Abstract
The matricellular glycoprotein SPARC (secreted protein acidic and rich in cysteine) has been accorded major roles in regulation of cell adhesion and proliferation, as well as tumorigenesis and metastasis. We have recently reported that in addition to its potent antiproliferative and proapoptotic functions, SPARC also abrogates ovarian carcinoma cell adhesion, a key step in peritoneal implantation. However, the underlying molecular mechanism through which SPARC ameliorates peritoneal ovarian carcinomatosis seems to be multifaceted and has yet to be delineated. Herein, we show that SPARC significantly inhibited integrin-mediated ovarian cancer cell adhesion to extracellular matrix proteins, as well as to peritoneal mesothelial cells. This counteradhesive effect of SPARC was shown to be mediated in part through significant attenuation of cell surface expression and clustering of αv-integrin subunit, αvβ3- and αvβ5-heterodimers, and β1-subunit, albeit to a lesser extent, in ovarian cancer cells. Moreover, SPARC significantly suppressed both anchorage-dependent and -independent activation of AKT and mitogen-acti-vated protein kinase survival signaling pathways in ovarian cancer cells in response to serum and epidermal growth factor stimulation. In summary, we have identified a novel role of SPARC as a negative regulator of both integrin-mediated adhesion and growth factor-stimulated survival signaling pathways in ovarian cancer.
Ovarian cancer is the leading cause of death from gynecological cancer in women in the United States. The progression of ovarian carcinoma involves growth of tumor cells on the surface of the ovary followed by shedding of cancer cells onto the mesothelial lining of the abdominal cavity, where these malignant cells survive as free-floating spheroids that may later attach and disseminate to peritoneal and extraperitoneal organs.1,2 Studies on normal and transformed cells suggest that on malignant transformation, striking changes occur in the ability of the cells to interact with their extracellular matrix (ECM) and other neighboring cells. Much interest in this respect has focused on the integrin family of cell surface receptors.
Integrins are a family of heterodimeric transmembrane glycoprotein receptors consisting of 18 different α-subunits and eight different β-subunits, which combine to form 24 different integrin receptors.3 As transmembrane receptors, integrins are integrated across the plasma membrane to provide bridges between the ECM and the cytoskeleton. Hence, integrins are not only implicated in the physical aspects of cell adhesion but also play a pivotal role in modulation of signaling pathways initiated by growth factor receptors, as well as in regulation of cell behavior, growth, survival, and acquisition of an invasive phenotype.4,5,6 The expression and the functional role of integrins in cancer tissues and cultured ovarian carcinoma cells have been correlated with their increased adhesion to the ECM components [collagen type I, laminin, and fibronectin (FN)] of the peritoneal surface. β1-Integrin subunits have been shown to mediate adhesion of ovarian carcinoma cells to the mesothelial lining of the peritoneum, and perturbations of this integrin subunit function have been implicated in diminutions of the invasive potential of these cancer cells.2,7,8,9,10 Moreover, the importance of α-integrin subunits, especially αv, in cell adhesion, proliferation, survival, migration, and invasion has been established in ovarian cancer.4,11,12,13 Studies of ovarian cancer specimens have shown that the expression of αv- and β1-integrin subunits is a frequent event in primary ovarian carcinoma and effusions, and is linked to the expression of other metastasis-associated molecules.2,13 Abrogation of tumor cell adhesion to ECM and inhibition of integrin-mediated outside-in signaling has been shown to subsequently inhibit phosphorylation (activation) of focal adhesion kinase (FAK), Src, AKT/protein kinase B (PKB), and mitogen-activated protein kinase (MAPK) 44/42, thereby inhibiting tumor cell invasion as well as survival.14,15,16
SPARC (also known as osteonectin and BM-40) is a secreted, calcium-binding matricellular glycoprotein that interacts with various ECM macromolecules.17 SPARC has been implicated in the regulation of cell adhesion, proliferation, and migration, as well as in processes requiring ECM turnover, such as wound healing and tumor progression.18 The mechanism through which SPARC modulates cancer progression is complex and depends on tumor cell type and the surrounding microenvironment. SPARC has been reported to promote melanoma, squamous cell tumor growth, and glioma invasion.19,20,21,22,23 SPARC has also been reported to serve as a chemoattractant for prostate and breast cancer cell lines, supporting their preferred migration and homing to bone.24,25 Conversely, mice with disrupted SPARC expression have been reported to support enhanced tumor growth of pancreatic and Lewis lung carcinoma cells.26,27 Decreased SPARC expression has also been associated with increased tumorigenicity and metastasis of human and murine ovarian carcinoma. Moreover, we have recently shown that SPARC ameliorates peritoneal ovarian carcinomatosis, at least in part, by abrogation of tumor cell adhesion to peritoneal surfaces.28,29
Because of the absence of specific diagnostic and/or prognostic markers of ovarian cancer, as well as the poor outcome of the treatment of patients in advanced stages of the disease, development of new therapeutic protocols is of great interest and depends primarily on improved knowledge of the molecular mechanisms controlling the pathogenic cascade, especially that of ECM-integrin interaction. In view of the central role of β1- and αv-integrin subunits in tumor cell-ECM adhesion and survival, we sought to determine whether the inhibitory effect of SPARC on ovarian carcinomatosis is in part mediated by these integrin subunits.
Materials and Methods
Cell Lines
Human ovarian cancer cell lines SKOV3 and NIH:OVCAR3 were purchased from American Type Culture Collection (Rockville, MD) and were maintained in McCoy’s medium and RPMI 1640 supplemented with 5 μg/ml bovine insulin, respectively. IGROV1 cell line was kindly provided by Dr. Franco Zunino (Instituto Nazionale Tumori Milano, Milan, Italy) and was maintained in RPMI 1640. LP9 primary human peritoneal mesothelial cells (Coriell Cell Repository, Cadmen, NJ) and Meso 301 immortalized human peritoneal mesothelial cell line (originally isolated from a premenopausal woman with endometrial cancer and immortalized with HPVE6E7; a kind gift of Dr. Samuel Mok, Harvard Medical School, Boston, MA) were maintained in MCDB106 and M199 (1:1). Unless stated otherwise, all media and supplements were purchased from Sigma, St. Louis, MO, and were supplemented with 5 to 15% fetal bovine serum (FBS) (Atlanta Biologicals, Norcross, GA) and 100 μU/ml penicillin and 100 μg/ml streptomycin.
Reagents and Antibodies
SPARC from mouse parietal yolk sac was purchased from Sigma, and human and bovine osteonectin were purchased from Hematological Technologies Inc. (Essex, VT). Collagen I (Col I) from rat tail, human plasma FN, and human plasma vitronectin (VN) were from BD Biosciences (Bedford, MA). Antibodies to integrin subunits β1 (P4C10), αvβ3 (LM609), αvβ5 (P1F6), α5β1 (JSB5), and β1-activating (P4G11), anti-αv (LM142), and anti-β3 (B3A) were from Chemicon International (Temecula, CA). Blocking antibody to αv (69-6-5) was a kind gift from Dr. Josè Luis (Unité Mixte de Recherche Centre National de la Recherche Scientifique 6032, Marseille, France). WOW-1, an antibody that recognizes activated αvβ3-integrin, was a kind gift of Dr. Sanford Shattil (University of California San Diego, La Jolla, CA). Monoclonal antibody mAb:VNR1 27.1, an αvβ3-blocking antibody, was a kind gift of Dr. M.H. Ginsberg (University of California San Diego, La Jolla, CA). The RGD hexapeptide GRGDSP was purchased from Sigma. Mouse monoclonal and rabbit polyclonal anti-AKT/PKB, anti-phospho-AKT/PKBSer473, anti-phospho-44/42-MAPK, and anti-44/42-MAPK, anti-phosphpo-EGFRTyr1068, anti-EGFR, anti-phospho-cSrcTyr416, anti-cSrc, anti-phospho FAKTyr576/577, and FAK antibodies were purchased from Cell Signaling (Beverly, MA) and Upstate Biotechnology (Lake Placid, NY). Horseradish peroxidase- and fluorescent-conjugated secondary antibodies were from Jackson ImmunoResearch (West Grove, PA). CellTracker Orange CMTMR [5-(and-6)-(((4-chlromethyl) benzoyl)amino) tetramethylrhodamine] was purchased from Molecular Probes Inc. (Eugene, OR); Hemacolor 3 stain was from Fisher Scientific (Fairlawn, NJ). Protease inhibitor cocktail mixture was from Roche Applied Sciences (Indianapolis, IN), and epidermal growth factor (EGF) was from R&D Systems, Inc. (Minneapolis, MN). All other chemicals were laboratory grade and were purchased from Sigma.
Adhesion Assays to ECM Proteins
Solid-phase assays in which 96-well tissue culture plates were coated with FN, Col I, and VN were performed as described earlier.29 One hundred μl (1 × 105 cells) of ovarian cancer cell lines was added to each well in the presence and absence of MnCl2 (1 to 5 mmol/L), β1-integrin activating antibody (P4G11, 5 to 10 μg/ml), blocking antibodies (1 to 5 μg/ml), vehicle control [phosphate-buffered saline (PBS) containing Ca2+ and Mg2+], or GRGDSP peptide. To determine whether SPARC inhibits integrin-mediated adhesion, cells were preincubated with SPARC (10 μg/ml) for 2 hours before integrin activation and addition to matrix-coated wells for a further 5 hours.
Adhesion of Human Ovarian Cancer Cells to Peritoneal Mesothelial Cells
Primary and/or immortalized human peritoneal mesothelial cells (PMCs) (HPMCs) were grown to confluence in 24-well plates. Ovarian cancer cell lines were stained with a 10 μmol/L solution of the fluorochrome CMTMR dissolved in appropriate serum-free medium for 30 minutes at 37°C per the manufacturer’s instructions. Three hundred μl of the cell suspension (3 × 105 cells) were coincubated with live mesothelial cell monolayers for 5 hours at 37°C in the presence or absence of αv-agonist (MnCl2) or β1-activating antibody (P4G11). The effect of SPARC on ovarian cancer cell adhesion to mesothelial cells was determined by preincubating ovarian cancer cell lines with SPARC (10 μg/ml) for 2 hours before incubation with integrin activators or agonists, as described for the solid-phase assays. After removing nonadherent cells by extensive washes in PBS, adhesion of ovarian cancer cell lines to HPMCs was determined by measuring the mean red fluorescent intensities in six fields per experimental condition using a fluorescent stereomicroscope equipped with a Q-Imaging digital camera (Leica Microsystems, Wetzlar, Germany).
Bromodeoxy Uridine (BrdU) Enzyme-Linked Immunosorbent Assay
Human ovarian cancer cell lines were allowed to attach to FN, VN, and Col I, as well as to uncoated 96-well plates for 4 hours in complete growth medium. They were then treated with SPARC (10 μg/ml) in the presence or absence of integrin activators and blocking antibodies for an additional 48 hours. Cell Proliferation Biotrak enzyme-linked immunosorbent assay kit (Amersham Biosciences Corp., Piscataway, NJ) was used according to the manufacturer’s instructions.
α/β-Integrin-Mediated Cell Adhesion Array
To investigate whether the counteradhesive effect of SPARC is attributable to its effect on cell surface integrin expression, SKOV3 cells were grown to confluence and starved overnight in serum-free medium followed by treatment with SPARC (10 μg/ml) for an additional 16 hours. Single-cell suspensions were then prepared using a nonenzymatic dissociation buffer (Sigma). One hundred μl (1 × 105 cells in adhesion buffer) was added to each well of α/β-integrin-mediated cell adhesion array kit (Chemicon). After incubation for 2 hours at 37°C/5% CO2, wells were washed and stained, and cell-bound stain was solubilized in extraction buffer. Integrin expression was determined by measuring the absorbance at 570 nm.
Flow Cytometry (FC)
Ovarian cancer cell lines were grown to confluence, starved overnight, and treated with SPARC (10 μg/ml) for 16 hours. Cells were harvested by mild trypsinization and were resuspended in 1 × 106 cells/ml in PBS/0.4% bovine serum albumin (adhesion buffer). They were then incubated for 1 hour on ice with P4G11, P4C10, B3A, LM 142, 69-6-5, LM609, VNR1 27.1, and P1F6 antibodies. After washing with PBS/0.2% bovine serum albumin/0.2% sodium azide, cells were incubated for 30 minutes on ice with appropriate fluorescent secondary antibodies. Cells were then analyzed with FACSCaliber flow cytometer (Becton Dickinson, Mountain View, CA). As negative controls, samples were incubated with the secondary antibody alone or with mouse, rat, or rabbit isotype-matched controls.
Immunocytochemistry
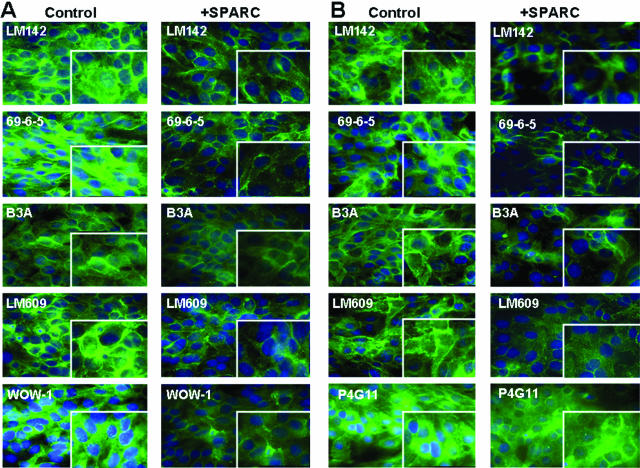
SKOV3 and OVCAR3 cells were plated on FN- and VN-coated eight-well slide chambers overnight at 4°C, fixed with 4% paraformaldehyde in PBS for 10 minutes at 4°C to maintain cell membrane integrity, followed by permeabilization with 0.1% Triton X-100. The following primary antibodies were used, LM142, 69-6-9, B3A, LM609, WOW-1, P1F5, and P4G11. Fluorescent-conjugated secondary antibodies were used as indicated. Appropriate isotype-matched secondary antibody controls were included to evaluate nonspecific binding.
Immunoblotting
Subconfluent monolayers of ovarian cancer cell lines were serum-starved for 20 hours and were treated with SPARC (20 μg/ml) for 2 hours. Cells were stimulated with PBS (control) or with 5% FBS and 40 ng/ml EGF for 5 and 15 minutes and harvested in lysis buffer (20 mmol/L Tris, pH 7.4, 150 mmol/L NaCl, 1 mmol/L ethylenediaminetetraacetic acid, 50 mmol/L NaF, 0.5% sodium deoxycholate, 1% Nonidet P-40, 1 mmol/L Na3VO4, and 1× protease inhibitor cocktail mixture). In some experiments, cells were starved overnight and plated on FN-coated plates or left in suspension for 4 hours, followed by treatment with SPARC (20 μg/ml) for 2 hours. Cells were then stimulated with FBS and EGF for 5 minutes. Lysates were cleared by centrifugation at 12,000 × g for 20 minutes at 4°C. Forty μg of cell lysates were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA). The membranes were incubated with phospho-specific antibodies against EGFR, 44/42 MAPK, AKT, FAK, and cSrc overnight at 4°C. Protein detection was performed using horseradish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibodies and a SuperSignal West Dura chemiluminescence kit (Pierce). Membranes were then stripped of immunoglobulins and reprobed with antibodies against total EGFR, MAPK, AKT, FAK, and cSrc to ensure equal protein loading.
Results
SPARC Inhibits αv- and β1-Integrin-Mediated Adhesion of Ovarian Cancer Cells to ECM
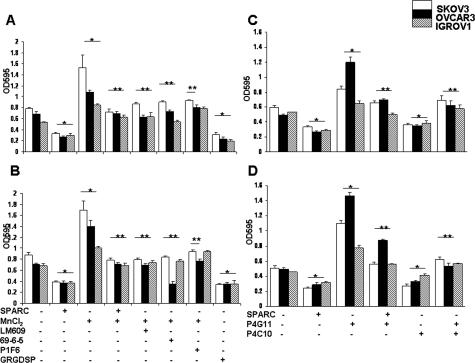
There is a strong body of evidence supporting the involvement of αv- and β1-integrins in anchorage of ovarian carcinoma cells to the ECM of the peritoneal mesothelium.9,10,30 We used FN, Col I, and VN in the present study because, in our earlier report,29 we found that these defined matrices permit maximal adhesion of ovarian cancer cell lines, compared with other tested matrices. Therefore, we sought to determine whether SPARC abrogates αv-mediated adhesion of ovarian cancer cells to peritoneal ECM in the presence of MnCl2—an αv agonist that has previously been reported to enhance ligand binding to αvβ3.15,31,32 Because Mn2+ is able to activate different types of integrins, we excluded the involvement of other major integrin subunits (ie, β1) by incorporating a β1-blocking antibody (P4C10) and using VN as a substrate. We found that MnCl2 increased adhesion of SKOV3, OVCAR3, and IGROV1 cell lines to FN by 96, 60, and 59% (Figure 1A) and to VN by 93, 97, and 47%, respectively (Figure 1B). This enhanced adhesion was significantly inhibited (up to 49%) by preincubation of cells with SPARC (10 μg/ml) and was comparable with the inhibitory effect of αvβ3- and αvβ5-integrin-blocking antibodies (LM609, 69-6-5, and P1F6). In addition to inhibition of adhesion mediated by activated αv-integrins, SPARC also significantly (>50%) suppressed the basal adhesion of all three ovarian cancer cell lines to FN and VN matrices, comparable with that of levels observed using the GRGDSP peptide (Figure 1, A and B). Activation of β1-integrin by P4G11 also increased the adhesion of SKOV3, OVCAR3, and IGROV1 to FN and Col I significantly, and SPARC (10 μg/ml) inhibited this adhesion by more than 40% (Figure 1, C and D). This inhibitory effect of SPARC was shown to be comparable with the effect of β1-blocking antibody P4C10. In our pilot studies, this anti-adhesive effect of SPARC was found to be concentration-dependent, with minimum inhibitory concentration of 10 μg/ml. At this concentration, together with the short duration of the adhesion assays (4 hours) used in our studies, SPARC neither adversely affected viability nor induced apoptosis [as determined by parallel trypan blue exclusion, and terminal dUTP nick-end labeling (TUNEL) staining; Promega] of ovarian cancer cell lines tested (data not shown).
Figure 1.
SPARC inhibits αv- and β1-integrin-mediated adhesion of ovarian cancer cells. Adhesion of SKOV3, OVCAR3, and IGROV1 cell lines to FN (A) and VN (B) was significantly increased by MnCl2 (2.5 mmol/L). Preincubation of the cells with SPARC (10 μg/ml) significantly decreased αv-mediated adhesion of all three cell lines to baseline levels. The specificity of Mn2+-induced αv-mediated adhesion was further verified with GRGDSP peptide and the blocking antibodies LM609 (αvβ3 blocking), 69-6-5 (αv blocking), and P1F6 (αvβ5 blocking). Adhesion of SKOV3, OVCAR3, and IGROV1 cell lines to FN (C) and Col (D) was significantly increased by the β1-integrin-activating antibody P4G11. Preincubation of the cells with SPARC significantly decreased β1-mediated adhesion. The inhibitory effect of SPARC was comparable with the effect of β1-integrin-blocking antibody P4C10. All assays were performed in quadruplicates, and results shown are representative of three independent experiments. *P < 0.05 from untreated control cells. **P < 0.05 from integrin-activated cells.
SPARC Inhibits Adhesion of Ovarian Cancer Cells to HPMCs
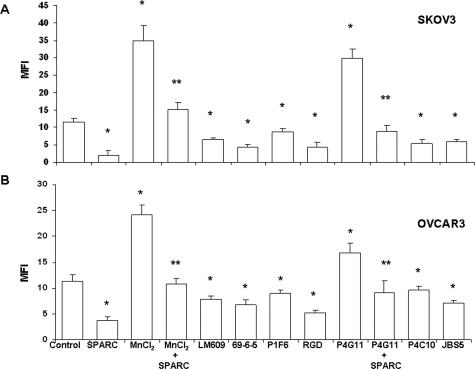
We used the highly invasive SKOV3 as well as the less invasive OVCAR3 cell lines to test the effect of SPARC on their adhesion to monolayers of HPMCs (Meso301 and LP9). We found that activation of αv- and β1-integrins increased adhesion of CMTMR-labeled SKOV3 to Meso301 by 2.5- and 1.8-fold, respectively (Figure 2A), and that of labeled OVCAR3 by 2.1- and 1.5-fold, respectively (Figure 2B). Similar results were obtained with primary, low-passage (two to four) LP9 cells (data not shown). Preincubation of SKOV3 and OVCAR3 cells with SPARC inhibited MnCl2- and β1-mediated adhesion by 1.3- and 2.0-fold for SKOV3, and 2.2- and 1.9-fold for OVCAR3, respectively. This anti-adhesive effect of SPARC was comparable with that of integrin-blocking antibodies and the GRGDSP peptide. These results suggest that the anti-adhesive effect of SPARC is attributable to its interference with αv- and β1-mediated anchorage of ovarian cancer cells to their respective matrices on peritoneal mesothelial cell monolayers.
Figure 2.
Influence of SPARC on ovarian cancer cell adhesion to HPMCs. CMTMR-labeled SKOV3 (A) and OVCAR3 (B) were co-incubated with confluent monolayers of Meso301. Activation of αv-integrins by MnCl2 and β1-integrins by P4G11 resulted in a substantial increase in the adhesion of SKOV3 and OVCAR3 to Meso301. This increase was significantly inhibited by preincubation of the cells with SPARC (10 μg/ml). The counteradhesive effect of SPARC was comparable with that of LM609, 69-6-5, P1F6, the RGD peptide (GRGDSP), P4C10, and JBS5. *P < 0.05 from untreated controls. **P < 0.05 from activated integrin subunits. Results shown are representative of three independent experiments performed in quadruplicates.
SPARC Inhibits Integrin-Dependent Proliferation of Ovarian Cancer Cell Lines
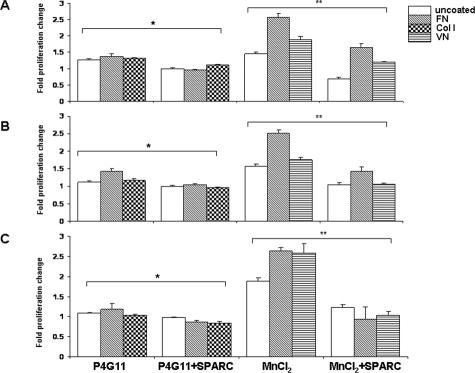
The antiproliferative effect of SPARC on human ovarian cancer cell lines in vitro and in vivo, as well as on a murine cell line in vitro, have been reported previously.28,29,33 In the present study, we show that activation of αv-integrin with 2.5 mmol/L MnCl2 significantly stimulated proliferation of SKOV3 (Figure 3A), OVCAR3 (Figure 3B), and IGROV1 (Figure 3C) cell lines plated on FN-coated matrices by up to 2.6-fold. The addition of exogenous SPARC (10 μg/ml) resulted in diminution of this MnCl2-induced proliferation by 0.9- to 1.0-fold. VN-coated and uncoated surfaces supported MnCl2-induced proliferation of these cell lines by ∼1.8- to 2.6-fold, and the addition of SPARC resulted in a statistically significant decrease in their rates of proliferation. On the other hand, whether the three cell lines were seeded on uncoated plates, Col I, or FN, β1-integrin activation by P4G11 increased their proliferation by ∼1.0- to 1.3-fold. SPARC significantly inhibited P4G11-induced proliferation of SKOV3 and OVCAR3; however, its effect on IGROV1 cell line was less pronounced. It is noteworthy to emphasize that the observed anti-proliferative effect of SPARC on ovarian cancer cell lines tested was not attributable to cytotoxicity (as determined by LIVE/DEAD viability/cytotoxicity kit; Invitrogen, Carlsbad, CA) (data not shown), major alterations in actin cytoskeleton and cell shape (as assessed by parallel F-actin staining and morphometric cell rounding-index calculations, data not shown), or early stages of apoptosis (as confirmed by parallel luminescent caspase activity assays and TUNEL staining in pilot studies, data not shown). Thus, these results suggest that SPARC inhibits integrin-mediated anchorage-dependent ovarian cancer cell proliferation.
Figure 3.
SPARC inhibits integrin-induced ovarian cancer cell proliferation. The proliferation in SKOV3, OVCAR3, and IGROV1 cells was determined by BrdU incorporation. Activation of β1-integrin by P4G11 (10 μg/ml) resulted in a significant increase in proliferation of SKOV3 (A), OVCAR3 (B), and IGROV1 (C) cell lines grown on FN-coated, Col I-coated, or uncoated plates. Treatment of the cells with SPARC (10 μg/ml) significantly decreased β1-induced proliferation. Likewise, activation of αv-integrins by MnCl2 (2.5 mmol/L) increased proliferation of the three cell lines, grown on FN-coated, VN-coated, or uncoated plates. SPARC also inhibited αv-induced proliferation. Note the augmented MnCl2-induced proliferation of all cell lines plated on FN compared with other matrices. Represented are the results of one experiment that was performed in quadruplicates per experimental condition and was repeated three times with reproducible results. Results are expressed as the mean ± SEM of the fold increase in cell proliferation as compared with isotype-treated or PBS-treated control cells (assigned a value of 1) and after subtraction of the background of control wells without cells. *P < 0.05 for β1-stimulated cells compared with unstimulated controls. **P < 0.05 for αv-stimulated cells compared with unstimulated controls.
SPARC Diminishes Cell Surface Expression, Activation, and Clustering of Integrin Subunits in Ovarian Cancer Cell Lines
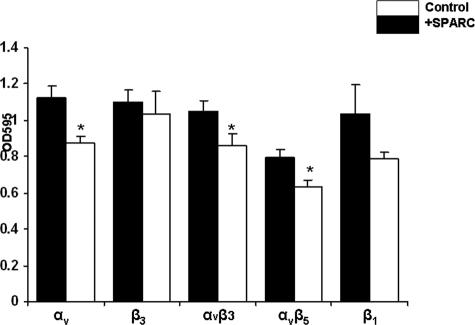
Having shown that the anti-adhesive and the antiproliferative effects of SPARC are mediated through antagonizing αv- and β1-integrins, we examined the effect of SPARC on the surface expression, and/or activation of these integrin subunits or heterodimers. First, using an integrin-mediated cell adhesion array, we found that preincubation of SKOV3 cells with SPARC for 16 hours resulted in a significant decrease in the expression levels of total αv (22%)-, αvβ3 (18%)-, and αvβ5 (19%)-integrins but not that of β3 (6%)- or the activated levels of β1 (24%)-integrins (Figure 4). In agreement with these results, FC revealed that SPARC significantly attenuated the expression and activation of αv-integrin subunit in SKOV3 cells by 35 and 33%, respectively (Table 1). The expression levels of total as well as activated αvβ3-heterodimers were significantly decreased by SPARC, as determined by LM609 (74%) and VNR1 27.1 (51%), and WOW-1 (29%) antibodies. SPARC also elicited significant diminutions in the levels of total αvβ5 (51%, as determined by P1F6)-, β1 (23%)-, and β5 (28%)-integrins (Table 1). Consistent with the integrin adhesion array results, SPARC-induced decrease (∼3%) in the cell surface expression levels of β3-integrin (as determined by B3A) or that of activated levels of β1-integrin subunit (as determined by P4G11) was not significant (Figure 4 and Table 1).
Figure 4.
SPARC decreases the level and activation of integrin subunits. Integrin expression on the cell surface of SKOV3 cells was assessed by an integrin-mediated cell adhesion array kit as described in Materials and Methods. Treatment of SKOV3 cells with SPARC (10 μg/ml) resulted in a significant decrease in the levels of αv, αvβ3, αvβ5, and, but not activated, β1- or β3-integrins. The results shown are representative of two independent experiments performed in triplicates with similar results. *P < 0.05, relative to PBS-treated controls.
Table 1.
Flow Cytometric Analysis of the Effect of SPARC on Cell Surface Expression of Total and Activated Integrin Subunits in Ovarian Cancer Cell Lines
| SKOV3
|
OVCAR3
|
IGROV1
|
||||
|---|---|---|---|---|---|---|
| Control | SPARC | Control | SPARC | Control | SPARC | |
| LM609 | 23,214 ± 976 | 6066 ± 250* | 4039 ± 196 | 3402 ± 178* | 23,435 ± 116 | 4190 ± 177* |
| 69-6-5 | 47,645 ± 2004 | 32,086 ± 1324* | 24,955 ± 1214 | 22,199 ± 393* | 51,092 ± 2267 | 47,809 ± 2022* |
| WOW-1 | 1287 ± 54 | 914 ± 37* | 4756 ± 231 | 4124 ± 216* | 1394 ± 61 | 1387 ± 58* |
| LM142 | 5880 ± 247 | 3864 ± 159* | 3880 ± 279 | 3201 ± 286* | 24,723 ± 1097 | 14,346 ± 60* |
| B3A | 9723 ± 408 | 9508 ± 392 | 2356 ± 114 | 2104 ± 110 | 25,105 ± 1114 | 8612 ± 364* |
| VNR | 8678 ± 365 | 4258 ± 175* | 10,842 ± 527 | 4836 ± 253* | 11,355 ± 503 | 7394 ± 312* |
| P1F6 | 11,362 ± 477 | 5149 ± 212* | 3847 ± 187 | 2086 ± 109* | ND | ND |
| P4C10 | 1382 ± 58 | 1065 ± 44* | 11,737 ± 571 | 1960 ± 102* | 2278 ± 101 | 2084 ± 88* |
| P4G11 | 790 ± 33 | 771 ± 31 | 2034 ± 83 | 1905 ± 43 | 2036 ± 90 | 1510 ± 63* |
| β5 | 4083 ± 171 | 2919 ± 120* | 3368 ± 163 | 2802 ± 147 | ND | ND |
Serum-starved subconfluent monolayers of cells were treated with SPARC (10 μg/ml) or PBS (control) for 16 hours before incubation with primary antibodies for total and activated integrin subunits, followed by allophycocyanin-conjugated secondary antibodies as described in Materials and Methods. The expression levels of integrin subunits and heterodimers were determined by mean fluorescent intensity measurements within the total cell population. The results shown are mean ± SD of two independent experiments performed in triplicates with similar results. ND, not detected.
P < 0.05 compared with isotype controls.
We also examined the effect of SPARC treatment on cell surface expression of various integrins on OVCAR3 and IGROV1 cell lines by FC (Table 1). Although the integrin repertoires of the three cell lines were different, SPARC consistently and significantly decreased the expression of total αvβ3 and αv as determined by LM609, LM142, 69-6-5, and VNR1 27.1 in all three cell lines. A significant decrease in the level of β3-subunit was only observed in IGROV1 but not in SKOV3 and OVCAR3 cells (despite a reproducible diminution). IGROV1 cells do not express αvβ5- nor β5-subunits. Similar to SKOV3, SPARC significantly decreased the level of β1-integrin in OVCAR3 and IGROV1 cell lines, but a significant decrease in the levels of activated β1-subunits was only observed in IGROV1 (Table 1). Taken together, it can be concluded that SPARC attenuates the surface expression and activation of αvβ3-, αvβ5-, αv-, β5-, and to a lesser extent β1-integrin, in the three ovarian carcinoma cell lines tested.
To substantiate these results, we studied the effect of SPARC on ligand-bound integrins by immunostaining of subconfluent SKOV3 and OVCAR3 cells grown on FN and VN because it has been previously shown that ligand binding to integrins promotes their activation and clustering on the cell surface.34 Integrin clustering was determined by dense punctate staining of αvβ3-heterodimer, as well as that of αv-, β1-, and β3-subunits in SKOV3 (Figure 5) and OVCAR3 cell lines (Supplemental Figure 1 at http://ajp.amjpathol.org). Consistent with our results from integrin cell adhesion array and FC studies, SPARC treatment of SKOV3 and OVCAR3 cells grown on FN or VN resulted in significant diminutions in the punctate staining of αv and αvβ3 (as determined by LM142 and LM609) as well as that of activated levels of αv and αvβ3 (as determined by 69-6-5 and WOW-1, respectively) but not that of β3-integrin subunit. A similar qualitative and quantitative decrease was noticed in total αvβ5 staining (as determined by P1F6) of SKOV3 and OVCAR3 cells treated with SPARC (Supplemental Figure 2 at http://ajp.amjpathol.org). Consistent with the FC and integrin adhesion array results, only a moderate, yet insignificant, attenuation of activated β1-integrin immunostaining was observed in SKOV3 and OVCAR3 cell lines (Figure 5 and Supplemental Figure 1 at http://ajp.amjpathol.org). These results further confirm that the effect of SPARC on ovarian cancer cell adhesion and proliferation is mediated, at least in part, through down-regulation of αv-, αvβ3-, and αvβ5-integrin expression and activation, as well as by an attenuated expression of β1-integrin.
Figure 5.
SPARC decreases the level and clustering of integrin subunits. SKOV3 cells were plated on VN-coated (A) and FN-coated (B) slide chambers for 24 hours before treatment with SPARC (10 μg/ml) for an additional 16 hours. Immunostaining with LM142 and 69-6-5 revealed clustering of αv-integrin in the form of a punctate, dense staining on the cell surface at the cell-cell junctions, and that clustering was disrupted with SPARC treatment of cells plated on either VN or FN. β3-Integrin exhibited a similar pattern of staining on both VN and FN in untreated control cells. Cells plated on VN and FN exhibited strong total αvβ3 staining (determined by LM609), whereas activated αvβ3 and αvβ5 (determined by WOW-1) was more pronounced on VN-plated untreated cells. Likewise, SPARC (10 μg/ml) treatment of SKOV3 cells resulted in a quantitative as well as a qualitative decrease in the staining pattern. Immunostaining with P4G11 detected β1-subunits in SKOV3 cells that were plated on FN. Fluorescent staining was mainly limited to the cell surface and cell-cell contacts. However, SPARC treatment of SKOV3 cells did not cause a significant qualitative or quantitative change in the staining pattern. Original magnifications, ×630.
SPARC Suppresses Survival Signaling in Ovarian Cancer Cells
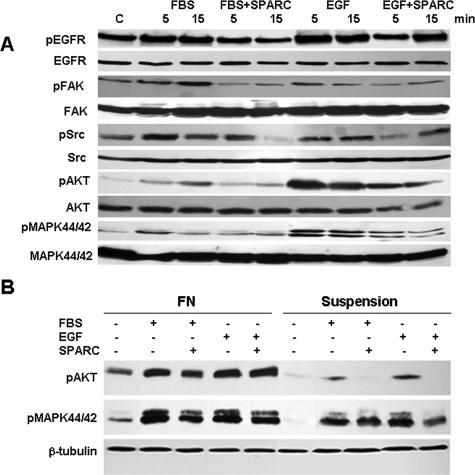
Integrin signaling may occur via several different pathways of which p44/42 MAPK is one of the best characterized. A collaboration between signaling by integrin engagement and growth factors is required to promote efficient activation (and autophosphorylation) of receptor tyrosine kinases (RTKs), such as EGF.3 The activation of p44/42 MAPK and AKT pathways was recently reported as a consequence of the adhesion of ovarian cancer cell lines to Col I, FN, and VN.35 These observations led us to investigate whether SPARC interferes with activation of survival pathways in ovarian cancer cell lines. We found that stimulation of SKOV3 cells with FBS not only resulted in pronounced phosphorylation of EGFR but also its downstream effectors FAK, Src, 44/42 MAPK, and AKT (Figure 6A). These effects occurred as early as 5 minutes and were sustained for 15 minutes. SPARC not only inhibited the FBS-induced EGFR phosphorylation but also inhibited activation of its downstream survival signaling molecules. Similar findings were observed in the less mestastatic OVCAR3 cell line (Supplemental Figure 4A at http://ajp.amjpathol.org) and IGROV1 cell line (which expresses αv β3-integrin as the sole αv-integrin subunit, Supplemental Figure 4B at http://ajp.amjpathol.org). However, the effect of SPARC on FBS- or EGF-induced FAK and Src phosphorylation was less pronounced (data not shown). Because serum contains an array of mitogenic growth factors, we hypothesized that SPARC could also be implicated in modulating the integrin growth factor survival signaling crosstalk. We focused on EGF-induced signaling because EGFR overexpression occurs in 35 to 70% of all primary ovarian cancers and is significantly associated with a high risk of progression in ovarian cancer patients.36 Stimulation of serum-starved SKOV3, OVCAR3, and IGROV1 cell lines with 40 ng/ml EGF resulted in a robust phosphorylation of EGFR, AKT, and 44/42 MAPK as early as 5 minutes and was sustained for at least 15 minutes in the three cell lines. Pretreatment of the cells with 20 μg/ml SPARC for 2 hours significantly inhibited phosphorylation of EGFR and its downstream effecter molecules (Figure 6A and Supplemental Figure 4, A and B, at http://ajp.amjpathol.org). The inhibitory effect of SPARC on EGF-induced phosphorylation of FAK and Src was more pronounced in SKOV3 (Figure 6A) compared with OVCAR3 and IGROV1 cells (data not shown). In an earlier report using the same cell lines,4 blockade of αv-integrin by the blocking antibody 69-6-5, neither affected FBS-induced 44/42 MAPK nor FAK phosphorylation, despite its inhibitory effect on cell-cycle progression and AKT phosphorylation. However, in SKOV3 cells grown on VN, we found that αv-integrins were clustered and co-localized with phosphorylated FAK on the cell surface. The addition of exogenous SPARC was shown to disrupt this clustering and co-localization significantly (Supplemental Figure 3 at http://ajp.amjpathol.org). The antiproliferative effect of SPARC on IGROV1 cell line, together with its inhibitory effect on EGFR, AKT, and 44/42 MAPK activation in, together with its effect on the cell surface expression of integrins suggest that the inhibitory effect of SPARC on survival signaling may be mediated mainly through αv-, and in part, β1-integrin-mediated signaling. To understand better the effect of SPARC on modulation of integrin-RTK survival signaling crosstalk, we examined which events were triggered by attachment of SKOV3 to FN, a ligand of a wide array of integrin subunits and heterodimers. Our studies revealed that SKOV3 cells plated on FN exhibited high basal levels of phosphorylated AKT and 44/42 MAPK compared with cells in suspension (Figure 6B). FBS and EGF stimulation elicited robust phosphorylation of AKT and 44/42 MAPK in FN-plated cells compared with suspended cells. Whether SKOV3 cells were plated on FN or kept in suspension, SPARC markedly inhibited FBS- and EGF-induced 44/42 MAPK phosphorylation—an effect that was more pronounced for cells in suspension. Interestingly, the inhibitory effect of SPARC on FBS- and EGF-induced AKT phosphorylation was more prominent in suspended cells, whereas EGF-induced AKT phosphorylation was not altered by SPARC in FN-anchored cells.
Figure 6.
SPARC inhibits anchorage-dependent and anchorage-independent survival signaling in ovarian cancer cells. Immunoblot analysis of p44/42 MAPK, PKB/AKTSer472 phosphorylation reveals that SPARC pretreatment of SKOV3 cells grown on uncoated tissue culture plates (A) for 2 hours inhibited FBS- and EGF-induced EGFR, FAK, Src, 44/42 MAPK, and PKB/AKTSer472 phosphorylation. SPARC inhibited FN-induced phosphorylation of 44/42 MAPK and PKB/AKTSer472 in SKOV3 cells (B) that were plated on FN for 4 hours compared with cells held in suspension. Pretreatment of cells with SPARC (20 μg/ml) attenuated FN-, FBS-, and EGF-induced activation of 44/42 MAPK, but not PKB/AKTSer472. In suspended cells, SPARC pretreatment markedly inhibited FBS- and EGF-induced phosphorylation of 44/42 MAPK and PKB/AKTSer472.
Discussion
The hallmark of epithelial ovarian cancer is a finely tuned multistep process that depends on the cooperative action of integrin-mediated tumor cell adhesion and growth factor-induced proliferation with concomitant increases in cell survival, migration, and invasion.4 Our interest in elucidating the role of SPARC in regulation of these cellular functions was prompted by the following recent findings: 1) anti-adhesive, antiproliferative, and proapoptotic effects of purified SPARC on ovarian cancer cell lines in culture29,33; 2) inverse correlation of the expression of SPARC in ovarian cancer cells and tumor tissues with the degree of malignancy33; 3) compromised ECM production and augmented matrix metalloproteinase (MMP) activity in tumor-bearing SP−/− mice18,29; and 4) enhanced tumor growth and metastasis in SP−/− mice.26,27,29
In this study, we provide evidence, for the first time, that the inhibitory effect of SPARC on adhesion of human ovarian cancer cell lines to ECM matrices, as well as to peritoneal mesothelial cells, is mediated in part through modulation of cell surface level and activity of αv- and β1-integrins; the crucial players in ovarian peritoneal carcinomatosis. Although SPARC decreased the surface expression level of β1-integrins in the three ovarian carcinoma cell lines tested and antagonized β1-integrin-induced ovarian cancer cell adhesion to the defined matrices and to PMCs, it neither had a significant effect on the activation state of β1-integrin in the highly invasive SKOV3 cell line nor in the less invasive OVCAR3. Conversely, SPARC exhibited a more pronounced inhibitory effect on the activation of β1-integrin in IGROV1 cell line. The substantial decrease in the expression of total and engaged αv-subunits in ovarian cancer cell lines after SPARC treatment could imply that the effect of SPARC on β1-induced adhesion is in part mediated through αvβ1- and/or α5β1-heterodimers, decreasing the available β1-subunits for dimerization (N.S. and K.M., unpublished results).
Integrins and RTKs exert a joint orchestrated control on tumor cell survival, proliferation, and migration/invasion. Striking activation of p44/42 MAPK has been reported in the presence of integrin aggregation and RTK ligands such as EGF, bFGF, vascular endothelial growth factor (VEGF), and platelet-derived growth factor (PDGF).3,37 Numerous observations have demonstrated that integrin-mediated cell anchorage can regulate the efficiency of signaling from RTKs to MAPK. Therefore, our findings that SPARC inhibited FAK, Src, AKT, and MAPK phosphorylation after stimulation with serum or EGF in ovarian cancer cells grown on uncoated plates, held in suspension, or anchored to FN not only reinforces the role of SPARC as an anti-adhesive molecule in ovarian cancer but also implicates SPARC as a negative modulator of integrin-growth factor survival signaling crosstalk. Furthermore, the inhibitory effect of SPARC on FN-, serum-, and EGF-induced survival signaling in ovarian cancer cells, strongly highlights its therapeutic potential, attributable to the fact that ovarian cancer cells escape anchorage-independent anoikis and have the ability to survive as floating spheroids in the peritoneal cavity.
The αvβ3-dependent adhesion, proliferation, and invasion have been reported in human ovarian cancer cell lines.4,38 However, the specific role of αvβ3-integrin in tumor invasion and metastasis is believed to be primarily attributable to its ability to interact with growth factors and their cognate receptors, particularly EGF/EGFRs and VEGF/VEGFRs, to recruit MMP-2 and MMP-9.3,24,39 We have recently reported that in the SP−/− syngeneic model of ovarian cancer, the expression and activity of VEGF and VEGFRs were significantly up-regulated, concomitant with increased expression and activity of MMP-2 and MMP-9 in tumor tissues and ascitic fluids.29 In this study, we report, for the first time, a significant suppression of EGFR activation in ovarian cancer cells by SPARC. Whereas a significant correlation between EGFR activation and ovarian carcinomatosis has been documented, the nature of the EGFR ligand that contributes to cancer progression has remained primarily unknown.36 The molecular mechanism of the interaction between SPARC and EGFR is currently being investigated. Taken together, the regulatory role of SPARC on tumor cell invasion could be attributed not only to the inhibitory effect of SPARC on expression level and clustering of integrin αv (αvβ3, αvβ5) and/or levels of β1 in tumor cells but also to the modulation of integrin crosstalk with VEGF and EGF growth factor receptors.
The underlying molecular mechanisms by which SPARC regulates the levels and clustering of integrin subunits, as well as integrin bidirectional signaling, are yet to be elucidated. Direct binding to integrins, disruption of the heterodimers, sequestration, or competitive inhibition of substrate binding should be considered as potential mechanisms of SPARC action. Other possibilities include a direct interaction with RTKs affecting phosphorylation and/or modulation of the crosstalk between the integrin-RTK signaling pathways. Moreover, the coincident localization and crosstalk of SPARC-ILK complex,40 together with the central role of ILK in αv- and β1-integrin signaling in ovarian cancer,4 highlight the involvement of ILK-SPARC interaction (N.S. and K.M., unpublished results). Further studies are warranted to definitively elucidate the role of SPARC in the paradigm of ECM-integrin-RTK crosstalk in tumorigenesis in general and in ovarian cancer in particular.
Supplementary Material
Acknowledgments
We thank Dr. Josè Luis for 69-6-5 antibody, Dr. Sanford Shattil for WOW-1 antibody, Dr. M.H. Ginsberg for mAb:VNR1 27.1, Dr. Franco Zunino for IGROV-1 cells, and Dr. Samuel Mok for Meso 301 peritoneal mesothelial cells.
Footnotes
Address reprint requests to Kouros Motamed, Vascular Biology Center, CB-3306, Medical College of Georgia, 1459 Laney Walker Blvd., Augusta, GA 30912. E-mail: kmotamed@mcg.edu.
Supported by the National Institutes of Health (grant 1K01CA89689) and The Georgia Cancer Coalition (grant GCC00023 to K.M.).
Supplemental material for this article can be found on http://ajp.amjpathol.org.
References
- Burleson KM, Casey RC, Skubitz KM, Pambuccian SE, Oegema TR, Jr, Skubitz AP. Ovarian carcinoma ascites spheroids adhere to extracellular matrix components and mesothelial cell monolayers. Gynecol Oncol. 2004;93:170–181. doi: 10.1016/j.ygyno.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Davidson B, Goldberg I, Reich R, Tell L, Dong HP, Trope CG, Risberg B, Kopolovic J. AlphaV- and beta1-integrin subunits are commonly expressed in malignant effusions from ovarian carcinoma patients. Gynecol Oncol. 2003;90:248–257. doi: 10.1016/s0090-8258(03)00321-4. [DOI] [PubMed] [Google Scholar]
- Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–826. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- Cruet-Hennequart S, Maubant S, Luis J, Gauduchon P, Staedel C, Dedhar S. Alpha(v) integrins regulate cell proliferation through integrin-linked kinase (ILK) in ovarian cancer cells. Oncogene. 2003;22:1688–1702. doi: 10.1038/sj.onc.1206347. [DOI] [PubMed] [Google Scholar]
- Attwell S, Mills J, Troussard A, Wu C, Dedhar S. Integration of cell attachment, cytoskeletal localization, and signaling by integrin-linked kinase (ILK), CH-ILKBP, and the tumor suppressor PTEN. Mol Biol Cell. 2003;14:4813–4825. doi: 10.1091/mbc.E03-05-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N, Riley C, Oliva K, Stutt E, Rice GE, Quinn MA. Integrin-linked kinase expression increases with ovarian tumour grade and is sustained by peritoneal tumour fluid. J Pathol. 2003;201:229–237. doi: 10.1002/path.1441. [DOI] [PubMed] [Google Scholar]
- Moser TL, Pizzo SV, Bafetti LM, Fishman DA, Stack MS. Evidence for preferential adhesion of ovarian epithelial carcinoma cells to type I collagen mediated by the alpha2beta1 integrin. Int J Cancer. 1996;67:695–701. doi: 10.1002/(SICI)1097-0215(19960904)67:5<695::AID-IJC18>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Cannistra SA, Ottensmeier C, Niloff J, Orta B, DiCarlo J. Expression and function of beta 1 and alpha v beta 3 integrins in ovarian cancer. Gynecol Oncol. 1995;58:216–225. doi: 10.1006/gyno.1995.1214. [DOI] [PubMed] [Google Scholar]
- Lessan K, Aguiar DJ, Oegema T, Siebenson L, Skubitz AP. CD44 and beta1 integrin mediate ovarian carcinoma cell adhesion to peritoneal mesothelial cells. Am J Pathol. 1999;154:1525–1537. doi: 10.1016/s0002-9440(10)65406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel T, Cannistra SA. Beta1-integrins partly mediate binding of ovarian cancer cells to peritoneal mesothelium in vitro. Gynecol Oncol. 1999;73:362–367. doi: 10.1006/gyno.1999.5388. [DOI] [PubMed] [Google Scholar]
- Carreiras F, Lehmann M, Sichel F, Marvaldi J, Gauduchon P, Le Talaer JY. Implication of the alpha v beta 3 integrin in the adhesion of the ovarian-adenocarcinoma cell line IGROV1. Int J Cancer. 1995;63:530–536. doi: 10.1002/ijc.2910630413. [DOI] [PubMed] [Google Scholar]
- Carreiras F, Cruet S, Staedel C, Sichel F, Gauduchon P. Human ovarian adenocarcinoma cells synthesize vitronectin and use it to organize their adhesion. Gynecol Oncol. 1999;72:312–322. doi: 10.1006/gyno.1998.5262. [DOI] [PubMed] [Google Scholar]
- Liapis H, Adler LM, Wick MR, Rader JS. Expression of alpha(v) beta3 integrin is less frequent in ovarian epithelial tumors of low malignant potential in contrast to ovarian carcinomas. Hum Pathol. 1997;28:443–449. doi: 10.1016/s0046-8177(97)90033-2. [DOI] [PubMed] [Google Scholar]
- Bao W, Stromblad S. Integrin alphav-mediated inactivation of p53 controls a MEK1-dependent melanoma cell survival pathway in three-dimensional collagen. J Cell Biol. 2004;167:745–756. doi: 10.1083/jcb.200404018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byzova TV, Kim W, Midura RJ, Plow EF. Activation of integrin alpha(V)beta(3) regulates cell adhesion and migration to bone sialoprotein. Exp Cell Res. 2000;254:299–308. doi: 10.1006/excr.1999.4765. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Pansino F, Baker M, Rice G, Quinn M. Association between alphavbeta6 integrin expression, elevated p42/44 kDa MAPK, and plasminogen-dependent matrix degradation in ovarian cancer. J Cell Biochem. 2002;84:675–686. doi: 10.1002/jcb.10080. [DOI] [PubMed] [Google Scholar]
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix communication. Matrix Biol. 2001;19:816–827. doi: 10.1016/s0945-053x(00)00133-5. [DOI] [PubMed] [Google Scholar]
- Framson PE, Sage EH. SPARC and tumor growth: where the seed meets the soil? J Cell Biochem. 2004;92:679–690. doi: 10.1002/jcb.20091. [DOI] [PubMed] [Google Scholar]
- Ledda MF, Adris S, Bravo AI, Kairiyama C, Bover L, Chernajovsky Y, Mordoh J, Podhajcer OL. Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat Med. 1997;3:171–176. doi: 10.1038/nm0297-171. [DOI] [PubMed] [Google Scholar]
- Aycock RL, Bradshaw AC, Sage EH, Starcher B. Development of UV-induced squamous cell carcinomas is suppressed in the absence of SPARC. J Invest Dermatol. 2004;123:592–599. doi: 10.1111/j.0022-202X.2004.23316.x. [DOI] [PubMed] [Google Scholar]
- Sturm RA, Satyamoorthy K, Meier F, Gardiner BB, Smit DJ, Vaidya B, Herlyn M. Osteonectin/SPARC induction by ectopic beta(3) integrin in human radial growth phase primary melanoma cells. Cancer Res. 2002;62:226–232. [PubMed] [Google Scholar]
- Ahmed N, Oliva K, Rice GE, Quinn MA. Cell-free 59 kDa immunoreactive integrin-linked kinase: a novel marker for ovarian carcinoma. Clin Cancer Res. 2004;10:2415–2420. doi: 10.1158/1078-0432.ccr-03-0042. [DOI] [PubMed] [Google Scholar]
- Schultz C, Lemke N, Ge S, Golembieski WA, Rempel SA. Secreted protein acidic and rich in cysteine promotes glioma invasion and delays tumor growth in vivo. Cancer Res. 2002;62:6270–6277. [PubMed] [Google Scholar]
- De S, Chen J, Narizhneva NV, Heston W, Brainard J, Sage EH, Byzova TV. Molecular pathway for cancer metastasis to bone. J Biol Chem. 2003;278:39044–39050. doi: 10.1074/jbc.M304494200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellahcène A, Castronovo V. Increased expression of osteonectin and osteopontin, two bone matrix proteins, in human breast cancer. Am J Pathol. 1995;146:95–100. [PMC free article] [PubMed] [Google Scholar]
- Brekken RA, Puolakkainen P, Graves DC, Workman G, Lubkin SR, Sage EH. Enhanced growth of tumors in SPARC null mice is associated with changes in the ECM. J Clin Invest. 2003;111:487–495. doi: 10.1172/JCI16804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puolakkainen PA, Brekken RA, Muneer S, Sage EH. Enhanced growth of pancreatic tumors in SPARC-null mice is associated with decreased deposition of extracellular matrix and reduced tumor cell apoptosis. Mol Cancer Res. 2004;2:215–224. [PubMed] [Google Scholar]
- Mok SC, Chan WY, Wong KK, Muto MG, Berkowitz RS. SPARC, an extracellular matrix protein with tumor-suppressing activity in human ovarian epithelial cells. Oncogene. 1996;12:1895–1901. [PubMed] [Google Scholar]
- Said N, Motamed K. Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. Am J Pathol. 2005;167:1739–1752. doi: 10.1016/S0002-9440(10)61255-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders RJ, Mainiero F, Giancotti FG. The role of integrins in tumorigenesis and metastasis. Cancer Invest. 1998;16:329–344. doi: 10.3109/07357909809084653. [DOI] [PubMed] [Google Scholar]
- Byzova TV, Plow EF. Activation of alpha Vbeta 3 on vascular cells controls recognition of prothrombin. J Cell Biol. 1998;143:2081–2092. doi: 10.1083/jcb.143.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood DA. Integrin activation. J Cell Sci. 2004;117:657–666. doi: 10.1242/jcs.01014. [DOI] [PubMed] [Google Scholar]
- Yiu GK, Chan WY, Ng SW, Chan PS, Cheung KK, Berkowitz RS, Mok SC. SPARC (secreted protein acidic and rich in cysteine) induces apoptosis in ovarian cancer cells. Am J Pathol. 2001;159:609–622. doi: 10.1016/S0002-9440(10)61732-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos AM, Arthur WT, Schneider P, Quest AF, Burridge K, Leyton L. Aggregation of integrins and RhoA activation are required for Thy-1-induced morphological changes in astrocytes. J Biol Chem. 2004;279:39139–39145. doi: 10.1074/jbc.M403439200. [DOI] [PubMed] [Google Scholar]
- Ahmed N, Riley C, Rice G, Quinn M. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin Exp Metastasis. 2005;22:391–402. doi: 10.1007/s10585-005-1262-y. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Hirata M, Yamazaki A, Kageyama T, Hasuwa H, Mizushima H, Tanaka Y, Yagi H, Sonoda K, Kai M, Kanoh H, Nakano H, Mekada E. Heparin-binding EGF-like growth factor is a promising target for ovarian cancer therapy. Cancer Res. 2004;64:5720–5727. doi: 10.1158/0008-5472.CAN-04-0811. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Gutkind J, Yamada K. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapke S, Kessler H, Luber B, Benge A, Hutzler P, Hofler H, Schmitt M, Reuning U. Ovarian cancer cell proliferation and motility is induced by engagement of integrin alpha(v)beta3/vitronectin interaction. Biol Chem. 2003;384:1073–1083. doi: 10.1515/BC.2003.120. [DOI] [PubMed] [Google Scholar]
- De S, Razorenova O, McCabe NP, O’Toole T, Qin J, Byzova TV. VEGF-integrin interplay controls tumor growth and vascularization. Proc Natl Acad Sci USA. 2005;102:7589–7594. doi: 10.1073/pnas.0502935102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker TH, Baneyx G, Cardo-Vila M, Workman GA, Weaver M, Menon PM, Dedhar S, Rempel SA, Arap W, Pasqualini R, Vogel V, Sage EH. SPARC regulates extracellular matrix organization through its modulation of integrin-linked kinase activity. J Biol Chem. 2005;280:36483–36493. doi: 10.1074/jbc.M504663200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.