Abstract
We compared the direct effects of selective EP4 and EP2 receptor agonists (EP4A and EP2A) with prostaglandin E2(PGE2) on the differentiation of cultured murine calvarial osteoblastic cells. EP4A increased alkaline phosphatase activity and osteocalcin mRNA levels in these cultures similar to PGE2. This effect was seen with both “direct plating” immediately after isolating the cells, or “indirect plating” in which the cells were grown to confluence and replated. EP2A had a smaller effect, significant only in “indirect plating” experiments. All three agents decreased the DNA and protein content in indirect plating experiments, but not in direct plating experiments. We conclude that the anabolic effect of PGE2 in calvarial osteoblastic cell cultures is largely mediated by activation of the EP4 receptor, while activation of the EP2 receptor is less effective.
Introduction
The ability of prostaglandins, particularly prostaglandin E2 (PGE2), to stimulate bone formation in vivo and to increase the differentiation of osteoblastic cells in vitro is well documented (1-3). Recent studies using animals with deletion of either the EP2 or EP4 receptors or using selective agonists for these receptors have suggested that both EP2 and EP4 can mediate anabolic responses to PGE2(4-10), although the EP1 receptor may also be involved (11). Activation of EP2 and EP4 receptors increases cAMP and can also induce COX-2, which can result in auto-amplification of PGE2 signaling (12). Selective EP2 receptor agonists appear to be more effective than EP4 agonists in activating cAMP and increasing COX-2 (12).
The present studies were undertaken to compare the direct effects of selective EP4 and EP2 receptor agonists on differentiation of cultured murine calvarial cells. Cultures were treated with a selective inhibitor of inducible cyclooxygenase (COX-2) to minimize the role of endogenous prostaglandins in the response. Our results indicate that a selective EP4 receptor agonist (EP4A) is more effective than an EP2 receptor agonist (EP2A) in increasing alkaline phosphatase activity and increasing osteocalcin RNA levels in calvarial osteoblasts. Studies were carried out both with “direct plating” of freshly isolated cells and with cells that had been cultured to confluence and then re-plated (“indirect plating”). The former, which we considered true primary cultures, showed a greater response to PGE2 and EP4A. However, directly plated cells showed a greater variability in response than with indirect plating.
Materials and Methods
Trypsin 0.25%/EDTA, Alpha MEM medium and Fetal Bovine Serum were obtained from Invitrogen Corp (Grand Island, NY). Collagenase P was obtained from Roche Diagnostics (Indianapolis, IN) . Phosphoascorbate was obtained from Wako Chemical Co. (Dallas, TX). NS-398 and Prostaglandin E2 were obtained from Cayman Chemical Corp. (Ann Arbor, MI). B-glycerol phosphate was obtained from Sigma Chemical Co. (St. Louis, MO) and EP2, EP4 agonists were obtained from ONO Pharmaceutical Co. (Cat.# EP2:AE1-259-01, EP4: AE1-329, Osaka, Japan). These compounds have been shown to have equal potency in increasing cAMP levels and inducing COX-2 in calvarial bone cells at concentrations of 10-6 to 10-8 M. However the EP2 agonist had greater efficacy for these parameters. Studies using cells from knockout animals or cells transfected with specific receptors have demonstrated that these agonists are highly selective (12,13).
Calvarial Osteoblast Cultures
Calvariae from CD-1 wild-type mice, 7-12 days old, were excised, rinsed in sterile PBS, and digested in a solution of Collagenase P, 0.25% Trypsin/EDTA, and sterile PBS, at 37°C, in an atmosphere of 5% CO2 in air. The first 10-minute digest was discarded and the next three 10 minute digests, and a final 90 minute digest were used to harvest cells. At the end of each digest, harvested cells were collected and passed through a 20μM nylon net filter (Millipore Corp.) to produce a single-cell suspension. Cells were centrifuged, and the resulting pellet resuspended in alpha MEM media supplemented with 10% heat-inactivated FCS, 50 μg/ml phosphoascorbate, penicillin 100 U/ml, and streptomycin 50 μg/ml. Cells from digests 2 through 5 were pooled, and an aliquot was counted, using a Beckman Coulter Z1 Particle Counter (Beckman Coulter, Fullerton, CA). These cells were then either cultured in 100mm plates, grown to confluence, harvested, and aliquoted to 6 well culture plates (indirect plating experiments), or added directly to 6 well culture dishes (direct plating experiments). Medium was changed every 3rd day. On Day 7, 10mM β-glycero-phosphate was added to the culture medium for the duration of the experiment. Most cultures were maintained for 14 days, at which time alkaline phosphatase activity, DNA, and protein content were measured. Cells were extracted and mRNA levels measured, as previously described.(12)
Direct Plating
Harvested cells from digests 2-5 were pooled, resuspended in media, and an aliquot taken for cell count, using a Beckman Coulter Z1 Particle counter. Cells were plated at a concentration of 5,000/cm2 in 6 well tissue culture dishes.
Indirect Plating
Following the primary osteoblast digest procedure, pooled cells were resuspended in media, and plated in 100 mm tissue culture dishes. Cells were allowed to grow to confluence, usually within 5-7 days, then trypsinized, counted, and replated at 5,000 cells/cm2 in 6 well tissue culture dishes.
Alkaline Phosphatase (AP) Assay
The culture medium was removed and cells were rinsed 4 times in PBS, and scraped into 10 mM Tris-HCL buffer, pH 7.5, plus 0.1% Triton-X. AP activity was assayed at 37° in a buffer of pH 10.5 containing 2 mM p-nitrophenol phosphate as substrate and 1 mM MgC12. Absorbance at 410 nm was measured approximately every 15 min. AP activity was normalized to the soluble protein content of the cell extracts and AP activity expressed as nM/min/μg protein.
Cell Count
In separate experiments, cell number was determined at the end of 7 days of culture. Cells were plated at a density of 5,000 cells/cm2 in 6 well tissue culture dishes. At takedown, medium was removed, the cells washed with PBS, and then trypsinized. Collected cells were spun down and resuspended in media. An aliquot was removed and counted, using a Beckman Coulter Z1 Particle Counter.
DNA Content
At the end of culture the cells were washed with PBS, and the treated with 0.5 ml of NaOH (0.5 M), the wells scraped, and the cells transferred to a glass tube. A second addition of 0.5 ml of NaOH with scraping and transfer was then carried out and pooled and stored overnight at 4°C. The samples were assayed using the DAPI (4,6 Diamindo-2-Phenylindole) method (14) and DNA content measured in a Dynatech Fluorolite 1000 Fluorimeter (Dynatech Laboratories, Chantilly, VA).
PGE2 and the selective PGE2 receptor agonists, EP2A and EP4A (kindly provided by Dr. Maruyama from ONO Pharmaceutical Co) were added to cultures treated with NS-398 to minimize endogenous prostaglandin synthesis and compared to NS-398 alone. In addition, some cultures were maintained without any treatment (untreated controls).
Statistical Analysis
Statistical significance of differences among means was assessed by analysis of variance with post-hoc comparison of more than two means by Bonferroni’s method, for each agonist separately, using the software program SigmaStat (San Rafael, CA)
RESULTS
Indirect Plating Experiments(Table 1)
Table 1.
Indirect Plating Experiments - Effects of PGE2, EP4A and EP2A on AP activity, DNA and protein content of cultured mouse calvarial cells that had been grown to confluence and then transferred to 6 well dishes and cultured in the presence of NS-398 (10-7).
| % of NS-398 treated cultures | |||
|---|---|---|---|
| AP Activity | DNA Content | Protein Content | |
| NS-398 | 100±3 | 100±3 | 100±4 |
| +PGE 10-7M | 195±31* | 73±4* | 75±6* |
| 10-6M | 268±35* | 58±5* | 59±5* |
| +EP4A 10-7M | 205±14* | 76±5* | 84±2 |
| 10-6M | 218±15* | 78±3* | 78±2* |
| +EP2A 10-7M | 173±10 | 71±1* | 67±3* |
| 10-6M | 158±9 | 61±5* | 66±3* |
| Untreated Control | 125±8 | 100±6 | 100±4 |
Vales are means ± SE for 9-17 cultures, pooled for 5 experiments in which the mean value for NS-398 treated cultures was set at 100%.
Significantly different from NS-398 P<0.01
PGE2 and EP4 both caused significant increases in AP activity of approximately two fold at 10-7 and 10-6 M. The effects of EP2A were smaller. All three agents produced decreases in DNA content of 20-42%, and protein content of 16 to 41%. In a separate experiment, cell counts were obtained at 7 days of culture and showed a 10% decrease with EP4A and 20% decreases with PGE2 and EP2A (data not shown). We used NS-398 to minimize any effects of these agents on endogenous prostaglandins since the agonists used have previously been shown to induce cyclooxygenase (12). At 10-7 M NS-398 fully inhibits prostaglandin synthesis from COX-2 (15). When compared to an untreated control, NS-398 caused a small, insignificant reduction in AP activity and no change in DNA or protein content.
Direct Plating Experiments(Table 2)
Table 2.
Effects of PGE2, EP4A and EP2A on AP activity and protein content of cultured mouse calvarial cells cultured in 6 well plates and treated with NS-398 (10-7M) immediately after collagenase digestion (direct plating)
| % of NS-398 treated cultures | ||
|---|---|---|
| AP Activity | Protein Content | |
| NS-398 | 100±10 | 100±7 |
| +PGE2 10-7M | 240±31* | 110±6 |
| 10-6M | 287±46* | 95±8 |
| +EP4A 10-7M | 206±24* | 123±7 |
| 10-6M | 324±19* | 121±7 |
| +EP2A 10-7M | 117±12 | 101±3 |
| 10-6M | 137±26 | 99±7 |
| Untreated Control | 116±9 | 103±5 |
Values are mean ± SE for 8-25 cultures pooled from 4 experiments, in which the mean value for NS-398 was set at 100%.
Significantly different from NS-398 alone, P<0.01
The effects of PGE2 EP4A and EP2A treatment when the cells were plated and treated directly after collagenase digestion are shown in Table 2. PGE2 and EP4A increased AP activity 2-3 fold at 10-7 and 10-6 M while the effect of EP2A was not significant. There were no significant changes in protein content in these experiments (Table 2) or in DNA content in a single experiment (data not shown).
Osteocalcin mRNA Expression
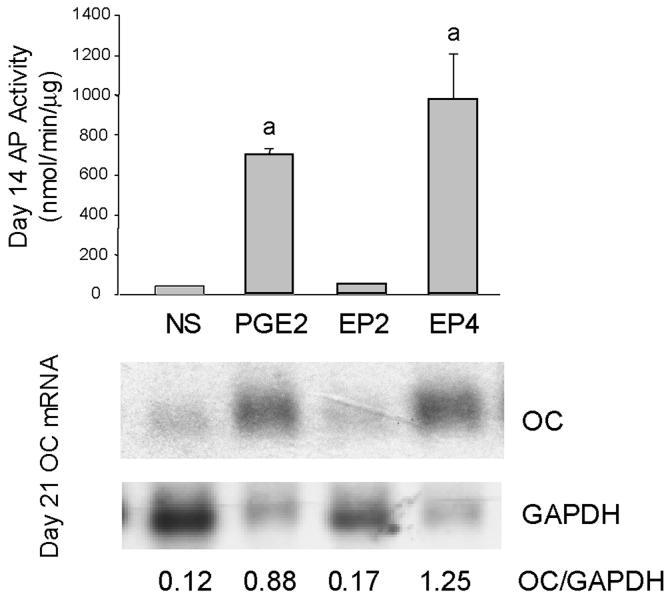
One reason for carrying out direct plating experiments was that we had previously observed that the expression of osteocalcin was greater in this culture system. Osteocalcin mRNA expression was measured after 14 days in culture (Figure 1). Both PGE2 and EP4A, but not EP2A increased osteocalcin mRNA levels. Large relative increases in AP activity were observed with PGE2 and EP4A in this experiment, perhaps due to the low activity in cultures treated with NS-398 alone.
Figure 1.
Effects of PGE2, EP2A and EP4A on AP activity and osteocalcin mRNA levels in a 14 day direct plating experiment. Note that PGE2 and EP4 produced both an absolute increase in OC/mRNA levels and even greater relative increase in OC/GAPDH ratio because of the decrease in GAPDH mRNA levels.
DISCUSSION
These studies demonstrate that PGE2 and a selective EP4 receptor agonist can enhance differentiation of cultured murine calvarial osteoblastic cells. A selective EP2 receptor agonist was less effective. None of the agonists increased cell DNA or protein content, rather there was a small inhibitory effect in the indirect plating cultures. We have not further explored this inhibitory effect, but it may be due to the fact that more differentiated cells are likely grow more slowly. Osteoblast proliferation is increased in COX-2 knockout cells and decreased when COX-2 is overexpressed (16). In direct plating experiments, PGE2 and EP4A also increased the expression of the mRNA levels for osteocalcin, further indicating an effect on differentiation. We have recently found that EP4A is also a potent stimulator of bone resorption in murine fetal calvarial organ cultures. In these studies we compared the response to PGE2 and EP4A in cells from wild type animals and from animals that were heterozygous for EP4 receptors and found that the response was significantly reduced even when the tissues were haploinsufficient for EP4 receptors (17). In preliminary studies we have found a similar effect of haploinsufficiency of the EP4 receptor, either when deletion is global or targeted to cells of the osteoblastic lineage (18).
Previous reports have demonstrated that EP2 selective agonists are anabolic in organ culture and in vivo (7,9,10). Yet we only observed small effects of EP2A in the present study. One explanation for this difference may be that in previous studies NSAIDS or COX-2 inhibitors were not used, since EP2 agonists could act, at least in part, by increasing endogenous prostaglandin synthesis (12). We have recently found a significant effect of EP2A on AP activity in directly plated cultures of both marrow stromal cells and calvarial cells, which was significantly diminished in cells from heterozygotes and abrogated in cells from mice lacking the EP2 receptor (S. Choudhary and C. Alander , unpublished observations).
We had previously shown that EP2A is more effective than EP4A in increasing cAMP and inducing COX-2, in the same cell preparation used in the present study. It was because of this that we elected to use NS-398, so that the results would reflect the direct effects of the agonists. The observation that EP4A is less effective as a stimulus for cAMP production and COX-2 induction, but more effective in differentiation, could indicate that there is a separate signal transduction pathway, not involving cAMP, or that there is a downstream separation of the cAMP dependent pathways initiated by activation of the EP2 and EP4 receptors. Since human studies using selective EP receptor agonists are now underway, it will be important to determine whether the differential effects that we have observed in murine cells are also obtained in human osteoblasts.
In conclusion, we have shown that PGE2 and a selective EP4 receptor agonist can increase the differentiation of murine calvarial osteoblastic cells. The EP2 receptor agonist was less effective. These effects may be important in both the physiologic and pathologic regulation of bone formation. Further study of selective prostaglandins agonists may lead to effective new approaches to anabolic therapy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flanagan AM, Chambers TJ. Stimulation of bone nodule formation in vitro by prostaglandins E1 and E2. Endocrinology. 1992;130(1):443–8. doi: 10.1210/endo.130.1.1309342. [DOI] [PubMed] [Google Scholar]
- 2.Nagata T, Kaho K, Nishikawa S, Shinohara H, Wakano Y, Ishida H. Effect of prostaglandin E2 on mineralization of bone nodules formed by fetal rat calvarial cells. Calcif Tissue Int. 1994;55(6):451–7. doi: 10.1007/BF00298559. [DOI] [PubMed] [Google Scholar]
- 3.Scutt A, Zeschnigk M, Bertram P. PGE2 induces the transition from non-adherent to adherent bone marrow mesenchymal precursor cells via a cAMP/EP2-mediated mechanism. Prostaglandins. 1995;49(6):383–95. doi: 10.1016/0090-6980(95)00070-q. [DOI] [PubMed] [Google Scholar]
- 4.Hagino H, Kuraoka M, Kameyama Y, Okano T, Teshima R. Effect of a selective agonist for prostaglandin E receptor subtype EP4 (ONO-4819) on the cortical bone response to mechanical loading. Bone. 2005;36(3):444–53. doi: 10.1016/j.bone.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Kaneki H, Takasugi I, Fujieda M, Kiriu M, Mizuochi S, Ide H. Prostaglandin E2 stimulates the formation of mineralized bone nodules by a cAMP-independent mechanism in the culture of adult rat calvarial osteoblasts. J Cell Biochem. 1999;73(1):36–48. [PubMed] [Google Scholar]
- 6.Li M, Healy DR, Li Y, et al. Osteopenia and impaired fracture healing in aged EP4 receptor knockout mice. Bone. 2005;37(1):46–54. doi: 10.1016/j.bone.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Li M, Ke HZ, Qi H, et al. A novel, non-prostanoid EP2 receptor-selective prostaglandin E2 agonist stimulates local bone formation and enhances fracture healing. J Bone Miner Res. 2003;18(11):2033–42. doi: 10.1359/jbmr.2003.18.11.2033. [DOI] [PubMed] [Google Scholar]
- 8.Machwate M, Harada S, Leu CT, et al. Prostaglandin receptor EP(4) mediates the bone anabolic effects of PGE(2) Mol Pharmacol. 2001;60(1):36–41. doi: 10.1124/mol.60.1.36. [DOI] [PubMed] [Google Scholar]
- 9.Paralkar VM, Borovecki F, Ke HZ. An EP2 receptor-selective prostaglandin E2 agonist induces bone healing. Proc Natl Acad Sci U S A. 2003;100(11):6736–40. doi: 10.1073/pnas.1037343100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raisz LG, Woodiel FN. Effects of selective prostaglandin EP2 and EP4 receptor agonists on bone resorption and formation in fetal rat organ cultures. Prostaglandins Other Lipid Mediat. 2003;71(34):287–92. doi: 10.1016/s1098-8823(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 11.Fujieda M, Kiriu M, Mizuochi S, Hagiya K, Kaneki H, Ide H. Formation of mineralized bone nodules by rat calvarial osteoblasts decreases with donor age due to a reduction in signaling through EP(1) subtype of prostaglandin E(2) receptor. J Cell Biochem. 1999;75(2):215–25. doi: 10.1002/(sici)1097-4644(19991101)75:2<215::aid-jcb4>3.3.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Sakuma Y, Li Z, Pilbeam CC, Alander CB, Chikazu D, Kawaguchi H, Raisz LG. Stimulation of cAMP production and cyclooxygenase-2 by prostaglandin E(2) and selective prostaglandin receptor agonists in murine osteoblastic cells. Bone. 2004;34(5):827–34. doi: 10.1016/j.bone.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Suzawa T, Miyaura C, Inada M, Maruyama T, Sugimoto Y, Uskikubi F, Ichikawa A, Narumiya S, Suda T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: an analysis using specific agonists for the respective EPs. Endocrinol. 2000;141(4):1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 14.Brunk CF, Jones KC, James TW. Assay for nanogram quantities of DNA in Cellular homogenates. Anal Biochem. 1979;92(2):497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- 15.Pilbeam CC, Fall PM, Alander CB, Raisz LG. Differential effects of nonstreroidal anti-inflammatory drugs on constitutive and inducible prostaglandin G/H synthase in cultured bone cells. J Bone Miner Res. 1997;12(8):1198–203. doi: 10.1359/jbmr.1997.12.8.1198. [DOI] [PubMed] [Google Scholar]
- 16.Xu Z, Voznesensky O, Choudhary S, Raisz LG, Pilbeam CC. Overexpression of Cyclooxygenase-2 decreases proliferation of osteoblastic cells. J Bone Miner Res. 2003;18(Suppl 2):S340. [Google Scholar]
- 17.Zhan P, Alander C, Kaneko H, Pilbeam CC, Guan Y, Zhang Y, Breyer MD, Raisz LG. Effect of deletion of the prostaglandin EP4 receptor on stimulation of calcium release from cultured mouse calvariae: impaired responsiveness in heterozygotes. Prostaglandins Other Lipid Mediat. 2005;78(14):19–26. doi: 10.1016/j.prostaglandins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Raisz LG, Alander CA, Zhan P, Breyer MD, Kream BE, Pilbeam CC. Effects of global or targed deletion of the EP4 receptor on the response of cultured osteoblasts to prostaglandin E2. J Bone Miner Res. 19(S1):S5. doi: 10.1016/j.bone.2009.03.667. [DOI] [PubMed] [Google Scholar]