Abstract
To study the effect of enhanced glucocorticoid signaling on T cells, we generated transgenic rats overexpressing a mutant glucocorticoid receptor with increased ligand affinity in the thymus. We found that this caused massive thymocyte apoptosis at physiological hormone levels, which could be reversed by adrenalectomy. Due to homeostatic proliferation, a considerable number of mature T lymphocytes accumulated in the periphery, responding normally to costimulation but exhibiting a perturbed T-cell repertoire. Furthermore, the transgenic rats showed increased resistance to experimental autoimmune encephalomyelitis, which manifests in a delayed onset and milder disease course, impaired leukocyte infiltration into the central nervous system and a distinct cytokine profile. In contrast, the ability of the transgenic rats to mount an allergic airway response to ovalbumin was not compromised, although isotype switching of antigen-specific immunoglobulins was altered. Collectively, our findings suggest that endogenous glucocorticoids impact T-cell development and favor the selection of Th2- over Th1-dominated adaptive immune responses.
Glucocorticoids (GCs) belong to a class of steroid hormones that are synthesized by the adrenal gland and released in response to stimuli such as stress and inflammation. Their secretion is under the control of the hypothalamus-pituitary-adrenal axis, a neuroendocrine cascade that involves positive and negative feedback loops. Once in the circulation, GCs exert pleiotropic effects ranging from the regulation of energy metabolism and the control of cognitive functions to the modulation of the immune system. Due to their lipophilic nature, they can passively diffuse into the cytoplasm and bind to the glucocorticoid receptor (GR). In turn, the GR translocates into the nucleus and interacts directly or indirectly via other transcription factors with promoter and enhancer elements of responsive genes.1,2 This ultimately leads to altered gene expression, forming the basis for most of the immunomodulatory activities of GCs. Although application of pharmacological doses of synthetic GCs has strong anti-inflammatory and immunosuppressive effects, endogenous GCs seem to modulate rather than outright suppress the immune system.3,4 The role of the GR in these processes has been investigated in cell culture and animal models, implicating it in lymphocyte development, apoptosis, and the control of innate and adaptive immunity.5,6 Nevertheless, many aspects of the function that endogenous GCs play in the thymus and the modulation of immune responses remain controversial.
In the thymus, immunocompetent T cells develop from pluripotent progenitors through a series of differentiation and selection steps.7 Whereas the ability of GCs to induce apoptosis in thymocytes is widely recognized, it is still controversial as to whether they are also involved in T-cell maturation.8,9 More than a decade ago, GR signaling was proposed to determine the outcome of positive and negative selection. Although mice expressing an antisense GR in the thymus were found to possess a T-cell repertoire with altered specificity, arguing that GC signaling impacts thymocyte selection, the analysis of hypomorphic GR knockout mice failed to provide any support for this model.10,11 Furthermore, there is also debate regarding the degree to which the thymus synthesizes GCs in addition to its common source, the adrenal gland.12 Corticosterone synthesis was demonstrated in the thymus,13,14,15 but studies using the inhibitor metyrapone led to ambiguous conclusions.16 Moreover, various functions were attributed to these GCs, ranging from T-cell development and thymic selection8,12 to the control of thymic involution.17 In summary, thymus-derived steroids and their relevance remain a matter of debate.
Beyond a role in T-cell development, it is also believed that GCs impact the type of immune responses generated.18 In particular, it was observed that elevated levels of endogenous GCs, such as experienced during prolonged periods of stress, can suppress cellular immunity while boosting humoral immunity. This has led to the concept that GCs govern the outcome of autoimmune and atopic diseases via their influence on cytokine production.19,20 A link between the activity of the hypothalamus-pituitary-adrenal axis and disease susceptibility is suggested by both animal experiments and human studies. Lewis rats, which have a hypoactive stress system, are extremely prone to the induction of Th1-mediated diseases such as experimental autoimmune encephalomyelitis.21,22 Conversely, women in the third trimester of pregnancy, who have increased levels of cortisol, often experience remission of Th1-mediated autoimmune diseases including multiple sclerosis and rheumatoid arthritis.22 This was explained by increased production of IL-4 and IL-10 and a reduction in IL-12. In line with this notion, Th2-mediated autoimmune disorders such as systemic lupus erythematosus can flare up under conditions of chronically elevated cortisol levels.18 In summary, despite good evidence that the strength of GR signaling impacts autoimmune and atopic diseases, the causal relationship to altered T-cell function is not yet well established.
Although many studies have addressed the question of what occurs when the GR is lacking, only a few reports have so far explored the physiological effects of increased GR levels in vivo.23,24 In an approach by Pazirandeh and colleagues, 25 GR overexpression was directed to the T-cell lineage, resulting in approximately twofold elevated receptor levels. This was accompanied by a moderate increase in GC sensitivity and a reduction in the thymic and peripheral T-cell pool. Analysis of aged mice further suggested that increased GC signaling interferes with thymic involution.17 Although these studies provided valuable new insight into the consequences of GR overexpression in T cells, the effects were comparably mild. Therefore, we decided to reevaluate the role that enhanced GR signaling plays in thymocyte development and adaptive immune responses by generating transgenic rats expressing a mutant GR with enhanced ligand affinity.26 These animals express strongly elevated receptor levels resulting in an extraordinarily high GC sensitivity. Consequently, thymocyte and mature T-cell numbers are strongly reduced, the T-cell repertoire perturbed, and the susceptibility to autoimmune and atopic diseases altered. This suggests that the modulation of T-cell development and the selection of Th2- over Th1-dominated adaptive immune responses are primary functions of endogenous GCs. In this sense, these rats represent a valuable model to assess effects of increased GR signaling such as observed during chronic stress.
Materials and Methods
Generation of Lentivirally Transduced Jurkat Cells
Two lentiviral vectors were cloned by inserting either the wild-type or a mutant mouse GR cDNA (carrying the point mutation C656G) along with an IRES-eGFP cassette into the plasmid FUW.27 Virus particles were generated following published protocols and were used to transduce Jurkat E6.1 cells by spinocculation.27 To obtain cell lines that uniformly express the wild-type GR (line J.Gr) or the mutant GR (line J.Gr*), the transduced cells were further sorted based on their eGFP levels by preparative flow cytometry using a FACSDiVa machine (BD Biosciences, San Jose, CA).
Generation of superTGR Transgenic Rats
Transgenesis was performed by pronucleus injection of a purified NotI fragment into (Crl:CDxLew/Crl)F1 zygotes as previously described.28,29 The transgene vector was constructed by cloning the mutant mouse GR (mGR C656G) into p1017, which consists of the proximal lck promoter and a human growth hormone (hGH) minigene.30 Analyses requiring a defined MHC haplotype were performed in rats that had been backcrossed to the inbred Lewis strain (RT1l) for a minimum of seven generations. All animal experiments were performed on male rats and approved by the Bavarian and Lower Saxony state authorities.
Antibodies
All antibodies were obtained from BD Biosciences unless otherwise indicated: Ox34 (CD2), Ox35 and Ox38 (CD4), Ox8 (CD8α), 3.4.1. (CD8β), JJ319 (CD28), Ox22 (CD45RC), Ox33 (CD45RA), P4/16 (RT6.1), Ox7 (Thy1), R73 (TCRβ), C-A11 (Vβ3.3), R78 (Vβ8.2), B73 (Vβ8.5), G101 (Vβ10), 18b1 (Vβ13), His42 (Vβ16) and V65 (TCRγδ). Yuggu-F6 (CD69) was a kind gift from Dr. Jung-Hyun Park (Experimental Immunology Branch, National Cancer Institute, Bethesda, MD). The polyclonal antibody against the GR was purchased from Santa Cruz Biotechnology (Heidelberg, Germany).
Proliferation Assay
Cells from the draining lymph nodes or magnetically purified T cells were cultured in 96-well plates in the presence of plate-bound anti-TCRβ (R73, 2 μg/ml) plus soluble anti-CD28 (JJ319, 0.5 μg/ml) monoclonal antibodies, ConA (2.5 μg/ml) or gpMBP (20 μg/ml). After 48 hours, 9.25 kBq of [3H]thymidine was added to the cultures, and 18 hours later the amount of incorporated radioactivity was determined using a β-plate reader.
CDR3 Spectratyping
Lymph node cDNA was amplified by PCR using a set of 23 different TCRBV primers together with a 6-FAM-tagged CB primer. All sequences have been reported previously.31 Cycling conditions for PCR were as follows: 95°C for 3 minutes and 30 seconds for denaturation, 57°C for 40 seconds for annealing, and 72°C for 1 minute for extension, followed by 34 cycles of 94°C for 40 seconds, 57°C for 40 seconds, and 72°C for 1 minute. PCR products were analyzed on a high-resolving polyacrylamide gel electrophoresis system in an automatic DNA sequencer model 377 (Applied Biosystems, Foster City, CA). The fluorescence-labeled DNA profile on the gel was analyzed using the Genescan software program (Applied Biosystems), which records the size and the intensity of each band. Spectratypes revealed by this analysis usually consist of five to seven bands.
Adrenalectomy (ADX)
The rats were weighed and anesthetized using ketamine and Rompun. For ADX, the adrenals were quickly removed through two small dorsal incisions, the wounds sealed with tissue glue, and the rats placed into their home cage. For sham surgery the same procedure was performed, except that the adrenals were merely exposed instead of removed. To compensate for the disturbed salt homeostasis, the rats were offered ad libitum a 0.9% NaCl solution for drinking.
In Vitro Apoptosis Assay
Total thymocytes or lymph node cells were cultured at 1 × 106 cells/ml RPMI containing 10% charcoal/dextran-treated FCS (HyClone, Logan, UT) in 48-well plates for 24 hours as described previously.32 The cells were analyzed by flow cytometry using Annexin V and monoclonal antibodies against TCRβ, CD4, and CD8.
Corticosterone RIA
Blood was collected retro-orbitally into precooled SST Microtainers (BD Biosciences) between 9:00 AM and 11:00 AM and kept on crushed ice. The samples were centrifuged and the serum stored at −20°C until analysis. The corticosterone RIA was performed according to the instructions of the manufacturer (MP Biomedicals, Eschwege, Germany).
Induction of Experimental Autoimmune Encephalomyelitis (EAE)
Active EAE in Lewis rats was induced as previously described.33 Briefly, transgenic rats and wild-type littermates were immunized subcutaneously with 100 μg of guinea pig MBP in 100 μg of CFA in the hind paws and limb. Disease severity was assessed as follows: 0 = no disease; 1 = limp tail; 2 = whole tail paralysis; 3 = beginning gait ataxia; 4 = more severe gait ataxia; 5 = mild paraparesis of the hind limbs; 6 = paraparesis of both hind limps or paraplegia of one hind limb; 7 = paraplegia of both hind limbs; 8 = mild tetraparesis; 9 = severe tetraparesis, moribund; and 10 = death.34
Histology
The rats were perfused with 4% paraformaldehyde and the spinal cords prepared, dehydrated, and embedded in paraffin as described.33 Five-micrometer sections were stained with hematoxylin and eosin (H&E), and infiltration was assessed by two independent investigators.
Cytokine Bead Array
Cytokine secretion by draining lymph node cells was determined by cytokine bead array (CBA, BD Biosciences) after culture for 72 hours in the presence of either ConA or gpMBP. Culture supernatant (50 μl) was incubated with beads specific for IFNγ, IL-4, or IL-10 according to the manufacturer’s instructions and analyzed using the supplied software.
Allergic Airway Hypersensitivity
Rats were sensitized using 2 mg/ml ovalbumin (OVA, grade V; Sigma Chemical, St. Louis, MO) in PBS, precipitated at a 1:1 ratio with alum (Sigma). One hundred micrograms of OVA/alum suspension was applied intraperitoneally. Four weeks later, the rats were challenged by two intranasal applications of 500 μg of OVA in PBS. Eighteen hours after the second treatment, the bronchoalveolar lavage and the draining lymph nodes were isolated. Serum was collected at weekly intervals over the whole experimental period to allow for the determination of the antibody titer.
Immunoglobulin Isotype ELISA
Serum titers of IgG1, IgG2a, IgG2b, and IgE OVA-specific antibodies were measured by ELISA. Ninety-six-well plates were coated with 10 μg/ml OVA in carbonate buffer. After blocking with 10% FCS, 1:1000 serum dilutions were added. OVA-specific immunoglobulins were detected using 1 μg/ml biotinylated anti-rat IgG1, IgG2a, IgG2b, and IgE antibodies (clones RG11/39.4, RG7/1.30, RG7/11.1, and B41-3; BD Biosciences) followed by incubation with streptavidin-AP conjugate (diluted 1:1000; Roche Diagnostics GmbH, Mannheim, Germany). The assay was developed using fast p-nitrophenyl phosphate (Sigma) and measured at 405 nm. OVA-specific positive and negative sera were used as controls and the results expressed as optical densities.
Statistical Analysis
The data were analyzed by Student’s t-test and are presented as the mean ± SEM (n.s., not significant, *P < 0.05, **P < 0.01).
Results
The Point Mutation C656G of the Glucocorticoid Receptor Renders T Cells Hypersensitive to GC-Induced Apoptosis
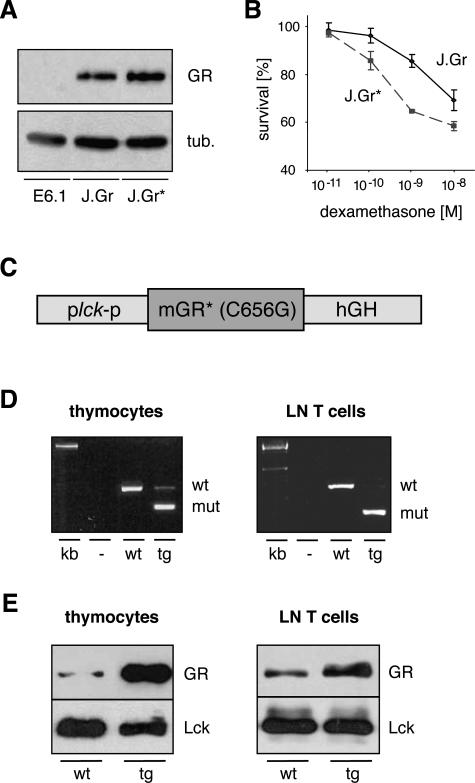
Residue C656 of the GR is involved in ligand binding and interpretation.26,35 When mutated to a glycine, the modified GR transactivates genes at lower hormone concentrations compared with the wild-type receptor and was therefore designated as a “super” GR. To investigate whether this mutation leads to a similar shift in the dose-response curve in the context of T-cell apoptosis, we tested its characteristics in cell culture. Jurkat E6.1 cells that are devoid of endogenous GR protein were transduced with lentiviruses coding for either the wild-type GR or the mutant GR(C656G). Analysis of the newly established cell lines J.Gr and J.Gr* by Western blot confirmed equal levels of GR expression (Figure 1A). To investigate their sensitivity to GC-induced apoptosis, the two cell lines were cultured in the presence of 10−11 to 10−8 mol/L dexamethasone (dex) for 48 hours and subsequently analyzed by flow cytometry using Annexin V staining. The dose-response curve for GC-induced cell death was shifted toward lower hormone concentrations, confirming that the point mutation C656G of the GR indeed increased the sensitivity of T cells to apoptosis (Figure 1B).
Figure 1.
Characterization of the GR(C656G) point mutation and generation of the superTGR rats. A: Western blot analysis of the three Jurkat cell lines E6.1, J.Gr, and J.Gr* for GR expression. β-Tubulin served as a loading control. B: The survival of J.Gr and J.Gr* cells cultured for 48 hours in the presence of serial dilutions of dexamethasone was determined by flow cytometry. Survival in control cultures was set at 100%. C: Structure of the transgene vector consisting of the proximal lck promoter, the mutant mGR(C656G) cDNA, and a hGH minigene. D: Analysis of GR mRNA expression in thymocytes and lymph node T cells from wild-type and transgenic rats. The cDNA was amplified using GR-specific primers and the PCR products digested with PstI. The smaller band is derived from the mutant GR encoded by the transgene (mut), whereas the larger band stems from the endogenous GR (wt). E: Western blot analysis of thymocytes and lymph node T cells using antibodies against GR and lck as a loading control.
Generation of Thymocyte-Specific GR(C656G)-Overexpressing Transgenic Rats
Having established that the C656G point mutation increases the GR’s sensitivity to GC-induced apoptosis, we questioned whether such a modified receptor would be constitutively active in the presence of basal GC levels when overexpressed in thymocytes. Therefore, we constructed a transgene vector encompassing the proximal lck promoter, the mouse GR cDNA carrying the point mutation C656G, and a human growth hormone minigene to allow for correct polyadenylation and splicing (Figure 1C). This construct was injected into Lew/CD F1 rat zygotes and the offspring screened for transgene integration. Two founder rats were identified, one of which was shown to have a high copy number and was used for further breeding and analysis (data not shown). These rats were designated superTGR in analogy with the initial description of the point mutation used to establish this transgenic line.26
First, we studied expression of the transgene in thymocytes and lymph node T cells by RT-PCR using primers derived from regions of the GR gene that were conserved between mouse, the origin of the transgene, and rat. A restriction site exclusively present in the amplicon derived from the transgene allowed us to determine the ratio between the mutant and the endogenous GR mRNA. Interestingly, the mutant receptor was predominantly expressed in both thymocytes and lymph node T cells (Figure 1D). Subsequently, we analyzed GR protein levels by Western blot. In transgenic thymocytes, the GR was dramatically overexpressed as compared with wild-type cells, whereas its increase in lymph node T cells from superTGR rats was more moderate (Figure 1E). This is in line with the initial goal to generate a thymus-specific transgenic rat.
Characterization of Thymocyte Development in superTGR Rats
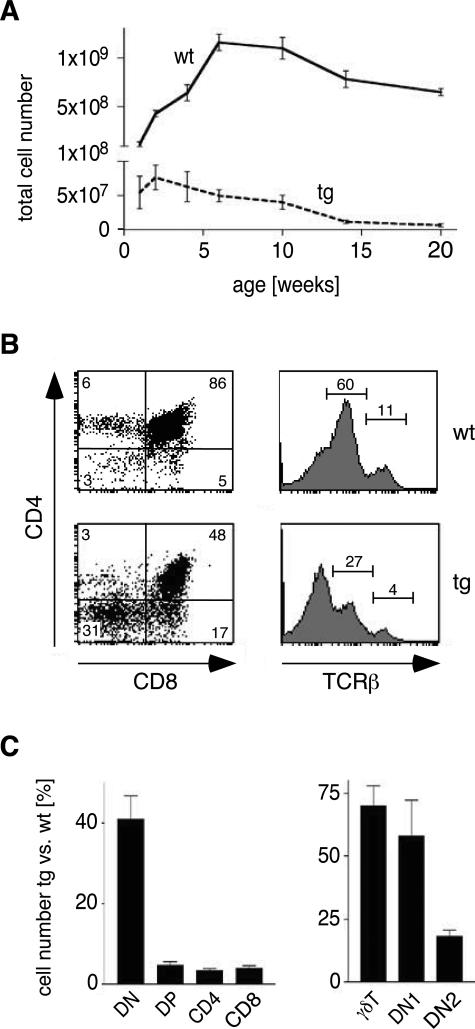
The impact of enhanced GR signaling on thymocyte apoptosis and development in superTGR rats was investigated by flow cytometry. Most impressively, the thymus as an anatomical structure was virtually absent in transgenic animals. Enumeration revealed that thymocyte cellularity was reduced by more than 90% in young adult superTGR rats (Figure 2A; note the split axis). This was accompanied by a strong reduction in the percentages of CD4+CD8+ DP, αβTCRint, and αβTCRhigh cells (Figure 2B). Because previous studies had indicated that GCs delay thymus involution,17 we extended our studies until 20 weeks of age. Despite its overall reduced size, the thymus in transgenic rats clearly underwent involution (Figure 2A). Importantly, this process appears to occur even earlier in superTGR rats compared with wild-type controls. Taken together, the mutant GR causes massive thymocyte apoptosis at basal hormone levels.
Figure 2.
Phenotypic characterization of thymocyte development. A: Total numbers of thymocytes in wild-type and transgenic rats during the first 20 weeks of age (note the split axis) (N = 5). B: Representative FACS analyses for CD4, CD8, and TCRβ expression on thymocytes in 10-week-old rats. The relative percentages within the quadrants/gates are indicated. C: Numbers of thymocyte subsets in 10-week-old rats. Depicted is the percentage of the absolute number of a cell population in transgenic rats compared with wild-type controls. γδT, γδTCR+ cells; DN1, CD45RC+CD2− DN cells; DN2, CD45RC+CD2+ DN cells.
Next, we analyzed the extent to which the different thymocyte subtypes were affected in young adult superTGR rats. First, we determined the absolute number of the four major thymocyte subpopulations in transgenic rats. Although the cellularity of the DN cell population was moderately affected (approximately 40% of control levels), there was a strong reduction in the number of DP, CD4 SP, and CD8 SP cells (Figure 2C). Second, we further dissected the effect on DN cells. The most immature cells in the rat thymus are CD45RC+CD2− DN cells (referred to as DN1), which subsequently progress to the CD45RC+CD2+ stage (referred to as DN2).36 This is also the time point when the lck promoter becomes active in the rat.28 The number of DN1 and γδ T cells was only mildly affected in superTGR rats, whereas considerably less DN2 cells were present in transgenic compared with wild-type animals (Figure 2C). Thus, enhanced GR signaling decreases the cellularity of all thymocyte subsets from the DN2 stage on, except for γδ T cells.
Characterization of Peripheral T Cells in superTGR Rats
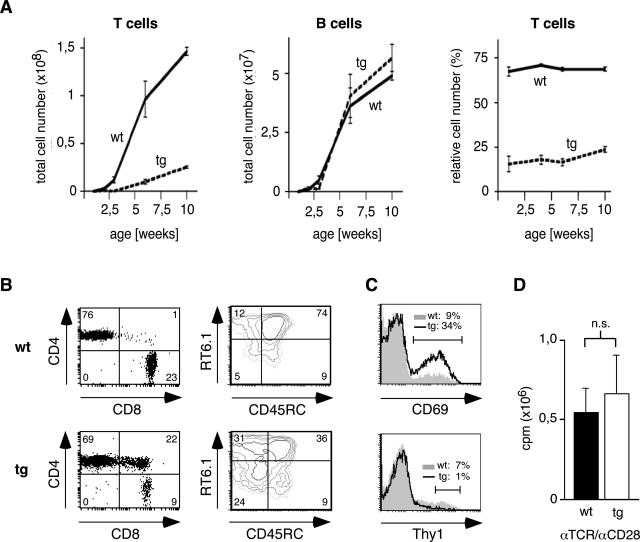
In light of the pronounced alterations observed in thymocytes, we wondered whether peripheral T lymphocytes were also affected in superTGR rats. The number of lymph node T cells was indeed diminished, although approximately 25% of wild-type levels were reached in older transgenic animals (Figure 3A). Because the number of B cells was similar in both genotypes, the relative number of lymph node T cells was also reduced in superTGR rats (Figure 3A). To characterize the peripheral T cells in more detail, we performed a number of flow cytometric analyses. CD8+ T cells were more affected by the expression of the mutant GR than CD4+ T cells (Figure 3B). In addition, a population of CD4+CD8+ DP cells was present in the lymph nodes of superTGR rats that express a CD8αα homodimer instead of a classic CD8αβ heterodimer (data not shown). This indicates that they represent activated CD4+ cells rather than being derived from DP thymocytes.37 In agreement with this finding, CD69+ cells were also more frequent in superTGR rats as compared with wild-type animals, indicating that the transgenic T cells were generally hyperactivated (Figure 3C).
Figure 3.
Phenotypic characterization of lymph node T and B cells. A: Enumeration of total lymph node T and B cells as well as the relative number of T cells in wild-type and transgenic rats during the first 10 weeks of age (N = 5). B: Representative FACS analyses of lymph node T cells in 10-week-old rats. Expression of CD4/CD8 was analyzed after gating on TCRβ+ cells; RT6.1/CD45RC was analyzed after gating on CD4+Thy1− cells. The relative percentages within the quadrants are indicated. C: Representative FACS analyses of lymph node T cells in 10-week-old rats. CD69 and Thy1 were analyzed after gating on CD4+ cells. The relative percentages within the gates are indicated. D: The proliferation of lymph node T cells was measured by [3H]thymidine incorporation assay after costimulation with anti-TCRβ and anti-CD28 monoclonal antibodies (N = 4).
Peripheral rat T cells can be classified on the basis of their Thy1, RT6.1, and CD45RC expression.38 Whereas so-called recent thymic emigrants are Thy1high, mature peripheral T cells that lack Thy1 expression are further subdivided into CD45RC+RT6.1+ naïve T cells, CD45RC−RT6.1− activated T cells, and two distinct populations of memory T cells. In line with the previous data, activated and memory T cells were increased in superTGR rats while the number of recent thymic emigrants and naïve T cells was strongly diminished (Figure 3, B and C). This indicates that the few mature thymocytes that manage to seed the peripheral lymphoid organs undergo vigorous homeostatic proliferation and consecutively acquire an activated phenotype that confers on them protection from GC-induced apoptosis.
To characterize the functional properties of the peripheral T lymphocytes that accumulate in superTGR rats, we investigated their proliferative response to costimulation. Lymph node T cells were purified from wild-type and transgenic rats and cultured in the presence of monoclonal anti-TCR and anti-CD28 antibodies. Two days later, a [3H]thymidine incorporation assay was performed. No difference in the mitogenic response was observed between both groups, suggesting that peripheral T cells in superTGR rats are able to proliferate normally (Figure 3D).
The Peripheral T-Cell Repertoire in superTGR Rats Is Altered
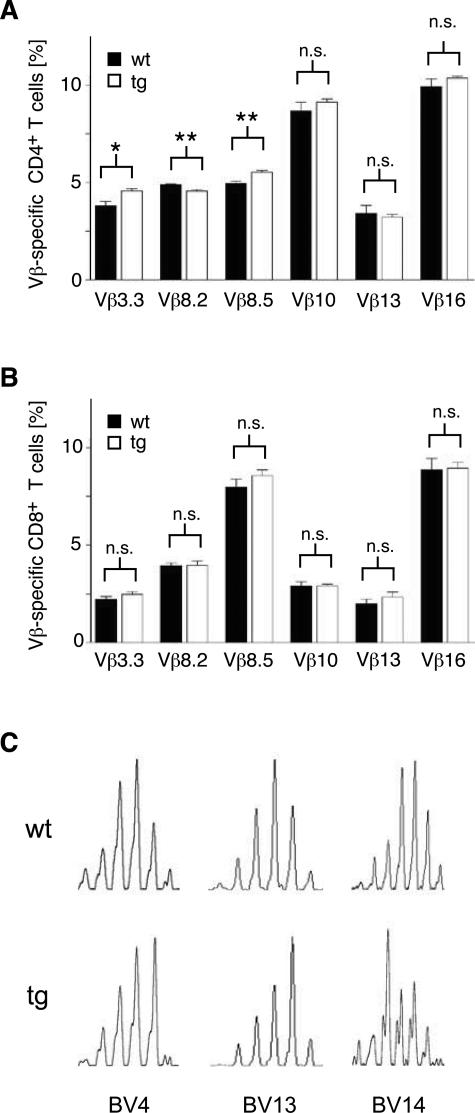
Controversial evidence suggests that endogenous GCs impact positive and negative selection, thereby altering the TCR repertoire of mature T lymphocytes.10,11 Thus, we reevaluated this issue in superTGR rats by analyzing the relative representation of six different TCR Vβ segments on lymph node T cells. Although endogenous superantigens have not been identified in rats, it is well established that the frequencies of Vβ segments on CD4+ and CD8+ cells result from differential thymic selection and are under strict genetic control.39 No differences were observed for the CD8 subset, whereas a small but significant shift could be demonstrated for the expression of three Vβ segments on CD4 cells (Figure 4, A and B).
Figure 4.
The TCR repertoire of peripheral T cells. A: CD4+ lymph node T cells were analyzed for the representation of various TCRβ V-segments by flow cytometry after staining with a combination of monoclonal antibodies against TCRβ and the respective V-segments (N = 7). B: CD8+ lymph node T cells were analyzed for the representation of various TCRβ V-segments by flow cytometry (N = 7). C: Examples for spectratypes of lymph nodes cells from wild-type and transgenic rats for the three different TCRβ chains BV4, BV13, and BV14.
To gain more detailed insight into the TCR repertoire, we performed a spectratype analysis of lymph node cells from superTGR and control rats. This PCR-based approach assesses the length of the CDR3 hypervariable region of the TCRβ chain, which correlates with the repertoire of antigen-specific cells.31,40 Wild-type rats showed a nearly Gaussian distribution of the CDR3 lengths for all 23 Vβ chains analyzed. In contrast, superTGR rats showed a perturbed spectrum for up to seven Vβ chains in individual transgenic animals. Examples of such abnormal distributions are given in Figure 4C. In summary, we conclude that enhanced GR signaling in superTGR rats alters the repertoire of peripheral T cells.
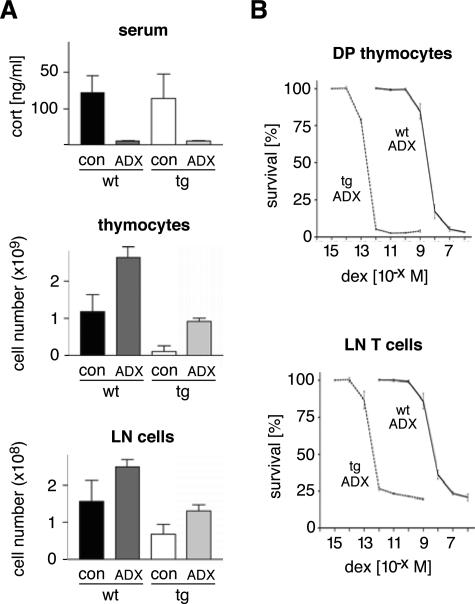
Adrenally Derived GCs Are Responsible for the Reduced Cellularity of the Thymus and the Lymph Nodes in superTGR Rats
There is a long-standing debate as to whether the adrenal gland is the exclusive source of GCs.12 This prompted us to investigate whether thymus-derived GCs might be involved in the induction of apoptosis in superTGR rats and thereby potentially contribute to the observed reduction in thymus and lymph node cellularity. To this end, we adrenalectomized wild-type and superTGR rats and enumerated thymocyte and T-cell numbers. Sham-operated animals served as controls for a potential influence from surgery. In addition, corticosterone levels were determined to confirm the complete absence of GCs after adrenalectomy (Figure 5A). Importantly, within 2 weeks the cellularity of the thymus and the lymph nodes in superTGR rats had increased almost 20-fold, although they still remained below the levels found in adrenalectomized wild-type controls (Figure 5A). In addition, the subtype composition of the thymocytes based on their CD4, CD8, and αβTCR expression profiles was indistinguishable between adrenalectomized wild-type and superTGR rats (data not shown). Thus, the phenotype of superTGR rats with regard to cellularity and cellular composition can be largely explained by the action of adrenally synthesized hormones. However, because the levels reached in adrenalectomized superTGR rats were not identical to those of controls, it cannot be formally excluded that thymus-derived GCs play a minor role in T-cell apoptosis.
Figure 5.
Effect of adrenalectomy on cellularity and the dose-response curve of GC-induced apoptosis. A: At the age of 5 weeks, wild-type and transgenic rats were bilaterally adrenalectomized (ADX). Fourteen days later serum corticosterone levels and the cellularity of the thymus and lymph nodes were determined. As a control, rats were sham-operated (con). B: Two weeks after surgery thymocytes and lymph node cells were recovered from adrenalectomized wild-type and transgenic rats. The cells were cultured for 24 hours in charcoal-treated medium in the presence of dexamethasone and the survival determined by flow cytometry using Annexin V in combination with monoclonal antibodies against CD4, CD8α, and TCRβ (N = 12).
To determine the in vitro sensitivity of the transgenic thymocytes and lymph node T cells to GC-induced apoptosis, we established dose-response curves using adrenalectomized rats. This should prevent any bias due to the selective survival of apoptosis-resistant cells in superTGR rats as a consequence of constitutive GR signaling in vivo. Significantly, the sensitivity to dex-induced apoptosis of both DP thymocytes and lymph node T cells from superTGR rats was increased by four orders of magnitude (Figure 5B). Transgenic cells underwent apoptosis at 10−12 mol/L dex, which corresponds to a hormone concentration much below the levels found in vivo, providing a convincing explanation for the dramatically reduced cellularity of the thymus. However, the finding that the number of mature peripheral T cells was around 25% of wild-type levels indicates that these cells are apparently protected from GC-induced apoptosis in vivo, presumably due to their activated phenotype.
superTGR Rats Are Partially Protected from EAE
EAE is a widely recognized animal model for multiple sclerosis (MS), a chronic inflammatory disease of the central nervous system.41 In view of the observation that increased GC signaling exerts a positive influence on the course of Th1-mediated diseases and therefore often leads to the remission of MS,18 we wondered whether superTGR rats may no longer succumb to EAE.
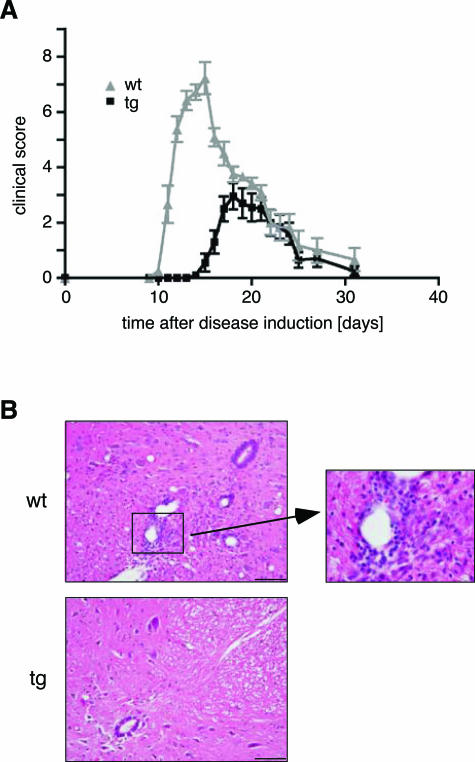
To address this question we induced a monophasic inflammatory EAE in Lewis rats by immunization with guinea pig MBP. Ten days after disease induction, the control rats showed the first signs of the disease and became severely affected within a few days, resulting in paraplegia in most of the animals (Figure 6A). Around day 16, the symptoms started to remit, and by day 30, the rats had almost completely recovered. In contrast, the onset of the disease in superTGR rats was delayed by 5 days, and the severity was strongly reduced (Figure 6A). This experiment was reproduced with cohorts of 3- and 6-month-old rats with identical results (data not shown). We conclude that alterations in the composition and function of the T-cell compartment in the transgenic rats delay the onset of EAE and prevent its exacerbation.
Figure 6.
Experimental autoimmune encephalomyelitis. A: Clinical disease scores for 3-month-old transgenic rats (black line, N = 10) and wild-type littermates (gray line, N = 9). One representative experiment of four is shown. B: H&E staining of paraffin sections from the lumbar spinal cord obtained at day 13 after EAE induction (N = 3). Scale bar = 100 μm. A highly infiltrated part of the wild-type spinal cord is enlarged.
To investigate the protective mechanism at work, we performed histological analyses on spinal cord sections from superTGR rats. At day 10, briefly before the onset of disease, no sign of inflammation was observed in the spinal cord of either genotype (data not shown). At day 13, at the peak of the disease in wild-type rats, their spinal cords were heavily infiltrated with leukocytes, most prominently around the blood vessels (Figure 6B). In contrast, in most superTGR rats no leukocytes were present in the spinal cord at this point (Figure 6B). Also at day 18, the peak of the disease in transgenic rats, only small meningeal and perivascular infiltrates were detected, mainly consisting of T cells and a few macrophages (data not shown). Moreover, the composition of the infiltrating cells was the same as in the control group at the peak of the disease (data not shown) and corresponds to what had been previously described using this EAE model.33 To exclude that leukocytes in transgenic rats migrate to the central nervous system (CNS) while being immediately cleared by apoptosis, we performed TUNEL staining. Whereas numerous apoptotic cells were detected in wild-type animals, they were extremely rare in the spinal cords of superTGR rats at all time points (data not shown). Taken together, these findings clearly indicate that transgenic rats owe the delayed onset and milder disease course of EAE to impaired infiltration of the CNS by leukocytes.
T-Cell Priming in superTGR Rats Occurs Normally While Cytokine Secretion Is Altered
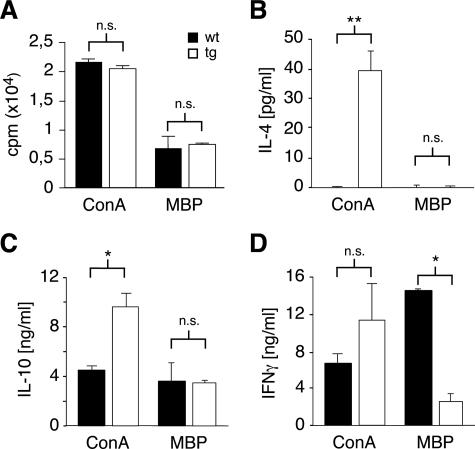
T-cell priming is an essential step in the development of an immune response.42 To investigate whether this was affected in superTGR rats, we isolated draining lymph node cells at day 13 after EAE induction and stimulated them with ConA or gpMBP. Both the mitogenic potential as well as the recall response to antigen were similar in wild-type and superTGR rats, suggesting that enhanced GR signaling does not compromise T-cell priming (Figure 7A). Consequently, sufficient numbers of antigen-specific cells should be present in superTGR rats to allow full EAE to develop.
Figure 7.
T-cell priming and cytokine production during EAE. A: Lymph node cells were recovered 13 days after EAE induction and stimulated with ConA or gpMBP. The mitogenic response was determined by [3H]thymidine incorporation assay (N = 4). B–D: The levels of IL-4, IL-10, and IFNγ were measured by cytokine bead array in the culture supernatant of lymph node cells 72 hours after stimulation with ConA or gpMBP, respectively (N = 3). Filled bars, wild type; open bars, transgenic.
Because the balance between Th1 and Th2 cytokines is known to impact the susceptibility to autoimmune diseases,20 we wondered whether the cytokines secreted during EAE were different in the two lines of rats. Therefore, we cultured lymph node cells recovered at day 13 after disease induction in the presence of ConA or gpMBP and studied cytokine release by flow cytometry. Most remarkably, cells from superTGR rats produced significant amounts of IL-4 after stimulation with ConA, whereas this Th2 cytokine was undetectable in culture supernatants from wild-type cells (Figure 7B). In addition, transgenic cells also produced higher amounts of IL-10 and IFN-γ under these conditions (Figure 7, C and D). To investigate cytokine production by the encephalitogenic T cells, restimulation was performed in the presence of gpMBP. The antigen-specific cells from superTGR rats turned out to produce significantly less IFNγ as compared with wild-type controls, whereas IL-10 secretion was unaltered and IL-4 undetectable (Figure 7, B–D). In summary, it appears that reduced IFNγ production by pathogenic T cells in combination with a less favorable cytokine environment as characterized by the presence of IL-4 may contribute to the decreased susceptibility of superTGR rats to EAE.
superTGR Rats Develop a Full Allergic Airway Response to Ovalbumin
In view of the fact that superTGR rats were T-lymphopenic, it was conceivable that they were generally compromised in their ability to mount an immune response. Therefore, we wondered how they would behave in the context of a Th2-dominated humoral immune reaction. Allergic airway inflammation represents such a prototypic Th2-dependent model disease. Repeated administration of ovalbumin induces the expansion of antigen-specific B cells, which ultimately leads to an inflammatory response following local antigen application.43,44,45 This involves the production of Th2-type cytokines that direct class switching of antigen-specific B cells. Although eosinophilia and IgE production, typical hallmarks of human asthma, are not observed in Lewis rats,45,46 challenge of the sensitized rats by intranasal application of ovalbumin still induces massive infiltration of the lungs by granulocytes and T cells.
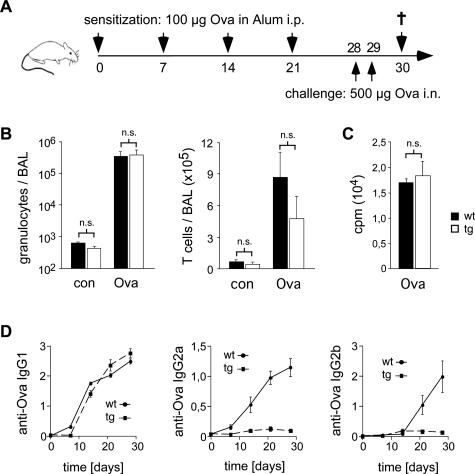
To study superTGR rats in this Th2 model disease, we sensitized them with ovalbumin in alum over a period of 4 weeks, followed by a challenge with two consecutive intranasal applications of ovalbumin (Figure 8A). Eighteen hours later, the rats were sacrificed and the bronchoalveolar lavage (BAL) analyzed by flow cytometry. In the case of nonimmunized rats, only a few leukocytes were detected in the BAL of either genotype (Figure 8B). In contrast, challenge of wild-type rats with ovalbumin induced a dramatic infiltration of the lung by both granulocytes and T cells, which resulted in an increase in the cellularity of the BAL by three orders of magnitude (Figure 8B). Importantly, in superTGR rats, infiltration of the lung by granulocytes and T cells was as pronounced as in the wild-type controls. This is in sharp contrast to the induction of EAE where almost no infiltration of the target organ was seen in the transgenic animals. We conclude that the development of a Th2-dominated immune response is not compromised in superTGR rats.
Figure 8.
Allergic airway hypersensitivity response. A: Experimental protocol: rats were sensitized by consecutive intraperitoneal injections of ovalbumin (Ova) in alum followed by intranasal challenge with Ova in PBS. On day 30, the rats were sacrificed. B: The infiltration of the lung was determined by analyzing the cellular content in the bronchoalveolar lavage (BAL) of untreated rats (con) and on day 30 of Ova-challenged rats. Granulocytes and T cells in the BAL were measured using the monoclonal antibodies His48 and R73 (N = 7). C: Proliferation was determined by [3H]thymidine incorporation assay after stimulating the cells with 100 μg/ml Ova for 48 hours. D: Time course of the Ova-specific IgG1, IgG2a, and IgG2b antibody titers in the serum during the sensitization phase. The relative OD units measured at 405 nm are depicted. One representative experiment of three is shown (N = 5).
Isotype Switching Is Altered in superTGR Rats
Despite the unaltered infiltration of the lung in superTGR rats, it was still conceivable that the characteristics of the immune response to ovalbumin were different as in wild-type animals. To address this issue, we cultured draining lymph node cells from intranasally challenged rats in the presence of ovalbumin and measured the recall response by [3H]thymidine incorporation assay (Figure 8C). Lymph node cells from both rat strains responded equally well to ovalbumin, indicating once more that T-cell priming is unaffected in superTGR rats.
The cytokines secreted during an immune response direct isotype switching by antigen-specific B cells. In the rat, Th1 cytokines favor the generation of IgG2a and IgG2b immunoglobulins, whereas under the influence of Th2 cytokines, IgG1 antibodies are preferentially produced.47,48 Similar to our findings in the EAE model, lymph node T cells from superTGR rats secreted high levels of IL-4 and reduced amounts of IFNγ after intranasal challenge with ovalbumin (data not shown). To investigate whether this Th2 bias impacts the production of ovalbumin-specific antibodies, we followed their time course in the serum over the time period of immunization. Interestingly, production of IgG1 antibodies was similar in both genotypes, whereas production of the Th1-type immunoglobulins IgG2a and IgG2b was completely abrogated in superTGR rats (Figure 8D). Thus, enhanced GR signaling does not impair Th2-dominated immune responses, although a different set of cytokines and immunoglobulins is produced under these conditions.
Discussion
The anti-inflammatory and immunosuppressive activity of pharmacological GCs is well established and used in the treatment of a variety of diseases.3,4,42 In contrast, the role that endogenous GCs play in T-cell development and adaptive immunity is far less understood.6 They have been implicated in thymocyte selection8 and are believed to be synthesized in situ.13,14,15 However, the evidence for this is contradictory. In addition, it is known that stress impacts the susceptibility to autoimmune and atopic diseases, but the mechanisms underlying this clinical observation have not been fully resolved.18 To investigate in vivo the role that the GR plays in these processes, we generated transgenic rats overexpressing a mutant receptor with increased ligand affinity. These animals are characterized by massive thymocyte apoptosis, a decrease in peripheral T-cell numbers, and altered T-cell function. This allowed us to investigate the consequences of enhanced GR signaling for thymus physiology and adaptive immune responses.
The GR point mutation C656G was selected for its remarkably strong transcriptional activity.26 Due to an increased ligand affinity, this GR becomes activated at hormone levels that are far below those present under physiological conditions. Consequently, the mutant GR in transgenic rats is constitutively active, leading to massive thymocyte apoptosis and an almost complete block in T-cell production. Only a small number of thymocytes manage to mature and seed the secondary lymphoid organs. Once in the periphery, however, the cells undergo vigorous homeostatic proliferation, which apparently protects them from apoptosis. This is presumably a consequence of their activated phenotype, because we have found elevated numbers of CD69+, CD8αα+, and CD45RC−RT6.1− cells in superTGR rats, all well-known markers of T-cell activation in the rat.37,38 In line with this interpretation, GCs were previously shown to induce CD8α on CD4+ T lymphocytes, leading to an accumulation of activated DP cells.37 Despite the altered composition of the T-cell compartment in transgenic rats, the peripheral T lymphocytes respond normally to costimulation. We conclude that constitutive GR signaling induces thymocyte apoptosis but still allows for a considerable number of mature T cells to accumulate in the periphery following homeostatic proliferation.
The impact of GR overexpression on T lymphocytes has been previously addressed in transgenic mice.25 In these animals, expression of the receptor was only twofold above normal, and consequently the effects on cellularity and apoptosis were much milder in comparison to superTGR rats. More importantly, the earlier study implicated GR overexpression in delaying age-associated thymic involution.17 In contrast, this process was not significantly different in superTGR rats as compared with controls, suggesting that enhanced GR signaling does not affect thymic involution.
Conflicting evidence has also been presented for intrathymic GC synthesis.13,14,15,16 Because the ligand affinity of the mutant GR in transgenic rats is dramatically increased, thymus-derived GCs should induce apoptosis even at minute amounts. Importantly, our experiments show that adrenalectomy of superTGR rats largely reversed the observed decrease in thymocyte and peripheral T-cell numbers as characterized by a 20-fold increase in thymocyte number within 2 weeks after surgery. However, it is noteworthy that the cellularity reached in adrenalectomized transgenic rats was still below the one in controls. Although this could be taken as evidence for thymus-derived GCs as concluded earlier from the analysis of conditionally GR overexpressing mice,49 we rather believe that the massive replenishment of the T-cell pool in adrenalectomized superTGR rats speaks in favor of a negligible role for extra-adrenal steroids.
Another long-standing debate concerns the potential impact of GCs on thymocyte selection and the TCR repertoire. Although some reports are in favor of such a role,10 other studies do not support this model.11 Moreover, the fact that some data had been obtained using a hypomorphic strain of GR knockout mice renders the situation even more confusing.23 Therefore, we re-evaluated this issue in superTGR rats. Importantly, the Vβ representation on CD4+ and CD8+ lymph node T cells as well as the spectrum of the CDR3 lengths showed small but significant differences between wild-type and superTGR rats. This result would be compatible with a role for GCs in thymic selection, if one assumes that this process is faithfully reflected by the repertoire of peripheral T cells. However, our finding could also be explained by oligoclonal expansion of individual T-cell clones, which is typically observed during homeostatic proliferation.50 We conclude that enhanced GR signaling impacts the TCR repertoire, although it remains unclear as to whether this actually occurs at the level of thymic selection.
Beside the thymus, our analyses revealed major functions for endogenous GCs in the control of adaptive immunity. It was previously reported that Th1-mediated autoimmune disorders such as multiple sclerosis improve under conditions of chronically elevated GC levels, whereas Th2-dependent diseases flare up.18 This clinical observation is mimicked by our superTGR rats, which on the one hand are largely protected from gpMBP-induced EAE but on the other hand are able to mount a full allergic airway response to ovalbumin. As a consequence of the compromised ability of pathogenic T cells to infiltrate the CNS, the onset of EAE in the transgenic rats is delayed, and the disease course is milder. Whereas the T lymphopenia seen in superTGR rats may contribute to this phenotype, we believe that it is at most a minor determinant. First, it was shown that thymectomy neither impacts the incidence nor the onset of EAE but even aggravates its pathology.51 This suggests that low T-cell numbers suffice to induce EAE. Second, recent evidence indicates that lymphopenia favors the development of autoimmune diseases due to exaggerated homeostatic proliferation.50 Thus, one would predict that superTGR rats are prone to the induction of EAE rather than being partially resistant. Third, our observation that an allergic airway response can be elicited in superTGR rats argues that these animals are not generally impaired in their ability to mount adaptive immune reactions. Therefore, we believe that it is primarily the altered functional properties of the transgenic T cells that influence disease susceptibility in superTGR rats.
Enhanced GR signaling has been hypothesized to alter the balance between Th1 and Th2 cytokines.20 This is now supported by our studies on EAE and allergic airway inflammation in superTGR rats. First, such a conclusion can be drawn from the analysis of rats undergoing EAE, a typical Th1-mediated disease. Following stimulation with ConA, transgenic lymph node cells synthesize significant amounts of IL-4 that is not seen in wild-type controls. In vivo this could be expected to affect negatively IFNγ production by MBP-specific T cells and thereby to impair their ability to infiltrate the CNS. Such a model is supported by the previous finding that encephalitogenic cells generated in the presence of dexamethasone and IL-4 secrete lower amounts of IFNγ and are unable to induce EAE after adoptive transfer.52 However, it is noteworthy that despite the observed Th2 shift, stimulation of T cells from superTGR rats with MBP did not result in IL-4 production. Thus, enhanced GR signaling in Lewis rats does not convert encephalitogenic Th1 cells into bona fide Th2 cells. In summary, our results suggest that GR signaling does not interfere with T-cell priming but rather affects the polarization of adaptive immune responses. At the same time, this would also explain why the allergic airway reaction was not affected in superTGR rats. In this model, a Th2-dependent humoral immune response to ovalbumin is elicited, characterized by antibody production and leukocyte infiltration of the lung.43,45 Although the latter occurred normally in the transgenic rats, analysis of antibody levels revealed an interesting effect on isotype switching. Ovalbumin-specific IgG1 antibodies were regularly made during the sensitization phase. In contrast, superTGR rats completely failed to produce any antigen-specific IgG2a and IgG2b immunoglobulins, two Th1-specific antibody classes.47 The previous finding in mice that IL-4 was able to suppress IgG2a and IgG2b class-switching provides a convincing explanation for this observation.53 We predict that the altered antibody class profile following enhanced GR signaling may significantly influence the quality of an immune response. This suggests that effects of GCs on humoral immunity may affect autoimmune and atopic disorders to an extent that had been previously underestimated. In summary, the analysis of superTGR rats has provided new answers as to the mechanism of endogenous GCs in thymocyte apoptosis and development, adaptive immune responses, and their influence on disease susceptibility.
Acknowledgments
We thank Katrin Voss, Melanie Schott, Christian Bauer, Silvia Seubert, and Christian Linden for expert technical help, and Dr. Ralf Linker for advice on histology.
Footnotes
Address reprint requests to Holger Reichardt, Institute for Virology and Immunobiology, University of Würzburg, Versbacher Strasse 7, 97078 Würzburg, Germany. E-mail: holger.reichardt@mail.uni-wuerzburg.de.
Supported by Deutsche Forschungsgemeinschaft, VolkswagenStiftung, Interdisziplinäres Zentrum für klinische Forschung Würzburg, and Gemeinnützige Hertie-Stiftung.
References
- Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- Zhou J, Cidlowski JA. The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids. 2005;70:407–417. doi: 10.1016/j.steroids.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci. 1998;94:557–572. doi: 10.1042/cs0940557. [DOI] [PubMed] [Google Scholar]
- Tuckermann JP, Kleiman A, McPherson KG, Reichardt HM. Molecular mechanisms of glucocorticoids in the control of inflammation and lymphocyte apoptosis. Crit Rev Clin Lab Sci. 2005;42:71–104. doi: 10.1080/10408360590888983. [DOI] [PubMed] [Google Scholar]
- Herold MJ, McPherson KG, Reichardt HM. Glucocorticoids in T cell apoptosis and function. Cell Mol Life Sci. 2006;63:60–72. doi: 10.1007/s00018-005-5390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winoto A, Littman DR. Nuclear hormone receptors in T lymphocytes. Cell. 2002;109(Suppl):S57–S66. doi: 10.1016/s0092-8674(02)00710-9. [DOI] [PubMed] [Google Scholar]
- Bommhardt U, Beyer M, Hünig T, Reichardt HM. Molecular and cellular mechanisms of T cell development. Cell Mol Life Sci. 2004;61:263–280. doi: 10.1007/s00018-003-3224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacchio MS, Ashwell JD. Glucocorticoids and thymocyte development. Semin Immunol. 2000;12:475–485. doi: 10.1006/smim.2000.0265. [DOI] [PubMed] [Google Scholar]
- Brewer JA, Kanagawa O, Sleckman BP, Muglia LJ. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J Immunol. 2002;169:1837–1843. doi: 10.4049/jimmunol.169.4.1837. [DOI] [PubMed] [Google Scholar]
- Tolosa E, King LB, Ashwell JD. Thymocyte glucocorticoid resistance alters positive selection and inhibits autoimmunity and lymphoproliferative disease in MRL-lpr/lpr mice. Immunity. 1998;8:67–76. doi: 10.1016/s1074-7613(00)80459-8. [DOI] [PubMed] [Google Scholar]
- Purton JF, Boyd RL, Cole TJ, Godfrey DI. Intrathymic T cell development and selection proceeds normally in the absence of glucocorticoid receptor signaling. Immunity. 2000;13:179–186. doi: 10.1016/s1074-7613(00)00018-2. [DOI] [PubMed] [Google Scholar]
- Jondal M, Pazirandeh A, Okret S. Different roles for glucocorticoids in thymocyte homeostasis? Trends Immunol. 2004;25:595–600. doi: 10.1016/j.it.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Vacchio MS, Papadopoulos V, Ashwell JD. Steroid production in the thymus: implications for thymocyte selection. J Exp Med. 1994;179:1835–1846. doi: 10.1084/jem.179.6.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazirandeh A, Xue Y, Rafter I, Sjovall J, Jondal M, Okret S. Paracrine glucocorticoid activity produced by mouse thymic epithelial cells. FASEB J. 1999;13:893–901. doi: 10.1096/fasebj.13.8.893. [DOI] [PubMed] [Google Scholar]
- Lechner O, Wiegers GJ, Oliveira-Dos-Santos AJ, Dietrich H, Recheis H, Waterman M, Boyd R, Wick G. Glucocorticoid production in the murine thymus. Eur J Immunol. 2000;30:337–346. doi: 10.1002/1521-4141(200002)30:2<337::AID-IMMU337>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Purton JF, Zhan Y, Liddicoat DR, Hardy CL, Lew AM, Cole TJ, Godfrey DI. Glucocorticoid receptor deficient thymic and peripheral T cells develop normally in adult mice. Eur J Immunol. 2002;32:3546–3555. doi: 10.1002/1521-4141(200212)32:12<3546::AID-IMMU3546>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Pazirandeh A, Jondal M, Okret S. Glucocorticoids delay age-associated thymic involution through directly affecting the thymocytes. Endocrinology. 2004;145:2392–2401. doi: 10.1210/en.2003-1660. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. 1999;10:359–368. doi: 10.1016/s1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- Hill N, Sarvetnick N. Cytokines: promoters and dampeners of autoimmunity. Curr Opin Immunol. 2002;14:791–797. doi: 10.1016/s0952-7915(02)00403-x. [DOI] [PubMed] [Google Scholar]
- Elenkov IJ. Glucocorticoids and the Th1/Th2 balance. Ann NY Acad Sci. 2004;1024:138–146. doi: 10.1196/annals.1321.010. [DOI] [PubMed] [Google Scholar]
- Wilder RL. Neuroendocrine-immune system interactions and autoimmunity. Annu Rev Immunol. 1995;13:307–338. doi: 10.1146/annurev.iy.13.040195.001515. [DOI] [PubMed] [Google Scholar]
- El-Etr M, Vukusic S, Gignoux L, Durand-Dubief F, Achiti I, Baulieu EE, Confavreux C. Steroid hormones in multiple sclerosis. J Neurol Sci. 2005;233:49–54. doi: 10.1016/j.jns.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Reichardt HM. Immunomodulatory activities of glucocorticoids: insights from transgenesis and gene targeting. Curr Pharm Des. 2004;10:2797–2805. doi: 10.2174/1381612043383575. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Umland T, Bauer A, Kretz O, Schütz G. Mice with an increased glucocorticoid receptor gene dosage show enhanced resistance to stress and endotoxic shock. Mol Cell Biol. 2000;20:9009–9017. doi: 10.1128/mcb.20.23.9009-9017.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazirandeh A, Xue Y, Prestegaard T, Jondal M, Okret S. Effects of altered glucocorticoid sensitivity in the T cell lineage on thymocyte and T cell homeostasis. FASEB J. 2002;16:727–729. doi: 10.1096/fj.01-0891fje. [DOI] [PubMed] [Google Scholar]
- Chakraborti PK, Garabedian MJ, Yamamoto KR, Simons SS., Jr Creation of “super” glucocorticoid receptors by point mutations in the steroid binding domain. J Biol Chem. 1991;266:22075–22078. [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- van den Brandt J, Kwon SH, Hünig T, McPherson KG, Reichardt HM. Sustained pre-TCR expression in Notch1IC-transgenic rats impairs T cell maturation and selection. J Immunol. 2005;174:7845–7852. doi: 10.4049/jimmunol.174.12.7845. [DOI] [PubMed] [Google Scholar]
- van den Brandt J, Wang D, Kwon S-H, Heinkelein M, Reichardt HM. Lentivirally generated eGFP-transgenic rats allow efficient cell tracking in vivo. Genesis. 2004;39:94–99. doi: 10.1002/gene.20037. [DOI] [PubMed] [Google Scholar]
- Chaffin KE, Beals CR, Wilkie TM, Forbush KA, Simon MI, Perlmutter RM. Dissection of thymocyte signaling pathways by in vivo expression of pertussis toxin ADP-ribosyltransferase. EMBO J. 1990;9:3821–3829. doi: 10.1002/j.1460-2075.1990.tb07600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf KL, Berne GP, Herrmann MM, Hansson GK, Olsson T, Weissert R. CDR3 sequence preference of TCRBV8S2+ T cells within the CNS does not reflect single amino acid dependent avidity expansion. J Neuroimmunol. 2005;166:47–54. doi: 10.1016/j.jneuroim.2005.05.004. [DOI] [PubMed] [Google Scholar]
- van den Brandt J, Wang D, Reichardt HM. Resistance of single-positive thymocytes to glucocorticoid-induced apoptosis is mediated by CD28 signaling. Mol Endocrinol. 2004;18:687–695. doi: 10.1210/me.2003-0390. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Metselaar JM, Wauben MH, Toyka KV, Storm G, Gold R. Drug targeting by long-circulating liposomal glucocorticosteroids increases therapeutic efficacy in a model of multiple sclerosis. Brain. 2003;126:1895–1904. doi: 10.1093/brain/awg176. [DOI] [PubMed] [Google Scholar]
- Hartung HP, Schafer B, Heininger K, Stoll G, Toyka KV. The role of macrophages and eicosanoids in the pathogenesis of experimental allergic neuritis: serial clinical, electrophysiological, biochemical and morphological observations. Brain. 1988;111:1039–1059. doi: 10.1093/brain/111.5.1039. [DOI] [PubMed] [Google Scholar]
- Sarlis NJ, Bayly SF, Szapary D, Simons SS., Jr Quantity of partial agonist activity for antiglucocorticoids complexed with mutant glucocorticoid receptors is constant in two different transactivation assays but not predictable from steroid structure. J Steroid Biochem Mol Biol. 1999;68:89–102. doi: 10.1016/s0960-0760(99)00021-7. [DOI] [PubMed] [Google Scholar]
- van den Brandt J, Voss K, Schott M, Hünig T, Wolfe MS, Reichardt HM. Inhibition of Notch signaling biases rat thymocyte development towards the NK cell lineage. Eur J Immunol. 2004;34:1405–1413. doi: 10.1002/eji.200324735. [DOI] [PubMed] [Google Scholar]
- Ramirez F, McKnight AJ, Silva A, Mason D. Glucocorticoids induce the expression of CD8 alpha chains on concanavalin A-activated rat CD4+ T cells: induction is inhibited by rat recombinant interleukin 4. J Exp Med. 1992;176:1551–1559. doi: 10.1084/jem.176.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan NL, Skandera CA, Montgomery PC. Lymphocyte lineages at mucosal effector sites: rat salivary glands. J Immunol. 2001;166:5522–5529. doi: 10.4049/jimmunol.166.9.5522. [DOI] [PubMed] [Google Scholar]
- Hünig T, Torres-Nagel N, Mehling B, Park HJ, Herrmann T. Thymic development and repertoire selection: the rat perspective. Immunol Rev. 2001;184:7–19. doi: 10.1034/j.1600-065x.2001.1840102.x. [DOI] [PubMed] [Google Scholar]
- Pannetier C, Cochet M, Darche S, Casrouge A, Zoller M, Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci USA. 1993;90:4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold R, Linington C, Lassmann H. Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain. 2006;129:1953–1971. doi: 10.1093/brain/awl075. [DOI] [PubMed] [Google Scholar]
- Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann NY Acad Sci. 2004;1024:124–137. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- Hylkema MN, Hoekstra MO, Luinge M, Timens W. The strength of the OVA-induced airway inflammation in rats is strain dependent. Clin Exp Immunol. 2002;129:390–396. doi: 10.1046/j.1365-2249.2002.01938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careau E, Sirois J, Bissonnette EY. Characterization of lung hyperresponsiveness, inflammation, and alveolar macrophage mediator production in allergy resistant and susceptible rats. Am J Respir Cell Mol Biol. 2002;26:579–586. doi: 10.1165/ajrcmb.26.5.4737. [DOI] [PubMed] [Google Scholar]
- Schneider T, van Velzen D, Moqbel R, Issekutz AC. Kinetics and quantitation of eosinophil and neutrophil recruitment to allergic lung inflammation in a brown Norway rat model. Am J Respir Cell Mol Biol. 1997;17:702–712. doi: 10.1165/ajrcmb.17.6.2849. [DOI] [PubMed] [Google Scholar]
- Singh P, Daniels M, Winsett DW, Richards J, Doerfler D, Hatch G, Adler KB, Gilmour MI. Phenotypic comparison of allergic airway responses to house dust mite in three rat strains. Am J Physiol. 2003;284:L588–L598. doi: 10.1152/ajplung.00287.2002. [DOI] [PubMed] [Google Scholar]
- Uhlig T, Cooper D, Eber E, McMenamin C, Wildhaber JH, Sly PD. Effects of long-term oral treatment with leflunomide on allergic sensitization, lymphocyte activation, and airway inflammation in a rat model of asthma. Clin Exp Allergy. 1998;28:758–764. doi: 10.1046/j.1365-2222.1998.00309.x. [DOI] [PubMed] [Google Scholar]
- Saoudi A, Bernard I, Hoedemaekers A, Cautain B, Martinez K, Druet P, De Baets M, Guery JC. Experimental autoimmune myasthenia gravis may occur in the context of a polarized Th1- or Th2-type immune response in rats. J Immunol. 1999;162:7189–7197. [PubMed] [Google Scholar]
- Pazirandeh A, Jondal M, Okret S. Conditional expression of a glucocorticoid receptor transgene in thymocytes reveals a role for thymic-derived glucocorticoids in thymopoiesis in vivo. Endocrinology. 2005;146:2501–2507. doi: 10.1210/en.2004-0943. [DOI] [PubMed] [Google Scholar]
- King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117:265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- Ben-Nun A, Ron Y, Cohen IR. Spontaneous remission of autoimmune encephalomyelitis is inhibited by splenectomy, thymectomy or aging. Nature. 1980;288:389–390. doi: 10.1038/288389a0. [DOI] [PubMed] [Google Scholar]
- Ramírez F, Mason D. Induction of resistance to active experimental allergic encephalomyelitis by myelin basic protein-specific Th2 cell lines generated in the presence of glucocorticoids and IL-4. Eur J Immunol. 2000;30:747–758. doi: 10.1002/1521-4141(200003)30:3<747::AID-IMMU747>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Severinson E, Fernandez C, Stavnezer J. Induction of germ-line immunoglobulin heavy chain transcripts by mitogens and interleukins prior to switch recombination. Eur J Immunol. 1990;20:1079–1084. doi: 10.1002/eji.1830200520. [DOI] [PubMed] [Google Scholar]