Abstract
When intraperitoneally injected into Swiss mice, Clostridium sordellii lethal toxin reproduces the fatal toxic shock syndrome observed in humans and animals after natural infection. This animal model was used to study the mechanism of lethal toxin-induced death. Histopathological and biochemical analyses identified lung and heart as preferential organs targeted by lethal toxin. Massive extravasation of blood fluid in the thoracic cage, resulting from an increase in lung vascular permeability, generated profound modifications such as animal dehydration, increase in hematocrit, hypoxia, and finally, cardiorespiratory failure. Vascular permeability increase induced by lethal toxin resulted from modifications of lung endothelial cells as evidenced by electron microscopy. Immunohistochemical analysis demonstrated that VE-cadherin, a protein participating in intercellular adherens junctions, was redistributed from membrane to cytosol in lung endothelial cells. No major sign of lethal toxin-induced inflammation was observed that could participate in the toxic shock syndrome. The main effect of the lethal toxin is the glucosylation-dependent inactivation of small GTPases, in particular Rac, which is involved in actin polymerization occurring in vivo in lungs leading to E-cadherin junction destabilization. We conclude that the cells most susceptible to lethal toxin are lung vascular endothelial cells, the adherens junctions of which were altered after intoxication.
Clostridium sordellii is a gram-positive spore-forming bacterium and obligatory anaerobe from the environment and occasionally from animal and human intestine. This Clostridium is responsible for myonecrosis and gangrene in humans, which once accounted for up to 4% of Clostridium myonecrosis.1 Today, myonecrosis attributable to C. sordellii is infrequent except in injection drug users.2 Infection by this bacterium is characterized by a marked local edema, no or weak hemolysis, variable production of gas, severe hypotension, and shock. Sporadic cases of C. sordellii infections are more common in women after postpartum wound, endometritis, or postabortion disease and, more recently, in fatal toxic shock syndrome after medical abortion characterized by irreversible hypotension, apyrexia, hemoconcentration with hyperproteinemia, leukocytosis, high hematocrit,3,4 pleural effusions, and sero-sanguinous ascites.5,6,7,8 Although rare, these cases are always dramatic. In addition, C. sordellii is responsible for bacteremia and arthritis resulting in a high rate of mortality.9,10 In contrast, C. sordellii diseases are more frequent in animals, leading to large outbreaks of enterotoxemia, mainly in sheep and lambs,11,12,13,14 and sporadic cases of necrotic and hemorrhagic enteritis in cattle.15 C. sordellii toxic infections in sheep and lambs most often result in sudden death, without characteristic postmortem abnormalities except a marked edema and emphysema of the abomasum wall in some animals.16 The most relevant feature of C. sordellii pathologies in humans and animals, whatever the initial site of infection, consists of a rapid and fatal toxic shock syndrome, indicating a major role for toxin(s) in the onset of the disease. However, the exact cause of death has not yet been deciphered.
C. sordellii produces several virulence factors, including lethal toxin (TcsL), hemorrhagic toxin, phospholipases, extracellular proteases, hemolysins, DNase, and cytotoxin, but the major one remains TcsL, also called edema-producing toxin.17 TcsL is responsible for fatal outcome after C. sordellii infections. It is one of the most potent toxins. Indeed, its mouse lethal dose (MLD)/kg was determined to be 150 ng.18 Only Clostridium botulinum and Clostridium tetani toxins were found to be more potent than TcsL (MLD/kg ∼1 ng).19 When injected intradermally, purified TcsL was shown to be erythematous and edematous. TcsL belongs to the family of large clostridial cytotoxins.20 It is a single chain protein with a molecular mass of 250 kd that is active intracellularly and contains three functional domains.21,22 The C-terminal domain contains multiple repeated sequences and is involved in cell surface receptor recognition. The central part contains a hydrophobic segment and mediates the translocation into the cytosol of the N-terminal part across the endosomal membrane. The N-terminal part contains the enzymatic site and supports the intracellular activity. TcsL catalyzes the glucosylation of small GTPases from UDP-glucose. The specific targets of TcsL from strain IP82 (TcsL-82) are Ras, which is inactivated on glucosylation at Thr-35, Rap, Ral, and Rac at Thr-37.23,24,25,26 The cellular effects of TcsL-82 have been studied using cell lines of various origins and all cell lines tested seemed sensitive to this toxin. So far, the cell modifications reported, cell rounding and depolymerization of actin cytoskeleton, involve the enzyme activity and the subsequent inactivation of small GTPases.
The present in vivo study was thereby designed to characterize the essential cause of the fatal issue in a mammalian organism intoxicated with purified TcsL-82 and to determine whether specific target cells for TcsL-82 exist in animals. We show that edema occurred mainly in lung and heart after TcsL-82 intoxication and that most symptoms arose from a major increase in vascular permeability observed at the lung level because of E-cadherin junction disorganization subsequent to in vivo glucosylation of small GTPases in lung.
Materials and Methods
Toxin
Toxin produced by C. sordellii IP82 (TcsL-82) was purified as previously described.18,27 TcsL-82 was diluted in Hanks’ balanced salt solution (HBSS) containing 0.1% bovine serum albumin and sterilized by filtration on a 0.22-μm Millex filter (Millipore S.A.S., Molsheim, France). MLD of the TcsL-82 preparation used in this study was assayed as 15 ng/animal.
Animals
Male Swiss mice, 3 to 4 weeks of age (16 g), were purchased from Charles Rivers (Les Oncins, France) and were kept for 2 weeks before use (approximate weight at 6 weeks = 20 g). Mice were injected intraperitoneally with 0.5 ml of HBSS supplemented with 0.1% bovine serum albumin containing 15 ng of TcsL-82. At varying times after intoxication, animals were euthanized by an intraperitoneal injection of 0.1 ml of a mixture made of 0.5 ml of Rompun 2% (xylazine; Roche Diagnostics, Indianapolis, IN) and 0.25 ml of Imalgene 1000 (ketamine chlorhydrate; Merial, Lyon, France) completed to 2 ml with phosphate-buffered saline (PBS). After animal opening, fluid present in the thoracic cavity was collected for analysis. Animals were terminally bled before organs were collected, and/or necropsies were performed. Blood samples were collected either on ethylenediaminetetraacetic acid (10 μl at 0.5 mol/L) or on dry tubes. Control mice were injected with the diluent only and processed as described above for intoxicated mice.
Histopathology and Immunohistochemistry
Histology was performed on each mouse. In mice euthanized 2, 6, and 16 hours after TcsL-82 injection, the main organs, including liver, spleen, lung, heart, thymus, adrenals, brain, mesenteric lymph nodes, and intestine, were collected. Organs were fixed in a freshly made 10% Zn2+ salt solution made of 0.1 mol/L Tris buffer containing calcium acetate (3.4 mmol/L) (pH 7.4). Fixed tissues were embedded in low-melting point fusion paraffin as described,28 cut in 3-μm sections, and stained with hematoxylin and eosin (H&E) for light microscopic examination by a board certified pathologist. To determine whether TcsL-82 would damage the brain-blood barrier, an experiment was performed to investigate whether brain tissue was a target for TcsL-82. In this experiment, organs, including brain, were fixed by intravenous injection of 4% paraformaldehyde (PFA) in PBS into anesthetized mice and then embedded in paraffin for routine histology.
For immunohistochemistry, lungs and hearts were embedded with OCT compound (Sakura Fineteck Europe, Zoeterwoude, The Netherlands). Both organs were plunged for ∼30 seconds into 2-methylbutane prechilled in liquid nitrogen. After freezing, organs were stored in a −80°C freezer until sectioning. Five-μm frozen organ sections were dried at room temperature and fixed by incubation in 4% paraformaldehyde for 20 minutes. After washes (3 × 5 minutes) in PBS, tissues were quenched for 10 minutes in 50 mmol/L NH4Cl in PBS and blocked in PBS containing 1 mmol/L Ca2+, 1% bovine serum albumin, and 0.2% gelatin. Tissues were then incubated with the primary antibodies diluted in the permeabilizing buffer (PBS with 1 mg/ml bovine serum albumin and 0.05% saponin) for 1 hour. Rat monoclonal antibodies specific for mouse VE-cadherin and anti CD-144 (Pharmingen, BD Biosciences Europe, Lille, France) were used as primary antibodies. After washes (3 × 5 minutes) in the permeabilizing buffer, proteins were revealed by incubation of the tissues for 1 hour with fluorescein isothiocyanate-conjugated goat anti-rat IgG (H+L) from Cappel Laboratories (Durham, NC) and tetramethyl-rhodamine isothiocyanate-phalloidin from Sigma when indicated, diluted in the permeabilizing buffer. Finally, the slides were washed in PBS (3 × 5 minutes), mounted in a Mowiol solution, and confocal microscopy was performed using a microscope (model TCS4D; Leica, Wetzlar, Germany) equipped with a ×40 lens. Z series of optical sections were acquired at 0.7 μm. Fluorescein isothiocyanate and tetramethyl-rhodamine isothiocyanate emissions were collected separately to avoid fluorescence passage from one channel to another.
Ratios of Wet/Dry Weights of Lungs and Hearts
After death, lungs and hearts from control and 6-hour intoxicated mice were collected and weighted on a precision balance. Organs were dried in an oven at 110°C overnight (until their weight stabilized). Ratios of wet-to-dry weights were calculated for lung and heart of each mouse.
Electron Microscopy
Blocks of tissue were fixed by immediate immersion after preparation in 2.5% glutaraldehyde in 0.1 mol/L cacodylate buffer. Fixation was continued overnight at 4°C. Samples were then rinsed three times with 0.1 mol/L cacodylate buffer, postfixed with 1% osmium tetroxide in 0.1 mol/L cacodylate buffer for 90 minutes, and afterward rinsed with distilled water. Samples were then dehydrated through a graded series of ethanol (25 to 100%), embedded in spur resin, and polymerized at 60°C for 48 hours. Ultrathin sections (70 to 80 nm) were cut with a diamond knife, stained with uranyl acetate and lead citrate, and viewed in a Jeol JEM 1010 transmission electron microscope (Tokyo, Japan) at 80 kv accelerating voltage. Images were recorded using an Eloise Mega View III camera and the Analysis Pro software version 3.1 (Eloïse SARL, Roissy, France). Histology and electron microscopy sections were analyzed for morphometry using the Histolab system (CE1750; Microvision Instruments, Evry, France) and AnalySIS Pro Software version 5.0 (Eloïse SARL).
In Vivo Glucosylation by TcsL-82 of Small GTP-Binding Protein
Lungs and livers from control and TcsL-82-intoxicated mice (15 ng i.p.) were homogenized in 1 and 2 ml, respectively, of lysis buffer, pH 7.4, containing 0.5% Triton X-100, 20 mmol/L 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 50 mmol/L NaCl, 5 mmol/L MgCl2, 1 mmol/L ethylene glycol bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 0.1 mmol/L orthovanadate, 10 μmol/L GDP, and protease inhibitor mixture (Complete; Boehringer Ingelheim France S.A.S., Paris, France). Organ lysates were centrifuged at 400 × g. In vitro glucosylation was achieved as previously reported.29 In brief, samples containing 50 μg of total protein were used with 7 μmol/L [14C]UDP-glucose (DuPont NEN, Boston, MA) and 1 μg of TcsL-82 in a final volume of 20 μl. After incubation (1 hour at 37°C), proteins were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (15%). After staining and destaining, the gel was dried and autoradiographed for several weeks.
Hematological Parameters and Blood Chemistry Analysis
Hematological parameters and differential leukocyte counts were analyzed on samples collected on ethylenediaminetetraacetic acid using the cell automated complete blood Vet’ABC counter (SCIL, Viernheim, Germany). After blood clotting, sera were collected by centrifugation for 10 minutes at 500 × g in a microcentrifuge (Eppendorf France SARL, Le Pecq, France), and serum chemistry analyses for renal (blood urea nitrogen and creatinine concentrations) and hepatic (alanine and asparagine transferase concentrations) functions were performed using a VetTest analyzer (IDEXX, Cergy-Pontoise, France). The same apparatus was used to measure albumin concentration in sera and thoracic fluids.
Vascular Permeability Assay
Evans blue dye (30 mg/kg in 100 μl of PBS) was injected into the tail vein of 18- to 20-g Swiss mice. Thirty minutes after injection of the dye, animals were sacrificed as detailed above. The volume of fluid present in the thoracic cavity was measured and then dried under vacuum in a Speedvac centrifuge (GMI, Inc., Ramsey, MI). The Evans blue dye present in the fluid was extracted overnight with 1 ml of formamide at 55°C and measured spectrophotometrically at 600 nm. Likewise, the background level was measured for each time point by similarly processing lung fluids from intoxicated animals that did not receive Evans blue dye. Control mice were intraperitoneally injected with 0.5 ml of toxin diluent (HBSS). One half-hour before sacrificing, mice (two control mice for each time point) were intravenously injected with Evans blue and further processed as intoxicated mice. Data are expressed as means ± SD.
Erythropoietin (EPO), Troponin-I, Chemokines, Cytokines, and Nitric Oxide Measurements
Aliquots of sera from mice treated for 0.5 to 6 hours with TcsL-82 were kept at −80°C until analysis. Lung and liver from the same animals were put in 1 and 2 ml of ice-cold Dulbecco’s modified Eagle’s medium, respectively, immediately homogenized on ice and centrifuged at 2000 × g for 30 minutes at +4°C. Supernatants were aliquoted and kept at −80°C until use. Mouse EPO, tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, KC, MIP-1α, MIP-2, and JE/MCP-1 enzyme-linked immunosorbent assay (ELISA) detection kits were from R&D Systems, mouse cardiac troponin-I (cTn-I) ELISA kit was from Life Diagnostics Inc. (West Chester, PA). All these molecules were measured in serum samples and/or in organs following the manufacturer’s instructions.
Nitrite (NO2−) and nitrate (NO3−) measurements were performed using the nitric oxide quantification kit (Active Motif, Carlsbad, CA), following the manufacturer’s instructions. All samples were filtered at +4°C through a 10,000-d micropore filter (Millipore) before assay and were diluted twofold in the assay buffer.
Statistical Analysis
Hematological and chemical data were analyzed using GraphPad Prism 4 (GraphPad Software Inc., San Diego, CA), and hematological data were expressed as mean ± SE. Statistical analyses were performed with the Wilcoxon signed-rank test arbitrarily using the median of controls as the hypothetical value for comparison. P values equal or smaller than 0.05 were considered as significant.
Results
In the present study, lethal effect of TcsL-82 was investigated in mice after an intraperitoneal injection of 1 MLD (a dose that kills a mouse in ∼24 hours).
Pathological and Histopathological Findings in Mice on Intraperitoneal Injection of TcsL-82
Pathology
As early as 6 hours after intoxication, six of eight mice were less mobile and displayed symptoms of ataxia. Eyes of these animals were deep set in the orbital, indicating dehydration (not shown). Five animals had darkened tails (Figure 1A). After 16 hours, one animal was normal, one was dead, and the other six had the same signs as after 6 hours.
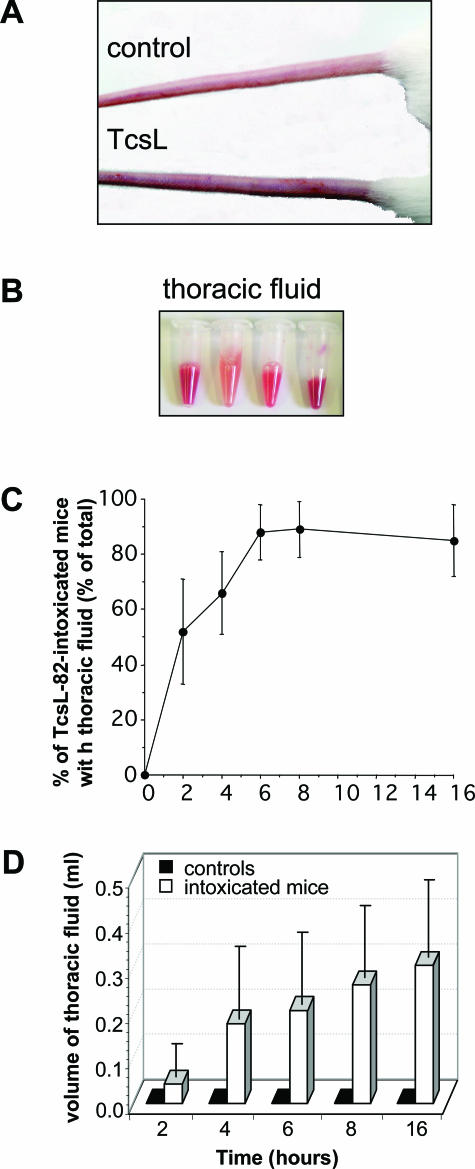
Figure 1.
TcsL-82 induces anoxia in mice and fluid accumulation in the thoracic cavity. After intoxication for 6 hours with intraperitoneally injected TcsL-82 (15 ng/mouse), mice exhibit signs of anoxia such as darkened extremities (A) and various volumes of serohemorrhagic fluid were collected in the thoracic cage (B). In three different experiments, six to eight mice were sacrificed at various times after intraperitoneal injection of TcsL-82. For each experiment, the percentage of mice with fluid in the thoracic cage was calculated (C), and the volume of fluid present in the thoracic cage was measured (open bars) (D). Two control mice, injected with the diluent only, were euthanized at each time point. No fluid was collected from the thoracic cage of control mice (black squares). Results in C and D represent the mean ± SEM of the three different experiments.
Histopathology
Macroscopic Findings: Starting at 2 hours after TcsL-82 intoxication, a clear cell-free and straw-colored serous fluid was collected from the thoracic cavity of 50% of the mice (Figure 1B). This fluid coagulated rapidly. Throughout time, the percentage of animals with fluid in the thoracic cavity increased, reaching 60% after 4 hours, 75% after 6 hours, and 87% later (Figure 1C). The volume of the thoracic cavity fluid was between 15 and 100 μl after 2 hours, it increased throughout time to 50 to 350 μl after 4 hours and reached 100 to 500 μl and 200 to 600 μl after 6 and 16 hours, respectively (Figure 1D). After 6 hours and more, the exudate was serohemorrhagic in several mice and contained varying amounts of red cells (Figure 1B). Local edema at the site of injection has been reported to be the main characteristic during C. sordellii infection, and the toxin responsible has been previously named edematous toxin.17 At 6 hours, fluid was collected also from the peritoneum cavity of three animals. Congestion of the parietal peritoneum was markedly greater at 16 hours. There was no gross lesion within thymus, lymph nodes, spleen, gastrointestinal system, brain, liver, or kidneys, and especially there were no hemorrhages, infarctions, or abscesses.
Microscopic Findings: All of the organs were examined in control and in mice intoxicated with TcsL-82 for 2, 6, or 16 hours. After intoxication, lesions occurred mainly in lung and heart as described below.
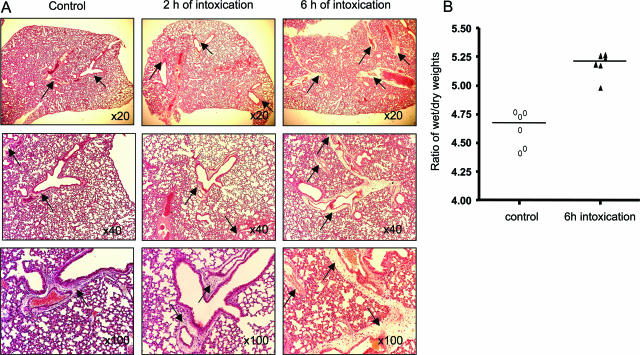
Lungs: As reported in Figure 2A, some edema was observed in control mice near a few large vessels, but fluid extravasation was very limited and likely attributable to the protocol of euthanasia. The most obvious lesion observed in mice receiving TcsL-82 was edema surrounding pulmonary vessels already after 2 hours, which became abundant and extended to most lung vessels after 6 hours of intoxication. To evaluate the importance of edema in lungs of intoxicated mice, the ratios between wet and dry weights of lungs were measured in control and 6-hour intoxicated mice as detailed in Materials and Methods. As shown in Figure 2B, the wet-to-dry weight ratio increased moderately in lungs of intoxicated mice compared with that of controls. This increase was statistically significant (P = 0.0156) using the Wilcoxon signed-rank test. This result fits with the absence of massive alveolar edema. To confirm further the extravasation of fluid in lungs observed at the periphery of vessels, the ratio of perivascular to vascular plus perivascular areas was measured in lung sections and compared with that of control mice. As reported in Table 1, a statistically significant increase in the ratio was observed already after 2 hours intoxication and was markedly greater after 6 hours, confirming that the extravasation process had already started at 2 hours. Another criterion was also used to measure the importance of edema in lung tissue, the light intensity (percentage of absorption), which inversely correlates with edema. It was measured in 20 fields for each time point. The mean of intensity was 71.4 ± 20.2 in control lung and decreased to 63.6 ± 15 and 69.7 ± 14.5 at 2 hours and 6 hours intoxication, respectively. However, in lung tissue, the difference in light intensity between control and intoxicated mice was not significant because of important variations among fields and to the limited alveolar edema that was primarily observed at the vessel periphery. In rare areas, an infiltrate of macrophages, monocytes, and polymorphonuclear cells was observed after 2 and 6 hours of intoxication, (data not shown). No other lung lesion, such as modification of bronchia, was observed at any time up to 16 hours of intoxication.
Figure 2.
Histopathological analysis of the lung of TcsL-82-treated Swiss mice. A: H&E staining of lungs from HBSS-injected control mice and from mice intoxicated for 2 and 6 hours with intraperitoneally injected TcsL-82 (15 ng/mouse). Lungs were examined with ×20, ×40, and ×100 lenses. Perivascular space corresponding to the area where exudate is observed after intoxication (×20 lens and higher magnifications) is very limited in control lung, increased already after 2 hours, and is massive after 6 hours of intoxication (arrows). B: Ratios of wet-to-dry weights of lung from control and intoxicated mice with TcsL-82 (15 ng/mouse, i.p.). Horizontal bars indicate the median values. The values of control versus intoxicated mice were analyzed by the Wilcoxon signed-rank test using arbitrarily the median of the control population as the hypothetical value for comparison; P = 0.0156.
Table 1.
Analysis of Modifications in Lung Tissue Observed in Histology and Electron Microscopy
| Analysis of lung edema in histological sections | Control (n = 13) | 2 hours intoxication (n = 10) | 6 hours intoxication (n = 11) |
|---|---|---|---|
| Ratio of perivascular area/vascular + perivascular area (mean ± SD)* | 0.133 ± 0.18 | 0.342 ± 0.25 | O.61 ± 0.19 |
| P (control/intoxicated mice)† | 0.0049 | 0.0005 | |
| Analysis of lung vessel endothelial cells (trans-electron microscopy) | Control | 6 hours intoxication | **P (control/intoxicated mice) |
| Length of cell-cell juxtaposition (nm) (mean ± SD) (See Figure 4, 6 hours of intoxication, high magnification) | (n = 22) 735.61 ± 337.46 | (n = 13) 1064.30 ± 508.24 | 0.0009 |
| Length of adherens junctions (high density) (nm) (mean ± SD) (see Figure 4, control, high magnification) | (n = 22) 458.30 ± 222.97 | (n = 13) 183.61 ± 141.84 | 0.0001 |
| Vesicles in 13 mm2 endothelial cell areas (mean ± SD) | (n = 15) 28 ± 6.8 | (n = 15) 56 ± 12.3 | <0.0001 |
The perivascular area corresponds to the edema area.
Determined by the Wilcoxon rank-signed test (control/intoxicated mice), using the control median as the hypothetical value.
n, number of measurements.
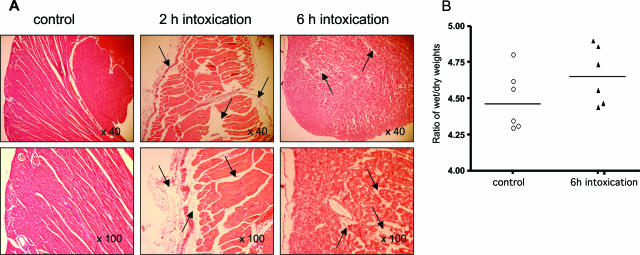
Heart: As reported in Figure 3, the structure of the heart appeared dilacerated already after 2 hours of intoxication. Edema was observed in the endocardium, and the pericardium was dissociated from the myocardium by fluid (Figure 3; ×40 and ×100, 2 hours). Edema was also observed after 6 hours of intoxication. At that time, most cells were dissociated by edema and the fibrillar structure of heart muscle was no longer observed (Figure 3; ×40 and ×100, 6 hours). In hearts, however, no edema was observed around vessels. Congestion and hemorrhages were observed in some area between myocytes (data not shown). Although cardiac lesions because of edema infiltration were observed in most intoxicated mice, their localization and extension varied among animals. It is noticeable that there was no myocardial infarction nor disseminated intravascular coagulation nor any thrombosis. Neither endothelium lesion nor contraction band in myocardium as observed in hearts suffering from hypoxia30 was evidenced. Evaluation of the importance of edema in hearts of intoxicated mice was also assessed by the ratio of wet-to-dry weights in control and 6-hour intoxicated mice. Although the ratio of wet-to-dry weights of heart increased moderately after intoxication (Figure 3B) reflecting the absence of a massive pericarditis, a statistically significant difference was observed between intoxicated mice and controls (P = 0.047). Light intensity was also measured in heart sections. Twenty different fields were analyzed for each time point. The mean of intensity ± SD which was 59.95 ± 5.7 in control, decreased in intoxicated mice to reach 56.6 ± 5.2 and 54.25 ± 5 after 2 and 6 hours of intoxication, respectively. The decrease in light intensity was statistically significant in intoxicated heart tissue (P = 0.03 and P = 0.001 after 2 and 6 hours of intoxication, respectively). Cardiac damages were further investigated by measuring the level of cardiac troponin-I (cTn-I) in the plasma of 16 mice intoxicated for 6 hours with TcsL-82. cTn-I, a serum marker with high sensitivity and specificity of cardiac myocyte injury,31 was not detectable in any control; in contrast, it was present in the plasma of 10 mice intoxicated with TcsL-82 for 6 hours, with values ranging from 0.45 to 280 ng/ml.
Figure 3.
Histopathological analysis of the hearts of TcsL-82-treated Swiss mice. A: H&E staining of hearts from HBSS-injected control mice as in Figure 2 and from mice intoxicated for 2 and 6 hours with intraperitoneally injected TcsL-82 (15 ng/mouse). Hearts were examined with ×40 and ×100 lenses. Exudate (arrows) is observed at the pericardium and endocardium levels and infiltrates heart muscle, which appears roughly dilacerated after 2 hours. After 6 hours of intoxication, myocardiocytes appear individualized by exudate. B: Ratios of wet-to-dry weight of lung from control and 6-hour intoxicated mice. Horizontal bars indicate the median values. The values of control versus intoxicated mice were analyzed by the Wilcoxon signed-rank test using arbitrarily the median of the control population as the hypothetical value for comparison; P = 0.047.
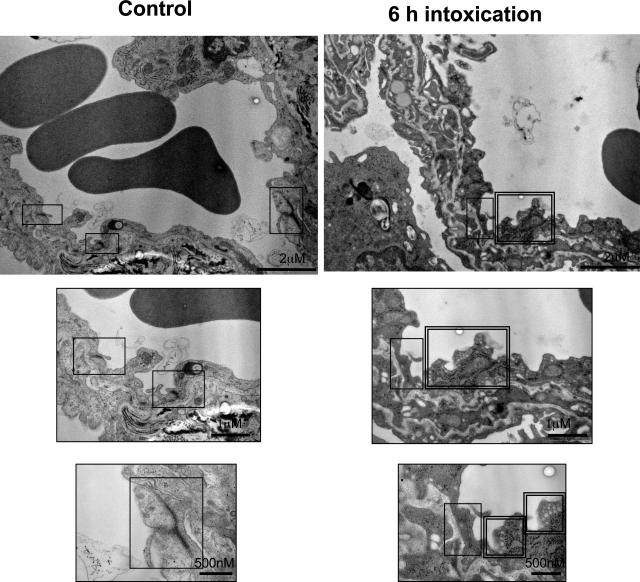
TcsL-82-dependent changes in lung vessels were further investigated by electron microscopy. After 6 hours of intoxication, major changes consisted in alterations of endothelial cells. At a small magnification (×24,000), lung vessel walls were more serrated with endothelial cells showing deeper crypts than in control (Figure 4, A and B). At a higher magnification (×100,000), intercellular junctions, which were well defined with a dense and continuous delineation between endothelial cells from control lung vessel, were faint and discontinuous in endothelial cells from TcsL-82-intoxicated mice (single frame). Moreover, after 6 hours of intoxication, endothelial cells presented a higher number of small vesicles (double frame) than that of control mice, suggesting an increase in intracellular membrane trafficking. As reported in Table 1, morphometric analysis of lung endothelial cells confirmed the disruption of adherens junctions and the increase in their number of vesicles.
Figure 4.
Transelectron microscopy analysis of lung vessels in a control mouse and a mouse intraperitoneally injected with TcsL-82 (15 ng/mouse) 6 hours before organ fixation (as in Figure 2). Top images are general views showing a highly modified aspect of the vessel after TcsL-82 intoxication. As visualized on enlarged endothelial structures (middle and bottom images), in the intoxicated mouse staining of intercellular junctions (single frame) is weaker than in control. Intracellular vesicles (double frame) are abundant in endothelial cells from intoxicated mouse. Original magnifications, ×24,000 (top).
TcsL-82 Induces Alteration of Hematological Parameters
As reported in Table 2, major hematological modifications in all parameters were observed in mice intoxicated for 6 hours with TcsL-82. Red cell counts, hemoglobin content, and hematocrit, all showed highly significant increases (P = 0.0002) after 6 hours of intoxication with TcsL-82 compared with control mice. Differential counts of white cells also showed a significant increase in granulocytes (P = 0.0002) and monocytes (P = 0.0005) and a decrease in the counts of platelets (P = 0.0012).
Table 2.
TcsL-82 Modifies Hematological Parameters
| Hematological parameters | Control mice (n = 10) | TcsL-82 intoxicated mice (n = 12) | Wilcoxon signed-rank test P value (two-tailed) |
|---|---|---|---|
| Hemoglobin (g/dl) | 11.06 ± 1.72 | 15.38 ± 2.06 | 0.0002 |
| Hematocrit (%) | 36.47 ± 6.39 | 50.71 ± 4.9 | 0.0002 |
| Red blood cells (103/mm3) | 6.22 ± 1.07 | 8.51 ± 0.72 | 0.0002 |
| White cells (103/mm3) | 5.40 ± 2.5 | 8.39 ± 2.8 | 0.0017 |
| Granulocytes (×103/mm3) | 1.22 ± 0.62 | 2.37 ± 1.08 | 0.0002 |
| Platelets (×103/mm3) | 1204 ± 388 | 644 ± 397 | 0.0012 |
| Monocytes (×103/mm3) | 0.23 ± 0.1 | 0.65 ± 027 | 0.0005 |
| Lymphocytes (×103/mm3) | 4.1 ± 1.8 | 5.5 ± 1.9 | 0.076 (NS) |
Hematological parameters and differential leukocyte counts were measured in control and in mice intoxicated for 6 hours with TcsL-82 (15 ng i.p.). For that purpose, blood samples were collected on EDTA and a cell automated complete blood Vet’ABC blood counter was used. n, the number of mice analyzed in each group. Results were analyzed by the Wilcoxon signed-rank test using arbitrarily the median of controls as the hypothetical value for comparison. The P value is given and considered as significant when equal or smaller than 0.05.
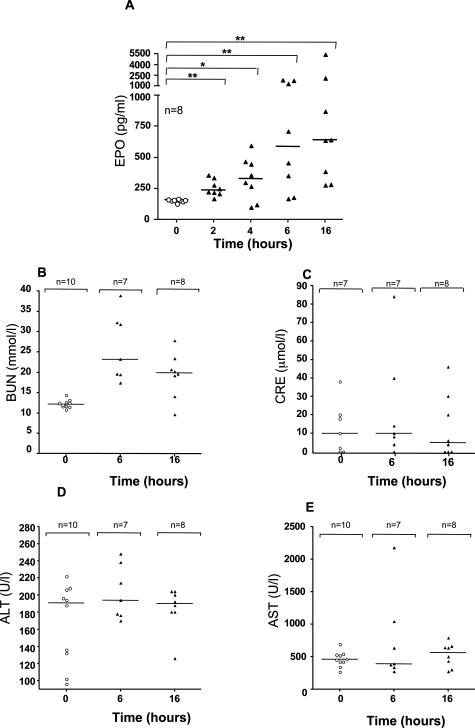
TcsL-82 Induces an Increase in the Level of Serum EPO, Does Not Modify Renal or Hepatic Functions, and Alters Vascular Permeability
Because mice showed signs of hypoxia and because major modifications concerned lung and heart, we investigated whether TcsL-82 intoxication induced a change in serum EPO content. As reported in Figure 5A, an increase in EPO was evidenced in a high percentage of mice intoxicated with TcsL-82. With time, the mean value of EPO increased (up to 6 hours). Statistical analysis indicated that EPO had already significantly increased 2 hours after intoxication (P = 0.0039), and the increase remained significant until and including 16 hours of intoxication.
Figure 5.
TcsL-82-induced plasma EPO increases and does not modify renal and hepatic functions. Mice were intraperitoneally injected with 0.5 ml of HBSS containing or not TcsL-82 (15 ng/mouse). At the indicated times, eight mice were euthanized, and sera were collected. The amount of EPO present in plasma of control or TcsL-82-intoxicated mice was estimated by ELISA using a R&D kit as indicated in the Materials and Methods section. Horizontal bars indicate the mean values. The Wilcoxon signed-rank test was performed between the values of the control versus intoxicated mice at each time point using arbitrarily the median of control batch as the hypothetical value for comparison. The probability P of a significant difference in TcsL-82-intoxicated mice is indicated: *0.05 > P > 0.01, **0.01 > P > 0.001 for each time point. Renal and hepatic functions were analyzed by measuring on the one hand BUN and CRE and, on the other hand, alanine aminotransferase and aspartate aminotransferase from serum at 0, 6, and 16 hours after intraperitoneal injection of 15 ng of TcsL-82. n represents the number of sera studied for each group.
Renal function was investigated by analysis of blood urea nitrogen (BUN) and serum creatinine (CRE) measurements. BUN was increased in TcsL-82-treated animals (Figure 5B). However, BUN changes independent of CRE are not indicative of renal dysfunction. Serum CRE is a more specific determinant of renal function, because unlike BUN level, which often reflects altered metabolism or urination, its level is not affected by protein metabolism or hydration status. No significant change in CRE was evidenced in TcsL-82-injected mice at the times studied, with only one mouse falling outside the 0.1 to 1.1 mg/dl serum CRE range (Figure 5C) indicating that renal function was not modified in intoxicated mice. Therefore, modification in BUN level in TcsL-82-intoxicated mice is likely to be the consequence of the major increase in vascular permeability and associated dehydration. Except in one mouse, no significant modification in the two liver function indicators, serum alanine aminotransferase and aspartate aminotransferase, was observed at 6 or 16 hours after intoxication with TcsL-82 compared with controls (Figure 5, D and E).
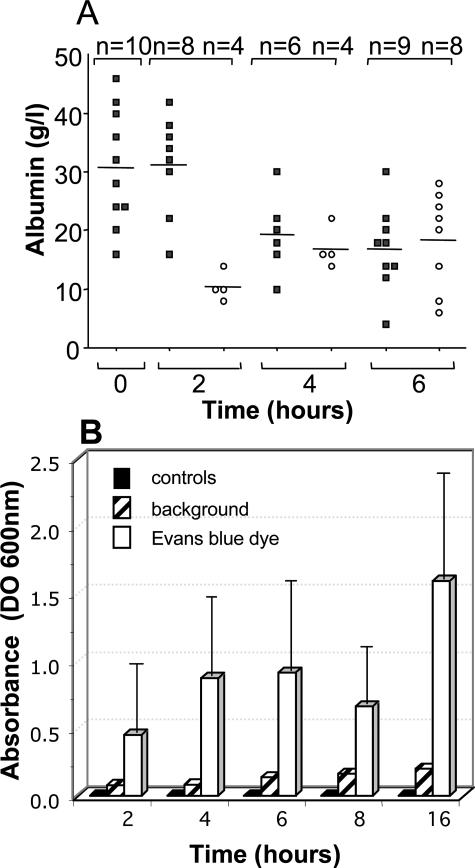
TcsL-82 effect on vascular permeability was investigated using two approaches. First of all, concentrations of albumin in serum and in lung fluid were measured throughout time. As shown in Figure 6A, albumin concentration in serum was stable for the first 2 hours and then decreased. After 6 hours of intoxication, serum albumin level was approximately half of that measured in control mice. Albumin level in lung fluid amounted to only one third of that in serum after 2 hours and increased progressively to reach the serum level after 6 hours. Second, we injected Evans blue dye in the tail vein 30 minutes before animal sacrifice to measure extravasation. In control mice, no fluid was collected and therefore Evans blue dye extravasation was considered as zero. All fluids collected from TcsL-82-intoxicated mice at various times contained Evans blue. As reported in Figure 6B, the amount of Evans blue dye present in the thoracic fluid increased progressively and approximately in parallel with the increase in lung fluid volume (Figure 1D) confirming that TcsL-82 induced an early increase in vascular permeability of the respiratory tract when intraperitoneally injected.
Figure 6.
Albumin in serum and thoracic fluid and vascular permeability measurements. A: Albumin was measured in the serum (S, squares) and in the thoracic fluid (L, circles) in animals injected with TcsL-82 (15 ng/mouse) 0, 2, 4, and 6 hours before sacrifice. The number (n) of sera and thoracic fluids analyzed ranged from 6 to 10 and 4 to 8, respectively, for each time point as indicated. The mean is indicated (horizontal bars). B: Vascular permeability was estimated by measuring the amount of Evans blue present in the thoracic fluid. For that purpose, 15 to 30 minutes before mouse sacrifice, Evans blue dye was injected intravenously, and its amount present in the thoracic fluid was quantified by spectrophotometric analysis at 600 nm (open bars). The relevant background was the spectrophotometric analysis of the thoracic fluid present in mice not injected with Evans blue dye (striped bars). For each time point, two control mice were injected with the diluent only and with Evans blue 30 minutes before being euthanized. No fluid was collected from the thoracic cage of control mice (see Figure 1D), and therefore no Evans blue was measurable (black squares). The graphs are the means ± SD of three different experiments with four mice at each time point.
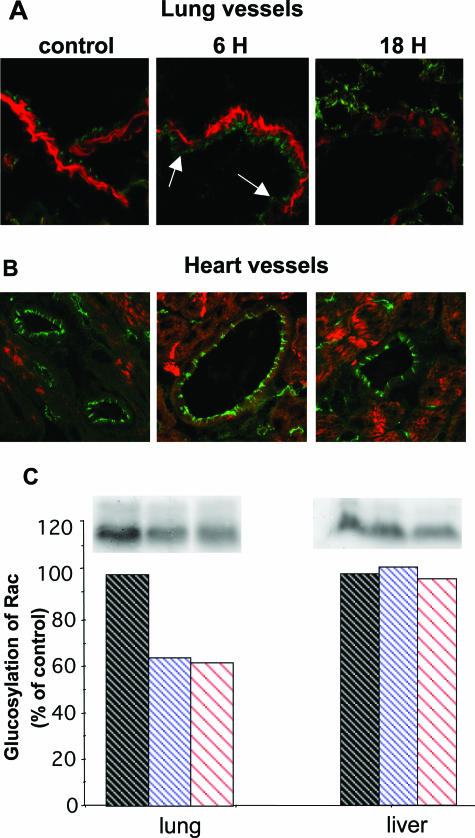
Because major modifications of lung vascular permeability seemed to be the cause of TcsL-82-induced edema, we further investigated whether TcsL-82 could induce modification of the intercellular endothelial cell junctions, by monitoring VE-cadherin immunostaining. In control lung VE-cadherin, a major protein of adherens junctions, regularly decorated cells along the borders of vessels. After 6 hours of intoxication, VE-cadherin immunostaining was irregular and diffuse while fluorescent intensity was weak (Figure 7A, arrows), probably reflecting a redistribution of VE-cadherin molecules from the junctional ring to the cytoplasm. After 16 hours, the pattern of VE-cadherin was markedly altered. Indeed, at that time, a diffuse and weak staining was observed in the cytosol. Such a change in VE-cadherin distribution suggests a major modification in adherens junctions. Moreover, muscle cells surrounding lung vessels and stained with phalloidin showed a marked decrease in actin filaments 16 hours after intoxication. In contrast, no obvious modification in endothelial cell decoration was observed in hearts of TcsL-82-treated mice throughout a period of 16 hours (Figure 7B).
Figure 7.
TcsL-82 modifies adherens junctions and actin cytoskeleton in lung vessels and glucosylates small GTPases in lung. Lung (A) and heart (B) vessel cryosections of control and TcsL-82-treated mice for 6 and 18 hours were analyzed with an anti-mouse VE-cadherin detected with a relevant secondary antibody coupled to fluorescein isothiocyanate, and with tetramethyl-rhodamine isothiocyanate phalloidin. C: Lungs and liver of mice intoxicated for 0, 6, and 16 hours with intraperitoneally injected TcsL-82 (15 ng/mouse) were homogenized and glucosylation with [14C]UDP-glucose in the presence of TcsL-82 was performed as previously reported.28 Autoradiograph of the gel shows the amount of in vivo glucosylated GTPases after TcsL-82 intoxication. Quantification of the radiolabeled bands was performed using Denylab 2.5.2. software (bottom; DynaLab Inc., Hong Kong). The experiments shown are representative of three different ones.
We also studied the in vivo level of small GTPase glucosylation in lung and liver. As shown in Figure 7C, control lung and liver preparations exhibited radiolabeled glucosylation of 20- to 22-kd proteins ranging for low-molecular mass GTPases. In contrast, weaker glucosylation (30 to 40% less) was observed in lung preparations from TcsL-82-intoxicated mice. These results indicated that in vivo TcsL-82 glucosylates small GTPases in lung, thus inhibiting incorporation of [14C]UDP glucose into those substrates during the subsequent in vitro incubation with the toxin. Lung, which is apparently the most sensitive organ to TcsL-82, includes several tissues and various cell types. Only a restricted percentage of GTPases were glucosylated in vivo after intraperitoneal intoxication, indicating that only a subpopulation of cells was highly sensitive to the toxin. In liver, which is an organ not damaged after intoxication, no change in the level of glucosylation was observed in preparations from intoxicated mice compared with that from control. Although TcsL-82 was shown to induce apoptosis,32 histological analysis revealed only a few apoptotic bodies in macrophages present in the thymus cortex and a limited number of apoptotic cells in alveolar tissue after 6 hours of TcsL-82 intoxication using terminal dUTP nick-end labeling (TUNEL) assay (data not shown). No major sign of necrosis was observed in other organs. Thus, apoptosis is unlikely to be a major process in death induced by TcsL-82.
TcsL-82 Triggers a Limited Inflammatory Response
Some bacterial toxins have been reported to generate inflammation. This is the case for various superantigen toxins that exhibit highly potent polyclonal lymphocyte-transforming properties and initiate massive release of proinflammatory cytokines,33 thus leading to a toxic shock syndrome that is occasionally fatal. Two other large clostridial toxins, toxin A (TcdA) and toxin B (TcdB) from C. difficile, the agent of pseudomembranous colitis and antibiotic-associated diarrhea, have been shown to be inflammatory enterotoxins in the intestine of several animal species and humans, respectively. Both toxins were reported to generate a local inflammation characterized by an intense inflammatory infiltrate with neutrophil recruitment34,35,36,37,38 and an early release of proinflammatory cytokines, both of which play an important role in the pathophysiology of C. difficile infection and tissue damage.36,39,40,41 TcdB is immunologically related to TcsL-8218 and shares the same glucosyl-transferase enzymatic activity.22,25 Moreover, several studies concerning the lethal toxin (LT) from Bacillus anthracis have suggested that this toxin killed animals by inducing nonspecific shock-like manifestations mainly related to the macrophage sensitivity and the production of TNF-α and IL-1β by these cells.42
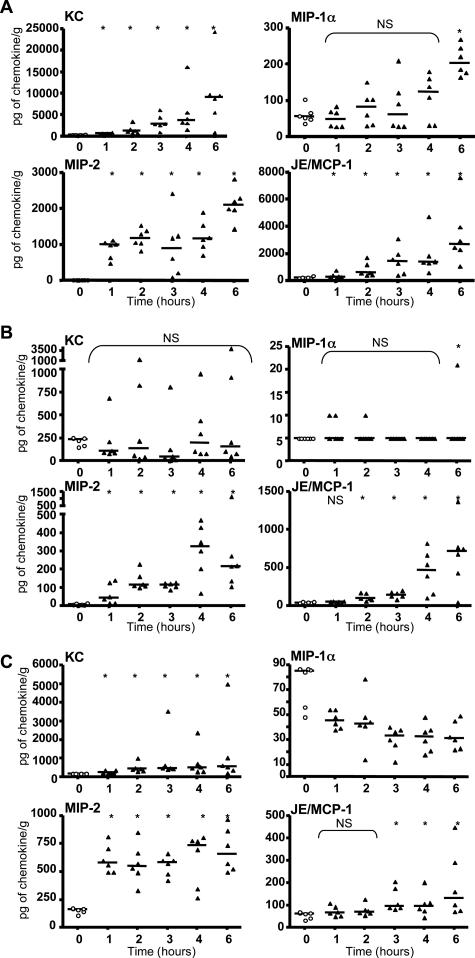
Because C. sordellii was reported to cause fatal infections with clinical features of a toxic shock,3,5,6,7 we investigated whether TcsL-82 induced a severe inflammatory response as a possible cause of shock and death. Chemokines for polymorphonuclear neutrophils, KC (CXCL1) and MIP-2 (CXCL2), and for monocytes, JE/MCP-1 (CCL2) and MIP-1α (CCL3), were assayed.43 It is worth noting that KC, MIP-2, and JE/MCP-1 can be produced by vascular endothelial cells. As shown in Figure 8A, KC, MIP-2, and JE/MCP-1 rapidly increased in lungs, their level being significantly different from that of the control group already 1 hour after TcsL-82 intoxication for KC and MIP-2 and after 2 hours for JE/MCP-1. The level of each cytokine increased at least up to 6 hours after intoxication to reach an average of ∼10,000, 2200, and 3400 pg/g of lung for KC, MIP-2, and JE/MCP-1, respectively (the average control levels being ∼300, 10, and 240 pg/g of lung, respectively). The same chemokines were also increased in the plasma and the liver but to a lesser extent (Figure 8, B and C). The increase in plasmatic KC was never significant because of important variations between mice (Figure 8B). No significant increase in MIP-1α level was observed in lung before 6 hours of intoxication (Figure 8A), a time at which vascular permeability and thoracic edema were maximal. This chemokine increased neither in plasma (Figure 8B) nor in liver. On the contrary, it showed a rapid and significant decrease in liver (Figure 8C).
Figure 8.
TcsL-82 increases the level of certain chemokines, primarily in lung and plasma and, to a lesser extent, in liver. Mice were injected intraperitoneally with 0.5 ml of HBSS containing or not TcsL-82 (15 ng/mouse). After the indicated times, mice were euthanized and sacrificed, blood, lung, and liver were collected. Plasma was sampled after centrifugation. After weighing, organs were homogenized and centrifuged, and the extracted supernatants were collected. KC, MIP-1α, MIP-2, and JE/MCP-1 were measured by ELISA using R&D kits in each sample from lungs (A), plasma (B), and liver (C). Chemokine measurements represent individual values of six samples for each time point. Horizontal bars represent the median value at each time point. The Wilcoxon signed-rank test was performed between the values of the control versus intoxicated mice at each time point using arbitrarily the median of the control population as the hypothetical value for comparison. The probability P of a significant difference in TcsL-82-intoxicated mice is indicated: *0.05 > P > 0.01; NS, nonsignificant.
To investigate further a possible inflammatory reaction in response to TcsL-82, three major early and potent proinflammatory cytokines, IL-1β, IL-6, and TNF-α, were measured in sera, as well as in lungs for TNF-α, in mice intoxicated for various length of time. No significant increase in any of these cytokines was detected in sera and lungs (data not shown), a result that excludes the possibility of even any partial relations between the lethal effect of TcsL-82 and a massive production of proinflammatory cytokines.
Nitric oxide (NO) and other reactive nitrogen species are thought to play an important role in the pathogenesis of pulmonary dysfunction associated with inflammation.44,45,46 Moreover, reactive nitrogen species were recently reported to be involved in the integrity of epithelial tight junctions.47 Accordingly, we investigated whether TcsL-82 intoxication could lead to generation of reactive nitrogen species (NO2− and NO3−). Measurements were performed in lung and blood samples of mice intoxicated for various times. However, compared with control mice no increase in NO2− and NO3− content was measured in sera and lungs of intoxicated mice at any time after TcsL-82 intraperitoneal injection (data not shown).
Discussion
When TcsL-82 was intraperitoneally injected in mice, it reproduced the major clinical and biological features reported in fatal human infections by C. sordellii, occurring postpartum, after abortion, and recently after medical abortion with RU-486.3,5,6,7,48,49 Indeed, these human infections attributable to C. sordellii were characterized by a toxic shock syndrome including hemoconcentration, a major increase in white blood cells with a decrease in platelet counts, and hypoalbuminemia but no liver dysfunction. Autopsy of these patients revealed in most cases massive pleural, pericardiac, and peritoneal effusion. All identified cases of C. sordellii infections had a fulminant course and a fatal outcome.
Is Massive Edema, Which Is a Consequence of Increased Vascular Permeability, the Unique Cause of the Lethal Effect of TcsL-82?
Pleural and pericardiac edema were observed shortly after TcsL-82 injection and appeared to be attributable to an increase in vascular permeability as assessed by the presence of Evans blue dye and albumin in pleural exudation. In the present work, we show that TcsL-82 modifies vascular endothelial cells, particularly at the cardiopulmonary level. Indeed, all of the effects of TcsL-82 observed in mice, including edema of lungs and myocardium, increased hematocrit, BUN modification in relation to dehydration, anoxia, and EPO production seemed to be the consequence of endothelial cell dysfunction. It is unlikely that another cause could participate in edema formation because no major histological lesion nor biochemical dysfunction has been observed in other organs such as kidney, liver, or brain even shortly before death. Moreover, the progressive decrease in serum albumin and the correlated increase in the amount of albumin in the thoracic fluid indicate that endothelium lesions from lung and heart and subsequent vascular permeability changes are the main causes of cardiopulmonary edema after TcsL-82 intoxication. Thus, the TcsL-82-induced death of mice seems to be mainly a consequence of edema. It remains unclear whether the massive edema in lung is sufficient for a fatal issue by suffocation or whether the edema in the heart participates in the death by inducing a cardiorespiratory failure.
Rac Inactivation by TcsL-82 Is Likely to Play a Role in Modifying Endothelial Cells Adherens Junctions
TcsL from C. sordellii strain IP82 glucosylates Rho and Ras family GTPases.23,24,25,26 The Rho GTPase, Rac, modified by TcsL-82 is involved in the cytoskeleton remodeling and induces lamellipodia.50 Actin cytoskeleton is in tight interaction with specific structures at the cell periphery involved in cell-cell contacts such as tight junctions and adherens junctions. The involvement of the Rho proteins in the regulation of cell-cell junctions in endothelial cells has been investigated either in ex vivo endothelial cells from human umbilical vein and porcine pulmonary arteria (human umbilical vein endothelial cells and porcine aortic endothelial cells)51,52,53,54 or in immortalized endothelial cell lines from human or murine myocardial vessels.55,56 Rho and Rac are the main regulators of the cell barrier integrity; Rho plays a critical role in the maintenance of tight junctions, a structure existing between endothelial cells, whereas Rac is a major regulator of the integrity of VE-cadherin junctions, mainly adherens junctions.57,58 In the present work, immunohistochemical analysis brought to light that VE-cadherin junctions were disrupted in vascular endothelium cells in lung of TcsL-82-intoxicated mice. Electron microscopy studies confirmed endothelial cell modifications, in particular at the level of adherens junctions in lung, generated by TcsL-82 that glucosylates and inactivates small GTPases. Moreover, in lung only a fraction of GTPases were glucosylated in vivo, suggesting that only a cell subpopulation was targeted by the toxin, probably the cells highly modified by TcsL-82, ie, vascular endothelial cells, which possess well-defined adherens junctions. In recent work, using a model of epithelial cells, we showed that TcsL-82 from C. sordellii mainly altered adherens junctions by removing E-cadherin-catenin complexes from the membrane to the cytosol.59 Similar effects on adherens junctions have been observed with other toxins that directly depolymerize actin filaments or indirectly alter the actin cytoskeleton through Rac. Thus, Rac, a major target for TcsL-82, is likely to play a central role in TcsL-82-dependent adherens junction alteration and subsequent edema formation without excluding a role of Ras family proteins modified by TcsL-82 in the cellular alterations. Indeed, Ras family GTPases, are also involved in the control of actin cytoskeleton, in particular of adherens junctions,60,61,62,63,64,65 in addition to their implication in the control of cell survival and cell cycle.
No Obvious Inflammatory Component Appears to Participate in the TcsL-82 Lethal Effect
Based on the present work, we can exclude major participation by an inflammatory process in the fatal issue induced by C. sordellii TcsL-82. Indeed, except for the local production of chemokines, which are likely responsible for the infiltrates of polymorphonuclear and monocytes in lungs, attempts to identify various types of inflammation were unsuccessful, including those caused by cytokine or NO production whether in the general circulation or in the lung, the main organ targeted by TcsL-82.
Death Induced by TcsL-82 from C. sordellii or by Anthrax Toxin fromB. anthracis Involves Different Mechanisms
In humans, clinical features of inhaled anthrax spores present similarities to those of C. sordellii infection such as depression of blood oxygen level, severe respiratory distress, and a septic shock. The major virulence factor of B. anthracis, anthrax toxin, consists of three proteins: one receptor binding subunit, protective antigen, and two catalytic subunits: edema factor and lethal factor. Edema factor and lethal factor, respectively, form LT and edema toxin in combination with protective antigen. LT, a metalloprotease, produces symptoms of anthrax in mice including hypoxia, circulatory collapse, and massive edema when intraperitoneally injected. It was first reported that the systemic shock induced by B. anthracis LT was the result of TNF-α production attributable to the lysis of infected macrophages.42,66 However, Moayeri and colleagues67 recently used two different mouse strains with anthrax-susceptible and -resistant macrophages to demonstrate that the mouse inflammatory response, whether in terms of TNF-α or cytokine production, was influenced by genetic factors but was not critical in LT-induced death. They clearly showed that the pathology induced by anthrax LT was related to extensive tissue necrosis and hypoxic damage in the liver, spleen, and bone marrow.67 It is noteworthy that both LT and TcsL-82 share a common effect in cells inhibiting the Ras MAP kinase pathway. TcsL-82 inactivates Ras through UDP glucosylation25 whereas LT proteolytically cleaves MAP kinases.68,69 Although in mice the clinical picture of anthrax LT (edema and hypoxia) is similar to that induced by C. sordellii TcsL-82, the origin of the edema is clearly different. In the first case edema has been related to liver failure, and in the present work we show that TcsL-82 increases vascular permeability by modifying pulmonary endothelial cells.
Recently it has been reported that death in BALB/cJ mice induced by purified edema toxin, an adenylyl cyclase, was attributable to multiorgan failure including adrenal glands, lymphoid organs, bone, bone marrow, gastrointestinal mucosa, heart, and kidney, and that it involved a production of several cytokines.70 On the contrary, after TcsL-82 intoxication the only organs modified by massive edema were lung and heart. Moreover, no cytokine production was observed after TcsL-82 intoxication.
What Are the Target Cells of TcsL-82 and How Does the Toxin Reach These Targets?
Peritoneum is a highly irrigated tissue, which can serve for various exchanges and has been used in patients to perform dialysis.71 Intraperitoneally injected molecules are delivered into the whole body after reabsorption by venous, arterial, and/or lymphatic vessels. So far, we do not know how TcsL-82 is captured in the peritoneum for delivery to its target cells. Based on the present work, it is likely that endothelial cells from pulmonary vessels and thoracic serous membranes are target cells when TcsL-82 is intraperitoneally injected. Large volumes of fluid were found in the pleural cavity of intoxicated mice. Indeed, edema was localized around pulmonary vessels, in pleura as well as in pericardium and endocardium, Several explanations may be proposed for why the first vascular lesions were observed in the lung and not in the peritoneum after TcsL-82 intraperitoneal injection. TcsL-82 is absorbed through blood vessels, but vessels from different organs express variable amounts of TcsL-82 receptors or different accessibility or affinity to the toxin, and TcsL-82 could be absorbed via venous circulation and thus reach heart and lung where it would be removed progressively from the peritoneum by lung endothelial cells. 125I-Labeled TcsL-82, when intraperitoneally injected, is progressively released into the circulation, reaching a maximum level in blood after 1.5 to 2 hours. Therefore, TcsL-82 is likely captured by cells with high-affinity receptors, probably pulmonary and serous membrane endothelial cells. We favor the latter hypothesis because the major histological lesions were observed only at the cardiopulmonary level, accompanied by massive fluid extravasation into the thoracic cage.
The systemic circulating biodistribution of intravenously injected, 125I-labeled TcsL-82 was extremely rapid, occurring within seconds (T1/2α = 90 seconds). Its circulatory half-life, T1/2β, was estimated as 4 hours. When intravenously injected, the heart was the first organ receiving high doses of the toxin. TcsL-82 probably killed mice by inducing heart lesions such as anoxia. Indeed, histological observation of H&E-stained heart tissue revealed many clear cardiomyocytes (data not shown). Intravenously injected TcsL-82 did not induce lung fluid effusion as it did after intraperitoneal injection. The clinical feature of intoxication observed after TcsL-82 intravenous injection in mice has never been reported during human infection by C. sordellii, an infection that, in contrast, seems similar to that observed in mice after intraperitoneal injection, ie, when the toxin is slowly released into the blood circulation.
A Proposed Mechanism for Intraperitoneally Injected TcsL-82 to Induce Death
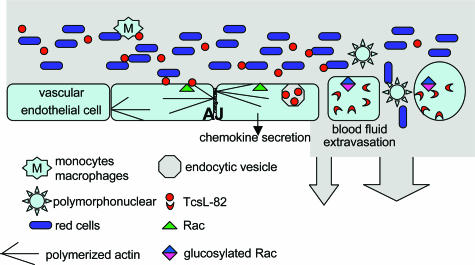
TcsL-82 is captured by endothelial cells from lung vessels. At this level most TcsL-82 molecules are cleared from the circulation and inactivate various small GTPases, in particular Rac, modifying adherens junctions (Figure 9) and thereby induce an increase in lung vascular permeability, whereas major fluid effusion in lung and pleura leads to modifications of hematological parameters and anoxia. As edema in heart was not observed around vessels, it is likely to be the result of a fluid effusion of endocardium and pericardium causing muscle cell dissociation.
Figure 9.
Diagram of TcsL-82 mechanism of action on lung vascular endothelial cells. TcsL-82 after intraperitoneal injection is recognized by a still unknown receptor at the surface vascular endothelial cells. This large clostridial toxin is then endocytosed, and its enzymatic domain (N-terminal part) is translocated into the cytosol72 where it glucosylates Rac and Ras proteins (Ras, Rap, Ral) and depolymerizes the actin cytoskeleton, thereby modifying adherens junctions (AJ).
In conclusion, death induced by TcsL-82 seems to be the consequence of an increase in vascular permeability—essentially at the lung level—resulting from modifications of endothelial cells. Extravasation of blood fluid into the pleural cavity leads to anoxia and finally to cardiorespiratory failure, in the absence of inflammation. Because the main effect of the TcsL-82 known so far is a glucosylation-dependent inactivation of small GTPases, in particular Rac, which is involved in actin polymerization, it can be concluded that when the toxin is intraperitoneally injected, the most susceptible cells for TcsL-82 are lung endothelial cells.
Acknowledgments
We thank Jean-Michel Alonso for stimulating discussion and helpful advice and Timothy Carlson for revising the English.
Footnotes
Address reprint requests to Blandine Geny, Institut Pasteur, 28, rue du Docteur Roux, 75724 Paris Cedex 15, France. E-mail: geny@pasteur.fr.
Supported by the Institut Pasteur (grant GPH no. 9) and Délégation Générale pour l’Armement (grant 03 34 046).
B.G. is senior scientist at Institut National de Santé et de Recherches Médicales (INSERM).
Current address of M.S.: Unité des Neisseria, Institut Pasteur, Paris, France.
References
- Cunniffe JG. Clostridium sordellii bacteraemia. J Infect. 1996;33:127–129. doi: 10.1016/s0163-4453(96)93100-x. [DOI] [PubMed] [Google Scholar]
- Kimura AC, Higa JI, Simpson G, Vargas Y, Vugia DJ. Outbreak of necrotizing fascillitis due to Clostridium sordellii among black-tar heroin users. Clin Infect Dis. 2004;38:e87–e89. doi: 10.1086/383471. [DOI] [PubMed] [Google Scholar]
- Fischer M, Bhatnagar J, Guarner J, Reagan S, Hacker JK, Van Meter SH, Poukens V, Whiteman DB, Iton A, Cheung M, Dassey DE, Shieh WJ, Zaki SR. Fatal toxic shock syndrome associated with Clostridium sordellii after medical abortion. N Engl J Med. 2005;353:2352–2360. doi: 10.1056/NEJMoa051620. [DOI] [PubMed] [Google Scholar]
- Miech RP. Pathophysiology of mifepristone-induced septic shock due to Clostridium sordellii. Ann Pharmacother. 2005;39:1483–1488. doi: 10.1345/aph.1G189. [DOI] [PubMed] [Google Scholar]
- Bitti A, Mastrantonio P, Spigaglia P, Urru G, Spano AI, Moretti G, Cherchi GB. A fatal postpartum Clostridium sordellii associated toxic shock syndrome. J Clin Pathol. 1997;50:259–260. doi: 10.1136/jcp.50.3.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rørbye C, Petersen IS, Nilas L. Postpartum Clostridium sordellii infection associated with fatal toxic shock syndrome. Acta Obstet Gynecol Scand. 2000;79:1134–1135. [PubMed] [Google Scholar]
- Sinave C, Le Templier G, Blouin D, Léveiller F, Deland E. Toxic shock syndrome due to Clostridium sordellii: a dramatic postpartum and postabortion disease. Clin Infect Dis. 2002;35:1441–1443. doi: 10.1086/344464. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Clostridium sordellii toxic shock syndrome after medical abortion with mifepristone and intravaginal miprostol—United States and Canada 2001–2005. Morb Mortal Wkly Rep. 2005;54:724. [PubMed] [Google Scholar]
- Abdulla A, Yee L. The clinical spectrum of Clostridium sordellii bacteraemia: two case reports and a review of the literature. J Clin Pathol. 2000;53:709–712. doi: 10.1136/jcp.53.9.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gredlein CM, Silverman ML, Downey MS. Polymicrobial septic arthritis due to Clostridium species: case report and review. Clin Infect Dis. 2000;30:590–594. doi: 10.1086/313686. [DOI] [PubMed] [Google Scholar]
- Richards SM. Clostridium sordellii in lambs. Vet Rec. 1982;111:22. doi: 10.1136/vr.111.1.22-a. [DOI] [PubMed] [Google Scholar]
- Al-Mashat RR, Taylor DJ. Bacteria in enteric lesions of cattle. Vet Rec. 1983;112:5–10. doi: 10.1136/vr.112.1.5. [DOI] [PubMed] [Google Scholar]
- Popoff MR. Bacteriological examination in enterotoxaemia of sheep and lamb. Vet Rec. 1984;114:324. doi: 10.1136/vr.114.13.324. [DOI] [PubMed] [Google Scholar]
- Lewis CJ, Naylor RD. Sudden death in sheep associated with Clostridium sordellii. Vet Rec. 1998;142:417–421. doi: 10.1136/vr.142.16.417. [DOI] [PubMed] [Google Scholar]
- Al-Mashat RR, Taylor DJ. Clostridium sordellii in enteritis in an adult sheep. Vet Rec. 1983;112:19. doi: 10.1136/vr.112.1.19. [DOI] [PubMed] [Google Scholar]
- Clark S. Sudden death in periparturient sheep associated with Clostridium sordellii. Vet Rec. 2003;153:340. [PubMed] [Google Scholar]
- Arseculeratne SN, Panabokké RG, Wijesundera S. The toxins responsible for the lesions of Clostridium sordellii gas gangrene. J Med Microbiol. 1969;2:37–53. doi: 10.1099/00222615-2-1-37. [DOI] [PubMed] [Google Scholar]
- Popoff MR. Purification and characterization of Clostridium sordellii lethal toxin and cross-reactivity with Clostridium difficile cytotoxin. Infect Immun. 1987;55:35–43. doi: 10.1128/iai.55.1.35-43.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill DM. Bacterial toxins: lethal amounts. Laskin AI, Lechevalier HA, editors. Cleveland: CRC Press; 1987:127–135. [Google Scholar]
- Bette P, Oksche A, Mauler F, von Eichel-Streiber C, Popoff MR, Habermann E. A comparative biochemical, pharmacological and immunological study of Clostridium novyi alpha-toxin, C difficile toxin B and C sordellii lethal toxin. Toxicon. 1991;29:877–887. doi: 10.1016/0041-0101(91)90224-f. [DOI] [PubMed] [Google Scholar]
- Thelen M, Wymann MP, Langen H. Wortmannin binds specifically to 1-phosphatidylinositol 3-kinase while inhibiting guanine nucleotide-binding protein-coupled receptor signaling in neutrophil leukocytes. Proc Natl Acad Sci USA. 1994;91:4960–4964. doi: 10.1073/pnas.91.11.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Just I, Hofmann F, Aktories K. Molecular mechanism of action of the large clostridial cytotoxins. Aktories K, Just I, editors. Berlin: Springer,; In Bacterial Protein Toxins. 2000:pp 307–331. [Google Scholar]
- Hofmann F, Rex G, Aktories K, Just I. The Ras-related protein Ral is monoglucosylated by Clostridium sordellii lethal toxin. Biochem Biophys Res Commun. 1996;227:77–81. doi: 10.1006/bbrc.1996.1470. [DOI] [PubMed] [Google Scholar]
- Just I, Selzer J, Hofmann F, Green GA, Aktories K. Inactivation of Ras by Clostridium sordellii lethal toxin-catalysed glucosylation. J Biol Chem. 1996;271:10149–10153. doi: 10.1074/jbc.271.17.10149. [DOI] [PubMed] [Google Scholar]
- Popoff MR, Chaves-Olarte E, Lemichez E, von Eichel-Streiber C, Thelestam M, Chardin P, Cussac D, Antonny B, Chavrier P, Flatau G, Giry M, de Gunzburg J, Boquet P. Ras, Rap, and Rac small GTP-binding proteins are targets for the Clostridium sordellii lethal toxin glucosylation. J Biol Chem. 1996;271:10217–10224. doi: 10.1074/jbc.271.17.10217. [DOI] [PubMed] [Google Scholar]
- Humeau Y, Popoff MR, Kojima H, Doussau F, Poulain B. Rac GTPase plays an essential role in exocytosis by controlling the fusion competence of release sites. J Neurosci. 2002;22:7968–7981. doi: 10.1523/JNEUROSCI.22-18-07968.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Eichel-Streiber C, Harperath U, Bosse D, Hadding U. Purification of two high molecular weight toxins of Clostridium difficile which are antigenically related. Microb Pathog. 1987;2:307–318. doi: 10.1016/0882-4010(87)90073-8. [DOI] [PubMed] [Google Scholar]
- Ferrero RL, Avé P, Radcliff FJ, Labigne A, Huerre M-R. Outbred mice with long-term Helicobacter felis infection develop both gastric lymphoid tissue and glandular hyperplastic lesions. J Pathol. 2000;191:333–340. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH619>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Barbier J, Popoff MR, Moglo J. Degeneration and regeneration of murine skeletal neuromuscular junctions after intramuscular injection with a sublethal dose of Clostridium sordellii lethal toxin. Infect Immun. 2004;72:3120–3128. doi: 10.1128/IAI.72.6.3120-3128.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SL. Hemodynamic disorders, thrombosis and shock. Cotran RS, Kumar V, Robbins SL, editors. Philadelphia: WB Saunders Co.; Pathologic Basis of Disease, ed 5, ch 4. 1994:pp 93–121. [Google Scholar]
- Smith SC, Ladenson JH, Mason JW, Jaffe AS. Elevations of cardiac troponin I associated with myocarditis. Circulation. 1997;95:163–168. [PubMed] [Google Scholar]
- Petit PX, Bréard J, Montalescot V, Ben El, Hadj N, Levade T, Popoff M, Geny B. Lethal toxin from Clostridium sordellii induces apoptotic cell death by disruption of the mitochondrial homeostasis in HL-60 cells. Cell Microbiol. 2003;5:761–771. doi: 10.1046/j.1462-5822.2003.00309.x. [DOI] [PubMed] [Google Scholar]
- Müller-Alouf H, Carnoy C, Simonet M, Alouf JE. Superantigen bacterial toxins: state of the art. Toxicon. 2001;39:1691–1701. doi: 10.1016/s0041-0101(01)00156-8. [DOI] [PubMed] [Google Scholar]
- Triadafilopoulos G, Pothoulakis C, O’Brien MJ, LaMont JT. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987;93:273–279. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]
- Mahida YR, Makh S, Hyde S, Gray T, Borriello SP. Effect of Clostridium difficile toxin A on human intestinal epithelial cells: induction of interleukin 8 production and apoptosis after cell detachment. Gut. 1996;38:337–347. doi: 10.1136/gut.38.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savidge TC, Pan W-H, O’Brien M, Anton PM, Pothoulakis C. Clostridium difficile toxin B is an inflammatory enterotoxin in human intestine. Gastroenterology. 2003;125:413–420. doi: 10.1016/s0016-5085(03)00902-8. [DOI] [PubMed] [Google Scholar]
- Anton PM, Gay J, Mykoniatis A, Pan A, O’Brien M, Brown K, Karalis K, Pothoulakis C. Corticotropin-releasing hormone (CRH) requirement in Clostridium difficile toxin A-mediated intestinal inflammation. Proc Natl Acad Sci USA. 2004;101:8503–8508. doi: 10.1073/pnas.0402693101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothoulakis C. Effects of Clostridium difficile toxins on epithelial cell barrier. Ann NY Acad Sci. 2000;915:347–356. doi: 10.1111/j.1749-6632.2000.tb05263.x. [DOI] [PubMed] [Google Scholar]
- Warny M, Keates AC, Keates S, Castagliuolo I, Zachs JK, Aboudola S, Qamar A, Potoulakis C, LaMont JT, Kelly C. p38 MAP kinase activation by Clostridium difficile toxin A mediates monocyte necrosis, IL-8 production and enteritis. J Clin Invest. 2000;105:1147–1156. doi: 10.1172/JCI7545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He D, Sougioultzis S, Hagen S, Liu J, Keates S, Keates AC, Pothoulakis C, LaMont JT. Clostridium difficile toxin A human colonocyte IL-8 release via mitochondrial oxygen radical generation. Gastroenterology. 2002;122:1048–1057. doi: 10.1053/gast.2002.32386. [DOI] [PubMed] [Google Scholar]
- Kim H, Rhee SH, Kokkotou E, Na X, Savidge T, Moyer MP, Pothoulakis C, LaMont JT. Clostridium difficile toxin A regulates inducible cyclooxygenase-2 and prostaglandin E2 synthesis in colonocytes via reactive oxygen species and activation of p38 MAPK. J Biol Chem. 2005;280:21237–21245. doi: 10.1074/jbc.M413842200. [DOI] [PubMed] [Google Scholar]
- Hanna PC, Acosta D, Collier RJ. On the role of macrophages in anthrax. Proc Natl Acad Sci USA. 1993;90:10198–10201. doi: 10.1073/pnas.90.21.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Pittet JF, Lu LN, Morris DG, Modelska K, Welch WJ, Carey HV, Roux J, Matthay MA. Reactive nitrogen species inhibit alveolar epithelial fluid transport after hemorrhagic shock in rats. J Immunol. 2001;166:6301–6310. doi: 10.4049/jimmunol.166.10.6301. [DOI] [PubMed] [Google Scholar]
- Sittipunt C, Steinberg KP, Ruzinski JT, Myles C, Zhu S, Goodman RB, Hudson LD, Matalon S, Martin TR. Nitric oxide and nitrotyrosine in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:503–510. doi: 10.1164/ajrccm.163.2.2004187. [DOI] [PubMed] [Google Scholar]
- Zhu S, Ware LB, Geiser T, Matthay MA, Matalon S. Increased levels on nitrate and surfactant protein A nitration in pulmonary edema fluid of patients with acute lung injury. Am J Respir Crit Care Med. 2001;163:166–172. doi: 10.1164/ajrccm.163.1.2005068. [DOI] [PubMed] [Google Scholar]
- Han X, Fink MP, Uchiyama T, Yang R, Delude RL. Increased iNOS activity is essential for pulmonary epithelial tight junctions dysfunction in endotoxemic mice. Am J Physiol. 2005;286:L259–L267. doi: 10.1152/ajplung.00187.2003. [DOI] [PubMed] [Google Scholar]
- McGregor JA, Soper DE, Lovell G, Lovell G, Todd JK. Maternal deaths associated with Clostridium sordellii infection. Am J Obstet Gynecol. 1989;161:987–995. doi: 10.1016/0002-9378(89)90768-0. [DOI] [PubMed] [Google Scholar]
- Sosolik RC, Savage BA, Vaccarello L. Clostridium sordellii toxic shock syndrome: a case report and review of the literature. Infect Dis Obstet Gynecol. 1996;4:31–35. doi: 10.1155/S1064744996000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- Vouret-Craviari V, Boquet P, Pouysségur J, Van Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell. 1998;9:2639–2653. doi: 10.1091/mbc.9.9.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J Cell Physiol. 1998;176:150–165. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Vouret-Craviari V, Grall D, Flatau G, Pouysségur J, Boquet P, Van Obberghen-Schilling E. Effect of cytotoxic necrotizing factor1 and lethal toxin on actin cytoskeleton and VE-cadherin localization in human endothelial cell monolayers. Infect Immun. 1999;67:3002–3008. doi: 10.1128/iai.67.6.3002-3008.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wójciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- Adamson RH, Curry FE, Adamson G, Liu B, Jiang Y, Aktories K, Barth H, Daigeler A, Golenhofen N, Ness W, Drenckhahn D. Rho and rho kinase modulation of barrier properties: cultured endothelial cells and intact microvessels of rats and mice. J Physiol. 2002;539:295–308. doi: 10.1113/jphysiol.2001.013117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke J, Baumgartner W, Adamson RH, Zeng M, Aktories K, Barth H, Wilde C, Curry FE, Drenckhahn D. Requirement of Rac activity for maintenance of capillary barrier properties. Am J Physiol. 2004;286:H394–H401. doi: 10.1152/ajpheart.00221.2003. [DOI] [PubMed] [Google Scholar]
- Jou T-S, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142:101–115. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaibuchi K. Regulation of cadherin mediated cell-cell adhesion by Rho family GTPases. Curr Opin Cell Biol. 1999;11:591–596. doi: 10.1016/s0955-0674(99)00014-9. [DOI] [PubMed] [Google Scholar]
- Boehm C, Gibert M, Geny B, Popoff M, Rodriguez P. Modification of epithelial cell barrier permeability and intercellular junctions by Clostridium sordellii lethal toxin. Cell Microbiol. 2006;8:1070–1085. doi: 10.1111/j.1462-5822.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, Waterfield MD, Ridley A, Downward J. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Bos JL. All in the family? New insights and questions regarding interconnectivity of Ras, Rap1 and Ral. EMBO J. 1998;17:6776–6782. doi: 10.1093/emboj/17.23.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accorsi K, Giglione C, Vanoni M, Parmeggiani A. The Ras GDP/GTP cycle is regulated by oxidizing agents at the level of Ras regulators and effectors. FEBS Lett. 2001;492:139–145. doi: 10.1016/s0014-5793(01)02251-7. [DOI] [PubMed] [Google Scholar]
- Campbell SL, Khosravi-Far R, Rossman KL, Clark GJ, Der CJ. Increasing complexity of Ras signaling. Oncogene. 1998;17:1395–1413. doi: 10.1038/sj.onc.1202174. [DOI] [PubMed] [Google Scholar]
- Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VMM, Birchmeier W, Fujita Y. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;24:6690–6700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LS, Hadjo-Milosinovic A, Zhao J, Zwartkruis FJT, Collard JG, Bos JL. Rap1 regulates E-cadherin-mediated cell-cell adhesion. J Biol Chem. 2004;279:35127–35132. doi: 10.1074/jbc.M404917200. [DOI] [PubMed] [Google Scholar]
- Park JM, Greten FR, Li Z-W, Karin M. Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science. 2002;297:2048–2051. doi: 10.1126/science.1073163. [DOI] [PubMed] [Google Scholar]
- Moayeri M, Haines D, Young HA, Leppla SH. Bacillus anthracis lethal toxin induces TNFα-independent hypoxia-mediated toxicity in mice. J Clin Invest. 2003;112:670–682. doi: 10.1172/JCI17991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzari R, Guidi-Rontani C, Vitale G, Mock M, Montecucco C. Anthrax lethal factor cleaves MKK3 in macrophages and inhibits the LPS/INFγ-induced release of NO and TNFα. FEBS Lett. 1999;462:199–204. doi: 10.1016/s0014-5793(99)01502-1. [DOI] [PubMed] [Google Scholar]
- Vitale G, Pellizzari R, Recchi C, Napolitani G, Mock M, Montecocco C. Anthrax lethal factor cleaves the N-terminus of MAPKKs and induces tyrosine/threonine phosphorylation of MAPKs in cultured macro-phages. Biochem Biophys Res Commun. 1998;248:706–711. doi: 10.1006/bbrc.1998.9040. [DOI] [PubMed] [Google Scholar]
- Firoved AM, Miller GF, Moayeri M, Kakkar R, Shen Y, Wiggins J, McNally EM, Tang W-J, Leppla SH. Bacillus anthracis edema toxin causes extensive tissue lesions and rapid lethality in mice. Am J Pathol. 2005;167:1309–1320. doi: 10.1016/S0002-9440(10)61218-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flessner MF. The transport barrier in intraperitoneal therapy. Am J Physiol. 2005;288:F433–F442. doi: 10.1152/ajprenal.00313.2004. [DOI] [PubMed] [Google Scholar]
- Pfeifer G, Schirmer J, Leemhuis J, Busch C, Meyer DK, Aktories K, Barth H. Cellular uptake of Clostridium difficile toxin B. J Biol Chem. 2003;278:44535–44541. doi: 10.1074/jbc.M307540200. [DOI] [PubMed] [Google Scholar]