Abstract
Despite metastasis as an important cause of death in colorectal cancer patients, current animal models of this disease are scarcely metastatic. We evaluated whether direct orthotopic cell microinjection, between the mucosa and the muscularis layers of the cecal wall of nude mice, drives tumor foci to the most relevant metastatic sites observed in humans and/or improves its yield as compared with previous methods. We injected eight animals each tested human colorectal cancer cell line (HCT-116, SW-620, and DLD-1), using a especially designed micropipette under binocular guidance, and evaluated the take rate, local growth, pattern and rate of dissemination, and survival time. Take rates were in the 75 to 88% range. Tumors showed varying degrees of mesenteric and retroperitoneal lymphatic foci (57 to 100%), hematogenous dissemination to liver (29 to 67%) and lung (29 to 100%), and peritoneal carcinomatosis (29 to 100%). Tumor staging closely correlated with animal survival. Therefore, the orthotopic cell microinjection procedure induces tumor foci in the most clinically relevant metastatic sites: colon-draining lymphatics, liver, lung, and peritoneum. The replication of the clinical pattern of dissemination makes it a good model for advanced colorectal cancer. Moreover, this procedure also enhances the rates of hematogenous and lymphatic dissemination at relevant sites, as compared with previously described methods that only partially reproduce this pattern.
Colorectal cancer cases represents 15% of all cancer types. Its poor prognosis and the consequence of its metastatic spread makes colorectal cancer the second most common cause of cancer death in western countries.1 However, genetically modified mouse models of colorectal cancer are scarcely or not metastatic.2,3 Moreover, more metastatic cancer models, such as surgical orthotopic implantation (SOI), experimental or spontaneous metastasis assays, and orthotopic cell injection also show limitations. Thus, although SOI of human colorectal cancer in nude mice yields liver metastasis,4,5 it does not generate lung metastasis, nor mesenteric or retroperitoneal lymphatic metastasis,6 and requires the previous expansion of the tumor in subcutaneous xenografts,7,8,9 which may alter its growth and dissemination capacities.10 On the other hand, the experimental metastasis assay or spontaneous metastasis assay, consisting of cell injection into the tail vein or footpad, are less physiological and usually generate tumor foci only at one single site.11,12,13,14,15,16 Moreover, injection of colorectal cancer cells in the ileocolic vein or in the apical lymphoid follicle12,17,18 limits metastases to liver and lymphatics, varying widely in their rate.
We tested whether direct orthotopic cell microinjection (OCMI), between the mucosa and the muscularis externa layers of the cecal wall of nude mice, induces tumor foci in the most relevant metastatic sites observed in humans and/or improves its yield compared with previous methods. This technique required the use of an especially designed pipette, under binocular guidance. The application of this procedure to the human colorectal cancer cell lines HCT-116, SW-620, and DLD-1 yielded high tumor take and dissemination rates, replicating the metastatic spread to lymph nodes, liver, lung, and peritoneum observed in advanced human colorectal cancer.
Materials and Methods
Cell Lines and Micropipette Construction
HCT-116, SW-620, and DLD-1 cell lines were purchased from the American Type Culture Collection, Rockville, MD, and maintained in RPMI or McCoy’s medium with 10% fetal bovine serum in 5% CO2 at 37°C. The micropipette used for cell injection was constructed using hemocytometric capillaries (1.55 × 70 mm; Vitrex Medical, Herlev, Germany). Two series of heat and stretch timing, pulling from the edges into opposite directions, elongated the tip of the pipette to achieve a 250-μm diameter. The unmodified end of the pipette was fitted into a pediatric butterfly 25 (19.1 × 0.5 mm) (Venisystems; Johnson & Johnson, Arlington, TX) (Figure 1A). Before its use, the pipette was cleaned with 90% ethanol, 70% ethanol, and sterile water, and exposed to UV for 24 hours.
Figure 1.
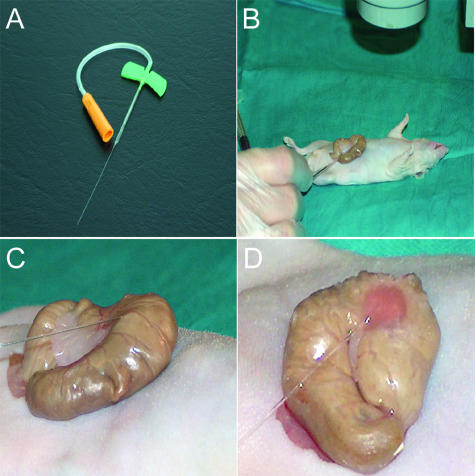
OCMI into the cecum of immunosuppressed mice. A: View of the micropipette made from Vitrex capillaries with a 250-μm-diameter tip. B: The cecum of anesthetized nude mice is exteriorized through a laparotomy. C: Two million human colorectal cancer cells, per animal, are injected, with a 30° angle under a binocular lens (×3). D: Reddish area depicts the tissue where tumor cell suspension has been injected.
Experimental Design
Four-week-old male Swiss Nu/Nu mice weighing 18 to 20 g (Charles River, Margate, Kent, UK) were used. Eight animals were injected with a cell suspension, for each of the HCT-116, SW-620, or DLD-1 colorectal cancer lines, to compare their dissemination pattern and survival time. Two additional animals per group were sacrificed 1 week after injection to analyze the distribution of the injected tumor cells within the colonic wall. Mice were housed in a sterile environment with water, bedding, and γ-ray-sterilized food. Experiments were approved by the Hospital de Sant Pau Animal Ethics Committee.
OCMI
Nude mice were anesthetized with ketamine and xylazine, exteriorizing their cecum by a laparotomy (Figure 1B). Cells (2 × 106) per cell line (HCT-116, SW-620, or DLD-1) were suspended in 50 μl of Dulbecco’s modified Eagle’s medium and placed into the sterile micropipette (Figure 1B). We slowly injected the cell suspension, under a binocular lens (×3 magnification), with an approximate 30° angle and its tip introduced 5 mm into the cecal wall (Figure 1, C and D). Afterward, we applied a slight pressure with a cotton stick at ∼2 mm from the injection point in the direction of the pipette axis. We pulled the pipette out and cleaned the area around the injection with 3% iodine to avoid seeding of unlikely refluxed tumor cells into the abdominal cavity. The small diameter and flexible tip of the pipette and the angular and slow rate of administration diminished resistance to the injection, limiting tissue damage and bleeding, ensuring the absence of cell reflux. After injection, the gut was returned to the abdominal cavity and closed with surgical grapes. Animals were kept until death because of their neoplastic process or until the end of the experiment (130 days).
Necropsy Procedure, Tumor Grading, and Staging
At animal death, a complete necropsy procedure was performed. Cervical, thoracic, abdominal, and pelvic organs were extracted en bloc. We assessed the presence of local tumor at the injection site, measured its two largest diameters, and recorded all macroscopic tumor deposits or abnormalities in any organ. The whole block of organs was fixed with buffered formalin for 48 hours, except for the lung, which was perfused and dissected and paraffin-embedded. Six-micron-thick sections were stained with hematoxylin and eosin. Tumor sections at the injection site and tumor deposits in other organs were stained with the per-iodine acid shift reaction.
Two general pathologists analyzed histopathologically the tumor at the injection site and at the end of the experiment. Tumors were classified into three different grades depending on necrotic extent, mitotic rate, and extent of gland-like structures.19 The presence of tumor cells in other organs was also recorded, especially those in which colorectal cancer foci were expected (mesenteric lymph nodes, liver, lung, and peritoneum). We calculated tumor take rate as the percentage of mice with local tumor growth with respect to the total number of injected mice. We monitored daily tumor progression by palpation and calculated the final tumor volume, using the formula: volume = (longer diameter) × (shorter diameter)2/2. Tumor growth rate was calculated dividing tumor volume by survival time.
In addition, we developed a four-stage system to stage the tumor in each animal. Stage I consisted in the presence of local tumor growth. Stage II was stage I plus peritoneal carcinomatosis (tumor in the parietal peritoneum of the abdominal wall, the diaphragm, or the visceral peritoneum of the digestive tract and liver). Stage III was stage I and/or II plus mesenteric lymph node and/or pancreatic foci (mouse pancreas is intraperitoneal). Stage IV was stage I, II, or III plus hepatic and/or lung foci.
Statistical Analysis
Differences in final tumor volume between HCT-116, SW-620, or DLD-1 groups were analyzed using the Mann-Whitney test. The likelihood of survival was estimated according to the Kaplan and Meier method20 and survival distributions compared using the log-rank test.21 Differences in take rates or in the presence of lymphatic, hepatic, or lung foci, or carcinomatosis between groups were compared using Fisher’s test. We considered the statistical differences significant at a value of P < 0.05.
Results
Localization of the Injected Cells
The main objective of the OCMI procedure was to deposit human colorectal cancer cells close to the colonic mucosa (Figure 1), where colorectal carcinomas are initiated,22,23 using a small caliber pipette (33-gauge) and a binocular lens to increase the precision of the injection. We histologically assessed the location of the injected cells in two animals per group, 1 week after injection, confirming their placement in the space between the mucosa and the muscularis externa layers of the cecal wall (Figure 2A). This was done safely because none of the animals showed any morbidity or died because of the procedure.
Figure 2.
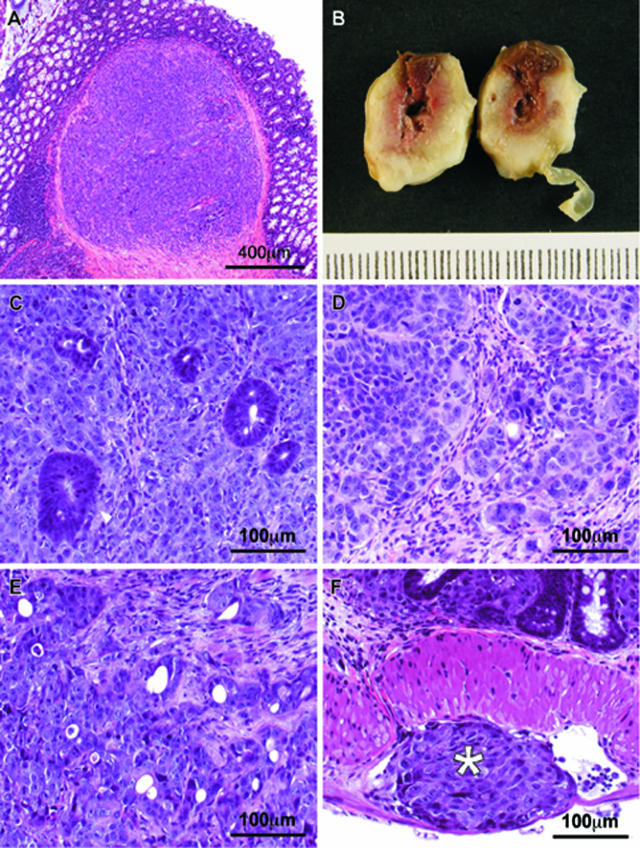
Site of injection and pattern of tumor growth and invasion. A: The site of injection, 1 week after the OCMI procedure, showed the tumor cells located between the mucosa and the muscularis externa layers of the cecal wall. B: Tumors grow, both tangentially and circumferentially, and protrude into the lumen of the large bowel obstructing it. C–E: Tumors derived from HCT-116 (C), SW-620 (D), or DLD-1 (E) cell lines are poorly differentiated adenocarcinomas with high cellularity, focal areas of necrosis, frequent mitoses, and highly atypical nuclei, showing invasion of all of the cecal layers. F: Tumor cells infiltrate the lymphatics of the cecal wall (white asterisk). A, C–F: H&E stains.
Take Rates, Local Tumor Growth, and Histopathology
We observed high tumor take rates for all cell lines (Table 1). HCT-116 or SW-620 cells generated tumors in six of eight (75%) animals, and DLD-1 cells generated tumors in seven of eight (88%) animals. Next, we evaluated the capacity of the OCMI procedure to replicate the histopathological appearance and clinical behavior of colorectal cancer in humans. Most HCT-116, SW-620, or DLD-1 tumors invaded the normal cecum in the tangential and transverse (circumferential growth) directions, yielding tumors that protruded into the cecal lumen (Figure 2B).
Table 1.
Local Tumor Growth and Dissemination Pattern in Animals Bearing Tumors Derived from OCMI-Implanted Human Colorectal Cancer Cell Lines
| Cell line | Mice (n) | Local tumor
|
Growth rate (cm3/day) | Dissemination site [mice (%)]†
|
||||
|---|---|---|---|---|---|---|---|---|
| Take rate [mice (%)]* | Final tumor volume (mean ± SE) (cm3) | Lymphatic | Hepatic | Lung | Carcinomatosis | |||
| HCT-116 | 8 | 6/8 (75) | 4.4 ± 0.1‡§ | 0.85 | 6/6 (100) | 4/6 (67)¶ | 3/6 (50) | 6/6 (100)|| |
| SW-620 | 8 | 6/8 (75) | 3.9 ± 0.2§ | 0.38 | 6/6 (100) | 0/6 (0)¶ | 1/6 (17) | 5/6 (83) |
| DLD-1 | 8 | 7/8 (88) | 3.7 ± 0.3‡ | 0.25 | 4/7 (57) | 2/7 (29) | 2/7 (29) | 2/7 (29)|| |
Mice with local tumor growth/total number of injected mice per group.
Mice with tumor foci at the particular distant (far away from the injection) site/total number of mice with local tumor growth per group.
Statistically significant differences at ‡P = 0.043 or at
P = 0.047 (Mann-Whitney test).
Statistically significant differences at ¶P = 0.030 or at
P = 0.016 (Fisher’s test).
The three cell lines gave rise to poorly differentiated adenocarcinomas. They were highly cellular, composed of atypical cells with pleomorphic nuclei, arranged mainly in sheets and solid nests, and forming diffuse fronts that invaded the normal colon. Only occasional glandular lumens were seen [Figure 2, C (HCT-116), D (SW-620), and E (DLD-1)]. Many necrotic areas were also found. The mitotic count was always higher than five mitoses per 10 high-power fields. The tumor cells invaded all of the cecal layers and were also found inside the lumen of lymphatic vessels of the cecal wall (Figure 2F).
Tumor volume and growth rates showed differences among groups. Mean tumor volumes in DLD-1 (3.7 ± 0.3 cm3) and SW-620 (3.9 ± 0.2 cm3) groups were similar; however, the mean HCT-116 tumor volume was significantly (P < 0.05) higher (4.4 ± 0.1 cm3) (Table 1). In addition, the HCT-116 tumor registered the highest growth rate (0.85 cm3/day), SW-620 growth rate was intermediate (0.38 cm3/day), and DLD-1 grew slowly (0.25 cm3/day) (Table 1). There was no relation between tumor take rate and growth rate in the studied groups.
Tumor Dissemination Sites and Tumor Staging
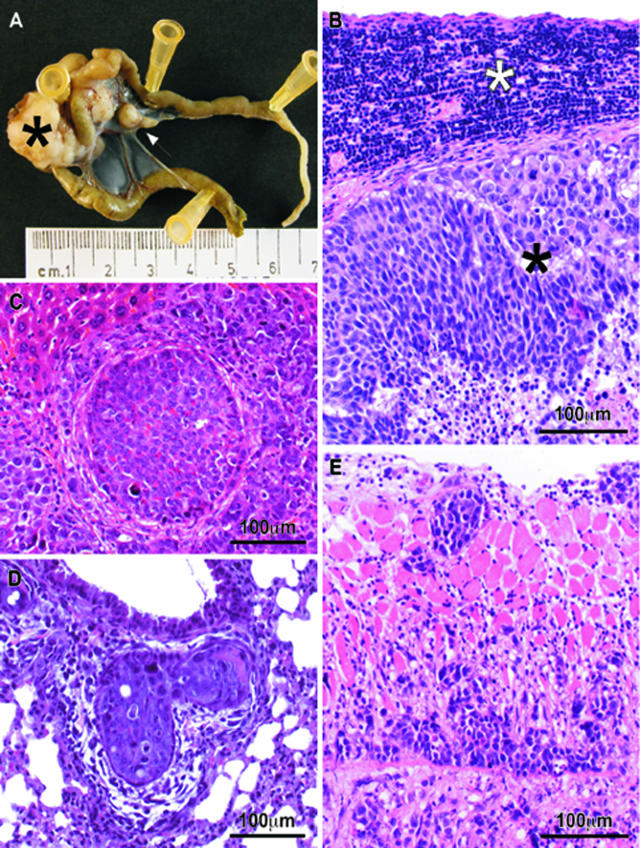
We also evaluated the usefulness of the OCMI procedure to study lymphatic and hematogenous dissemination and carcinomatosis. Mesenteric lymphatic foci occurred in almost all of the animals, with the highest frequencies in the HCT-116 or SW-620 tumor-bearing mice (six of six, 100%) and the lowest in DLD-1 (four of seven, 57%) (Table 1). Tumor foci were located in the mesenteric lymph nodes draining the cecal area (Figure 3A) or in retroperitoneal lymph nodes. Microscopically, the affected nodes showed a rim of lymphocytes under the capsule (Figure 3B). Tumor foci were also frequent in pancreatic lymphatics.
Figure 3.
Lymphatic, hematogenous, and peritoneal tumor spread in nude mice bearing tumors derived from OCMI-implanted cell lines A: Out of the local tumor (black asterisk), tumor cells spread to the mesenteric lymph nodes (white arrow). B: Microscopically, tumor foci in the mesenteric lymph nodes showed a rim of lymphocytes (white asterisk) compressed against the capsule by tumor cells (black asterisk). C: Representative foci of hematogenous dissemination to the liver, observed in the HCT-116 cell line. D: Tumor cells at the lung were found inside peribronchial vessels. E: Tumor cells invading the muscle of the diaphragm from its peritoneal surface. B–E: H&E stains.
We also studied the presence of liver or lung tumor foci because these are the most common sites for blood-borne metastases in human colorectal cancer.24 We observed liver foci in the HCT-116 (four of six, 67%) and DLD-1 (two of seven, 29%) tumor-bearing animals (Table 1), with tumor cells within and around blood vessels invading the liver parenchyma (Figure 3C). However, tumors derived from the SW-620 cell line did not generate foci in the liver. Tumor microfoci in the lung (Figure 3D) were detected in 50% (three of six) of the animals bearing HCT-116 tumors, 29% (two of seven) of the DLD-1 mice, and 17% (one of six) of the SW-620 mice (Table 1). In addition, tumor deposits infiltrating the surface of the visceral and parietal peritoneum (Figure 3E) were found in six of six (100%) HCT-116 mice, five of six (83%) SW-620 mice, and two of seven (29%) DLD-1 mice (Table 1). The three different cell lines varied widely in their capacity of inducing different disease stages (Table 2). Whereas the HCT-116 cell line yielded mainly stage IV tumors (67%), most of the SW-620 tumors were at stage III (67%). In addition, whereas no early tumors were recorded in the HCT-116 or SW-620 cell lines, most of the DLD-1 tumors were at stage I of the disease (42%) (Table 2).
Table 2.
Tumor Staging and Survival Time in Animals Bearing Tumors Derived from OCMI-Implanted Human Colorectal Cancer Cell Lines
| Cell line | Tumor staging (% of mice)
|
Survival time (days, mean ± SE) | |||
|---|---|---|---|---|---|
| Stage I | Stage II | Stage III | Stage IV | ||
| HCT-116 | 0/6 (0%) | 0/6 (0%) | 2/6 (33%) | 4/6 (67%) | 39 ± 4† |
| SW-620 | 0/6 (0%) | 0/6 (0%) | 5/6 (67%) | 1/6 (17%) | 73 ± 10*† |
| DLD-1 | 3/7 (42%) | 0/7 (0%) | 2/7 (29%) | 2/7 (29%) | 110 ± 7*† |
Statistically significant differences at *P = 0.0002 or at
P = 0.00116 (log-rank test).
Correlation between Tumor Staging and Animal Survival
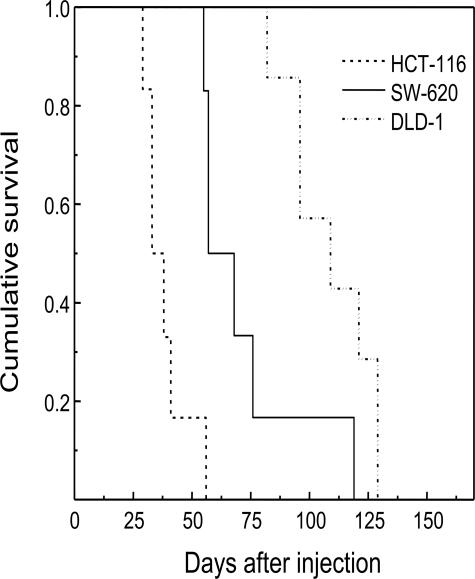
Survival time differed widely (4 to 16 weeks range) among groups (P = 0.032) (Table 2 and Figure 4); it was the shortest in HCT-116 tumor-bearing mice (39 ± 4 days); SW-620 mice showed intermediate survival times (73 ± 10 days), and DLD-1 mice had the longest survival (110 ± 7 days). Cell lines varied widely in their capacity to induce hematogenous or lymphatic dissemination. As described in Tables 1 and 2, there was a clear correlation between staging and animal survival in the three studied groups. Thus, the HCT-116 cell line gave rise to highly aggressive and disseminating tumors, which reached stage IV in most cases (67%) and caused death in only 39 ± 4 days. The SW-620 cell line gave rise mostly (67%) to stage III tumors, causing death in 73 ± 10 days. Finally, the DLD-1 cell line gave rise mostly (42%) to stage I tumors, which caused death only after 110 ± 7 days (see Figure 4).
Figure 4.
Survival curves of animals from the three studied groups. Survival time was the shortest in HCT-116 tumor-bearing mice, intermediate in SW-620 animals, and the longest in DLD-1 mice. Cumulative survival values were estimated using the Kaplan and Meier method and plotted versus time after injection. The log-rank test showed statistically significant differences in survival among groups (see also Table 2).
Discussion
The OCMI procedure—the injection of human colorectal cancer cell lines between the mucosa and the muscularis externa layers of the cecal wall of nude mice—generates models in which the dissemination pattern closely replicates all relevant metastatic sites observed in humans and enhances their dissemination rate compared with previous methods. We chose to assess the HCT-116,25,26 SW-620,27 and DLD-125,28 cell lines because of the previous characterization of their capacity to disseminate.
Replication of Human Colorectal Cancer Stages, Dissemination Pattern, and Aggressiveness
As observed in humans, tumors generated using the OCMI procedure show circumferential growth, finally protruding into the lumen.24 They also replicate the pattern of lymphatic spread, disseminating to the mesenteric lymph nodes draining the cecal area and retroperitoneal lymph nodes. Moreover, hematogenous dissemination involves the liver and the lung in the HCT-116- and DLD-1-derived tumors. Only SW-620 tumors did not generate tumor foci in the liver, most likely because this cell line does not express EGFR,27,29 a requirement for colorectal cancer metastasis in the liver.30 In addition, we observed a clear correlation between tumor staging and animal survival in the three studied groups, with HCT-116 being the most aggressive and DLD-1 the least aggressive, whereas tumor grading did not relate to survival. These results are consistent with the highly significant prognostic value of the tumor staging systems, and the lower prognostic value of tumor grade, in human colorectal cancer.24
The OCMI Model Improves Previous Colorectal Cancer Models
OCMI generates significantly more tumor foci in organs distant from the injection site than transgenic, knockout, or knockin mice generate metastatic tumor foci. In addition, genetically modified mice show tumor dissemination at significantly longer time periods (1.5 to 2 years), develop tumors in the small rather than in the large bowel, and show secondary mutations different from these found in humans.31 These differences could be related to a distinct transformation capacity between human and mouse cells32 because OCMI uses human tumor cells whereas in genetically modified mice tumors spontaneously arise out of mouse cells. In contrast, to generate an OCMI model takes only 4 to 8 weeks and uses fewer animals than the genetically modified models because of its higher dissemination rate.
On the other hand, experimental metastasis assay or spontaneous metastasis assay, which inject cells in the tail vein or footpad, are less physiological, generate metastases mostly at one single site, and require the use of selected metastatic variants or irradiation to enhance their efficiency.33,34 In contrast, the OCMI method induces tumor colonization in all relevant sites and does not require any previous selection procedure. Table 3 compares the procedures and dissemination rates of the most relevant orthotopic colorectal cancer models. Orthotopic cell injection of colon carcinoma cells into the apical lymphoid follicle achieves a widely variable rate of lymphoid or hepatic tumor foci12,17,18; however, its lymph node dissemination rates are lower than those achieved by OCMI using the same cell line (SW-620).27,35,36 Moreover, these models do not yield lung metastases or carcinomatosis (Table 3).18 Likewise, the intrasplenic or ileocolic vein injections of colon cancer cells generate liver deposits37,38,39,40,41 but do not produce tumor foci in lymphoid or lung tissue nor carcinomatosis.
Table 3.
Comparison of Procedures and Dissemination Rates at Different Sites for the OCMI and Other Orthotopic Implantation Methods
| Procedure | OCMI | SOI | Cell injection methods | |||
|---|---|---|---|---|---|---|
| Reference number | (4) | (17) | (12) | (18) | ||
| Implanted colorectal Cancer cells | Human carcinoma cells | Disaggregated human tumors grown SC | Murine MCA-38 | Murine 51B | Murine Colon 26 | Human HT-29 MM |
| Point of entry in cecum | Submucosal side | Serosal side | Lymphoid follicle | Serosal side | Lymphoid follicle | Lymphoid follicle |
| Dissemination sites | Dissemination rates | |||||
| Colon-draining lymphatics | 10/13 (77%) | 0% | – | – | 0/5 (0%) | 2/5–3/3 (40 to 100%) |
| Liver-draining lymphatics | 0% | 100%† | – | – | – | – |
| Hepatic | 6/13 (46%)* | 100%† | (65%) | 4/21 (19%) | 1/5 (20%) | 0/6 (0%) |
| Lung | 5/13 (38%)* | 0% | – | 1/21 (5%) | – | – |
| Carcinomatosis | 8/13 (62%) | – | – | – | – | – |
SW-620 was excluded to calculate dissemination rate to liver or lung because this cell line does not express EGFR and does not generate these metastases (see Discussion).
Highly metastatic cells to the liver selected by consecutive passages in nude mice.
SOI, surgical orthotopic implantation of tumor tissue; OCMI, orthotopic cell microinjection of tumor cells.
In addition, OCMI shows differences in dissemination capacity with the SOI of tumor fragments. Thus, despite SOI closely replicating early metastatic events, because all spreading tumor cells detach from the primary tumor tissue, it only yields tumor foci in the liver and liver-draining lymphatics;6,42,43,44 however, it does not induce the dissemination to the lung or to colon-draining lymphatics. In contrast, the OCMI procedure allows tumor cells to infiltrate the lymphatics of the intestinal wall, disseminating first into the mesenteric lymph nodes, to spread through the bloodstream into the liver and lung, and to form foci in the peritoneum, as observed in human colorectal cancer.24,45,46,47,48 Again, OCMI gives rise to higher dissemination rates without requiring the selection of aggressive metastatic variants as SOI does.4,49 Therefore, OCMI seems to be more physiological than SOI to study the latter processes of the metastatic cascade, including lymphatic or hematogenous dissemination and organ colonization.
Morikawa et al50 were the first to report a mouse cancer model applying the intracecal injection of human colorectal cancer cells (using essentially the same procedure as OCMI). However, this article and the work that followed18,51,52,53 did not perform a systematic study of the tumor sites, distant to the injection site, the method yielded, as the one we are presenting here. Instead, they focused on studying the influence of organ microenvironment (comparing the effect of subcutaneous, splenic, or orthotopic implantation) on tumor growth and dissemination capacity. They were mainly aimed at selecting cell lines, through successive splenic passages, with enhanced rate of hepatic metastasis. Consequently, they did not report the ability of their model to generate tumor foci in the lung or in the peritoneum (carcinomatosis).
Importance of the Cell Injection Site and Handling Procedure
Placing the cells in the space between the mucosa and the muscularis externa layers of the cecal wall could be accomplished because OCMI uses a flexible and small-diameter micropipette (33-gauge), which allows injecting a limited volume of the cell suspension that could be accommodated within the cecal wall. This could be done, with expert handling, without significant tissue damage and avoiding cell reflux, which could mask the carcinomatosis data. Some of the factors that may have contributed to increase the tumor take rates, to ensure the induction of tumor foci in all clinically relevant metastatic sites and to improve dissemination rates, compared with previous procedures, include the following: 1) the orthotopic placement of the suspended tumor cells directly into the submucosal compartment, close to the mucosa, the site of initiation of human colon cancer.22,23 This may increase the likelihood that tumor cells replicate the pattern of successive interactions with the extracellular matrix (ECM) of the lymphatic system, vasculature, and target organ site, along the dissemination route, each playing a significant role in determining migration, intravasation, or tumor growth at the distant site.54,55,56,57 2) The deposit of a high number of suspended cells that, once established the correct interactions, are free to migrate and easily reach the abundant lymphatic and blood vessels of the submucosal cecal wall,24 enhancing tumor spreading. In contrast, SOI attaches tumor tissue to the serosal side of the cecum, which could diminish their capacity to reach the lymphatic and blood vessels located in the submucosa. This argument is consistent with different techniques yielding distinct metastatic rates for the same cell lines.8
OCMI, a Novel and Improved Model for Advanced Colorectal Cancer
In summary, the OCMI procedure is an easy and useful method to generate advanced stages of colorectal cancer, which replicates in a mouse model, with high take rates and in a short time (1 to 4 months), its clinical behavior, including its pattern of local tumor growth, invasion of mesenteric lymphatics, hematogenous dissemination to the liver and lung, and peritoneal carcinomatosis. Therefore, this procedure complements currently available colorectal cancer models, widening the number of affected tumor dissemination sites and their yield. Thus, OCMI specially facilitates the study of the latter processes of the metastatic cascade, including lymphatic or hematogenous dissemination and organ colonization, which is important because growth at the distant sites seem to be a rate-limiting step in the metastatic cascade.58 Moreover, our characterization of the pattern of dissemination for the HCT-116, SW-620, and DLD-1 colorectal cancer cells, using OCMI, will allow the ex vivo manipulation of particular genes to evaluate their involvement in each of these processes, using a low number of animals. We are applying it now to the study of the Ras and Rho family members (C. Espina, M.V. Céspedes, M.A. Garcia-Cabezas, M.T. Gomez del Pulgar, A. Boluda, L. García-Droz, P. Cajas, M. Nistal, R. Mangues, J.C. Lacal, manuscript in preparation). Finally, the OCMI-derived models might also be used in the development and testing of novel therapies for advanced disease and could be applied to the development of models for advanced cancer in other organs.
Acknowledgments
We want to thank Judith Darrical (Hospital de Sant Pau), Dr. Carmen Cavada (School of Medicine, Universidad Autónoma de Madrid), and the Photography Unit of the Instituto de Investigaciones Biomédicas for their technical assistance.
Footnotes
Address reprint requests to R. Mangues, Ph.D., Laboratori d’Investigació Gastrointestinal, Institut de Recerca, Hospital de Sant Pau. Av. Sant Antoni M Claret, 167, 08025 Barcelona, Spain. E-mail: rmangues@santpau.es.
Supported in part by the Government of Spain (grants SAF03-07437, Fondo de Investigaciones Sanitarias PI052591, Agaur SGR-01050, and La Caixa BM05-258-0 to R.M.), the National Health System (grant FIS 98/3197 to R.M.), and the Instituto Carlos III (C03/10 and PI051162 to the research team belonging to the Network of Cooperative Research on Cancer).
R.M. is a Catalonian Public Health Researcher and a National Health System Researcher (FIS 98/3197).
References
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Corpet DE, Pierre F. Point: from animal models to prevention of colon cancer. Systematic review of chemoprevention in min mice and choice of the model system. Cancer Epidemiol Biomarkers Prev. 2003;12:391–400. [PMC free article] [PubMed] [Google Scholar]
- Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- Hoffman RM. Orthotopic metastatic mouse models for anticancer drug discovery and evaluation: a bridge to the clinic. Invest New Drugs. 1999;17:343–359. doi: 10.1023/a:1006326203858. [DOI] [PubMed] [Google Scholar]
- Fu XY, Besterman JM, Monosov A, Hoffman RM. Models of human metastatic colon cancer in nude mice orthotopically constructed by using histologically intact patient specimens. Proc Natl Acad Sci USA. 1991;88:9345–9349. doi: 10.1073/pnas.88.20.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashidi B, Gamagami R, Sasson A, Sun FX, Geller J, Moossa AR, Hoffman RM. An orthotopic mouse model of remetastasis of human colon cancer liver metastasis. Clin Cancer Res. 2000;6:2556–2561. [PubMed] [Google Scholar]
- Kuo TH, Kubota T, Watanabe M, Furukawa T, Teramoto T, Ishibiki K, Kitajima M, Moossa AR, Penman S, Hoffman RM. Liver colonization competence governs colon cancer metastasis. Proc Natl Acad Sci USA. 1995;92:12085–12089. doi: 10.1073/pnas.92.26.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilbaud N, Kraus-Berthier L, Meyer-Losic F, Malivet V, Chacun C, Jan M, Tillequin F, Michel S, Koch M, Pfeiffer B, Atassi G, Hickman J, Pierre A. Marked antitumor activity of a new potent acronycine derivative in orthotopic models of human solid tumors. Clin Cancer Res. 2001;7:2573–2580. [PubMed] [Google Scholar]
- Flatmark K, Maelandsmo GM, Martinsen M, Rasmussen H, Fodstad O. Twelve colorectal cancer cell lines exhibit highly variable growth and metastatic capacities in an orthotopic model in nude mice. Eur J Cancer. 2004;40:1593–1598. doi: 10.1016/j.ejca.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Farré L, Casanova I, Guerrero S, Trias M, Capella G, Mangues R. Heterotopic implantation alters the regulation of apoptosis and the cell cycle and generates a new metastatic site in a human pancreatic tumor xenograft model. FASEB J. 2002;16:975–982. doi: 10.1096/fj.01-0973com. [DOI] [PubMed] [Google Scholar]
- Giavazzi R, Jessup JM, Campbell DE, Walker SM, Fidler IJ. Experimental nude mouse model of human colorectal cancer liver metastases. J Natl Cancer Inst. 1986;77:1303–1308. [PubMed] [Google Scholar]
- Bresalier RS, Hujanen ES, Raper SE, Roll FJ, Itzkowitz SH, Martin GR, Kim YS. An animal model for colon cancer metastasis: establishment and characterization of murine cell lines with enhanced liver-metastasizing ability. Cancer Res. 1987;47:1398–1406. [PubMed] [Google Scholar]
- Garofalo A, Chirivi RG, Scanziani E, Mayo JG, Vecchi A, Giavazzi R. Comparative study on the metastatic behavior of human tumors in nude, beige/nude/xid and severe combined immunodeficient mice. Invasion Metastasis. 1993;13:82–91. [PubMed] [Google Scholar]
- Wang Y, Liang X, Wu S, Murrell GA, Doe WF. Inhibition of colon cancer metastasis by a 3′-end antisense urokinase receptor mRNA in a nude mouse model. Int J Cancer. 2001;92:257–262. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1178>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Katoh M, Neumaier M, Nezam R, Izbicki JR, Schumacher U. Correlation of circulating tumor cells with tumor size and metastatic load in a spontaneous lung metastasis model. Anticancer Res. 2004;24:1421–1425. [PubMed] [Google Scholar]
- Chen X, Su Y, Fingleton B, Acuff H, Matrisian LM, Zent R, Pozzi A. Increased plasma MMP9 in integrin alpha1-null mice enhances lung metastasis of colon carcinoma cells. Int J Cancer. 2005;116:52–61. doi: 10.1002/ijc.20997. [DOI] [PubMed] [Google Scholar]
- Goldrosen MH. Murine colon adenocarcinoma: immunobiology of metastases. Cancer. 1980;45:1223–1228. doi: 10.1002/1097-0142(19800315)45:5+<1223::aid-cncr2820451330>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Schackert HK, Fidler IJ. Development of an animal model to study the biology of recurrent colorectal cancer originating from mesenteric lymph system metastases. Int J Cancer. 1989;44:177–181. doi: 10.1002/ijc.2910440131. [DOI] [PubMed] [Google Scholar]
- Hamilton SR, Aaltonen LA, editors. IARC Press: Lyons,; Pathology and genetics of tumours of the digestive system. World Health Organization Classification of Tumours. 2000 [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- Spratt JS, Jr, Spjut HJ. Prevalence and prognosis of individual clinical and pathologic variables associated with colorectal carcinoma. Cancer. 1967;20:1976–1985. doi: 10.1002/1097-0142(196711)20:11<1976::aid-cncr2820201125>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Safford KL, Spebar MJ, Rosenthal D. Review of colorectal cancer in patients under age 40 years. Am J Surg. 1981;142:767–769. doi: 10.1016/0002-9610(81)90331-7. [DOI] [PubMed] [Google Scholar]
- De Vita V. Philadelphia: Lippincott,; CancerPrinciples and Practice of Oncology. (ed 7) 2005 [Google Scholar]
- Tsuiji H, Hayashi M, Wynn DM, Irimura T. Expression of mucin-associated sulfo-Lea carbohydrate epitopes on human colon carcinoma cells. Jpn J Cancer Res. 1998;89:1267–1275. doi: 10.1111/j.1349-7006.1998.tb00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Albuquerque Garcia Redondo P, Nakamura CV, de Souza W, Morgado-Diaz JA. Differential expression of sialic acid and N-acetylgalactosamine residues on the cell surface of intestinal epithelial cells according to normal or metastatic potential. J Histochem Cytochem. 2004;52:629–640. doi: 10.1177/002215540405200507. [DOI] [PubMed] [Google Scholar]
- Hewitt RE, McMarlin A, Kleiner D, Wersto R, Martin P, Tsokos M, Stamp GW, Stetler-Stevenson WG. Validation of a model of colon cancer progression. J Pathol. 2000;192:446–454. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH775>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Honda K, Yamada T, Hayashida Y, Idogawa M, Sato S, Hasegawa F, Ino Y, Ono M, Hirohashi S. Actinin-4 increases cell motility and promotes lymph node metastasis of colorectal cancer. Gastroenterology. 2005;128:51–62. doi: 10.1053/j.gastro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Murphy LD, Valverius EM, Tsokos M, Mickley LA, Rosen N, Bates SE. Modulation of EGF receptor expression by differentiating agents in human colon carcinoma cell lines. Cancer Commun. 1990;2:345–355. doi: 10.3727/095535490820874092. [DOI] [PubMed] [Google Scholar]
- Radinsky R, Risin S, Fan D, Dong Z, Bielenberg D, Bucana CD, Fidler IJ. Level and function of epidermal growth factor receptor predict the metastatic potential of human colon carcinoma cells. Clin Cancer Res. 1995;1:19–31. [PubMed] [Google Scholar]
- Kobaek-Larsen M, Thorup I, Diederichsen A, Fenger C, Hoitinga MR. Review of colorectal cancer and its metastases in rodent models: comparative aspects with those in humans. Comp Med. 2000;50:16–26. [PubMed] [Google Scholar]
- Hamad NM, Elconin JH, Karnoub AE, Bai W, Rich JN, Abraham RT, Der CJ, Counter CM. Distinct requirements for Ras oncogenesis in human versus mouse cells. Genes Dev. 2002;16:2045–2057. doi: 10.1101/gad.993902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Radinsky R, Fan D, Tsan R, Bucana CD, Wilmanns C, Fidler IJ. Organ-specific modulation of steady-state mdr gene expression and drug resistance in murine colon cancer cells. J Natl Cancer Inst. 1994;86:913–920. doi: 10.1093/jnci/86.12.913. [DOI] [PubMed] [Google Scholar]
- Ozawa Y, Sugi NH, Nagasu T, Owa T, Watanabe T, Koyanagi N, Yoshino H, Kitoh K, Yoshimatsu K. E7070, a novel sulphonamide agent with potent antitumour activity in vitro and in vivo. Eur J Cancer. 2001;37:2275–2282. doi: 10.1016/s0959-8049(01)00275-1. [DOI] [PubMed] [Google Scholar]
- Zirvi KA, Atabek U. In vitro response of a human colon tumor xenograft and a lung adenocarcinoma cell line to alpha-difluoromethylornithine alone and in combination with 5-fluorouracil and doxorubicin. J Surg Oncol. 1991;48:34–38. doi: 10.1002/jso.2930480107. [DOI] [PubMed] [Google Scholar]
- Witty JP, McDonnell S, Newell KJ, Cannon P, Navre M, Tressler RJ, Matrisian LM. Modulation of matrilysin levels in colon carcinoma cell lines affects tumorigenicity in vivo. Cancer Res. 1994;54:4805–4812. [PubMed] [Google Scholar]
- Goldrosen MH, Paolini N, Jr, Holyoke ED. Description of a murine model of experimental hepatic metastases. J Natl Cancer Inst. 1986;77:823–828. doi: 10.1093/jnci/77.3.823. [DOI] [PubMed] [Google Scholar]
- Bresalier RS, Rockwell RW, Dahiya R, Duh QY, Kim YS. Cell surface sialoprotein alterations in metastatic murine colon cancer cell lines selected in an animal model for colon cancer metastasis. Cancer Res. 1990;50:1299–1307. [PubMed] [Google Scholar]
- Goldrosen MH, Biddle WC, Pancook J, Bakshi S, Vanderheyden JL, Fritzberg AR, Morgan AC, Jr, Foon KA. Biodistribution, pharmacokinetic, and imaging studies with 186Re-labeled NR-LU-10 whole antibody in LS174T colonic tumor-bearing mice. Cancer Res. 1990;50:7973–7978. [PubMed] [Google Scholar]
- Bresalier RS, Schwartz B, Kim YS, Duh QY, Kleinman HK, Sullam PM. The laminin alpha 1 chain Ile-Lys-Val-Ala-Val (IKVAV)-containing peptide promotes liver colonization by human colon cancer cells. Cancer Res. 1995;55:2476–2480. [PubMed] [Google Scholar]
- Bresalier RS, Mazurek N, Sternberg LR, Byrd JC, Yunker CK, Nangia-Makker P, Raz A. Metastasis of human colon cancer is altered by modifying expression of the beta-galactoside-binding protein galectin 3. Gastroenterology. 1998;115:287–296. doi: 10.1016/s0016-5085(98)70195-7. [DOI] [PubMed] [Google Scholar]
- An Z, Wang X, Willmott N, Chander SK, Tickle S, Docherty AJ, Mountain A, Millican AT, Morphy R, Porter JR, Epemolu RO, Kubota T, Moossa AR, Hoffman RM. Conversion of highly malignant colon cancer from an aggressive to a controlled disease by oral administration of a metalloproteinase inhibitor. Clin Exp Metastasis. 1997;15:184–195. doi: 10.1023/a:1018461112732. [DOI] [PubMed] [Google Scholar]
- Rho YS, Lee KT, Jung JC, Yoon C, An Z, Hoffman RM, Chang SG. Efficacy of new platinum analog DPPE in an orthotopic nude mouse model of human colon cancer. Anticancer Res. 1999;19:157–161. [PubMed] [Google Scholar]
- Sun FX, Sasson AR, Jiang P, An Z, Gamagami R, Li L, Moossa AR, Hoffman RM. An ultra-metastatic model of human colon cancer in nude mice. Clin Exp Metastasis. 1999;17:41–48. doi: 10.1023/a:1026442321295. [DOI] [PubMed] [Google Scholar]
- Gabriel WB, Dukes C, Bussey HJR. Lymphatic spread in cancer of the rectum. Br J Surg. 1935;23:395–413. [Google Scholar]
- Grinnell RS. The grading and prognosis of carcinoma of the colon and rectum. Ann Surg. 1939;109:500–533. doi: 10.1097/00000658-193904000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Watson C, Dukes C. The radium problem: III. The treatment of carcinoma of the rectum with radium, with an introduction on the spread of cancer of the rectum. Br J Surg. 1930;17:643–649. [Google Scholar]
- Umpleby HC, Williamson RC. Carcinoma of the large bowel in the first four decades. Br J Surg. 1984;71:272–277. doi: 10.1002/bjs.1800710407. [DOI] [PubMed] [Google Scholar]
- Hoffman RM. Orthotopic metastatic (MetaMouse) models for discovery and development of novel chemotherapy. Methods Mol Med. 2005;111:297–322. doi: 10.1385/1-59259-889-7:297. [DOI] [PubMed] [Google Scholar]
- Morikawa K, Walker SM, Jessup JM, Fidler IJ. In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res. 1988;48:1943–1948. [PubMed] [Google Scholar]
- Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48:6863–6871. [PubMed] [Google Scholar]
- Nakajima M, Morikawa K, Fabra A, Bucana CD, Fidler IJ. Influence of organ environment on extracellular matrix degradative activity and metastasis of human colon carcinoma cells. J Natl Cancer Inst. 1990;82:1890–1898. doi: 10.1093/jnci/82.24.1890. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Orthotopic implantation of human colon carcinomas into nude mice provides a valuable model for the biology and therapy of metastasis. Cancer Metastasis Rev. 1991;10:229–243. doi: 10.1007/BF00050794. [DOI] [PubMed] [Google Scholar]
- Tlsty TD. Stromal cells can contribute oncogenic signals. Semin Cancer Biol. 2001;11:97–104. doi: 10.1006/scbi.2000.0361. [DOI] [PubMed] [Google Scholar]
- Versteeg HH, Spek CA, Peppelenbosch MP, Richel DJ. Tissue factor and cancer metastasis: the role of intracellular and extracellular signaling pathways. Mol Med. 2004;10:6–11. doi: 10.2119/2003-00047.versteeg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- Felding-Habermann B. Integrin adhesion receptors in tumor metastasis. Clin Exp Metastasis. 2003;20:203–13. doi: 10.1023/a:1022983000355. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]