Abstract
The immune response that accompanies spinal cord injury contributes to both injury and reparative processes. It is this duality that is the focus of this review. Here we consider the complex cellular and molecular immune responses that lead to the infiltration of leukocytes and glial activation, promote oxidative stress and tissue damage, influence wound healing, and subsequently modulate locomotor recovery. Immunomodulatory strategies to improve outcomes are gaining momentum as ongoing research carefully dissects those pathways, which likely mediate cell injury from those, which favor recovery processes. Current therapeutic strategies address divergent approaches including early immunoblockade and vaccination with immune cells to prevent early tissue damage and support a wound-healing environment that favors plasticity. Despite these advances, there remain basic questions regarding how inflammatory cells interact in the injured spinal cord. Such questions likely arise as a result of our limited understanding of immune cell/neural interactions in a dynamic environment that culminates in progressive cell injury, demyelination, and regenerative failure.
Keywords: leukocyte, matrix metalloproteinase, spinal cord, regeneration
Introduction
Leukocytes are key responders to spinal cord injury. Their actions are immune-cell specific and driven by a dynamic environment where early cell injury, axonal degeneration, and demyelination become integrated into complex wound healing events including angiogenesis and glial scar formation. In this review we consider spinal cord injury in the context of innate and adaptive immunity and address the molecular mechanisms that govern leukocyte recruitment and activation as well as leukocyte-mediated cell injury and repair processes. Lastly, we consider the controversy of immunomodulation by vaccination with immune cells as a strategy for the treatment of spinal cord injury.
Immune privilege and the spinal cord
The central nervous system (CNS) has been considered immune privileged because of its inability to mount an immune response and process antigens (1). However, we now know that the CNS, when challenged by injury and systemic infections, has the ability to mount a well-organized immune response (2). Evidence over the past two decades has thus redefined the CNS from ‘immunologically privileged’ to an ‘immunologically quiescent’ site (3). This quiescent state is dramatically altered in the injured spinal cord where there is an orchestrated invasion of circulating immune cells, activation of resident microglia and astrocytes, and expression of classic immune and inflammatory mediators including complement, cytokines, and chemokines (4).
Inflammation and the “Uniqueness” of the spinal cord
The immune response in the nervous system varies according to location, with differences noted between the injured peripheral nerve relative to the injured brain (5, 6), and between the injured brain and spinal cord (7–9).
Central versus peripheral nervous system
In both brain and peripheral nerve injury, axonal degeneration is evident within several days post insult (5). However, the time course and the role of inflammatory cells in the ensuing degradation of myelin and removal of cellular debris differ between these regions. Axonal debris is rapidly cleared in the peripheral nerve within several weeks after injury (5), whereas in the brain similar processes may extend over a period of months (5). This slower removal of debris may be attributed to the kinetics of the immune response, particularly with regard to macrophages. Macrophages infiltrate the degenerating peripheral nerve in a matter of days after axotomy and, along with Schwann cells, play a significant role in both degradation and removal of debris (5, 6). These findings contrast to brain injury, where an increase in mononuclear phagocytes is more delayed in onset (5, 6) and oligodendrocytes, unlike Schwann cells, remain quiescent during Wallerian degeneration (5). The persistence of myelin debris, which has growth inhibitory properties, likely, contributes to an environment that is nonpermissive to regeneration.
Brain versus spinal cord
Injury to the spinal cord results in a more robust inflammatory response than seen in the brain (7, 8). Following a mechanical injury or injection of proinflammatory cytokines, neutrophil recruitment is significantly greater in the spinal cord as well as more widespread within the cord parenchyma relative to the adult brain, which is nearly refractory to leukocyte infiltration (7–9). This pattern of neutrophil recruitment is attributed to differences in the expression of cytokine-induced neutrophil chemoattractant chemokines (9).
Collectively, these findings demonstrate that inflammation in the nervous system exhibits at least some degree of site specificity. This regional specificity may confer a distinguishing “signature” and as such offer clues as what factors govern immune-mediated events and how immunomodulation may be best exploited to restore function after injury.
Innate and adaptive immunity
The immune system affords two types of defenses (Figure 1): one that is non-specific for any particular antigen (innate immunity) and one that is specific (adaptive/acquired immunity), where specificity is related to antigenic recognition and response (10).
Figure 1. Characterization of immunity.
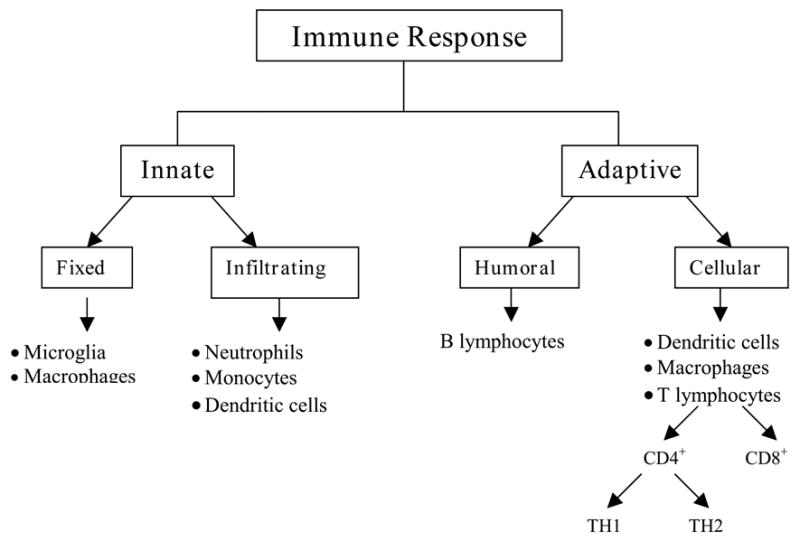
In general, cells associated with innate immunity are either tissue specific or derived from a circulating pool of inflammatory cells. The adaptive response consists of both humoral and cellular components. The humoral response is typified by the production of antibodies and is mediated by B-lymphocytes. The cellular response is mediated by T-lymphocytes, which are further classified into cytotoxic, CD8+ and helper cells, CD4+. CD4+ cells give rise to either TH1 or TH2 types of responses. TH1 cells produce pro-inflammatory cytokines such as IFN-γ, TNF-agr;, and IL-6, whereas TH2 cells secrete anti-inflammatory cytokines including IL-4, IL-10 and TGF-β. Abbreviations: Interferon-gamma - IFN-γ; tumor necrosis factor-alpha - TNF-agr;; interleukin-6 - IL-6; interleukin-4 - IL-4; interleukin-10 - IL-10; and transforming growth factor-beta - TGF-β.
Innate immunity
The innate immune response, typically triggered shortly after injury, involves resident microglia/macrophages, neutrophils, and dendritic cells that infiltrate into the injured parenchyma. In general, macrophages and neutrophils perform a variety of functions that include destruction of offending microbes and phagocytosis of cellular debris (11, 12). Dendritic cells have a dual role in the immune reaction. Immature dendritic cells are characterized by their phagocytic behavior, motility and expression of chemokine receptors (13). These characteristics contrast that of mature dendritic cells which participate in adaptive immunity through their ability to present antigens to T lymphocytes, resulting in their activation (14).
In the injured spinal cord, microglia, macrophages and dendritic cells function as antigen presenting cells by signaling through Toll-like receptors. These receptors, expressed at the surface of antigen presenting cells, bind to specific components of the pathogen, the pathogen-associated molecular patterns, which in turn leads to activation of antigen presenting cells (2). Once antigen presenting cells are activated they phagocytose debris, express major histocompatibility complex II (MHC II) molecules on their surface, and present peptides that have been degraded to helper T-lymphocytes. Binding of pathogenic components to the Toll-like receptors leads to the production of cytokines by circulating monocytes/macrophages, microglia, and neutrophils, resulting in a pro-inflammatory reaction.
Adaptive immunity
Antigen presenting cells function as a crucial bridge through their ability to present specific antigens to cells that participate in adaptive immunity. Unlike innate immunity, adaptive immunity exhibits specificity, diversity, memory, self-/non-self-recognition, and involves the upregulation of chemokines and cytokines with subsequent recruitment of lymphocytes. The adaptive immune system is classified into humoral and cellular responses (Figure 1). The humoral response is a second line of defense and is mediated by B-lymphocytes, which release antibodies. The cellular response is executed by T-lymphocytes (cytotoxic, CD8+ and helper cells, CD4+) that are activated in response to specific antigenic signals. CD4+ T cells are classified into two subtypes based on the cytokines they secrete upon activation. TH1 cells produce pro-inflammatory cytokines such as interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-agr;), and interleukin-6 (IL-6), whereas TH2 cells secrete anti-inflammatory cytokines such as interleukin-4 (IL-4), interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β).
Leukocytes, microglia/macrophages, and dendritic cells as mediators of cell injury and repair processes
The inflammatory reaction in the acutely injured cord is an essential host defense mechanism, which functions to eliminate invading pathogens and clear debris. Inflammatory cells also promote wound-healing events that support recovery. These beneficial events in both the acutely injured spinal cord and during wound healing may be overshadowed by an excessive accumulation of toxic molecules produced by inflammatory cells that damage otherwise intact tissue. As such, inflammation in the injured spinal cord has been regarded as a “two-edged sword” (15).
Neutrophils
Neutrophils are well-differentiated cells that have an intravascular half-time of approximately 9 hours (16). Their persistence in inflamed tissue reflects a balance between recruitment and apoptosis (11). Neutrophils accumulate within hours after spinal cord injury (17–19), reaching a peak at 3 days post-injury followed by a second peak several weeks later (11) (Figure 2).
Figure 2. Schematic representation of timing of infiltration of inflammatory cells relative to secondary pathogenesis and wound healing.
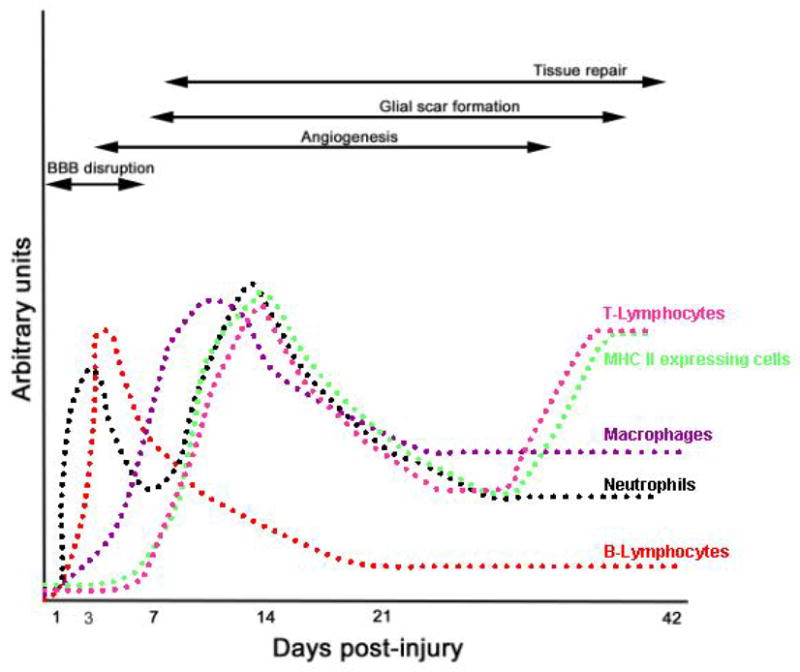
Inflammatory cells participate in both early tissue injury and in wound healing events. This summarizes data from several studies that have addressed the timing of infiltration relative to early injury and wound healing events. Peak periods of infiltration coincide with barrier disruption (91, 92), angiogenesis (93–95), and glial scar formation (11). Note that the magnitude of infiltration is expressed as arbitrary units for each of the cell types, and as such comparisons cannot be made between groups.
Neutrophils support recovery processes through their ability to phagocytose cellular debris. In addition, they summon macrophages into the damaged tissue (11). These macrophages function not only as phagocytes (20) but also serve as a reservoir for cholesterol derived from ingested myelin, which can then be used during remyelination of regenerating axons (21).
Neutrophils release reactive oxygen and nitrosyl radicals as well as cytokines, chemokines, and a variety of proteases, including metalloproteinases and neutrophil elastase, and as such are key determinants in secondary tissue damage (Table 1) (22–25). The damaging effects of neutrophils in spinal cord injury are perhaps best illustrated in studies showing that either depletion of circulating neutrophils, inhibition of neutrophil-related proteolytic enzyme activities (19, 23), or inhibition of neutrophil adhesion (26) confer neuroprotection.
Table 1.
Summary of immune cells and their roles in inflammation.
| Cell Type | Type of Immunity | Pro-inflammatory molecules | Mediators of cell injury/death | Pro-regeneration/wound healing events |
|---|---|---|---|---|
| Neutrophils | Innate (41) | Express receptors for various chemokines and cytokines, MMP-9 (23) | Produce metalloproteinases (23), reactive oxygen and nitrosyl radicals (64), neutrophil elastase (19) | Phagocytosis |
| Dendritic cells | Innate and adaptive (34) | Produce TNF-agr;, IL-1, IL-2, IL-6, IL-12, 1L-18, IFN-γ (34) | Function as antigen presenting cells | Production of neurotrophin-3 (47) |
| Monocytes/Macrophages | Innate (41) | Produce TNF-agr;, IL-1, IL-2, IL-6, IL-12, 1L-18 (34) | Produce reactive oxygen species and nitrosyl radicals (17) | Phagocytosis, production of trophic factors, IL-10, TGF-β (41) |
| Microglia/Macrophages | Innate and adaptive (41) | Produce TNF-agr;, IL-1, IL-2, IL-6, IL-12, 1L-18 (34) | Function as antigen presenting cells; produce reactive oxygen species and nitrosyl radicals (17) | Phagocytosis, production of trophic factors, IL-10, TGF-β (41) |
| B-Lymphocytes | Adaptive (41) | Express receptors for various chemokines and cytokines (41) | Produce antibodies | Unknown |
| T-Lymphocytes | Adaptive (41) | Express receptors for various chemokines and cytokines, IFN-γ, TGF-β (41) | Pro-inflammatory cytokines and chemokines (41) | Production of trophic factors, IL-10, IL-4, IL-13 (41) |
Abbreviations: matrix metalloproteinase-9 - MMP-9; tumor necrosis factor-alpha - TNF-agr;; interleukin - IL; interferon-gamma - IFN-γ; transforming growth factor-beta - TGF-β;
Activated microglia
Upon spinal cord injury, microglia become activated as evidenced by retraction of processes, enlargement of their soma, up-regulation of surface antigens, and production of innate cytokines and chemokines (Table 1). Activated microglia are evident by the first day after spinal cord injury, increase in numbers by 7 days, and then plateau between 2–4 weeks post injury (27).
A recent review highlights the multiple phenotypes of microglia and their corresponding functions in tissue repair and/or damage (28). Microglia participate in the removal of myelin debris, produce growth factors including glial cell line derived neurotrophic factor (29) that are favorable for neurite growth and regeneration (30), and express transforming growth factor-beta1 (TGF-beta1), a cytokine/growth factor that inhibits the release of cytotoxic molecules, decreases astrocyte proliferation, and promotes neuronal survival (31).
Microglia are also implicated in necrotic and apoptotic neuronal cell death (32) and are integral in the early response to axonal damage in human spinal cord injury (33). They produce TNF-agr;, IL-1, reactive free radicals, and nitric oxide (17, 34), each of which can be damaging to the spinal cord, as well as keratan sulfate proteoglycans, which form inhibitory boundaries to extending neurites (35).
Monocytes/macrophages
Monocytes/macrophages are apparent in the injured spinal cord within the first week after injury (27) (36) and macrophage activation is maximal between 7–14 days post-injury (Figure 2) (11). Macrophages support wound healing events in part by functioning as phagocytes. However, they are also sources of pro-inflammatory cytokines and neurotoxins including reactive oxygen species (37–39) and inducible nitric oxide synthase (40), implicating them in cell injury (Table 1). As is the case with other inflammatory cells, their relative contribution to either injury or repair is determined by the collective impact of these opposing processes (41).
Dendritic cells
Dendritic cells, which arise from the differentiation of microglia (42) or from a circulating pool (43), are antigen presenting cells and hence express high levels of MHC II and pro-inflammatory cytokines (34) (Table 1). As such, they support the ongoing inflammatory response, which may exacerbate secondary injury. Their impact on wound healing and recovery remains a matter of debate (44–46) in part because of their ability to produce growth factors, including neurotrophin-3, and enhance neurogenesis (47).
B-Lymphocytes
B-lymphocytes are produced and mature within the bone marrow and their numbers decrease in the peripheral lymph nodes and spleen after spinal cord injury (48). They are present in the vicinity of the lesion site within hours after spinal cord injury and persist for up to 1 week post-injury (Figure 2) (48, 49). B-lymphocytes produce antibodies in response to injury and as such are responsible for memory in adaptive immunity (Table 1). There is very little known about the involvement of B-cells in the injured spinal cord. In experimental allergic encephalomyelitis, they are known to promote demyelination (50). Given the paucity of data, it is premature to suggest that they function in a similar manner in the injured spinal cord.
T- Lymphocytes
T-lymphocytes, produced in the bone marrow, mature in the thymus. They are classified as either CD4+ or CD8+ cells based on their phenotype and function (Figure 1). T-lymphocytes are present in low numbers in the uninjured spinal cord (36) and progressively increase, in parallel with the activation of microglia and influx of peripheral macrophages, within the first week post injury(32). Even though T-lymphocytes are typically fewer in number than the invading macrophages or activated microglia, they have the potential for promoting greater tissue damage. They do so through their ability to recognize specific antigens, such as myelin basic protein, and proliferate in response to those antigens (51). These cells have an estimated life span of 1-2 months and thus their presence is well into the recovery/regeneration phase after injury (52). The magnitude and timing of their infiltration into the injured spinal cord is species/strain dependent (36) (11). In mice there is a biphasic peak of infiltration, the first occurring between 7–14 days and a second at 42 days (Figure 2). Similar kinetics apply to both CD4+ and CD8+ cells, though the former occurs in higher numbers and typically resides in a centralized zone of fibrosis (11).
T-lymphocytes likely play complex roles in both injury and repair mechanisms. Similar to other inflammatory cells, T- cell functions are determined by molecular signals that attract them to the injury site and by the microenvironment they encounter. Chemokines are responsible for T-cell migration and modulate their activation and effector potential at sites of inflammation (53, 54). There is evidence that T-lymphocytes participate in both injury and recovery processes. Upon their activation, T-lymphocytes may kill target cells and produce cytokines (55, 56) (Table 1). In addition, chronic T-cell activation precipitates in pathological fibrosis and scarring (57). Such detrimental interactions are countered by studies supporting a neuroprotective role in models of CNS injury and neurodegeneration (58, 59). Thus, how T-lymphocytes influence spinal cord integrity and function are determined in part by the antigens targeted by the T-cell population (55).
Modulators of leukocyte infiltration
Specific adhesion proteins on both endothelial cells and leukocytes orchestrate the infiltration of leukocytes into the CNS.
Adhesion molecules
Intercellular adhesion molecule (ICAM-1) and vascular adhesion molecule-1 (VCAM-1) facilitate cell-to-cell interactions among astrocytes, endothelium, microglia, and effector cells of the peripheral immune system such as T-lymphocytes, macrophages, and neutrophils. Initial rolling of leukocytes is mediated by the selectins, while interactions of ICAM-1 and leukocyte β2 integrin complex (CD11/CD18) are responsible for leukocyte adhesion and diapedesis (60). The physical interaction mediated by ICAM-1, VCAM-1, and other cell adhesion molecules forms an integral component of the effector phase of immunological responses in the CNS. Supporting this idea is the observation that antibodies specific for cell adhesion molecules are neuroprotective in spinal cord injury (26).
ICAM-1 serves as a ligand for CD11/CD18 (61). Beta2 integrins play an essential role in leukocyte trafficking and activation, and arbitrate cell-cell interactions during inflammation. The beta2 integrin, alphaDbeta2, expressed on monocytes/macrophages and neutrophils, binds to VCAM-1. Increased expression of VCAM-1 promotes leukocyte extravasation. Blocking the interaction between alphaDbeta2 and VCAM-1 attenuates the inflammatory response in the injured spinal cord (62), resulting in decreased oxidative damage and lipid peroxidation, and improves neurological function (63–65).
Cytokines and chemokines
Cytokines are regulatory proteins whose plieotropic actions govern the amplitude and the duration of the immune response (Table 1). They are usually released within minutes after challenge because they are stored intracellularly as precursor proteins, which can then be modified into active molecules. Cytokines are broadly classified into five major groups: interleukins, growth factors, interferons, chemokines, and tumor necrosis factor. Tumor necrosis factor is the prototypic proinflammatory cytokine, as it is primarily responsible for initiating the cascade of other cytokines in the classic immune response.
The kinetics of chemokine expression parallels the influx of immune cells after spinal cord injury (20, 25, 27). Chemokines are small, secreted molecules that have very specific conserved cysteine residues in their amino acid sequences. Chemokines are expressed early after injury and are thought to play a role in the recruitment of inflammatory cells to the lesion site (66, 67). There is a close correlation between infiltration of hematogenous cells and the expression of soluble mediators, particularly chemokines, in injured tissue. Chemokine CXCL10, involved in the recruitment of T-lymphocytes, is upregulated in the injured spinal cord and contributes to post-traumatic tissue loss (56). Chemokine production is temporally regulated after spinal cord injury and is associated with T-lymphocyte recruitment (55). Blocking the interaction of a chemokine with its receptor by using specific antibodies, antagonists, antisense oligoneucleotides, or blocking peptides limits the inflammatory response (68–73). Whether chemokines predispose the spinal cord to T-cell mediated injury or repair will depend on the antigen specificity of the responding T-lymphocytes and the timing, magnitude, and composition of the chemokines produced.
Therapeutic vaccination: good or bad?
Historically, administration of high-dose methylprednisolone (MP), a glucocorticoid steroid, acutely after SCI has been considered the standard of care in the United States. The principal mechanism by which MP confers neuroprotection is likely through its ability to inhibit post-traumatic lipid peroxidation. Although clinical results were initially promising, there have been growing concerns that the modest neurological improvements seen with high-dose MP treatment in injured patients are not worth the associated risks (74). Therefore, there is a critical need to develop new pharmacologic therapies for treatment of SCI. A number of strategies that non-selectively suppress inflammation have had varying effects on outcome after experimental spinal cord injury over the last decade (Table 2). This variability may be in part attributed to unique roles of inflammatory cells in both injury and recovery processes (10, 31, 75, 76).
Table 2.
Summary of anti-inflammatory therapeutics.
| Anti-inflammatory Agents | Type of Action | Locomotor Function (relative to controls) | References |
|---|---|---|---|
| Antibody to the CD11d integrin | Blocks infiltration of leukocytes | Improves motor recovery | (63) |
| Methylprednisolone | General anti-inflammatory agent | No difference | (96) |
| Prednisolone derivative NCX1015 | General anti-inflammatory agent | Improves motor recovery | (97) |
| Methylprednisolone + antibody to the CD11d integrin | General anti-inflammatory agent + blocks infiltration of leukocytes | No difference | (98) |
| GM6001 | General matrix metalloproteinase inhibitor | Improves motor recovery | (23) |
| Interleukin-10 (IL-10) | Reduces inflammation | Improves motor recovery | (99, 100) |
| MP + IL-10 | General anti-inflammatory agents | No difference | (101) |
| Interleukin-1 (IL-1) receptor antagonist | Inhibits binding of IL-1β | Not studied | (102) |
| Interleukin-6 (IL-6) receptor antagonist | Inhibits IL-6 binding | Improves motor recovery | (103) |
| Cyclooxygenase-2 inhibitors | Inhibits cyclooxygenase enzyme secreted by macrophages | Improves motor recovery | (104–106) |
| Antibody to CD95 | Inhibit T cell apoptosis | Improves motor recovery | (107) |
| Minocycline | General anti-inflammatory agent, partially inhibits mitochondrial cytochrome C release | Improves motor recovery | (108, 109) |
| Antibody to chemokine CXCL10 | Inhibits chemokine function and hence T cell recruitment | Improves motor recovery | (56, 110) |
| Antibody to integrins | Inhibits leukocyte infiltration | Improves motor recovery | (63) |
| Neutrophil elastase inhibitor | Inhibits neutrophil elastase | Improves motor recovery | (111, 112) |
| Iloprost | Blocks leukocyte accumulation | Improves motor recovery | (113) |
| Antibody to P-selectin | Blocks neutrophil adhesion to endothelial cells | Improves motor recovery | (114) |
| FK 506 | Inhibits T cell activation | Improves motor recovery | (115) |
| Antibody to ICAM-1 | Inhibits neutrophil infiltration | Improves motor recovery | (26) |
| Antisense oligoneucleotides to inducible nitric oxide synthase | Decreases neutrophil infiltration and astrogliosis | Not studied | (116) |
Over the past decade, a number of anti-inflammatory approaches have had varying success in improving functional recovery after spinal cord injury.
Abbreviations: interleukin - IL; intercellular adhesion molecule-1 - ICAM-1
Recent studies, targeting T-lymphocytes, highlight the complexity of developing immunotherapies for spinal cord injury. It has been argued that autoreactive T-lymphocytes exacerbate injury to axons and induce demyelination leading to functional loss (10). Autoreactive lymphocytes are present in the CNS and may react with CNS proteins (77). They play a prominent role in demyelinating disorders (78) and participate in brain and spinal cord injury. There is an abundance of myelin reactive T-cells in spinal cord injured and stroke patients (79–81). Experimental studies are only now beginning to explore their function in spinal cord injury. CNS-reactive T-cells exacerbate axonal injury, demyelination, and functional loss after experimental spinal cord injury (51, 82, 83). Moreover, mice with a transgenic T-cell receptor specific to myelin exhibit worse functional outcomes after spinal cord injury than mice of the same genetic background. This difference may be attributed to altered chemokine expression (55, 82).
Counter to this detrimental role is the view that macrophages are required for repair, and that activated T-cells directed against CNS antigens are needed for defense and protection (84). Consistent with this position, immunization with myelin epitopes concomitant with spinal cord injury enhances axonal growth and recovery of motor function (85). Therefore, boosting the immune responses to CNS injury under certain conditions may be more beneficial than harmful to functional regeneration (58). Implantation of activated macrophages (86) or T-lymphocyte mediated immune activity, achieved by either adoptive transfer or active immunization (87–90), enhances recovery from spinal cord injury by conferring neuroprotection or regeneration. Although the concept of neuroprotective autoimmunity is intriguing, there is no definitive mechanism that explains its efficacy.
Conclusion
Spinal cord injury initiates both innate and adaptive immune responses, which participate in early secondary pathogenesis and wound healing events. These immune responses function in an environment in which cell injury and reparative processes co-exist. The extent to which immune cells promote cellular injury or support recovery depends upon the timing and magnitude of their appearance and the nature of their interactions with one another as well as with both injured and intact cells. Various strategies to selectively block the early innate response yield the most consistent findings, namely that of neuroprotection and functional recovery. However, we have yet to understand how such manipulations alter adaptive immunity. Moreover, we are only beginning to address the role of inflammation in wound healing events. We are moving beyond the important role that macrophages play in tissue remodeling to the more complex interactions of lymphocytes in white matter injury and remyelination. This exciting direction affords us the opportunity to examine novel interactions that will ultimately provide greater insight into how we view immunomodulation as a strategy to improve recovery in the spinal cord injured patient.
This review illustrates the complexity of manipulating the immune system to repair the injured spinal cord. We are just beginning to appreciate that immune cells and mediators of the immune system can have divergent effects in the injured spinal cord. Until there is clarity on the mechanisms of this divergence, there is likely to be continued discordance in the scientific community regarding the development of immune-based therapeutic vaccinations.
Acknowledgments
This research was supported by NIH NINDS NS039278 and a Linker Foundation Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cserr HF, Knopf PM. Cervical lymphatics, the blood-brain barrier and the immunoreactivity of the brain: a new view. Immunol Today. 1992;13(12):507–12. doi: 10.1016/0167-5699(92)90027-5. [DOI] [PubMed] [Google Scholar]
- 2.Nguyen MD, Julien JPSR. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3(3):216–27. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 3.Prins RMLML. Immunology and immunotherapy in neurosurgical disease. Neurosurgery. 2003;53(1):144–52. doi: 10.1227/01.neu.0000068865.34216.3a. [DOI] [PubMed] [Google Scholar]
- 4.Stoll G, Jander S, Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv Exp Med Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- 5.Hughes PM, et al. Comparison of matrix metalloproteinase expression during Wallerian degeneration in the central and peripheral nervous systems. Neuroscience. 2002;113(2):273–87. doi: 10.1016/s0306-4522(02)00183-5. [DOI] [PubMed] [Google Scholar]
- 6.Lawson LJ, et al. Quantification of the mononuclear phagocyte response to Wallerian degeneration of the optic nerve. J Neurocytol. 1994;23(12):729–44. doi: 10.1007/BF01268086. [DOI] [PubMed] [Google Scholar]
- 7.Schnell L, et al. Acute inflammatory responses to mechanical lesions in the CNS: differences between brain and spinal cord. Eur J Neurosci. 1999;11(10):3648–58. doi: 10.1046/j.1460-9568.1999.00792.x. [DOI] [PubMed] [Google Scholar]
- 8.Schnell L, et al. Cytokine-induced acute inflammation in the brain and spinal cord. J Neuropathol Exp Neurol. 1999;58(3):245–54. doi: 10.1097/00005072-199903000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Campbell SJ, et al. Altered chemokine expression in the spinal cord and brain contributes to differential interleukin-1beta-induced neutrophil recruitment. J Neurochem. 2002;83(2):432–41. doi: 10.1046/j.1471-4159.2002.01166.x. [DOI] [PubMed] [Google Scholar]
- 10.Popovich PG, Jones TB. Manipulating neuroinflammatory reactions in the injured spinal cord: back to basics. Trends Pharmacol Sci. 2003;24(1):13–7. doi: 10.1016/s0165-6147(02)00006-8. [DOI] [PubMed] [Google Scholar]
- 11.Kigerl KA, McGaughy VM, Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol. 2006;494(4):578–94. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Castro R, Jr, et al. Evidence that infiltrating neutrophils do not release reactive oxygen species in the site of spinal cord injury. Exp Neurol. 2004;190(2):414–24. doi: 10.1016/j.expneurol.2004.05.046. [DOI] [PubMed] [Google Scholar]
- 13.Sallusto F, Lanzavecchia A. Understanding dendritic cell and T-lymphocyte traffic through the analysis of chemokine receptor expression. Immunol Rev. 2000;177:134–40. doi: 10.1034/j.1600-065x.2000.17717.x. [DOI] [PubMed] [Google Scholar]
- 14.Cella M, et al. Inflammatory stimuli induce accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388(6644):782–7. doi: 10.1038/42030. [DOI] [PubMed] [Google Scholar]
- 15.Bethea JR. Spinal cord injury-induced inflammation: a dual-edged sword. Prog Brain Res. 2000;128:33–42. doi: 10.1016/S0079-6123(00)28005-9. [DOI] [PubMed] [Google Scholar]
- 16.Parwaresch MR, Walle AJ, Voigt J. An unusual labeling index pattern of neutrophilic granulocytes after a 3H-thymidine flash labeling injection. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;33(2):177–85. doi: 10.1007/BF02899180. [DOI] [PubMed] [Google Scholar]
- 17.Carlson SL, et al. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 1998;151(1):77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- 18.Chatzipanteli K, et al. Posttraumatic hypothermia reduces polymorphonuclear leukocyte accumulation following spinal cord injury in rats. J Neurotrauma. 2000;17(4):321–32. doi: 10.1089/neu.2000.17.321. [DOI] [PubMed] [Google Scholar]
- 19.Taoka Y, Okajima K. Spinal cord injury in the rat. Prog Neurobiol. 1998;56(3):341–58. doi: 10.1016/s0301-0082(98)00049-5. [DOI] [PubMed] [Google Scholar]
- 20.Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2(4):299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- 21.Boyles JK, et al. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J Clin Invest. 1989;83(3):1015–31. doi: 10.1172/JCI113943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiss SJ. Tissue destruction by neutrophils. N Engl J Med. 1989;320(6):365–76. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 23.Noble LJ, et al. Matrix metalloproteinases limit functional recovery after spinal cord injury by modulation of early vascular events. J Neurosci. 2002;22(17):7526–35. doi: 10.1523/JNEUROSCI.22-17-07526.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bethea JR, et al. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci. 1998;18(9):3251–60. doi: 10.1523/JNEUROSCI.18-09-03251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dusart I, Schwab ME. Secondary cell death and the inflammatory reaction after dorsal hemisection of the rat spinal cord. Eur J Neurosci. 1994;6(5):712–24. doi: 10.1111/j.1460-9568.1994.tb00983.x. [DOI] [PubMed] [Google Scholar]
- 26.Hamada Y, et al. Involvement of an intercellular adhesion molecule 1-dependent pathway in the pathogenesis of secondary changes after spinal cord injury in rats. J Neurochem. 1996;66(4):1525–31. doi: 10.1046/j.1471-4159.1996.66041525.x. [DOI] [PubMed] [Google Scholar]
- 27.Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377(3):443–64. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz M, et al. Microglial phenotype: is the commitment reversible? Trends Neurosci. 2006;29(2):68–74. doi: 10.1016/j.tins.2005.12.005. Epub 2006 Jan 6. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto M, et al. Inflammation-induced GDNF improves locomotor function after spinal cord injury. Neuroreport. 2005;16(2):99–102. doi: 10.1097/00001756-200502080-00004. [DOI] [PubMed] [Google Scholar]
- 30.Schwartz M. Sell Memorial Lecture. Helping the body to cure itself: immune modulation by therapeutic vaccination for spinal cord injury. J Spinal Cord Med. 2003;26(Suppl 1):S6–10. [PubMed] [Google Scholar]
- 31.McTigue DM, et al. Strategies for spinal cord injury repair. Prog Brain Res. 2000;128:3–8. doi: 10.1016/S0079-6123(00)28002-3. [DOI] [PubMed] [Google Scholar]
- 32.Popovich PG. Immunological regulation of neuronal degeneration and regeneration in the injured spinal cord. Prog Brain Res. 2000;128:43–58. doi: 10.1016/S0079-6123(00)28006-0. [DOI] [PubMed] [Google Scholar]
- 33.Yang L, et al. Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine. 2004;29(9):966–71. doi: 10.1097/00007632-200405010-00004. [DOI] [PubMed] [Google Scholar]
- 34.Town T, Nikolic V, Tan J. The microglial “activation” continuum: from innate to adaptive responses. J Neuroinflammation. 2005;2:24. doi: 10.1186/1742-2094-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones LL, Tuszynski MH. Spinal cord injury elicits expression of keratan sulfate proteoglycans by macrophages, reactive microglia, and oligodendrocyte progenitors. J Neurosci. 2002;22(11):4611–24. doi: 10.1523/JNEUROSCI.22-11-04611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sroga JM, et al. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 2003;462(2):223–40. doi: 10.1002/cne.10736. [DOI] [PubMed] [Google Scholar]
- 37.Blight AR. Macrophages and inflammatory damage in spinal cord injury. J Neurotrauma. 1992;9 (Suppl 1):S83–91. [PubMed] [Google Scholar]
- 38.Blight AR. Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. Neuroscience. 1994;60(1):263–73. doi: 10.1016/0306-4522(94)90220-8. [DOI] [PubMed] [Google Scholar]
- 39.Popovich PG, et al. Elevation of the neurotoxin quinolinic acid occurs following spinal cord trauma. Brain Res. 1994;633(1–2):348–52. doi: 10.1016/0006-8993(94)91560-1. [DOI] [PubMed] [Google Scholar]
- 40.Minc-Golomb D, et al. In vivo expression of inducible nitric oxide synthase in cerebellar neurons. J Neurochem. 1996;66(4):1504–9. doi: 10.1046/j.1471-4159.1996.66041504.x. [DOI] [PubMed] [Google Scholar]
- 41.Profyris C, et al. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15(3):415–36. doi: 10.1016/j.nbd.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 42.Karman J, et al. Dendritic cells in the initiation of immune responses against central nervous system-derived antigens. Immunol Lett. 2004;92(1–2):107–15. doi: 10.1016/j.imlet.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 43.Newman TA, et al. Blood-derived dendritic cells in an acute brain injury. J Neuroimmunol. 2005;166(1–2):167–72. doi: 10.1016/j.jneuroim.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 44.David S, et al. Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron. 1990;5(4):463–9. doi: 10.1016/0896-6273(90)90085-t. [DOI] [PubMed] [Google Scholar]
- 45.Streit WJ. Microglial-neuronal interactions. J Chem Neuroanat. 1993;6(4):261–6. doi: 10.1016/0891-0618(93)90047-8. [DOI] [PubMed] [Google Scholar]
- 46.Schwartz M, et al. Potential repair of rat spinal cord injuries using stimulated homlogous macrophages. Neurosurgery. 1999;44(5):1041–5. doi: 10.1097/00006123-199905000-00057. discussion 1045–6. [DOI] [PubMed] [Google Scholar]
- 47.Mikami Y, et al. Implantation of dendritic cells in injured adult spinal cord results in activation of endogenous neural stem/progenitor cells leading to de novo neurogenesis and functional recovery. J Neurosci Res. 2004;76(4):453–65. doi: 10.1002/jnr.20086. [DOI] [PubMed] [Google Scholar]
- 48.Popovich PG, et al. Alterations in immune cell phenotype and function after experimental spinal cord injury. J Neurotrauma. 2001;18(9):957–66. doi: 10.1089/089771501750451866. [DOI] [PubMed] [Google Scholar]
- 49.Schnell L, et al. Lymphocyte recruitment following spinal cord injury in mice is altered by prior viral exposure. Eur J Neurosci. 1997;9(5):1000–7. doi: 10.1111/j.1460-9568.1997.tb01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chavarria A, Alcocer-Varela J. Is damage in central nervous system due to inflammation? Autoimmun Rev. 2004;3(4):251–60. doi: 10.1016/j.autrev.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Popovich PG, Stokes BT, Whitacre CC. Concept of autoimmunity following spinal cord injury: possible roles for T lymphocytes in the traumatized central nervous system. J Neurosci Res. 1996;45(4):349–63. doi: 10.1002/(SICI)1097-4547(19960815)45:4<349::AID-JNR4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 52.Velardo MJ, et al. Patterns of gene expression reveal a temporally orchestrated wound healing response in the injured spinal cord. J Neurosci. 2004;24(39):8562–76. doi: 10.1523/JNEUROSCI.3316-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sallusto F, Lanzavecchia A, Mackay CR. Chemokines and chemokine receptors in T-cell priming and Th1/Th2-mediated responses. Immunol Today. 1998;19(12):568–74. doi: 10.1016/s0167-5699(98)01346-2. [DOI] [PubMed] [Google Scholar]
- 54.Sallusto F, Mackay CR, Lanzavecchia A. The role of chemokine receptors in primary, effector, and memory immune responses. Annu Rev Immunol. 2000;18:593–620. doi: 10.1146/annurev.immunol.18.1.593. [DOI] [PubMed] [Google Scholar]
- 55.Jones TB, Hart RP, Popovich PG. Molecular control of physiological and pathological T-cell recruitment after mouse spinal cord injury. J Neurosci. 2005;25(28):6576–83. doi: 10.1523/JNEUROSCI.0305-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez R, et al. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp Neurol. 2003;184(1):456–63. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- 57.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4(8):583–94. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwartz M, Kipnis J. Protective autoimmunity: regulation and prospects for vaccination after brain and spinal cord injuries. Trends Mol Med. 2001;7(6):252–8. doi: 10.1016/s1471-4914(01)01993-1. [DOI] [PubMed] [Google Scholar]
- 59.Kipnis J, et al. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc Natl Acad Sci U S A. 2002;99(24):15620–5. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67(6):1033–6. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 61.Springer TA. Leucocyte adhesion to cells. Scand J Immunol. 1990;32(3):211–6. doi: 10.1111/j.1365-3083.1990.tb02912.x. [DOI] [PubMed] [Google Scholar]
- 62.Mabon PJ, Weaver LC, Dekaban GA. Inhibition of monocyte/macrophage migration to a spinal cord injury site by an antibody to the integrin alphaD: a potential new anti-inflammatory treatment. Exp Neurol. 2000;166(1):52–64. doi: 10.1006/exnr.2000.7488. [DOI] [PubMed] [Google Scholar]
- 63.Gris D, et al. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 2004;24(16):4043–51. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bao F, et al. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004;88(6):1335–44. doi: 10.1046/j.1471-4159.2003.02240.x. [DOI] [PubMed] [Google Scholar]
- 65.Bao F, Dekaban GA, Weaver LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J Neurochem. 2005;94(5):1361–73. doi: 10.1111/j.1471-4159.2005.03280.x. Epub 2005 Jun 30. [DOI] [PubMed] [Google Scholar]
- 66.McTigue DM, et al. Selective chemokine mRNA accumulation in the rat spinal cord after contusion injury. J Neurosci Res. 1998;53(3):368–76. doi: 10.1002/(SICI)1097-4547(19980801)53:3<368::AID-JNR11>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 67.Lee YL, et al. Cytokine chemokine expression in contused rat spinal cord. Neurochem Int. 2000;36(4–5):417–25. doi: 10.1016/s0197-0186(99)00133-3. [DOI] [PubMed] [Google Scholar]
- 68.Sozzani S, et al. Chemokines as targets for pharmacological intervention. Prog Drug Res. 1996;47:53–80. doi: 10.1007/978-3-0348-8998-8_2. [DOI] [PubMed] [Google Scholar]
- 69.Howard OM, Ben-Baruch A, Oppenheim JJ. Chemokines: progress toward identifying molecular targets for therapeutic agents. Trends Biotechnol. 1996;14(2):46–51. doi: 10.1016/0167-7799(96)80920-6. [DOI] [PubMed] [Google Scholar]
- 70.Adams DH, Lloyd AR. Chemokines: leucocyte recruitment and activation cytokines. Lancet. 1997;349(9050):490–5. doi: 10.1016/s0140-6736(96)07524-1. [DOI] [PubMed] [Google Scholar]
- 71.Ghirnikar RS, Lee YL, Eng LF. Chemokine antagonist infusion promotes axonal sparing after spinal cord contusion injury in rat. J Neurosci Res. 2001;64(6):582–9. doi: 10.1002/jnr.1110. [DOI] [PubMed] [Google Scholar]
- 72.Ghirnikar RS, Lee YL, Eng LF. Chemokine antagonist infusion attenuates cellular infiltration following spinal cord contusion injury in rat. J Neurosci Res. 2000;59(1):63–73. [PubMed] [Google Scholar]
- 73.Ghirnikar RS, et al. Chemokine inhibition in rat stab wound brain injury using antisense oligodeoxynucleotides. Neurosci Lett. 1998;247(1):21–4. doi: 10.1016/s0304-3940(98)00268-7. [DOI] [PubMed] [Google Scholar]
- 74.Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004;1(1):80–100. doi: 10.1602/neurorx.1.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schwartz M, et al. The remedy may lie in ourselves: prospects for immune cell therapy in central nervous system protection and repair. J Mol Med. 1999;77(10):713–7. doi: 10.1007/s001099900047. [DOI] [PubMed] [Google Scholar]
- 76.Schwartz M, et al. Innate and adaptive immune responses can be beneficial for CNS repair. Trends Neurosci. 1999;22(7):295–9. doi: 10.1016/s0166-2236(99)01405-8. [DOI] [PubMed] [Google Scholar]
- 77.Pette M, et al. Myelin autoreactivity in multiple sclerosis: recognition of myelin basic protein in the context of HLA-DR2 products by T lymphocytes of multiple-sclerosis patients and healthy donors. Proc Natl Acad Sci U S A. 1990;87(20):7968–72. doi: 10.1073/pnas.87.20.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–87. doi: 10.1146/annurev.iy.10.040192.001101. [DOI] [PubMed] [Google Scholar]
- 79.Kil K, et al. T cell responses to myelin basic protein in patients with spinal cord injury and multiple sclerosis. J Neuroimmunol. 1999;98(2):201–7. doi: 10.1016/s0165-5728(99)00057-0. [DOI] [PubMed] [Google Scholar]
- 80.Wang WZ, et al. Myelin antigen reactive T cells in cerebrovascular diseases. Clin Exp Immunol. 1992;88(1):157–62. doi: 10.1111/j.1365-2249.1992.tb03056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Becker KJ, et al. Immunologic tolerance to myelin basic protein decreases stroke size after transient focal cerebral ischemia. Proc Natl Acad Sci U S A. 1997;94(20):10873–8. doi: 10.1073/pnas.94.20.10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jones TB, et al. Pathological CNS autoimmune disease triggered by traumatic spinal cord injury: implications for autoimmune vaccine therapy. J Neurosci. 2002;22(7):2690–700. doi: 10.1523/JNEUROSCI.22-07-02690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jones TB, et al. Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J Neurosci. 2004;24(15):3752–61. doi: 10.1523/JNEUROSCI.0406-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwartz M. Beneficial autoimmune T cells and posttraumatic neuroprotection. Ann N Y Acad Sci. 2000;917:341–7. doi: 10.1111/j.1749-6632.2000.tb05400.x. [DOI] [PubMed] [Google Scholar]
- 85.Huang DW, et al. A therapeutic vaccine approach to stimulate axon regeneration in the adult mammalian spinal cord. Neuron. 1999;24(3):639–47. doi: 10.1016/s0896-6273(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 86.Rapalino O, et al. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4(7):814–21. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- 87.Hauben E, et al. Passive or active immunization with myelin basic protein promotes recovery from spinal cord contusion. J Neurosci. 2000;20(17):6421–30. doi: 10.1523/JNEUROSCI.20-17-06421.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hauben E, et al. Posttraumatic therapeutic vaccination with modified myelin self-antigen prevents complete paralysis while avoiding autoimmune disease. J Clin Invest. 2001;108(4):591–9. doi: 10.1172/JCI12837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Moalem G, et al. Production of neurotrophins by activated T cells: implications for neuroprotective autoimmunity. J Autoimmun. 2000;15(3):331–45. doi: 10.1006/jaut.2000.0441. [DOI] [PubMed] [Google Scholar]
- 90.Moalem G, et al. Autoimmune T cells protect neurons from secondary degeneration after central nervous system axotomy. Nat Med. 1999;5(1):49–55. doi: 10.1038/4734. [DOI] [PubMed] [Google Scholar]
- 91.Noble LJ, Wrathall JR. Blood-spinal cord barrier disruption proximal to a spinal cord transection in the rat: time course and pathways associated with protein leakage. Exp Neurol. 1988;99(3):567–78. doi: 10.1016/0014-4886(88)90173-2. [DOI] [PubMed] [Google Scholar]
- 92.Mautes AE, et al. Vascular events after spinal cord injury: contribution to secondary pathogenesis. Phys Ther. 2000;80(7):673–87. [PubMed] [Google Scholar]
- 93.Casella GT, et al. New vascular tissue rapidly replaces neural parenchyma and vessels destroyed by a contusion injury to the rat spinal cord. Exp Neurol. 2002;173(1):63–76. doi: 10.1006/exnr.2001.7827. [DOI] [PubMed] [Google Scholar]
- 94.Whetstone WD, et al. Blood-spinal cord barrier after spinal cord injury: relation to revascularization and wound healing. J Neurosci Res. 2003;74(2):227–39. doi: 10.1002/jnr.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Loy DN, et al. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 2002;445(4):308–24. doi: 10.1002/cne.10168. [DOI] [PubMed] [Google Scholar]
- 96.Mu X, Azbill RD, Springer JE. Riluzole and methylprednisolone combined treatment improves functional recovery in traumatic spinal cord injury. J Neurotrauma. 2000;17(9):773–80. doi: 10.1089/neu.2000.17.773. [DOI] [PubMed] [Google Scholar]
- 97.Mallei A, et al. The nitrosteroid NCX 1015, a prednisolone derivative, improves recovery of function in rats after spinal cord injury. Brain Res. 2005;1062(1–2):16–25. doi: 10.1016/j.brainres.2005.08.057. Epub 2005 Nov 2. [DOI] [PubMed] [Google Scholar]
- 98.Weaver LC, et al. Methylprednisolone causes minimal improvement after spinal cord injury in rats, contrasting with benefits of an anti-integrin treatment. J Neurotrauma. 2005;22(12):1375–87. doi: 10.1089/neu.2005.22.1375. [DOI] [PubMed] [Google Scholar]
- 99.Bethea JR, et al. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 1999;16(10):851–63. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- 100.Jackson CA, et al. Enhanced functional recovery from spinal cord injury following intrathecal or intramuscular administration of poliovirus replicons encoding IL-10. Virology. 2005;336(2):173–83. doi: 10.1016/j.virol.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 101.Takami T, et al. Methylprednisolone and interleukin-10 reduce gray matter damage in the contused Fischer rat thoracic spinal cord but do not improve functional outcome. J Neurotrauma. 2002;19(5):653–66. doi: 10.1089/089771502753754118. [DOI] [PubMed] [Google Scholar]
- 102.Nesic O, et al. IL-1 receptor antagonist prevents apoptosis and caspase-3 activation after spinal cord injury. J Neurotrauma. 2001;18(9):947–56. doi: 10.1089/089771501750451857. [DOI] [PubMed] [Google Scholar]
- 103.Okada S, et al. Blockade of interleukin-6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. J Neurosci Res. 2004;76(2):265–76. doi: 10.1002/jnr.20044. [DOI] [PubMed] [Google Scholar]
- 104.Hains BC, Yucra JA, Hulsebosch CE. Reduction of pathological and behavioral deficits following spinal cord contusion injury with the selective cyclooxygenase-2 inhibitor NS-398. J Neurotrauma. 2001;18(4):409–23. doi: 10.1089/089771501750170994. [DOI] [PubMed] [Google Scholar]
- 105.Resnick DK, et al. Role of cyclooxygenase 2 in acute spinal cord injury. J Neurotrauma. 1998;15(12):1005–13. doi: 10.1089/neu.1998.15.1005. [DOI] [PubMed] [Google Scholar]
- 106.Yamamoto T, Nozaki-Taguchi N. Analysis of the effects of cyclooxygenase (COX)-1 and COX-2 in spinal nociceptive transmission using indomethacin, a non-selective COX inhibitor, and NS-398, a COX-2 selective inhibitor. Brain Res. 1996;739(1–2):104–10. doi: 10.1016/s0006-8993(96)00817-7. [DOI] [PubMed] [Google Scholar]
- 107.Demjen D, et al. Neutralization of CD95 ligand promotes regeneration and functional recovery after spinal cord injury. Nat Med. 2004;10(4):389–95. doi: 10.1038/nm1007. Epub 2004 Mar 7. [DOI] [PubMed] [Google Scholar]
- 108.Teng YD, et al. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci U S A. 2004;101(9):3071–6. doi: 10.1073/pnas.0306239101. Epub 2004 Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stirling DP, et al. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24(9):2182–90. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Glaser J, et al. Neutralization of the chemokine CXCL10 enhances tissue sparing and angiogenesis following spinal cord injury. J Neurosci Res. 2004;77(5):701–8. doi: 10.1002/jnr.20204. [DOI] [PubMed] [Google Scholar]
- 111.Taoka Y, et al. Role of neutrophil elastase in compression-induced spinal cord injury in rats. Brain Res. 1998;799(2):264–9. doi: 10.1016/s0006-8993(98)00459-4. [DOI] [PubMed] [Google Scholar]
- 112.Tonai T, et al. A neutrophil elastase inhibitor (ONO-5046) reduces neurologic damage after spinal cord injury in rats. J Neurochem. 2001;78(5):1064–72. doi: 10.1046/j.1471-4159.2001.00488.x. [DOI] [PubMed] [Google Scholar]
- 113.Taoka Y, et al. Reduction of spinal cord injury by administration of iloprost, a stable prostacyclin analog. J Neurosurg. 1997;86(6):1007–11. doi: 10.3171/jns.1997.86.6.1007. [DOI] [PubMed] [Google Scholar]
- 114.Taoka Y, et al. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 1997;79(4):1177–82. doi: 10.1016/s0306-4522(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 115.Lopez-Vales R, et al. FK 506 reduces tissue damage and prevents functional deficit after spinal cord injury in the rat. J Neurosci Res. 2005;81(6):827–36. doi: 10.1002/jnr.20605. [DOI] [PubMed] [Google Scholar]
- 116.Pearse DD, et al. Comparison of iNOS inhibition by antisense and pharmacological inhibitors after spinal cord injury. J Neuropathol Exp Neurol. 2003;62(11):1096–107. doi: 10.1093/jnen/62.11.1096. [DOI] [PubMed] [Google Scholar]


