miao Z, Wang Y, Premack B, Howard MC, Schall TJ. Proteolytic activation of alternative CCR1 ligands in inflammation. J Immunol. 2005;174:7341–7451. doi: 10.4049/jimmunol.174.11.7341. [DOI] [PubMed] [Google Scholar]
- Chertov O, Ueda H, Xu LL, Tani K, Murphy WJ, Wang JM, Howard OMZ, Sayers TJ, Oppenheim JJ. Identification of human neutrophil-derived cathepsin G and azurocidin/CAP37 as chemoattractants for mononuclear cells and neutrophils. J Exp Med. 1997;186:739–747. doi: 10.1084/jem.186.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck LW, Balckburn WD, Irwin MH, Abrahamson DR. Degradation of basement membrane laminin by human neutrophil elastase and cathepsin G. Am J Pathol. 1990;136:1267–1274. [PMC free article] [PubMed] [Google Scholar]
- Raptis SZ, Shapiro SD, Simmons PM, Cheng AM, Pham CTN. Serine protease cathepsin G regulates adhesion-dependent neutrophil effector functions by modulating integrin clustering. Immunity. 2005;22:679–691. doi: 10.1016/j.immuni.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Richter R, Bistrian R, Escher S, Forssmann WG, Vakili J, Henschler R, Spodsberg N, Frimpong-Boateng A, Forssmann U. Quantum proteolytic activation of chemokine CCL15 by neutrophil granulocytes modulates mononuclear cell adhesiveness. J Immunol. 2005;175:1599–1608. doi: 10.4049/jimmunol.175.3.1599. [DOI] [PubMed] [Google Scholar]
- Saunders WB, Bayless KJ, Davis GE. MMP-1 activation by serine proteases and MMP-10 induces human capillary tubular network collapse and regression in 3D collagen matrices. J Cell Sci. 2005;118:2325–2340. doi: 10.1242/jcs.02360. [DOI] [PubMed] [Google Scholar]
- Weiss SJ, Curnutte JT, Regiani S. Neutrophil-mediated solubilization of the subendothelial matrix: oxidative and nonoxidative mechanisms of proteolysis used by normal and chronic granulomatous disease phagocytes. J Immunol. 1986;136:636–641. [PubMed] [Google Scholar]
- MacIvor DM, Shapiro SD, Pham CT, Belaaouaj A, Abraham SN, Ley TJ. Normal neutrophil function in cathepsin G-deficient mice. Blood. 1999;94:4282–4293. [PubMed] [Google Scholar]
- Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- Tkalcevic J, Novelli M, Phyactides M, Iredale JP, Segal AW, Roes J. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–210. doi: 10.1016/s1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- El-Sawy T, Miura M, Fairchild R. Early T cell response to allografts occurring prior to alloantigen priming up-regulates innate mediated inflammation and graft necrosis. Am J Pathol. 2004;165:147–157. doi: 10.1016/s0002-9440(10)63283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita K, Miura M, Paolone DR, Engeman TM, Kapoor A, Remick DG, Fairchild RL. Early chemokine cascades in murine cardiac grafts regulate T cell recruitment and progression of acute allograft rejection. J Immunol. 2001;167:2979–2984. doi: 10.4049/jimmunol.167.5.2979. [DOI] [PubMed] [Google Scholar]
- Araki M, Fahmy N, Zhou L, Kumon H, Krishnamurthi V, Goldfarb D, Modlin C, Flechner S, Novick AC, Fairchild RL. Expression of IL-8 during reperfusion of renal allografts is dependent on ischemic time. Transplantation. 2006;81:783–788. doi: 10.1097/01.tp.0000198736.69527.32. [DOI] [PubMed] [Google Scholar]
- Belperio JA, Keane MP, Burdick MD, Gomperts B, Xue YY, Hong K, Mestas J, Ardehali A, Mehrad B, Saggar R, Lynch JP, Ross DJ, Strieter RM. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest. 2005;115:1150–1162. doi: 10.1172/JCI24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sawy T, Belperio JA, Strieter RM, Remick DG, Fairchild RL. Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation. 2005;112:320–331. doi: 10.1161/CIRCULATIONAHA.104.516708. [DOI] [PubMed] [Google Scholar]
- Reutershan J, Morris MA, Burcin TL, Smith DF, Chang D, Saprito MS, Ley K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen CA, Campbell EJ. The cell biology of leukocyte-mediated proteolysis. J Leukoc Biol. 1999;65:137–150. doi: 10.1002/jlb.65.2.137. [DOI] [PubMed] [Google Scholar]
- Scapini P, Lapinet-Vera Z, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- Daemen MA, de Vries B, van’t Veer C, Wolfs TG, Buurman WA. Apoptosis and chemokine induction after renal ischemia-reperfusion. Transplantation. 2001;17:1007–1011. doi: 10.1097/00007890-200104150-00032. [DOI] [PubMed] [Google Scholar]
- de Vries B, Matthijsen RA, van Bijnen AA, Wolfs TG, Buurman WA. Lysophophatidic acid prevents renal ischemia-reperfusion injury by inhibition of apoptosis and complement activation. Am J Pathol. 2003;163:47–56. doi: 10.1016/S0002-9440(10)63629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri A, Alcott SG, Elouardighi H, Pak E, Derian C, Andrade-Gordon P, Kinnally K, Steinberg SF. Neutrophil cathepsin G promotes de-tachment-induced cardiomyocyte apoptosis via a protease-activated receptor-independent mechanism. J Biol Chem. 2003;278:23944–23954. doi: 10.1074/jbc.M302718200. [DOI] [PubMed] [Google Scholar]
- Askari AT, Brennan M-L, Zhou X, Drinko J, Morehead A, Thomas JD, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197:615–624. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissers MC, Pullar JM, Hampton MB. Hypochlorous acid causes caspase activation and apoptosis or growth arrest in human endothelial cells. Biochem J. 1999;344:443–449. [PMC free article] [PubMed] [Google Scholar]
- Persy VP, Verhulst A, Ysebaert DK, De Greef KE, De Broe ME. Reduced postischemic macrophage infiltration and interstitial fibrosis in osteopontin knockout mice. Kidney Int. 2003;63:543–553. doi: 10.1046/j.1523-1755.2003.00767.x. [DOI] [PubMed] [Google Scholar]
- Yang B, Jain S, Pawluczyk IZA, Imtiaz S, Bowley L, Ashra SY, Nicholson ML. Inflammation and caspase activation in long-term renal ischemia/reperfusion injury and immunosuppression in rats. Kidney Int. 2005;68:2050–2067. doi: 10.1111/j.1523-1755.2005.00662.x. [DOI] [PubMed] [Google Scholar]