Abstract
The inactivation of the von Hippel–Lindau (VHL) tumor suppressor gene predisposes affected individuals to the human VHL cancer syndrome and is associated with sporadic renal cell carcinomas (RCC) and brain hemangioblastomas. VHL-negative 786–0 RCC cells are tumorigenic in nude mice which is suppressed by the reintroduction of VHL. Remarkably, this occurs without affecting the growth rate and cell cycle profile of these cells in culture. The 786–0 cell line, like many cancer cells, fails to exit the cell cycle upon serum withdrawal. Here, it is shown that reintroduction of the wild-type VHL gene restores the ability of VHL-negative RCC cancer cells to exit the cell cycle and enter G0/quiescence in low serum. Both VHL-positive and VHL-negative RCC cells exit the cell cycle by contact inhibition. The cyclin-dependent kinase inhibitor, p27, accumulates upon serum withdrawal, only in the presence of VHL, as a result of the stabilization of the protein. We propose that the loss of wild-type VHL gene results in a specific cellular defect in serum-dependent growth control, which may initiate tumor formation. This is corrected by the reintroduction of wild-type VHL, implicating VHL as the first tumor suppressor involved in the regulation of cell cycle exit, which is consistent with its gatekeeper function in the kidney.
Inactivation of the von Hippel–Lindau tumor suppressor gene (VHL) is responsible for the hereditary human VHL cancer syndrome predisposing affected individuals to a variety of different tumors in specific target organs, as well as for more than 80% of sporadic RCC (1–3). The VHL gene was proposed to have a gatekeeper function in the kidney, because a VHL patient’s kidney may contain up to 600 independent solid tumors and 1,100 cysts. The VHL gene product (pVHL = 30 kDa) associates in the cell with elongin B and C (VBC), two small proteins of 18 and 15 kDa, respectively (4–6). The trimeric VBC complex associates in vivo and in vitro with Hs-CUL-2 (7). Hs-cul-2 is a member of a multigene family, the cullins, which have been implicated in the regulation of the cell cycle exit through the ubiquitin-mediated degradation of cyclin-dependent kinase inhibitors (8–10). The VHL-negative human 786–0 RCC cell line is tumorigenic in nude mice, which is suppressed by the reintroduction of wild-type VHL (11). Interestingly, both VHL-negative and VHL-positive 786–0 cells are indistinguishable in their growth rates and cell cycle profiles when grown in standard culture conditions (i.e., 10% fetal calf serum). This demonstrates that the reexpression or overexpression of wild-type VHL does not simply inhibit cell growth or lead to cell death in VHL-negative RCC cells (11).
It is a physiological characteristic of normal and immortalized cells to exit the cell cycle upon withdrawal of serum or contact inhibition in culture. The majority of cancer cells lose this ability and continue to proliferate in low serum or at high density. This suggests that the inability of cells to exit the cell cycle either by serum deprivation or contact inhibition might be a prerequisite for malignant transformation. Therefore, we wanted to test whether VHL plays a role in regulating cell cycle exit either by serum deprivation or contact inhibition. We show here that VHL-negative RCC cells continue to grow in low serum. This defect is restored by the reintroduction of VHL, enabling these cells to exit the cell cycle upon serum withdrawal.
MATERIALS AND METHODS
Cells and Cell Culture.
The human sporadic renal cell carcinoma (RCC) 786–0 cell line lacks one VHL allele and express a truncated VHL protein (AA 1–104) from the second allele (11). These cells were transfected with a cytomegalovirus promoter-based expression plasmid containing either polylinker sequence 786–0(v), a naturally occurring cancer predisposing VHL nonsense mutation, which truncates the protein after amino acid 115 786–0(mt), or wild-type human VHL 786–0(wt) followed by selection in G418 as described (11). HeLa cell lines are described in ref. 7. A498 RCC cells were established and maintained like the 786-0 cells. Cell lines were maintained in DMEM containing 10% FCS.
Flow Cytometry, TUNEL Assay.
Cells were plated at either low density (2–3 × 105 cells per 15-cm dish) or high density (2–3 × 106 cells per 15-cm dish) and incubated for 16 h in DMEM/10% FCS. Cells were then incubated in either DMEM/10% FCS, DMEM/0.1%FCS, DMEM/0%FCS, or DMEM/ITS (insulin, transferrin, and selenium; GIBCO/BRL) for 48–96 h. Cells were trypsinized, washed several times in PBS, and incubated in a PBS containing 0.1% Triton-X100/RNAse A (50 mg/ml) for 30 min at 4°C. Propidium iodide (50 mg/ml) was added and the cells were incubated for another 30 min at 4°C. DNA content analysis was performed on a Becton Dickinson FACScan by using the manufacturer’s protocol. The percentage of cells in S-phase was also quantified by bromodeoxyuridine (BrdU) labeling. Cells were plated on coverslips and incubated for 20 min in the presence of 10 μM BrdU. Cells were fixed in 70% ethanol in 50 mM glycine (pH 2.0) for 30 min at −20°C. The coverslips were covered with a solution containing an anti-BrdU antibody (Boehringer Mannheim) for 30 min at 37°C, washed in PBS, and incubated with an anti-mouse secondary antibody labeled with FITC. Coverslips were counterstained with Hoechst reagent to identify all nuclei, and the percentage of BrdU-labeled cells (FITC/Hoechst) was quantified by using a fluorescence microscope. Experiments were performed in triplicate, and three coverslips were analyzed per experiment. Approximately 200 cells were randomly selected per experiment. TUNEL assay was performed as described in the manufacturer’s protocol (Promega).
Western Blot Analysis.
Cells were released from the Petri dish with a cell scraper and lysed in 2× SDS sample buffer (5 vol), and the DNA was sheared by passage through a 20-gauge needle. Protein concentrations were measured by using the BCA assay (Pierce), and 50 μg of protein was analyzed on SDS-polyacrylamide gels. Proteins were transferred to an Immobilon P membrane (Millipore), blocked for 30 min in Tris buffered saline (TBS; 10 mM Tris⋅HCl, pH 7.5/150 mM NaCl) containing 5% (wt/vol) nonfat dry milk (Giant Food, Bethesda, MD), 0.1% Tween 20, followed by a 12-h incubation with one of the three antibodies against p27 (C-19, 1:500, Santa Cruz Biotechnology; Ab-2, 1:500, Oncogene Science; or p27 antibody, 1:2500, Transduction Laboratories, Lexington, KY), p21 (Ab-3, 1:100, Oncogene Science), or p57 (Ab-1, 1:100, Oncogene Science). The blots were washed in TBS/0.1% Tween followed by a 30-min incubation in horse radish peroxidase-conjugated anti-mouse or anti-rabbit secondary antibody. Signals were detected using an ECL detection system (Pierce).
RESULTS
VHL Is Required for Cell Cycle Exit in Low Serum.
We wanted to test the hypothesis that VHL might play a role in the regulation of cell cycle exit either by serum deprivation or contact inhibition. To do so, we used cell clones derived from the human 786–0 RCC cell line, which does not express functional VHL protein (11) and the human cervix carcinoma HeLa cell line, which expresses endogenous wild-type VHL (data not shown). 786–0 cells were stably transfected with vector 786–0(v), vector expressing wild-type VHL 786–0(wt), or a vector expressing a naturally occurring mutant of VHL truncating the protein at amino acid 115 786–0(mt). HeLa cells were transfected with vector HeLa(v) or with a vector-expressing wild-type VHL HeLa(wt). The 786–0(wt) and HeLa(wt) cells expressed comparable levels of exogenous pVHL, which was approximately 5- to 10-fold overexpressed compared with endogenous pVHL in HeLa, 293, and Cos-7 cells as determined by immunoblot analysis (data not shown).
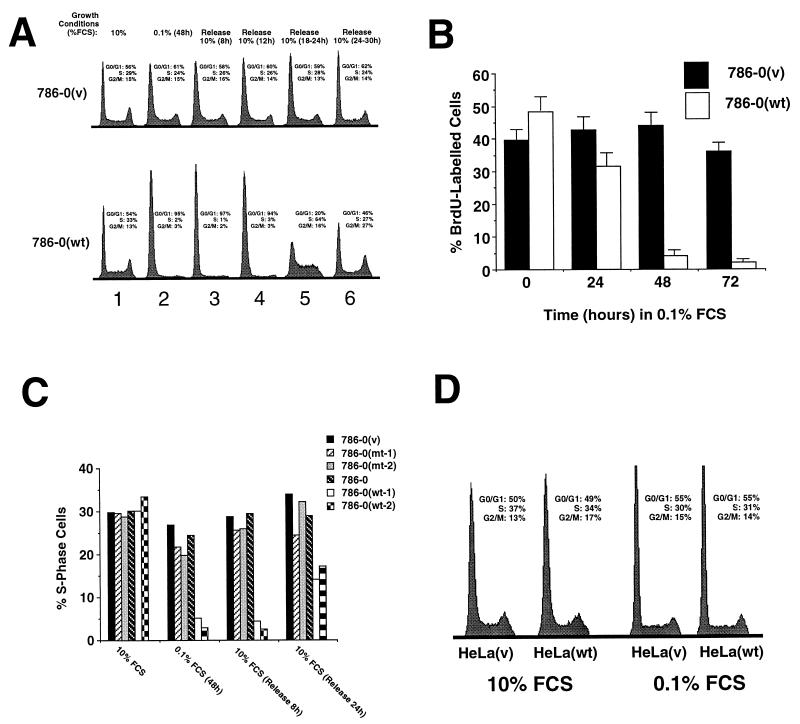
In 10% serum, when cells were growing exponentially, no difference in DNA content was observed between 786–0(v) and 786–0(wt), confirming the similar growth characteristics for both cell lines (Fig. 1A, lane 1). However, when cells were switched to 0.1% serum for 48 h, the DNA content of 786–0(v) cancer cells was not significantly changed (Fig. 1A, lane 2), whereas a dramatic increase in G0/G1 DNA content was observed with the 786–0(wt) cells (lane 2). These results show that 786–0(v) cells continue to grow in low serum at low density whereas 786–0(wt) cells ceased proliferating. Similar results were obtained by BrdU labeling of these cells (Fig. 1B).
Figure 1.
Growth characteristics of different cells in 10% and 0.1% serum. (A) Flow cytometry analysis of DNA content of cells incubated in different growth conditions. Cells were plated at low density (2–3 × 105 cells per 15-cm dish) and incubated for 16 h in 10% serum (lane 1), followed by incubation for 48 h in 0.1% serum (lane 2), followed by incubation in 10% serum for the indicated times (lanes 3–6) and were analyzed by flow cytometry after staining with propidium iodide. The percentage of cells in different phases of the cell cycle is indicated for each DNA content profile. (B) BrdU labeling analysis of 786–0(wt) and 786–0(v) cells that were plated at low density and incubated for 16 h in 10% serum followed by a 48- to 72-h incubation in 0.1% serum. Cells were incubated in the presence of 10 mM of BrdU for 20 min and analyzed as described in Methods. Approximately 200 cells were counted from three independent experiments. (C) 786–0(wt), 786–0(v), 786–0(mt), and the parental 786–0 cell lines were plated at low density and incubated in 10% serum for 16 h followed by a 48-h incubation in 0.1% FCS, followed by the addition of 10% FCS for 8 and 24 h. Percentage of S phase cells was obtained by flow cytometry such as in A. (D) Flow cytometry analysis of DNA content of HeLa cells incubated in different growth conditions. Cells were plated at low density (2–3 × 105 cells per 15-cm dish) and incubated for 16 h in 10% serum (lane 1), followed by incubation for 48 h in 0.1% serum (lane 2) and were analyzed by flow cytometry after staining with propidium iodide. The percentage of cells in different phases of the cell cycle is indicated for each DNA content profile.
G0 arrested cells can be distinguished from G1 arrested cells by the time that is required to reenter S phase after serum restimulation (12). In general, G1 arrested cells need only 1–6 h to initiate S phase, whereas G0 cells require 12–24 h. This additional time is required for the synthesis of molecules necessary for growth and cell division that are not present in quiescent cells (12). When 10% serum was added to G0/G1 growth-arrested 786–0(wt) cells at low cell density, they required 18–24 h to reenter S phase as a synchronized population, indicating they were quiescent and not blocked in G1 (Fig. 1A, 786–0(wt), lanes 3–6). In contrast, the readdition of 10% serum did not significantly alter the DNA content of 786–0(v) cells, suggesting continuous cycling 786–0(v) (lanes 3–6). These experiments were repeated with a larger panel of clones (Fig. 1C), confirming that only the 786–0(wt) cells are capable of cell cycle exit upon serum withdrawal, whereas VHL-negative or VHL-mutant cells continue to grow. An independently derived VHL negative human RCC cell line, A498, continued to grow in low serum, but was able to exit the cell cycle when wild-type VHL was reintroduced (unpublished observation). This cell clone expressed levels of introduced VHL similar to that seen for the endogenous protein. Thus, the effect of reintroducing wt VHL is not peculiar to 786-O cells and to the level of exogenous VHL expression. Overexpression of wild-type VHL in VHL-positive HeLa cancer cells had no measurable effect on the flow cytometry profiles, and the HeLa(wt) continued to proliferate in low serum such as HeLa(v) (Fig. 1D). These results demonstrate that the simple overexpression of VHL has no effect on growth in low serum in cells already expressing endogenous wild-type VHL. It is likely that a VHL-independent function in the pathway regulating serum dependent growth is lost in HeLa cells.
VHL Is Not Required for Cell Cycle Exit by Contact Inhibition.
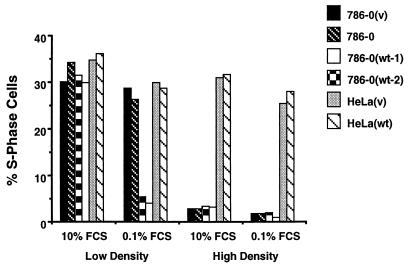
Because mammalian cells can exit the cell cycle by serum withdrawal or by contact inhibition, we evaluated whether or not VHL is responsible for cell cycle exit through contact inhibition. To test this, cells were plated at either high or low cell density in 10% serum and allowed to attach and proliferate for 16 h. The cells that were plated at high density showed no increase in numbers after 24–36 h in 10% serum (data not shown), suggesting they were growth arrested by contact inhibition. The cells were then either further incubated in 10% serum (changing the serum every 12 h for 48 h) or switched to 0.1% serum for 48 h. Cells were then analyzed by flow cytometry to determine the percentage of cells in S phase (Fig. 2). In all the 786–0-derived cells the percentage of S phase cells plummeted, confirming the observed growth arrest at high cell density, regardless of either serum concentration or VHL status. When these arrested cells were detached by EDTA treatment and replated in 10% serum at low cell density they entered the cell cycle within 24 h in a synchronized manner (not shown). These experiments demonstrate that 786–0-derived cells can exit the cell cycle and enter G0 through contact inhibition in a VHL-independent manner. In contrast, both HeLa lines continued to grow in either high or low density at 10% or 0.1% serum, suggesting that they lost both pathways to exit the cell cycle and the overexpression of VHL had no effect (Fig. 2).
Figure 2.
Effect of cell density on growth. Two clones of 786–0(wt), one 786–0(v), and the parental 786–0 cell line as well as HeLa(v) and HeLa(wt) cell lines were plated at low (2–3 × 105 cells per 15-cm dish) or high (2–3 × 106 cells per 15-cm dish) cell density in the presence of 10% serum for 36 h. The cells were then either incubated in 10% serum (changing the serum every 12 h for 48 h) or switched to 0.1% serum for 48 h. Percentages of S phase cells were assessed by flow cytometry such as in A.
VHL Is Required for Serum-Dependent Growth.
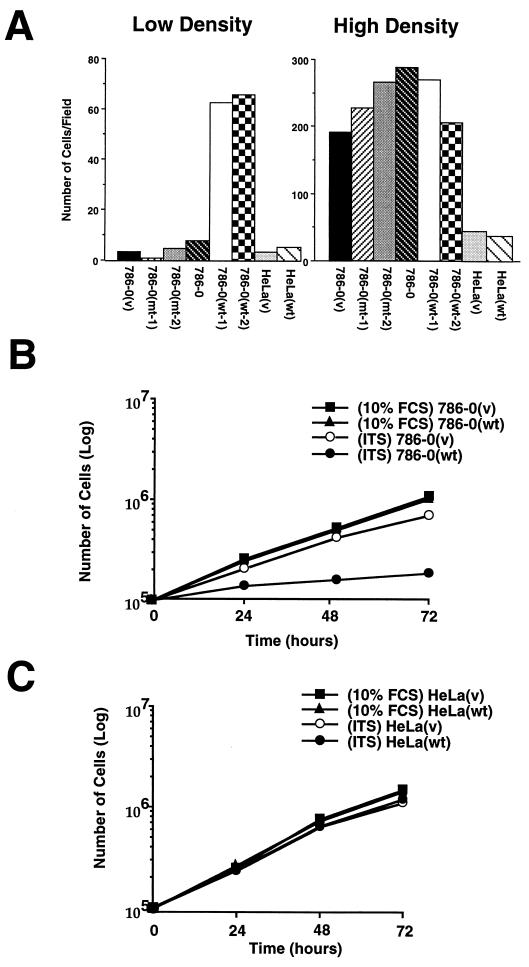
It is well known that prolonged incubation of transformed cells in absence of serum will result in cell death through apoptosis, whereas normal cells exit the cell cycle and survive these conditions longer (13–15). 786–0(v) cells continued to cycle 48 h after a switch to 0% serum, but after 72 h most of the 786–0(v) cells were detached and showed pycnotic nuclei, whereas 786–0(wt) cells survived for more than 1 week (Fig. 3A; data not shown). The pycnotic nuclei of 786–0(v) cells were confirmed as apoptotic by the TUNEL assay (data not shown). Two 786–0 cell lines expressing the mutant VHL were indistinguishable from 786–0(v) cells and died after 72 h of incubation in the absence of serum (Fig. 3A). In contrast, if the cells were first grown to high density before being incubated in 0% serum, all cell lines survived, regardless of their VHL status (Fig. 3A). HeLa(v) cells and HeLa(wt) died through apoptosis in low serum regardless of cell density, suggesting that they cannot exit the cell cycle either under conditions of serum withdrawal or contact inhibition, and the introduction of wild-type VHL cannot rescue this defect (Fig. 3A).
Figure 3.
Effect of cell density on cell death induced by the absence of serum. (A) Cells were plated to low or high density in 10% serum for 16 h followed by a 48- to 72-h incubation in DMEM/0% serum. Cells were then incubated for 10 min with Hoechst #33325, which stains nuclei of living cells. The numbers of live cells were determined by counting round, nonpycnotic nuclei per field. Experiments were performed with two wild-type clones, one 786–0(v), two 786–0(mt), and the parental 786–0 cell lines as well as HeLa(v) and HeLa(wt) cell lines. (B) Effect of ITS on the growth of 786–0(v) and 786–0(wt) cells in serum free media. Cells were plated at low density in DMEM/10% serum for 16 h followed by an incubation in either DMEM/10%FCS or DMEM/ITS. (C) Growth curves of HeLa(v) and HeLa(wt) cell lines incubated in either DMEM/10%FCS or DMEM/ITS. Cells were washed several times with DMEM, detached by trypsinization, and counted with a hemocytometer. Number of cells per 10-cm Petri dish was counted at the indicated time. The average of two independent experiments is shown.
For many cancer cells, insulin alone is sufficient to proliferate and survive under serum-free conditions. Normal or immortalized cells enter quiescence under the same conditions (13–15) and need additional growth factors to proliferate. Therefore, we tested the ability of the 786–0 lines to grow in media supplemented only with ITS (insulin, transferrin, selenium) in the absence of serum. 786–0(wt) cells exited the cell cycle and entered G0 after 24–48 h of incubation in serum free media containing ITS (data not shown), whereas 786–0(v) cells continued to cycle for more than 72 h (Fig. 3B). In contrast to 0% serum, where 786–0(v) cells died after 72 h, these cells continued to grow in the presence of ITS without undergoing apoptosis (Fig. 3B). These results further substantiate that 786–0(wt) cells enter quiescence/G0 upon serum withdrawal protecting them from cell death, whereas 786–0(v) cells lose this ability as observed with many cancer cell lines. Both HeLa lines continued to grow in the presence of ITS for more than 72 h (Fig. 3C).
p27 Accumulates in Low Serum Only in VHL-Positive Cells.
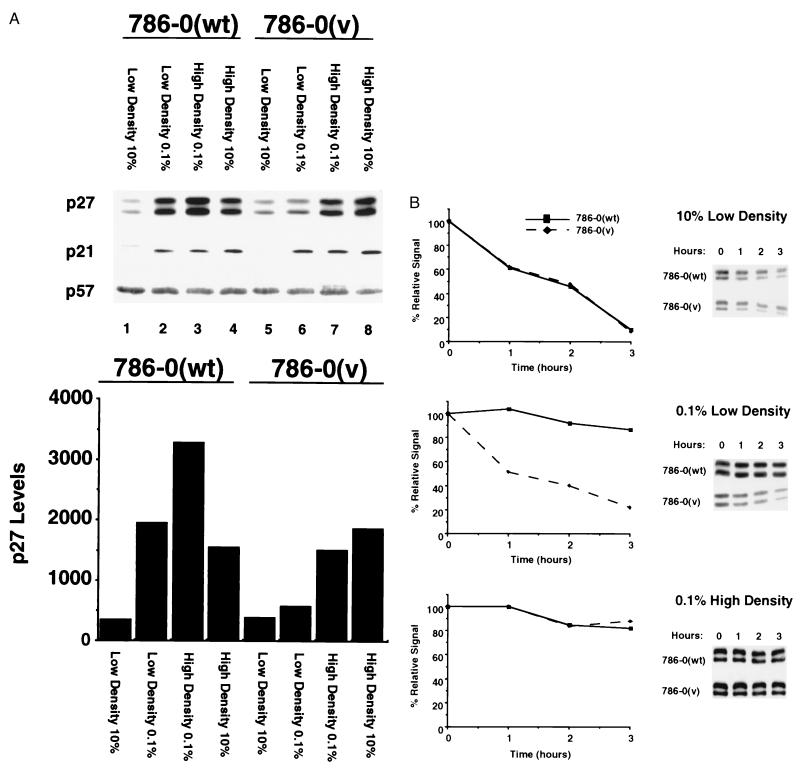
p27 is a cyclin-dependent kinase inhibitor (CKI) that has been implicated in cell cycle exit in response to serum deprivation and contact inhibition (16, 17). p27 levels increase in quiescent cells and rapidly decrease after serum re-stimulation. Moreover, constitutive expression of p27 in cells induces cell cycle exit, and loss of p27 via antisense oligonucleotides in certain cells results in failure to growth arrest and enter G0 mimicking the phenotype observed in VHL-negative cell lines (18, 19). To test whether p27 might be regulated in a VHL-dependent manner, 786–0(wt) and 786–0(v) cells were incubated at low or high density in either 10% or 0.1% serum. In cells growing in 10% serum, p27 protein levels were low regardless of the presence of VHL (Fig. 4A). Strikingly, a 6-fold increase in p27 levels was observed in serum-deprived 786–0(wt) cells, but no significant increase of p27 was detected in either 786–0(v) (Fig. 4A) or in 786–0(mt) cells (data not shown) upon serum deprivation. In 786–0(wt) cells p27 levels increased 10-fold under conditions of contact inhibition in 0.1% serum and 5-fold when 10% serum was added to these growth inhibited cells. In 786–0(v) cells, p27 levels increased 5-fold under contact inhibition in either 0.1 or 10% serum. The additive effects of serum deprivation and contact inhibition seen in 786–0(wt) cells suggests two distinct and additive pathways for p27 regulation. One, because of serum deprivation, is VHL-dependent and the other, because of cell-cell contact, appears to be VHL-independent. We also examined the regulation of two other characterized p27 family members, p21 and p57. Neither p21 nor p57 appeared to be regulated in a VHL-dependent manner in RCC cells.
Figure 4.
Assessment of CKI levels and stability in 786–0(v) and 786–0(wt) cells. (A) 786–0(wt) and 786–0(v) cells were plated either at low or high cell density in 10% serum and incubated for 16–24 h followed by an incubation for 48 h in 0.1% or 10% serum. p27, p21 and p57 levels were analyzed by immunoblot analysis, and p27 levels were quantitated by densitometry of different exposures of autoradiographs. Immunoblotting with several anti-p27 antibodies revealed a p27 doublet. Both of these bands behaved identically in all experiments. (B) 786–0(wt) and 786–0(v) cells were plated in 10% serum and incubated for 48 h at low density in 10% FCS (Top), low density in 0.1% FCS (Middle), or high density in 0.1% FCS (Bottom). p27 levels were analyzed by immunoblot analysis. p27 stability was assessed by incubation of the cells in 100 μg/ml of cycloheximide for the indicated time periods. p27 levels were quantitated by densitometry of different exposures of autoradiographs.
The p27 protein is known to be regulated at the level of protein stability (20, 21). Therefore, we assessed p27 stability under different growth conditions. In growing cells (low density, 10% serum) p27 was short-lived independent of the VHL status (Fig. 4B). In contrast, p27 stability was increased in serum-starved 786–0(wt) cells but not in 786–0(v) cells plated at low density. p27 stability increased in contact-inhibited cells regardless of VHL status. Hence, the reintroduction of VHL into these RCC cells enables them to stabilize p27 in response to serum deprivation at low cell density, which may promote cell cycle exit (18, 19).
DISCUSSION
Cell cycle exit is a physiological response of normal cells to serum withdrawal and contact inhibition in culture, and this is often lost in malignant cells (22). We hypothesize that the RCC cells used in this study originate from normal renal cells that quiesce in low serum. These cells may have acquired a defect in exiting the cell cycle in response to serum withdrawal by the inactivation of their functional VHL alleles. This particular defect can be restored by the reintroduction of wild-type VHL into these RCC cells, regardless of expression levels of reintroduced VHL, because the same phenotype is observed with both 786–0 and A498 cells expressing wild-type VHL at levels similar to those of endogenous VHL (unpublished observations). Overexpression of oncogenes such as myc, ras, src, and E1A induces entry into the cell cycle from G0 in the absence of growth factors demonstrating a dominant effect of these genes (22). Reintroduction of wild-type VHL does not create a dominant phenotype of growth arrest because VHL-expressing 786–0 cells are indistinguishable from VHL-negative 786–0 cells in their growth rate in 10% serum. However, it appears that VHL activities are induced only in low serum conditions, suggesting a growth factor-regulated mechanism. Other cancer cells such as HeLa also have a defect in the ability to exit the cell cycle, which is not caused by a loss of the VHL gene and therefore cannot be corrected by its overexpression. Perhaps other proteins in the VHL pathway or a different pathway involved in the serum-dependent growth control in HeLa cells have been lost during malignant transformation. Therefore, the simple overexpression of wild-type VHL in HeLa is insufficient to restore the loss of function that has led to serum-independent growth.
It is intriguing that the majority of the VBC complex is associated with Hs-CUL-2, a homologue of yeast Cdc53 (ref. 7; unpublished observations). Disruption of cdc53 results in a failure to degrade the CKI p40, resulting in a G1 arrest (23, 24). It is tempting to speculate that the VBC-CUL-2 complex regulates the stability of p27. This protein is a target of oncogenic events because adenoviral E1A and papillomaviral E7 as well as c-myc proteins inactivate p27 activity, dissociating it from cyclin–cdk complexes, which would explain why virus-infected cells like HeLa or 293 cannot exit the cell cycle (25–27). Recently, several reports have shown that p27 levels are often found to be down-regulated in human breast and colon carcinomas because of an increase in p27 protein degradation activity in these cancer cells (28–30). It was proposed that an activity that stabilizes p27 might be a target of genetic alteration in human cancers. In this report we demonstrate a link between VHL and p27 stabilization that provides a working model to further investigate VHL-mediated cell cycle exit.
Acknowledgments
We are grateful to Othon Iliopoulos and Bill Kaelin (Dana–Farber Cancer Institute, Boston) for the kind gift of the 786–0 RCC and A498 RCC cloned lines that were used in this study. We thank Edward Kipreos (University of Georgia, Athens) for helpful discussions. We also thank Drs. G. Storz, J. Bonifacino, J. Harford, J. Lipincott-Schwartz, and J. Humphrey for critical reading of the manuscript. We thank M.-C. Fournier and Heather Kontaxis for excellent technical assistance. We thank W. M. Linehan for financial support. A.P. is supported by a fellowship from the Deutsche Forschungsgemeinschaft.
ABBREVIATIONS
- VHL
von Hippel–Lindau
- VBC
pVHL-elongin B-C
- RCC
renal cell carcinoma
- FCS
fetal calf serum
- CKI
cyclin-dependent kinase inhibitor
- ITS
insulin-transferrin-selenium
References
- 1.Gnarra J R, Tory K, Weng Y, Schmidt L, Wei M H, et al. Nat Genet. 1994;7:85–90. doi: 10.1038/ng0594-85. [DOI] [PubMed] [Google Scholar]
- 2.Latif F, Tory K, Gnarra J, Yao M, Duh F M, et al. Science. 1993;260:1317–1320. doi: 10.1126/science.8493574. [DOI] [PubMed] [Google Scholar]
- 3.Gnarra J R, Duan D R, Weng Y, Humphrey J S, Chen D Y, et al. Biochim Biophys Acta. 1996;1242:201–210. doi: 10.1016/0304-419x(95)00012-5. [DOI] [PubMed] [Google Scholar]
- 4.Duan D R, Pause A, Burgess W H, Aso T, Chen D Y, Garrett K P, Conaway R C, Conaway J W, Linehan W M, Klausner R D. Science. 1995;269:1402–1406. doi: 10.1126/science.7660122. [DOI] [PubMed] [Google Scholar]
- 5.Duan D R, Humphrey J S, Chen D Y, Weng Y, Sukegawa J, Lee S, Gnarra J R, Linehan W M, Klausner R D. Proc Natl Acad Sci USA. 1995;92:6459–6463. doi: 10.1073/pnas.92.14.6459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kibel A, Iliopoulos O, DeCaprio J A, Kaelin W G., Jr Science. 1995;269:1444–1446. doi: 10.1126/science.7660130. [DOI] [PubMed] [Google Scholar]
- 7.Pause A, Lee S, Worrell R A, Chen D Y, Burgess W H, Linehan W M, Klausner R D. Proc Natl Acad Sci USA. 1997;94:2156–2161. doi: 10.1073/pnas.94.6.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kipreos E T, Lander L E, Wing J P, He W W, Hedgecock E M. Cell. 1996;85:829–839. doi: 10.1016/s0092-8674(00)81267-2. [DOI] [PubMed] [Google Scholar]
- 9.Mathias N, Johnson S L, Winey M, Adams A E, Goetsch L, Pringle J R, Byers B, Goebl M G. Mol Cell Biol. 1996;16:6634–6643. doi: 10.1128/mcb.16.12.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willems A R, Lanker S, Patton E E, Craig K L, Nason T F, Mathias N, Kobayashi R, Wittenberg C, Tyers M. Cell. 1996;86:453–463. doi: 10.1016/s0092-8674(00)80118-x. [DOI] [PubMed] [Google Scholar]
- 11.Iliopoulos O, Kibel A, Gray S, Kaelin W G., Jr Nat Med. 1995;1:822–826. doi: 10.1038/nm0895-822. [DOI] [PubMed] [Google Scholar]
- 12.Pardee A B. Science. 1989;246:603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- 13.Eilers M, Schirm S, Bishop J M. EMBO J. 1991;10:133–141. doi: 10.1002/j.1460-2075.1991.tb07929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evan G I, Wyllie A H, Gilbert C S, Littlewood T D, Land H, Brooks M, Waters C M, Penn L Z, Hancock D C. Cell. 1992;69:119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- 15.Harrington E A, Bennett M R, Fanidi A, Evan G I. EMBO J. 1994;13:3286–3295. doi: 10.1002/j.1460-2075.1994.tb06630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sherr C J, Roberts J M. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 17.Elledge S J, Harper J W. Curr Opin Cell Biol. 1994;6:847–852. doi: 10.1016/0955-0674(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 18.Coats S, Flanagan W M, Nourse J, Roberts J M. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- 19.Rivard N, L’Allemain G, Bartek J, Pouyssegur J. J Biol Chem. 1996;271:18337–18341. doi: 10.1074/jbc.271.31.18337. [DOI] [PubMed] [Google Scholar]
- 20.Hengst L, Reed S I. Science. 1996;271:1861–1864. doi: 10.1126/science.271.5257.1861. [DOI] [PubMed] [Google Scholar]
- 21.Pagano M, Tam S W, Theodoras A M, Beer-Romero P, Del Sal G, Chau V, Yew P R, Draetta G F, Rolfe M. Science. 1995;269:682–685. doi: 10.1126/science.7624798. [DOI] [PubMed] [Google Scholar]
- 22.Varmus H, Weinberg R, A. Genes and the Biology of Cancer. New York: Scientific American Library; 1993. [Google Scholar]
- 23.Goebl M G, Yochem J, Jentsch S, McGrath J P, Varshavsky A, Byers B. Science. 1988;241:1331–1335. doi: 10.1126/science.2842867. [DOI] [PubMed] [Google Scholar]
- 24.Schwob E, Bohm T, Mendenhall M D, Nasmyth K. Cell. 1994;79:233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 25.Mal A, Poon R Y, Howe P H, Toyoshima H, Hunter T, Harter M L. Nature (London) 1996;380:262–265. doi: 10.1038/380262a0. [DOI] [PubMed] [Google Scholar]
- 26.Zerfass-Thome K, Zwerschke W, Mannhardt B, Tindle R, Botz J W, Jansen-Durr P. Oncogene. 1996;13:2323–2330. [PubMed] [Google Scholar]
- 27.Vlach J, Hennecke S, Alevizopoulos K, Conti D, Amati B. EMBO J. 1996;15:6595–6604. [PMC free article] [PubMed] [Google Scholar]
- 28.Porter P L, Malone K E, Heagerty P J, Alexander G M, Gatti L A, Firpo E J, Daling J R, Roberts J M. Nat Med. 1997;3:222–225. doi: 10.1038/nm0297-222. [DOI] [PubMed] [Google Scholar]
- 29.Loda M, Cukor B, Tam S W, Lavin P, Fiorentino M, Draetta G F, Jessup J M, Pagano M. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 30.Catzavelos C, Bhattacharya N, Ung Y C, Wilson J A, Roncari L, Sandhu C, Shaw P, Yeger H, Morava-Protzner I, Kapusta L, Franssen E, Pritchard K I, Slingerland J M. Nat Med. 1997;3:227–230. doi: 10.1038/nm0297-227. [DOI] [PubMed] [Google Scholar]