Abstract
Essential mixed cryoglobulinemia in humans is strongly associated with chronic hepatitis C virus infection. It remains controversial whether liver injury in hepatitis C is primarily attributable to direct viral cytopathic effect or to an immune-mediated response. We characterized the role of cryoglobulinemia in the development of liver disease in thymic stromal lymphopoietin (TSLP) transgenic mice that produce mixed cryoglobulinemia and develop hepatitis. The role of immune complexes in this animal model was evaluated using techniques of light, immunofluorescence, and electron microscopy. To assess the role of Fc receptor engagement in mediation of the disease, TSLP transgenic mice were crossbred with mice deficient for immunoglobulin-binding receptor γ IIb (FcγRIIb). Livers from the TSLP transgenic animals showed mild to moderate liver injury, minimal to mild fibrosis, and deposition of immunoglobulin around the portal tracts. TSLP transgenic mice deficient in inhibitory FcγRIIb had more severe hepatitis and accelerated mortality. TSLP-associated hepatitis bears strong similarity to hepatitis C virus-related hepatitis as it occurs in humans, making this a valuable model system of chronic hepatitis and fibrosis to study therapies aimed at manipulating immune responses. Periportal immune complex deposition may play an important role in the pathogenesis of hepatitis occurring in the setting of systemic cryoglobulinemia.
According to the World Health Organization, ∼170 million people worldwide are infected with hepatitis C virus (HCV).1 It has been recognized that HCV has lymphotropic properties, as shown by the presence of viral replication in peripheral lymphocytes of infected patients.2,3 The great majority of cases of essential mixed cryoglobulinemia, up to 70 to 100% in various series, are associated with chronic HCV infection,4,5,6,7,8 possibly as a consequence of lymphocytic infection. A recent meta-analysis of 19 studies published between 1994 and 2001 indicated that 44% of patients with chronic HCV infection also had detectable cryoglobulinemia, indicating the magnitude of this association.9 Although this relationship between HCV and mixed cryoglobulinemia is now well established,6,7,8 the potential role of immune complex deposition in conjunction with cryoglobulinemia in the pathogenesis of liver injury has not been defined. Cryoglobulins are immunoglobulins or immune complexes that precipitate in cold and redissolve after rewarming.4 The current classification of cryoglobulins distinguishes three types: type I, consisting of monoclonal immunoglobulin (IgG or IgM); type II, composed of monoclonal IgM with rheumatoid factor activity that binds to polyclonal IgG; and type III, which is a mixture of polyclonal IgM and IgG.4,7 Although there have been attempts to create a small animal model to study the pathophysiology of HCV-associated diseases, a good experimental model of liver injury consequent to HCV infection has yet to be established.10,11,12 To date, no animal model exists that develops cryoglobulinemia after viral infection, and the role of immune complexes in the pathogenesis of HCV-related diseases is poorly understood.
The present studies use a mouse model of cryoglobulinemia in which mice overexpressing thymic stromal lymphopoietin (TSLP), an interleukin-7-like cytokine with B-cell-promoting properties, produce large amounts of circulating cryoglobulins of mixed IgG-IgM composition.13 Development of mixed cryoglobulinemia in these animals results in systemic inflammatory disease involving kidneys, liver, lungs, spleen, and skin. The renal involvement closely resembles human cryoglobulinemic glomerulonephritis as it occurs in patients infected with HCV.14,15,16 We have also shown that the deletion of the inhibitory immunoglobulin-binding receptor γ IIb (FcγRIIb) in these animals leads to aggravated renal injury with accelerated morbidity and mortality.17
Here, we extend these observations to the hepatitis that develops in TSLP transgenic mice, which despite the absence of hepatic infection by HCV, closely resembles the histological appearance of hepatitis encountered in patients with HCV infection. Accordingly, this may be a useful model to study mechanisms underlying the immune and inflammatory components of chronic hepatitis and fibrosing injury. Aggravation of liver injury in TSLP transgenic mice deficient in the inhibitory FcγRIIb indicates a role for immune complex activation of leukocytes via Fc receptors in the pathogenesis of liver injury associated with cryoglobulinemia.
Materials and Methods
Animal Study and Experimental Design
This study was originally designed to evaluate renal involvement by membranoproliferative glomerulonephritis in mice transgenic for TSLP and, subsequently, to test the role of Fc receptors in immune complex-mediated renal injury. The results of these studies have been published previously.13,17 The opportunity to study morphological changes in liver developed after animals were sacrificed. The experimental protocol for these studies was reviewed and approved by the Animal Care Committee of the University of Washington in Seattle. The TSLP transgenic mouse strain has been described previously13 as has the combined TSLP transgenic and FcγRIIb knockout (TSLP/FcγRIIb−/−).17 Mice were housed in the animal care facility of the University of Washington under standardized specific pathogen-free conditions (25°C, 50% humidity, 12-hour dark/light cycle) with access to food and water ad libitum.
A total of 130 animals were enrolled in this study, including 55 TSLP transgenic mice (20 females and 35 males), 10 TSLP/FcγRIIb−/− (five females and five males), 55 wild-type (20 females and 35 males), and 10 FcγRIIb−/− (five females and five males) controls. There were five animals in each group at each given time point. TSLP transgenic females and their wild-type controls were sacrificed at 2-week intervals from birth to 2.5 months of age. Males were sacrificed at monthly intervals up to 7 months of age. Females and males with combined TSLP/FcγRIIb−/− were sacrificed at a single time point (females at 50 days of age, males at 120 days of age), which has been shown in earlier studies to be the time when the renal and systemic manifestations of cryoglobulinemic disease are fully developed, with female mice demonstrating faster progression of the disease than males. Observations throughout a later time frame were precluded because of high mortality of the animals at these later times, attributed to concurrent severe lung involvement.
At the time points indicated, mice were anesthetized, blood was collected by cardiac puncture or retro-orbital bleeding, and organs were harvested. Hepatic tissue was fixed in 10% neutral buffered formalin for standard histology and fixed in half-strength Karnovsky’s solution for electron microscopy (1% paraformaldehyde and 1.25% glutaraldehyde in 0.1mol/L sodium cacodylate buffer, pH 7.0). A portion of the liver was snap frozen in liquid nitrogen for immunofluorescence study.
Tissue Preparation and Histological Staining
Formalin-fixed tissue was processed and embedded in paraffin using routine protocols. Tissue blocks were sectioned at 4-μm thickness for routine staining with hematoxylin and eosin (H&E), periodic acid-Schiff with diastase treatment, Masson’s trichrome stain, Sirius red, and immunohistochemistry. Immunofluorescence staining was performed on snap-frozen livers, sectioned at 6 μm and fixed in ice-cold acetone for 10 minutes.
Immunohistochemistry
Formalin-fixed tissue sections were processed for immunohistochemistry according to routine protocols, which have been previously described in detail.13 B cells were detected using a monoclonal anti-CD45RA antibody (Pharmingen, San Diego, CA), and T cells were detected using a monoclonal rat anti-CD3 antibody (clone number CD302; Serotec, Raleigh, NC). Macrophages were detected with Mac-2 antibody (Cederlane, Ontario, ON, Canada). To detect the presence of lymphatic endothelial cells, the sections were stained with goat anti-mouse LYVE antibodies (R&D Systems, Inc., Minneapolis, MN). To identify follicular dendritic cells, frozen sections of liver tissue were stained with rat anti-mouse follicular dendritic cell antibodies (clone FDC-M1; BD Biosciences, San Jose, CA). The 2-μm sections from paraffin-embedded tissues were deparaffinized in xylene and rehydrated in graded ethanol. Antigen retrieval was performed by heating tissue sections in antigen unmasking solution (Vector Laboratories, Burlingame, CA). Endogenous peroxidases were blocked in 3% hydrogen peroxide and endogenous biotin was blocked using the avidin/biotin blocking kit from Vector Laboratories. Slides then were incubated with the primary antibody diluted in phosphate-buffered saline (PBS) containing 1% bovine serum albumin (Sigma, St. Louis, MO) for 1 hour at room temperature. The sections then were washed repeatedly and incubated with the appropriate secondary antibody. The ABC-Elite reagent (Vector Laboratories) was used for signal amplification, and 3,3′-diaminobenzidine with nickel enhancement was used as chromogen, resulting in black color product. Slides were counterstained in methyl green, dehydrated, and coverslipped.
Immunofluorescence
Acetone-fixed frozen sections were rehydrated in PBS, blocked with normal rabbit serum, and then incubated with fluorescein-conjugated antibodies against IgG, IgM, IgA, and complement factor C3 (Cappel Pharmaceuticals, Aurora, OH). Slides were subsequently coverslipped with Vectashield mounting medium (Vector Laboratories) and viewed with a fluorescence microscope (Zeiss, Thornwood, NY). A semiquantitative score was used to describe the fluorescence intensity (0, negative; 1, weak; 2, moderate; 3, strong).
Electron Microscopy
A detailed protocol for tissue preparation has been described elsewhere.18 Grids were scanned and photographed using a Philips 410 electron microscope (Philips Export BV, Eindhoven, The Netherlands).
Serum Cryoglobulin Isolation
After collection, blood was allowed to clot at 37°C and then was centrifuged at 2800 rpm for 2 minutes. The collected serum was kept at 4°C for several days. The formation of cryoprecipitates was identified visually after 3 to 5 days. The detailed procedure for cryoglobulin characterization has been described previously.13 In brief, the cryoprecipitates were washed and resuspended in sodium chloride solution at 37°C. The components of the cryoprecipitates were evaluated by agarose gel electrophoresis and immunofixation for IgG, IgM, and κ and λ light chains. The involved immunoglobulin isotypes were evaluated using a mouse monoclonal antibody isotyping kit (Life Technologies, Inc., Gaithersburg, MD).
Quantitative Analysis and Statistics
Tissue sections stained with H&E, diastase-treated periodic acid-Schiff, and Masson’s trichrome stains were used for histological assessment of liver injury. To assess the grade of inflammation and stage of the liver fibrosis, the tissue sections were evaluated according to the modified histological activity index of Knodell (also known as the Ishak score).19,20 According to this system, grading of inflammatory activity includes assessment of portal inflammation (scale 0 to 4; 0 = none; 1 = mild, some or all portal tracts; 2 = moderate, some or all portal tracts; 3 = moderate/marked, all portal tracts; 4 = marked, all portal tracts); periportal injury (scale 0 to 4; 0 = none; 1 = mild, focal, few portal tracts; 2 = mild/moderate, focal, most portal tracts; 3 = moderate, continuous around in <50% portal tracts or septa; 4 = marked, continuous around in >50% portal tracts or septa); assessment of confluent necrosis (scale 0 to 6; 0 = none; 1 = focal; 2 = zone 3, some; 3 = zone 3, most; 4 = zone 3 and occasional portal-central bridging, 5 = zone 3 and multiple portal-central bridging, 6 = panacinar/multiacinar); and parenchymal injury (scale 0 to 4; 0 = none; 1 = mild, ≤focus per ×10 objective; 2 = moderate, two to four foci per ×10 objective; 3 = moderate, 5 to 10 foci per ×10 objective; 4 = marked, >10 foci per ×10 objective). The schema for staging fibrosis is based on semiquantitative scale from 0 to 6 (0 = none; 1 = portal, some; 2 = portal, most; 3 = occasional bridging; 4 = marked bridging; 5 = incomplete cirrhosis; 6 = established cirrhosis).19,20 Statistical analysis was performed using t-test without assuming equal variance. Quantitative results were expressed as mean ± SEM. A P value of ≤0.05 was considered statistically significant.
Results
TSLP Transgenic Mice Develop Mixed Cryoglobulinemia and Hepatitis Reminiscent of Human Chronic Hepatitis C
Sera from 95% of TSLP transgenic animals and 100% of TSLP/FcγRIIb−/− animals contained visible cryoprecipitates, whereas all of the samples from the wild-type and FcγRIIb−/− animals lacked such precipitate. The cryoprecipitates from nine selected cases were analyzed and found to contain polyclonal IgM and polyclonal IgG (type III) cryoglobulins. All cryoprecipitates contained IgG1, IgM, and κ and λ light chains. IgG3 was present in two cases, and IgG2A and IgA were detectable in two cases.13,17
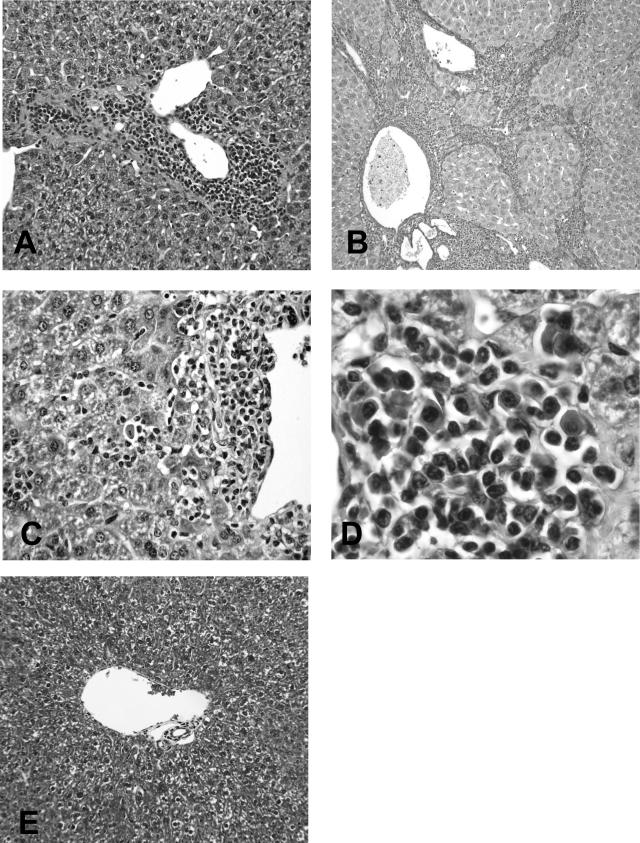
All TSLP transgenic animals sacrificed at 50 days (females) and 120 days (males) showed liver injury that was characterized by the presence of portal and periportal inflammation and foci of parenchymal injury (Figure 1, A–D). The portal inflammation ranged from mild (9% females, 64% males), moderate (36% females, 18% males), moderate/marked (45% females, 9% males), to marked (10% females, 9% males). Periportal injury, characterized by the accumulation of inflammatory cells invading the hepatic parenchyma at the limiting plate with a consequent disappearance of periportal hepatocytes (piecemeal necrosis, interface hepatitis), was mild in 27% of females and 55% of males, mild to moderate in 18% of females and 18% of males, and moderate in 55% of females and 9% of males, and marked in 0% of females and 9% of males. Areas of confluent necrosis were absent in all of the cases. The degree of parenchymal injury varied from mild to marked and was predominately moderate (64%) in females (mild in 18% and marked in 18%) and mostly mild (36%) in males (no parenchymal injury in 27%, moderate in 27%, and marked in 9%). Periportal and lobular infiltrates were composed of mixed inflammatory cells, predominately lymphocytes and occasional plasma cells (Figure 1, C and D). The mononuclear cells within portal areas frequently showed lymphoid follicle-like organization reminiscent of what is seen in chronic HCV hepatitis. No significant steatosis was present in any case.
Figure 1.
A: TSLP transgenic mouse showing prominent portal and periportal mononuclear inflammatory cell infiltrate. B: In some TSLP/FcγRIIb−/− animals, there was significant fibrosis associated with distortion of the parenchyma (cirrhosis). C: Rare necrotic hepatocytes in areas of periportal inflammation. D: High-power magnification of the inflammatory infiltrate shows the presence of lymphocytes and plasma cells. E: For comparison, the trichrome stain of the liver from a wild-type control with normal portal tract without inflammation or fibrosis. A, C, D: H&E stain; B: Sirius red stain; E: Masson trichrome stain. Original magnifications: ×200 (A, E); ×100 (B); ×400 (C); ×600 (D).
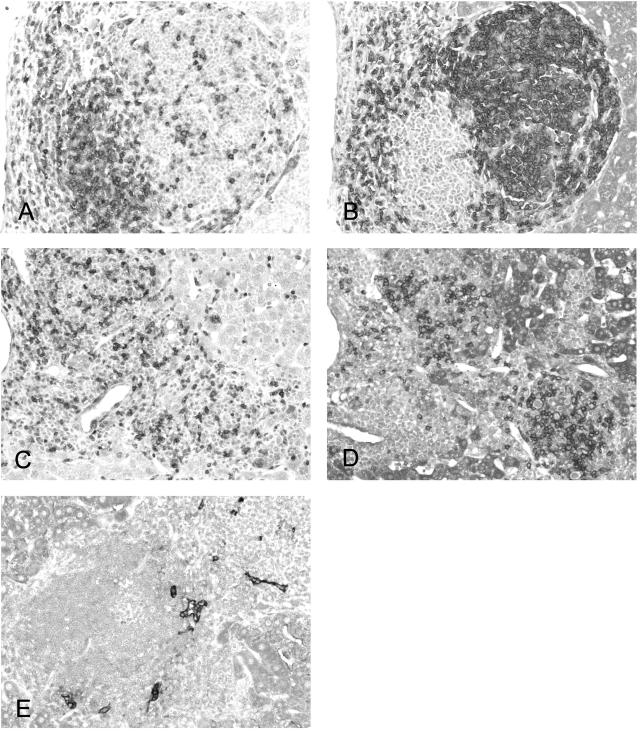
Immunohistochemical studies revealed that the portal areas contained an approximately equal number of CD3-expressing T cells (Figure 2A) and CD45RA-expressing B cells; however, the areas of follicle-like formation were composed mainly of B cells (Figure 2B), whereas more dispersed lymphocytes within the liver parenchyma expressed T-cell marker (Figure 2A). Numerous cells within the portal tracts were labeled with macrophage marker (Mac-2). In areas of lymphoid follicle-like organization, the small capillaries expressed a marker of high endothelial venules (LYVE; Figure 2E), typically found in such follicles in humans. Compared with the control section of the spleen, only occasional cells within portal tracts were labeled with an antibody to the follicular dendritic cell marker FDC-M1; overall, this was a rare finding.
Figure 2.
Immunohistochemical staining in TSLP transgenic mice demonstrates numerous CD3-expressing T cells in portal and periportal areas (A) and a significant subset of CD45RA-expressing B cells concentrated in areas of follicle-like formation (B). The infiltrates in the TSLP/FcγRIIb−/− animals show more numerous portal and parenchymal infiltrating T lymphocytes (C) and less follicle formation (D). In areas of lymphoid follicle organization, the small capillaries express LYVE, a marker of high endothelial venules (E) typically found in follicles in humans. Original magnifications: ×200 (A–D); ×400 (E).
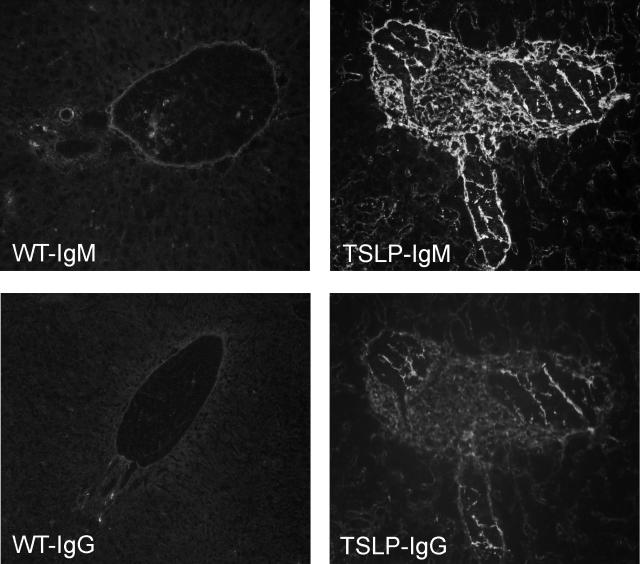
Immunofluorescence studies revealed strong, predominately portal, deposition of IgM, with lesser deposition of IgG and IgA (Figure 3). No significant deposition of complement factor C3 was observed. The control wild-type animals showed no significant hepatic pathology (Figure 1E) and no detectable deposition of these immune reactants (Figure 3). Despite the strong positivity for immunoglobulin heavy and light chains detected by immunofluorescence studies, we were not able to visualize immune-type dense deposits by electron microscopy that corresponded to the immunofluorescence findings.
Figure 3.
Immunofluorescence studies show strong portal and periportal staining for IgM and less staining for IgG in the same location. In comparison, there is no significant standing for either IgG or IgM in livers of wild-type controls. Original magnifications, ×200.
TSLP Transgenic Mice Do Not Develop Cirrhosis
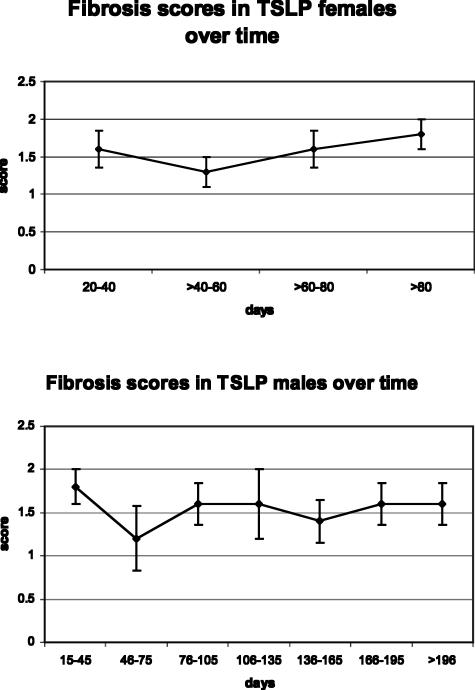
Examination of the Masson trichrome- and Sirius red-stained slides revealed mild to moderate deposition of collagen within some (36% females, 45% males) or most (45% females, 54% males) portal tracts (Figure 1B). Only one TSLP male, at the age of 4 months, showed the presence of focal bridging. There was no evidence of cirrhosis. Overall, the fibrosis was present in 81% of females and 64% of males. The overall degree of fibrosis was similar in animals at different time points, without significant worsening throughout the time course studied (Figure 4). Neither females nor males, at the given time points up to 75 and 210 days, respectively, developed significant fibrosis. Further observation beyond this time frame was precluded by the high mortality of the animals attributable to pulmonary involvement. Features of hepatitis, already present at the age of 30 days, remained stable thereafter for the remaining lifespan of these animals.
Figure 4.
Staging of fibrosis in TSLP transgenic mice demonstrates similar degrees of fibrosis during the time course in both males and females.
Deletion of FcγRIIb in TSLP Transgenic Mice Results in More Severe Liver Involvement by Hepatitis and Increase in Fibrosis
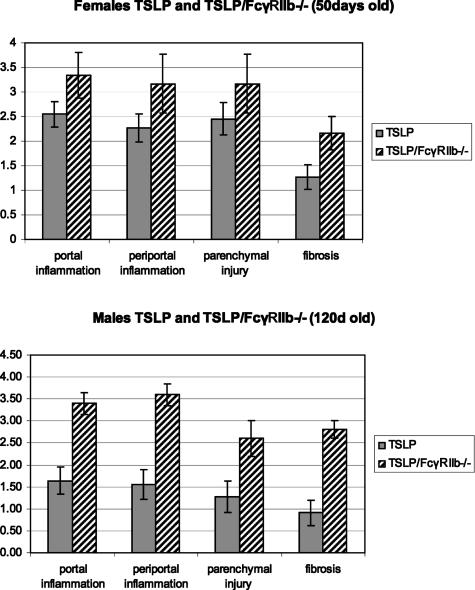
Animals transgenic for TSLP and additionally deficient in the inhibitory FcγRII showed a greater degree of liver injury. The groups of animals were compared at 50 days for females and 120 days for males. As revealed by the modified histological activity index scoring system, both female and male TSLP/FcγRIIb−/− mice, compared with animals transgenic for TSLP, showed a significant increase in portal inflammation (females: 2.5 ± 0.3 versus 3.3 ± 0.4, P < 0.05; males: 1.64 ± 0.3 versus 3.4 ± 0.2, P < 0.001), periportal inflammation (females: 2.3 ± 0.3 versus 3.2 ± 0.6, P < 0.05; males: 1.55 ± 0.3 versus 3.6 ± 0.2, P < 0.001), parenchymal injury (females: 2.4 ± 0.3 versus 3.1 ± 0.6, P = 0.11; males; 1.27 ± 0.4, P < 0.03), and degree of fibrosis (females: 1.3 ± 0.2 versus 2.2 ± 0.3, P < 0.03; males: 0.9 ± 0.3 versus 2.8 ± 0.2, P < 0.001) (Figure 5). Most noteworthy was an increase in fibrosis from mainly portal involvement in TSLP animals to bridging fibrosis present in 30% of TSLP/FcγRIIb−/− females and 80% of TSLP/FcγRIIb−/− males.
Figure 5.
Grading of inflammation and staging of fibrosis, according to the modified histological activity index of Knodell,19,20 in TSLP and TSLP/FcγRIIb−/− animals shows significant worsening of inflammation and fibrosis in TSLP/FcγRIIb−/− animals.
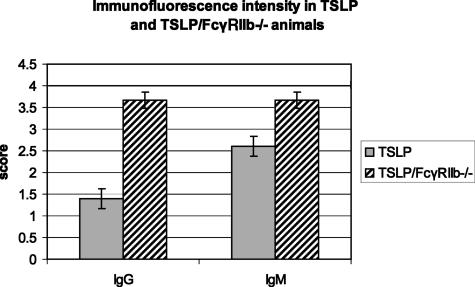
The infiltrates in the TSLP/FcγRIIb−/− animals showed overall less follicle formation (and overall less B cells) and more numerous portal and parenchymal infiltrating T lymphocytes (Figure 2, B and C) compared with TSLP transgenic mice. Immunofluorescence staining of livers of TSLP/FcγRIIb−/− animals, compared with the TSLP transgenic animals, revealed an increased portal deposition of IgG and IgM (1.4 ± 0.2 versus 3.7 ± 0.2, P < 0.001, and 2.6 ± 0.2 versus 3.7 ± 0.2, P < 0.03) (Figure 6). Similar to the TSLP transgenic animals, no discrete electron-dense immune-type deposits could be ultrastructurally identified in portal or periportal areas of livers from TSLP/FcγRIIb−/− animals.
Figure 6.
Immunofluorescence staining in TSLP and TSLP/FcγRIIb−/− animals shows significant increase in IgG and IgM deposition in TSLP/FcγRIIb−/− animals.
Discussion
We have shown for the first time that the animals transgenic for TSLP with systemic mixed cryoglobulinemia but without known infection develop liver injury with histological features very similar to that seen in humans infected with HCV. This injury is further aggravated by the deletion of the inhibitory immunoglobulin-binding receptor FcγRIIb, pointing to the direct role of immune complexes in the pathogenesis of this process.
Features of human HCV-associated hepatitis that are specifically replicated in these mice include portal and periportal inflammation with features of piecemeal necrosis, the presence of lymphoid aggregates in portal tracts, and lymphocytic aggregation within the lobules. Although the features of late stages of fibrosis are not well developed in all animals, it is possible that the mice need more time to develop severe fibrosis, in view of the situation of human disease in which HCV-infected patients may require ∼20 years to develop cirrhosis.21 It is unfortunate that the relatively short lifespan of TSLP transgenic and TSLP/FcγRIIb−/− mice precludes further studies of the progression of fibrosis.
An important feature of the hepatitis in TSLP mice is that immunohistochemical labeling reveals that a significant number of the portal and periportal inflammatory infiltrates in this animal model is composed of CD45RA-expressing B cells. However, in humans with HCV-induced liver disease, the majority of the infiltrating cells consist of T lymphocytes.22,23 The role of localized B cells in this model is not clear, but follicular aggregates of B cells in visceral organs such as liver and kidneys are often encountered in biopsies from patients with chronic immune inflammatory diseases such as systemic lupus erythematosus. Their significance in these settings is also unknown. This model may be a useful tool to study the contribution of B cells in the development of this aspect of liver disease.
It has been shown that the nonenveloped HCV core protein is a constitutive component of cryoprecipitable immune complexes in patients with type II cryoglobulinemia.7 There is some evidence that the presence of cryoglobulins in patients infected with HCV correlates with the development of cirrhosis.9 These findings point to an important role of cryoglobulins in the development and progression of HCV-related liver disease, but how this process evolves remains unknown.
Our findings are in agreement with the studies on human patients that suggest a relationship between the presence of circulating cryoglobulins and the severity and the stage of liver disease.9,24,25 A recent review of such studies concluded that the presence of circulating cryoglobulins in patients with HCV infection is significantly associated with cirrhosis, independently of age, gender, and duration of the disease.9 Treatment of HCV-associated cryoglobulinemia with an anti-CD20 antibody directed against B cells has been reported in two short, uncontrolled studies.7,26 This intervention resulted in significant remission in cryoglobulinemia and significant clinical improvement of purpura, arthralgia, and peripheral neuropathy despite increased or stable viral load. The liver function remained stable in patients followed for up to 18 months after therapy, but liver biopsies to evaluate histological changes have not been reported to date. Such studies support the premise that amelioration of cryoglobulinemia may lead to improvement or stabilization of concurrent liver injury in affected patients. The availability of an animal model to test therapies with such an approach is a new and valuable resource that has emerged from these studies.
We have shown, using immunofluorescence microscopy, that the liver injury in TSLP transgenic mice is associated with deposition of immune complexes containing IgM and IgG within portal tracts. However, electron microscopic examination did not reveal cor-responding discrete electron-dense, immune-type deposits. We are unable to offer a convincing explanation for this phenomenon. Our interpretation that these are immune complexes is based on the observation that corresponding electron-dense deposits are present concurrent with IgG and IgM in kidneys of these mice13 and on the observation that similar discrepancies are occasionally encountered in human glomerulonephritis, which is still considered of immune complex type. We nonetheless recognize that extrapolating these findings to the liver without better evidence is potentially inaccurate. We have considered the possibility that our immunofluorescence microscopy findings could represent direct binding to local antigens or some other mechanism of entrapment. This interpretation, although possible, seems unlikely because no significant staining was present in the control animals including wild type and FcγRIIb−/−.
It is likely that these complexes play a role in the pathogenesis of liver injury in these animals. This hypothesis was further studied by modifying this animal model of systemic cryoglobulinemia to create mice additionally deficient in the inhibitory Fc receptor γ. This receptor, which normally acts to dampen inflammatory responses by leukocytes, is engaged by the Fc component of deposited immunoglobulins.27,28 In addition, it has been recently shown to provide a peripheral checkpoint limiting the accumulation of autoreactive plasma cells, and its absence can augment the production of IgG antibodies in disease states.29 The deletion of the FcγRIIb in the TSLP transgenic mice has been previously shown to exacerbate the kidney damage in this model.17 Here, we show this mutation, which prevents amelioration of immune complex-induced inflammation and which may augment production of pathogenic IgG and IgG-containing immune complexes, results in significant worsening of liver injury, characterized by increased portal and periportal inflammation, parenchymal injury, and worsening of fibrosis.
The exact mechanism by which cryoglobulins may cause liver injury is uncertain. In addition to leukocyte engagement through Fc receptors, it is possible that the local deposition of immune complexes may elicit activation of the complement cascade.30 Our studies, which fail to detect significant deposition of complement in conjunction with immunoglobulins, do not support such a scenario. Rather, previously reported studies in TSLP mice made doubly transgenic for the complement regulatory protein Crry31 also support the hypothesis that the major system regulating local inflammation in this model is the Fc receptor system, with complement activation playing a minor role at best.
In summary, this study establishes a new model of chronic hepatitis in mice with systemic cryoglobulinemia and provides evidence for a direct role of immune complexes in the development, and possibly progression, of liver injury. This model provides an exceptional opportunity to study further the mechanism of liver injury associated with systemic cryoglobulinemia and to define optimal interventional strategies to ameliorate liver injury in these animals.
Footnotes
Address reprint requests to Jolanta Kowalewska, University of Washington Medical Center, Department of Pathology, Box 356100, 1959 NE Pacific St., Seattle, WA 98195. E-mail: jkowal@u.washington.edu.
References
- Wasley A, Alter MJ. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin Liver Dis. 2000;20:1–16. doi: 10.1055/s-2000-9506. [DOI] [PubMed] [Google Scholar]
- Ferri C, Monti M, La Civita L, Longombardo G, Greco F, Pasero G, Gentilini P, Bombardieri S, Zignego AL. Infection of peripheral blood mononuclear cells by hepatitis C virus in mixed cryoglobulinemia. Blood. 1993;82:3701–3704. [PubMed] [Google Scholar]
- Zignego AL, Macchia D, Monti M, Thiers V, Mazzetti M, Foschi M, Maggi E, Romagnani S, Gentilini P, Brechot C. Infection of peripheral mononuclear blood cells by hepatitis C virus. J Hepatol. 1992;15:382–386. doi: 10.1016/0168-8278(92)90073-x. [DOI] [PubMed] [Google Scholar]
- Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol. 2002;55:4–13. doi: 10.1136/jcp.55.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garini G, Allegri L, Vaglio A, Buzio C. Hepatitis C virus-related cryoglobulinemia and glomerulonephritis: pathogenesis and therapeutic strategies. Ann Ital Med Int. 2005;20:71–80. [PubMed] [Google Scholar]
- Agnello V, Chung RT, Kaplan LM. A role for hepatitis C virus infection in type II cryoglobulinemia. N Engl J Med. 1992;327:1490–1495. doi: 10.1056/NEJM199211193272104. [DOI] [PubMed] [Google Scholar]
- Dammacco F, Sansonno D, Piccoli C, Tucci FA, Racanelli V. The cryoglobulins: an overview. Eur J Clin Invest. 2001;31:628–638. doi: 10.1046/j.1365-2362.2001.00824.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Perrin L, Giostra E, Schifferli JA. Hepatitis C virus in patients with cryoglobulinemia type II. J Infect Dis. 1990;162:569–570. doi: 10.1093/infdis/162.2.569. [DOI] [PubMed] [Google Scholar]
- Kayali Z, Buckwold VE, Zimmerman B, Schmidt WN. Hepatitis C, cryoglobulinemia, and cirrhosis: a meta-analysis. Hepatology. 2002;36:978–985. doi: 10.1053/jhep.2002.35620. [DOI] [PubMed] [Google Scholar]
- Fimia GM, Tripodi M, Alonzi T. Transgenic models for hepatitis C virus pathogenesis. Cell Death Differ. 2003;10(Suppl 1):S16–S18. doi: 10.1038/sj.cdd.4401114. [DOI] [PubMed] [Google Scholar]
- Ramos E, Drachenberg CB, Papadimitriou JC, Hamze O, Fink JC, Klassen DK, Drachenberg RC, Wiland A, Wali R, Cangro CB, Schweitzer E, Bartlett ST, Weir MR. Clinical course of polyoma virus nephropathy in 67 renal transplant patients. J Am Soc Nephrol. 2002;13:2145–2151. doi: 10.1097/01.asn.0000023435.07320.81. [DOI] [PubMed] [Google Scholar]
- Mercer DF, Schiller DE, Elliott JF, Douglas DN, Hao C, Rinfret A, Addison WR, Fischer KP, Churchill TA, Lakey JR, Tyrrell DL, Kneteman NM. Hepatitis C virus replication in mice with chimeric human livers. Nat Med. 2001;7:927–933. doi: 10.1038/90968. [DOI] [PubMed] [Google Scholar]
- Taneda S, Segerer S, Hudkins KL, Cui Y, Wen M, Segerer M, Wener MH, Khairallah CG, Farr AG, Alpers CE. Cryoglobulinemic glomerulonephritis in thymic stromal lymphopoietin transgenic mice. Am J Pathol. 2001;159:2355–2369. doi: 10.1016/S0002-9440(10)63085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarantino A, De Vecchi A, Montagnino G, Imbasciati E, Mihatsch MJ, Zollinger HU, Di Belgiojoso GB, Busnach G, Ponticelli C. Renal disease in essential mixed cryoglobulinaemia. Long-term follow-up of 44 patients. Q J Med. 1981;50:1–30. [PubMed] [Google Scholar]
- D’Amico G, Fornasieri A. Cryoglobulinemic glomerulonephritis: a membranoproliferative glomerulonephritis induced by hepatitis C virus. Am J Kidney Dis. 1995;25:361–369. doi: 10.1016/0272-6386(95)90095-0. [DOI] [PubMed] [Google Scholar]
- D’Amico G. Renal involvement in hepatitis C infection: cryoglobulinemic glomerulonephritis. Kidney Int. 1998;54:650–671. doi: 10.1046/j.1523-1755.1998.00028.x. [DOI] [PubMed] [Google Scholar]
- Mühlfeld AS, Segerer S, Hudkins K, Carling MD, Wen M, Farr AG, Ravetch JV, Alpers CE. Deletion of the fcgamma receptor IIb in thymic stromal lymphopoietin transgenic mice aggravates membranoproliferative glomerulonephritis. Am J Pathol. 2003;163:1127–1136. doi: 10.1016/s0002-9440(10)63472-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpers CE, Hudkins KL, Pritzl P, Johnson RJ. Mechanisms of clearance of immune complexes from peritubular capillaries in the rat. Am J Pathol. 1991;139:855–867. [PMC free article] [PubMed] [Google Scholar]
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, Phillips MJ, Portmann BG, Poulsen H, Scheuer PJ, Schmid M, Thaler H. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- Ishak KG. Pathologic features of chronic hepatitis. A review and update. Am J Clin Pathol. 2000;113:40–55. doi: 10.1309/42D6-W7PL-FX0A-LBXF. [DOI] [PubMed] [Google Scholar]
- Ryder SD, Irving WL, Jones DA, Neal KR, Underwood JC. Progression of hepatic fibrosis in patients with hepatitis C: a prospective repeat liver biopsy study. Gut. 2004;53:451–455. doi: 10.1136/gut.2003.021691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann-Haefelin C, Blum HE, Chisari FV, Thimme R. T cell response in hepatitis C virus infection. J Clin Virol. 2005;32:75–85. doi: 10.1016/j.jcv.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Penna A, Missale G, Lamonaca V, Pilli M, Mori C, Zanelli P, Cavalli A, Elia G, Ferrari C. Intrahepatic and circulating HLA class II-restricted, hepatitis C virus-specific T cells: functional characterization in patients with chronic hepatitis C. Hepatology. 2002;35:1225–1236. doi: 10.1053/jhep.2002.33153. [DOI] [PubMed] [Google Scholar]
- Donada C, Crucitti A, Donadon V, Tommasi L, Zanette G, Crovatto M, Santini GF, Chemello L, Alberti A. Systemic manifestations and liver disease in patients with chronic hepatitis C and type II or III mixed cryoglobulinaemia. J Viral Hepat. 1998;5:179–185. doi: 10.1046/j.1365-2893.1998.00097.x. [DOI] [PubMed] [Google Scholar]
- Saadoun D, Asselah T, Resche-Rigon M, Charlotte F, Bedossa P, Valla D, Piette JC, Marcellin P, Cacoub P. Cryoglobulinemia is associated with steatosis and fibrosis in chronic hepatitis C. Hepatology. 2006;43:1337–1345. doi: 10.1002/hep.21190. [DOI] [PubMed] [Google Scholar]
- Roccatello D, Baldovino S, Rossi D, Mansouri M, Naretto C, Gennaro M, Cavallo R, Alpa M, Costanzo P, Giachino O, Mazzucco G, Sena LM. Long-term effects of anti-CD20 monoclonal antibody treatment of cryoglobulinaemic glomerulonephritis. Nephrol Dial Transplant. 2004;19:3054–3061. doi: 10.1093/ndt/gfh469. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Ravetch JV, Luster AD, Weinshank R, Kochan J, Pavlovec A, Portnoy DA, Hulmes J, Pan YC, Unkeless JC. Structural heterogeneity and functional domains of murine immunoglobulin G Fc receptors. Science. 1986;234:718–725. doi: 10.1126/science.2946078. [DOI] [PubMed] [Google Scholar]
- Fukuyama H, Nimmerjahn F, Ravetch JV. The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat Immunol. 2005;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- Liszewski MK, Farries TC, Lublin DM, Rooney IA, Atkinson JP. Control of the complement system. Adv Immunol. 1996;61:201–283. doi: 10.1016/s0065-2776(08)60868-8. [DOI] [PubMed] [Google Scholar]
- Mühlfeld AS, Segerer S, Hudkins K, Farr AG, Bao L, Kraus D, Holers VM, Quigg RJ, Alpers CE. Overexpression of complement inhibitor Crry does not prevent cryoglobulin-associated membranoproliferative glomerulonephritis. Kidney Int. 2004;65:1214–1223. doi: 10.1111/j.1523-1755.2004.00495.x. [DOI] [PubMed] [Google Scholar]