Abstract
Background
It has been suggested that cognitive changes in response to T supplementation may occur within an ideal range. The objective of this study was to compare the cognitive responses of older, eugonadal men in whom moderate or large increases in serum testosterone levels was induced by exogenous testosterone supplementation.
Design
Randomized, double-blind, placebo-controlled study with subsequent grouping of participants according to average increase in circulating T from baseline.
Setting
Community dwelling participants.
Participants
Fifty-seven healthy, eugonadal, community dwelling male volunteers, mean age 67 years (± 11 years).
Interventions
Participants were randomized to receive weekly intramuscular (I.M.) injections of either 50, 100 or 300mg T enanthate or placebo (saline) injection for six weeks. Cognitive evaluations using a battery of neuropsychological tests were conducted at baseline, weeks 3 and 6 of treatment and after 6 weeks of wash-out.
Main Outcome Measures
Performance on cognitive tests of verbal and spatial memory.
Results
Men with moderate increases in serum T and/or its metabolites demonstrated significant improvements in verbal and spatial memory. In contrast, men with large or low increases in circulating T levels, failed to demonstrate significant changes in memory.
Conclusion
These results suggest that in healthy older men, beneficial changes in cognitive function induced by T supplementation are most evident with moderate increases in T levels. Large or no to low increases in T levels do not appear to appreciably effect cognition.
Keywords: Testosterone, Estradiol, Aging, Cognition, Human, Dose response
INTRODUCTION
Age-related decline in testosterone (T) is associated with several physiologic changes including decreased muscle mass and strength, osteoporosis and reduced sexual activity (Matsumoto 2002, Swerdloff & Wang 1993). The age-related decline in testosterone is also associated with a progressive decline in cognitive abilities. Several retrospective epidemiological studies have found declines in bioavailable or free testosterone to be associated with decrements in cognitive functioning (Barrett-Connor et al. 1999, Moffat et al. 2002, Yaffe et al. 2002).
Studies examining T supplementation in older men have found evidence for a beneficial effect on cognition (Cherrier et al. 2001, Cherrier et al. 2003, Cherrier et al. 2005, Gray et al. 2005, Janowsky et al. 2000, Janowsky et al. 1994, Kenny et al. 2002). In contrast, other studies have found no support for beneficial effects on cognition (Kenny et al. 2004, O'Connor et al. 2001, Sih et al. 1997, Wolf et al. 2000). It has been suggested that a positive relationship between testosterone and cognition may exhibit an inverted U shape dose-response, such that changes in cognition occur within a moderate dose range but not at high or low levels (Gouchie & Kimura 1991, Hampson & Moffat 1994, Hogervorst et al. 2005, Moffat & Hampson 1996, Muller et al. 2005, Shute et al. 1983). In the present study, we directly examined the role of dose effects of T supplementation on cognition in a sample of healthy older men.
METHODS
Participants
Participants were healthy older men between the ages of 50 and 90 mean age 67 years (± 11 years) recruited from the community through flyers. The study protocol was approved by the University of Washington Institutional Review Board and approved informed consent procedures were followed. Participants underwent a screening visit to determine eligibility including a physical exam, psychiatric and laboratory evaluation to exclude any significant physical or medical illness. This included tests of liver function, hypertension, and prostate disease (prostate specific antigen (PSA) level, and digital rectal exam (DRE)). Participants with abnormal findings were excluded from the study. Participants also underwent a cognitive screening examination consisting of the Mattis Dementia Rating Scale (DRS) to ensure that participants were within the normal range for their age (Folstein et al. 1975, Mattis 1988). Participants with scores at or below the recommended cut-off score (DRS 130 or lower) were excluded from the study. Participants with a history of significant alcohol abuse, psychiatric illness, head injury with loss of consciousness greater than 1 hour, or who were taking lupron, finasteride, spironolactone or cimetidine, testosterone or dutasteride were excluded. Participants with previous or current prostate cancer, elevated PSA levels, history of myocardial infarction, abnormal renal or hepatic disease, sleep apnea, previous testosterone or other androgen treatment, or other gonadal endocrine disorders were also excluded.
Procedures
Study Design
Participants who were not excluded from screening criteria, were randomly assigned to one of four treatment groups. Assignment for each consecutively enrolled participant was made by research pharmacists using a pre-determined assignment sheet that was created using a random number generator. Study personnel, investigators and subjects were blind to treatment condition.
Fifty-seven healthy older men who met screening criteria reported to the University of Washington General Clinical Research Unit or the Clinical Research Unit of the Veterans Administration Puget Sound Health Care System (VAPSHCS) and received weekly intramuscular (I.M.) gluteal injections of 50,100 or 300 mg testosterone enanthate (Delatestryl, Manufactured for BTG Pharmaceuticals Corporation by Bristol-Myers Squibb, Princeton, NJ 08543) or placebo (saline) for six weeks. Cognitive testing was conducted at baseline and repeated at weeks 3 and 6 of treatment and again after six weeks of no medication (washout). Blood samples were taken to measure serum testosterone and estradiol levels by IFMA and RIA (see below) Blood sampling and testing sessions occurred within 24-48 hours following testosterone or placebo injection to capture peak testosterone levels. Therefore, cognitive performance results reflect the effects of peak testosterone levels. Endogenous testosterone levels measured at baseline, prior to the start of the study were in the slightly low to normal range (5.4 - 34 nmol/L) and were not significantly different between groups. PSA and hematocrit levels were measured at screening, week 4 of treatment and again at washout. Mean PSA level of 1.54 (±1.2) ng/ml for all participants at the start of the study was within normal limits (0-4 ng/ml). DRE was also conducted at screening and at the washout visit with no abnormal findings.
Neuropsychological Test Measures
The cognitive test battery included a measure of verbal and spatial memory. To help control for practice and learning effects, randomized, comparable versions of each test were administered at each time point and participants underwent an identical pre-baseline testing session prior to the actual baseline session. Psychometrists, participants and investigators were blind to the treatment condition.
Puget Sound Route Learning Test
This test measured the ability to navigate a short route within a room (Tiernan et al. 2004). The task used a 6'×24' piece of black flooring on which a diamond pattern was placed using bright yellow tape. A particular route was indicated using a bright red ribbon and the subject walked the route as shown. The ribbon was removed, and the subject was asked to immediately retrace the route. Three trials were administered followed by three trials of a new route using pictures placed on the floor as landmarks. A delayed recall is administered after twenty minutes. Performance was assessed by calculating the number of correct sequential units recalled summed across all landmark trials.
Word List Recall (Word List)
Participants listened to a list of 12 words from the same semantic category (e.g., articles of clothing), and then recalled as many of these words as possible. The task was adapted from a previous task and allows for examination of both memory and interference effects (Moscovitch 1994). The procedure was repeated for a total of 4 trials, each containing different words drawn from the same semantic category. For the fifth trial 12 words from a new semantic category (e.g., types of furniture) were read and participants were asked to recall these words. The total number of words recalled correctly on each trial was recorded. Two aspects of the test were used in the analysis, total words recalled for last half of the first four lists was used as a measure of memory and list one minus list four as a measure of interference effects. Reliability of the test is generally good, including validity studies conducted with brain injured patients and controls (Lezak 1995).
Hormone Assays
Blood samples were drawn within 48 hours of injection to capture a peak blood level at the time of cognitive testing. Samples were kept frozen in a −70 C degree freezer until the completion of the study when all samples were included in the assays and processed by Dr. Bremner's laboratory at the University of Washington. Serum estradiol and total testosterone levels were analyzed with radioimmunoassay (RIA) and ImmunoFluorimetric assay (IFMA) respectively, according to standard procedures for each commercial kit. Although studies examining the relationship between endogenous testosterone levels and cognition have frequently used measures of free testosterone, we chose to measure total testosterone as this has been shown previously to be a sensitive and valid measure for hormone manipulation studies. Serum total testosterone was measured using the DELFIA IFMA kit (Wallac OY, Turku, Finland) with sensitivity of 0.5 nmol/L and 4.5% intra assay co-efficient of variation (CV) for a mid-range pool of samples. The normal range for the assay in Dr. Bremner's laboratory is 7.6 – 22 nmol/L. Serum estradiol was measured by 3rd Generation Estradiol RIA Kit (DSL-39100), from Diagnostic Systems Laboratory, in Webster, Texas with sensitivity of 5.5 pmol/L and intra and inter-assay CVs for midrange measurements were 3.6 and 6.0%. The normal range for the assay is 76 - 353 pmol/L. Samples from each participant were run in duplicate in the same assay to avoid inter-assay variability. Hormone levels reported were the average of the duplicate samples.
Statistical Analyses
A mixed model ANOVA with original group assignment (placebo, 50, 100, 300mg) as the between factor and total testosterone levels at baseline, treatment (weeks 3 and 6 averaged) and washout as the repeated factor revealed a significant increase in T levels in the 100mg group (F (8, 31) = 5.19, p<.01) and 300mg group (F (8, 31) = 22.14, p<.01) compared to baseline and compared to placebo (F (3, 38) = 11.6, p<.01). Neither the placebo group nor the 50mg group demonstrated a significant change in T levels compared to baseline and the 50mg group did not have a significant difference in T levels compared to placebo. Due to this lack of increase in the 50mg group and variability with regard to T levels with some participants in the 50 mg group demonstrating a small increase from baseline with others demonstrating no increase, participants were divided according to actual changes in serum T level from baseline. Baseline T levels were subtracted from the average of serum T at weeks 3 and 6 to calculate the average increase in serum T concentration achieved during treatment. Subjects were categorized as having no increase (change in serum T of 0 - 10 nmol/L, N= 22), a moderate increase (change in serum T of 11 - 50 nmol/L, N=22), or a large increase (change serum T of > 51 nmol/L, N=13) in serum T level during treatment. These cut-off values for the groups were determined by using the mean plus one standard deviation for serum T change scores for the placebo group (value of 10) and the 100mg group (value of 50). The no-change group was comprised of 18 placebo and four 50mg subjects, the moderate-change group was comprised of seventeen 100mg, two 300mg, three 50mg and the high change group was comprised of eleven 300mg and two 100mg subjects. Although tertiles or quartiles is a method that is typically used with studies of endogenous T levels, this method was not optimal for the present study as tertiles and quartiles resulted groups containing both placebo participants along with participants with significant changes in T levels from baseline. Confirmation of our method for group assignment was provided by a regression analysis using age, baseline T levels, original assigned condition, tertile and quartile group membership and group membership using our informed method described above as predictor variables and change from baseline in serum T levels as the dependent variable. Only our revised group membership variable was a significant predictor of serum T change t=2.89, p<.01, ß=.642.
A repeated measures, Multivariate Analysis of Variance (MANOVA) was used with group as the independent factor (moderate, large or no increase in T); weeks (baseline, treatment, washout) as the repeated factor; and spatial memory (Route Test,) and verbal memory (word list total and interference) as the dependent measures. Planned comparisons of treatment compared to baseline were performed and post hoc comparisons were subjected to Bonferroni correction. A MANOVA with the original group assignments (placebo, 50mg, 100mg and 300mg) as the group factor with weeks (baseline, treatment, washout) as the repeated factor; and spatial memory (Route Test,) and verbal memory (word list) as dependent measures was also conducted. A separate repeated measure MANOVA with groups as defined above and T, estradiol, hematocrit and PSA (baseline, week 4 and week 12) as dependent measures with post hoc comparisons subject to Bonferroni correction were performed to determine changes in serum hormone levels and safety measures. Cognitive test scores, serum T and estradiol values for weeks 3 and 6 were averaged to create a single on treatment value. We did not expect large differences between weeks 3 and 6 of treatment for these variables, and a statistical examination of changes between week 3 and 6 confirmed this.
A curve fit regression analysis was also performed to assess the linear and quadratic relationships between T, estradiol and cognitive performance changes. A regression analysis with age, education, change in T and estradiol as predictor variables and change verbal or spatial memory performance as the dependent variable was conducted to examine strength of association and predictive power for these variables.
RESULTS
Sixty five men gave informed consent and were screened for the study. Five participants evidenced an elevated PSA, one participant evidenced an elevated calcium level, two subjects declined to continue after screening visit due to scheduling or other conflicts. Fifty-seven men with a mean age of 67 ± 11 years (range 50 – 85) met screening criteria and were randomized. Mean education level was 16 years ± 2 years (range 12 – 19) and mean DRS score was 140 points ± 3 points (range 137 – 144). There were no significant differences between the groups for age, education, or DRS.
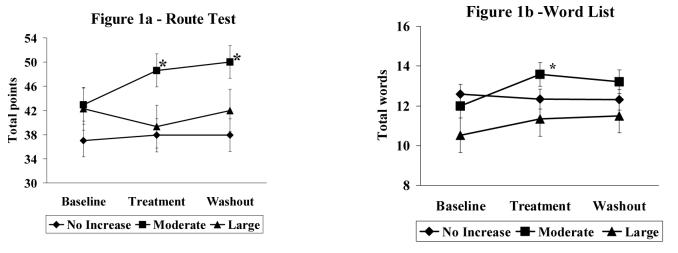
MANOVA with (no change, moderate and high change groups) revealed an overall time by condition interaction (F (8, 216) = 2.14, p<.05, partial eta squared =.08). Men who had a moderate increase in serum T demonstrated a significantly better performance for both verbal and spatial memory during treatment compared to the other groups (no change and large change groups) and compared to baseline. This was due to a significant change over time in the moderate group only (F (4, 51) = 5.81, p<.01, partial eta squared=.32) due to an increase (p<.05) during treatment and washout compared to baseline for total points on the Route Test (Figure 1a) and an increase (p<.05) during treatment compared to baseline for total words on the Word List test (Figure 1b). The large and no increase groups did not demonstrate a significant change from baseline for verbal or spatial memory. A significant between group difference was also evident with the moderate increase group out-performing the no increase group during treatment for both the Route Test (spatial memory) (F (2, 54) = 6.05, p<.01, partial eta squared=.18) (Figure 1a) and Word List test (verbal memory) (F (2, 54) = 2.98, p<.05, partial eta squared=.09) (Figure 1b). Interference effects (word list one minus four) did not demonstrate a significant change from baseline or interaction effect for any groups. MANOVA with original groups (placebo, 50, 1000 and 300mg) revealed a significant change over time for the 300mg (F (4, 50) = 2.94, p<.05, partial eta squared=.19) and 100mg (F (4, 50) = 2.55, p<.05, partial eta squared=.17) groups due to a significantly better performance on the route test for the 100mg group during treatment compared to baseline (p<.05) and better performance on the word list recall during treatment compared to baseline in the 300mg group (p<.05).
Figure 1a.
Mean total points correct on the Route Test, a measure of spatial and navigational memory. Figure 1b. Mean total points correct on Word List recall, a measure of verbal memory. Diamond represents men who demonstrated no change in serum T (0-10 nmol/L) during treatment. Square represents men who demonstrated a moderate increase in serum T (11-50 nmol/L) during treatment. Triangle represents men who demonstrated a large increase in serum T (> 50 nmol/L) during treatment. Error bars represent standard error of measurement. Men with a moderate increase in serum T levels evidenced a significant improvement on the Route test during treatment and at washout compared to baseline (*p<.05) and compared to men who had no increase or a large increase in serum T levels (*p<.05) (1a). Men with a moderate increase in serum T levels during treatment also demonstrated a significant improvement on the Word List test compared to baseline (*p<.05) (1b). There was no significant change from baseline for Route Test or Word list in the no or large increase groups.
T and estradiol levels increased over time in the moderate (F (8, 32) = 5.4, p<.01) and large (F (8, 32) = 24.9, p<.01) change groups. There was no significant change in T and estradiol levels from baseline in the no-change group. There was a significant interaction effect (F (16, 66) = 5.13, p<.01) due to the increases in T and estradiol in the moderate and large increase groups with no change in the no-increase group. Between group differences were also evident during treatment for T during treatment (F (2, 39) = 32.12, p<.01) which was due to higher T levels in the moderate and large increase groups compared to no-increase group (p<.01) and for estradiol (F (2, 39) = 19.74, p<.01) which was due to higher estradiol levels in the moderate and large increase groups compared to no-increase group (p<.01). PSA levels did not increase significantly at week 4 or washout compared to baseline. Hematocrit increased significantly from baseline in the moderate and high groups (p<.01). (See Table 1).
Table 1.
Hormone, PSA, Hematocrit and Cognitive Test Results. Means, Standard Deviations and Range at baseline, during treatment and washout. Mean total testosterone (nmol/L) levels at peak period (24-48) hours following injection, normal range = 10-30 nmol/L; Mean total estradiol (pmol/L) levels at peak period (24-48) hours following T injection, normal range = 76 – 353 pmol/L; Mean PSA- prostate specific antigen (ng/mL); Mean Hematocrit (percent of total); Route Test mean number of points correct (66 possible); Word List mean number of words recalled (24 possible). Asterisks denote significant increases in testosterone and estradiol for the moderate and large increase groups compared to baseline and compared to the no increase group, a significant increase in hematocrit for the moderate and large groups compared to baseline, and a significant increase in total points on the Route test and total words on the Word List test in the moderate group compared to baseline
| Group | Baseline | Treatment | Washout | p | |
|---|---|---|---|---|---|
| Testosterone | No Increase | 12.7 (3.8) 7.2 - 24.0 | 15.1 (5.2) 8.8 – 27.5 | 13.6 (5.1) 7.3 – 25.0 | |
| nmol/L | Moderate | 14.6 (6.9) 6.8 – 34.0 | 41.1** (15.4) 22.5 – 59.5 | 11.3 (3.9) 5.4 – 18.1 | <.01 |
| Large | 12.3 (3.8) 5.4 – 27.2 | 109.4** (58.4) 60.0 – 273.0 | 12.3 (4.1) 8.4 – 19.0 | <.01 | |
| Estradiol | No Increase | 146.4 (60.4) 66.0 – 265.0 | 164.9 (59.3) 63.0 – 276.5 | 154.1 (61.3) 64.0 – 303. 0 | |
| pmol/L | Moderate | 161.2 (54.6) 100.0 – 295. 0 | 312.4** (169.9) 76.5 – 661.5 | 160.9 (52.7) 65.0 – 281.0 | <.01 |
| Large | 136.9 (33.7) 94.0 – 195.0 | 507.7** (174.7) 288.0 - 837.5 | 135.9 (44.5) 96.0 – 261.0 | <.01 | |
| Hematocrit | No Increase | 42.8 (3.5) 36.5 - 49.2 | 43.9 (2.8) 39.0 – 48.0 | 44.4 (3.4) 37.0 – 49.0 | |
| % | Moderate | 43.2 (4.1) 36.4 – 49.4 | 45.3** (4.3) 38.0 – 51.0 | 44.0 (5.2) 36.0 – 51.0 | <.01 |
| Large | 43.5 (4.1) 36.0 – 51.0 | 45.8** (4.4) 38.0 – 54.0 | 45.6** (4.3) 38.0 – 51.0 | <.01 | |
| PSA | No Increase | 1.5 (1.1) 0.30 – 3.50 | 1.4 (1.0) 0.30 – 3.40 | 1.5 (1.3) 0.30 – 5.30 | |
| ng/mL | Moderate | 1.5 (1.2) 0.30 – 5.60 | 1.5 (1.2) 0.60 – 8.60 | 1.5 (1.2) 0.30 – 6.50 | |
| Large | 2.0 (1.2) 0.40 – 3.70 | 2.1 (1.1) 0.50 – 3.50 | 2.0 (1.2) 0.30 – 4.10 | ||
| Route Test | No Increase | 37.0 (12.8) 10.0 – 54.0 | 38.1 (10.7) 16.0 – 54.5 | 40.1 (14.7) 12.0 –65.0 | |
| Total Points | Moderate | 42.9 (12.1) 16.0 – 66.0 | 49.0* (10.6) 23.5 – 61.5 | 53.4* (9.4) 31.0 – 66.0 | <.05 |
| Large | 42.3 (13.4) 30.0 – 61.0 | 37.6 (11.3) 19.5 – 59.0 | 42.5 (14.9) 34.0 – 60.0 | ||
| Word List | No Increase | 12.5 (3.5) 5.0 – 18.0 | 12.3 (3.3) 6.0 – 18.5 | 12.3 (3.7) 8.0 – 18.0 | |
| Total words | Moderate | 12.0 (2.6) 5.0 – 15.0 | 13.5* (2.1) 10.0 – 18.0 | 13.2 (2.6) 6.5 – 19.0 | <.05 |
| Large | 10.1 (3.1) 4.5 – 13.0 | 11.1 (1.6) 8.0 – 14.0 | 11.7 (4.0) 4.0 – 17.0 | ||
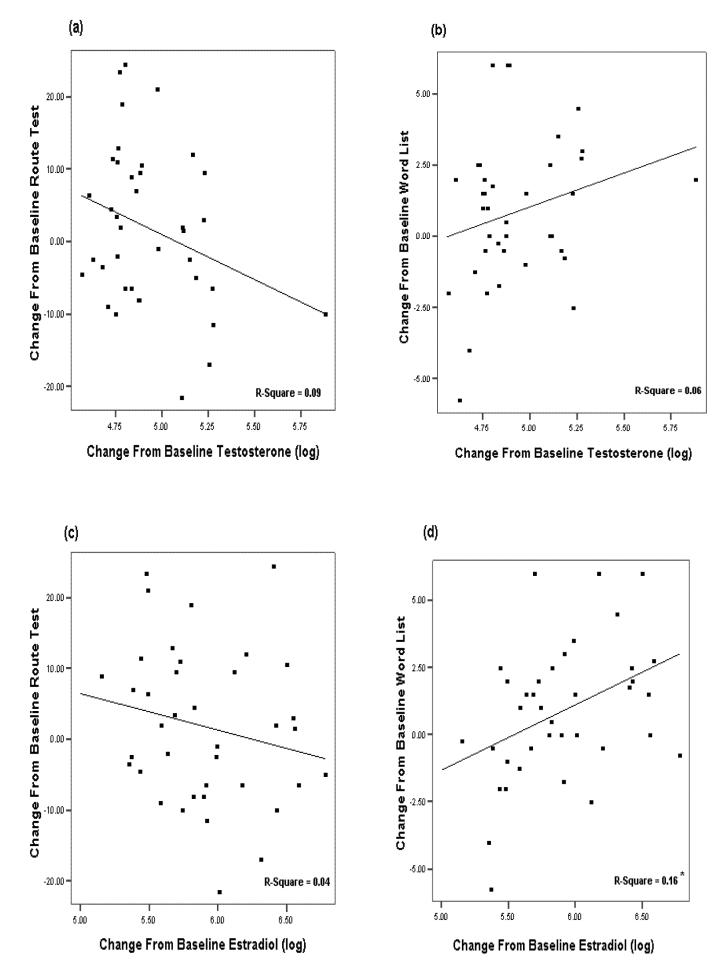
Regression analyses revealed estradiol as a significant predictor of verbal memory (F (4, 37)=2.893, p<.05, ß=.481) with age, education, baseline T and change in T as non-significant predictors. Regression analysis with change in spatial memory did not indicate a significant predictor amongst age, education, baseline T, change in T or estradiol. A curve-fit analysis revealed significant linear F(1,36) = 6.7, p<.01, multiple R = .397 and quadratic (slight inverted U shape) F(2,35) = 5.14, p<.01, multiple R = .476 associations between change scores for Word List recall and estradiol (log transformed) ß=.397-linear and ß=8.1 quadratic, and a trend linear association (p<.07) between change scores for Route Test and testosterone (log transformed). Scatter plots with best fit linear regression lines are shown in Figure 2 (a-d). Change scores for cognitive tests were calculated by subtracting baseline scores from on treatment average (week 3 and 6).
Figure 2.
(a-d). Scatter- plots and best fit regression lines depicting the relationship between the change in testosterone from baseline (log transformed) for the top two figures and change from baseline performance on (a) Route test (top left) and (b) Word list (top right). For the bottom two figures, change in estradiol from baseline (log transformed) and change from baseline performance on (c) Route test (bottom left) and (d) Word list (bottom right). Asterisk indicates significant linear regression line F(1,36) = 6.7, p<.01, multiple R = .397.
DISCUSSION
In this study of brief T supplementation, improvements in verbal and spatial memory were evident in men who demonstrated moderate increases in T levels during exogenous T administration. In men with moderate increases, T levels were raised to the normal to high-normal range for young men. Significant changes in cognition were not observed for the groups with large or no to low increases in T levels,. Serum T levels in the large increase group were raised into the supraphysiological range (Table 1).
Studies of endogenous testosterone and cognition in young men and women have reported better performance in men with low compared to high T levels on spatial tasks such as paper folding, mental rotation and mathematical reasoning and in women with high compared to low T levels on these same tasks (Gouchie & Kimura 1991, Moffat & Hampson 1996). These previous studies along with another previous report of a significant quadratic association between endogenous T levels and spatial abilities, in the shape of an inverted U, have led many to suggest that there is a non-linear dose-response relationship between T and cognitive abilities (Gouchie & Kimura 1991, Hampson & Moffat 1994, Moffat & Hampson 1996, Muller et al. 2005, Shute et al. 1983). The inverted U-shaped relationship is described as optimal performance occurring within a specified mid-range of T, but not at higher or lower levels.
Our results indicated a significant quadratic relationship between Word List recall and change in estradiol levels, the linear relationship between these variables was also strong and only the linear relationship between Route test and testosterone approached significance. Thus, we did not find robust support for a quadratic or inverted U shaped relationship between change in cognitive performance and change in T or estradiol levels. It is possible that previous studies have found an inverted U shaped relationship due to the use of younger study samples of combined men and women (Gouchie & Kimura 1991, Moffat & Hampson 1996). Also it is important to consider the absolute hormone levels as these previous studies suggesting an inverted U relationship used only endogenous levels which are no where near the range of T levels in the present study achieved through supplementation. The range of testosterone levels in these previous study samples is somewhat bi-modal with the endogenous T levels of women clustering in the lower range and the T levels of the healthy young men clustering in the upper range. In addition, many previous studies have examined endogenous hormone levels and cognitive performance at one point in time. In contrast, the present study examined change scores of T and estradiol in relationship to the change in cognitive performance in a sample of healthy older men.
Studies of young, eugonadal men who are given exogenous T supplementation, thus raising their T levels to the supraphysiologic range have been mixed with reports of improved verbal fluency and selective attention (Cherrier et al. 2002, O'Connor et al. 2001), decreased spatial reasoning (O'Connor et al. 2001) or no change (Alexander et al. 1998, Bhasin et al. 2001). In contrast, studies of T supplementation in young hypogonadal men have reported improved verbal fluency (Alexander et al. 1998), and spatial and verbal memory (Cherrier et al. 2003). Studies of older men, who may have lower T levels than young men have reported improved spatial reasoning (Cherrier et al. 2001, Janowsky et al. 1994), working memory (Janowsky et al. 2000), spatial and verbal memory (Cherrier et al. 2001, Cherrier et al. 2005) or no change (Haren et al. 2005, Kenny et al. 2002). Discrepant findings from studies that utilize hypogonadal versus eugonadal and young versus old subjects may be due to the large variation in the end hormonal concentrations during treatment. T supplementation in hypogonadal men generally results in a eugonadal state whereas T supplementation in healthy men may result in a supraphysiological state. Also, the slow and progressive decline in hormone levels in older adults may make some target organs such as the brain more responsive to hormone supplementation in comparison to a young adult population. Gradually declining serum T levels over time are associated with cognitive deficits in men, independent of education and health status (Moffat et al. 2002).
Our results represent one of the earliest studies to characterize cognitive response to variable increases in serum T levels in a sample of healthy older men. Our results suggest that moderate increases in T levels and/or its metabolites results in beneficial effects on verbal and spatial memory. Larger or supraphysiological increases in T levels did not result in further improvements in cognition. These results have implications for future studies of T supplementation in older hypogonadal men and men with existing cognitive difficulties such as mild cognitive impairment or Alzheimer's disease. Use of a T dosage that produces moderate increases in circulating T levels into the physiological or modest supraphysiological range may also help to reduce potential side effects such as polycythemia (Basaria & Dobs 1999). Overall, adverse effects of T supplementation, for this brief treatment period were minimal. Hematocrit increased significantly during treatment in both men who demonstrated moderate and large increases in serum T. PSA did not change significantly in any group. Both PSA and hematocrit should always be included in studies or clinical applications of T supplementation as safety measures, and increases for either or both would suggest strong consideration for stopping treatment.
Although findings of improved verbal and spatial memory in men who had moderate increases in T levels induced by T treatment is promising, these results will need to be replicated in a larger sample size. Our study examined healthy older men, at the period of peak testosterone levels, and who had a relatively short period of treatment. Thus, it is possible that longer-term T supplementation in a group of older hypogonadal men may result in even greater cognitive improvements. Use of a T formulation with more stable pharmacokinetics such as gel formulations may result in greater cognitive improvements as the serum T level would be elevated or stable for a longer period of time. As recommended by a recent Institute of Medicine report, both short- and long-term studies are needed to assess the overall physiological and clinical consequences of testosterone supplementation on safety and biological outcome variables as well as cognitive function, quality of life and other clinical outcomes (Liverman & Blazer 2004).
Acknowledgements
This research was supported in part by NIA award #K01AG00858, American Federation for Aging Research, and Veterans Administration Puget Sound Health Care System. Serum T and E samples were processed in Dr. William Bremner's laboratory at University of Washington. A portion of this work was conducted through the General Clinical Research Center at the University of Washington and supported by the National Institutes of Health, grant M01RR-00037.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GM, Swerdloff RS, Wang C, Davidson T, McDonald V, Steiner B, Hines M. Androgen-behavior correlations in hypogonadal men and eugonadal men. II. Cognitive abilities. Horm Behav. 1998;33(2):85–94. doi: 10.1006/hbeh.1998.1439. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Goodman-Gruen D, Patay B. Endogenous sex hormones and cognitive function in older men. J Clin Endocrinol Metab. 1999;84(10):3681–5. doi: 10.1210/jcem.84.10.6086. [DOI] [PubMed] [Google Scholar]
- Basaria S, Dobs AS. Risks versus benefits of testosterone therapy in elderly men. Drugs Aging. 1999;15(2):131–42. doi: 10.2165/00002512-199915020-00006. [DOI] [PubMed] [Google Scholar]
- Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281(6):E1172–81. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Anawalt BD, Herbst JK, Amory JK, Craft S, Matsumoto A, Bremner WJ. Cognitive Effects of Short Term Manipulation of Serum Sex Steroids in Healthy Young Men. Journal of Clinical Endocrinology and Metabolism. 2002;87:3090–3096. doi: 10.1210/jcem.87.7.8570. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Asthana S, Baker LD, Plymate S, Matsumoto A, Peskind E, Raskind MA, Brodkin K, Bremner W, Petrova A, Latendresse S, Craft S. Testosterone supplementation improves spatial and verbal memory in healthy older men. Neurology. 2001;57:80–88. doi: 10.1212/wnl.57.1.80. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Craft S, Matsumoto AH. Cognitive changes associated with supplementation of testosterone or dihydrotestosterone in mildly hypogonadal men: a preliminary report. J Androl. 2003;24(4):568–76. doi: 10.1002/j.1939-4640.2003.tb02708.x. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Ahmed S, Bremner W, Peskind ER, Raskind MA, Johnson M, Craft S. The Role of Aromatization in Testosterone Supplementation: Effects on Cognition in Older Men. Neurology. 2005;64:290–296. doi: 10.1212/01.WNL.0000149639.25136.CA. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gouchie C, Kimura D. The relationship between testosterone levels and cognitive ability patterns. Psychoneuroendocrinology. 1991;16(4):323–334. doi: 10.1016/0306-4530(91)90018-o. [DOI] [PubMed] [Google Scholar]
- Gray PB, Singh AB, Woodhouse LJ, Storer TW, Casaburi R, Dzekov J, Dzekov C, Sinha-Hikim I, Bhasin S. Dose-Dependent Effects of Testosterone on Sexual Function, Mood and Visuospatial Cognition in Older Men. J Clin Endocrinol Metab. 2005 doi: 10.1210/jc.2005-0247. [DOI] [PubMed] [Google Scholar]
- Hampson E, Moffat SD. Is Testosterone Related to Spatial Cognition and Hand Preference in Humans? Brain and Cognition. 1994;26:255–266. doi: 10.1006/brcg.1994.1060. [DOI] [PubMed] [Google Scholar]
- Haren MT, Wittert GA, Chapman IM, Coates P, Morley JE. Effect of oral testosterone undecanoate on visuospatial cognition, mood and quality of life in elderly men with low-normal gonadal status. Maturitas. 2005;50(2):124–33. doi: 10.1016/j.maturitas.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Hogervorst E, Bandelow S, Moffat SD. Increasing Testosterone Levels and Effects on Cognitive Functions in Elderly Men and Women: A Review. Current Drug Targets- CNS & Neurological Disorders. 2005;4:531–540. doi: 10.2174/156800705774322049. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Chavez B, Orowoll E. Sex steroids modify working memory. Journal of Cognitive Neuroscience. 2000;12(3):407–414. doi: 10.1162/089892900562228. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES. Testosterone influences spatial cognition in older men. Behavioral Neuroscience. 1994;108(2):325–332. doi: 10.1037//0735-7044.108.2.325. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Bellantonio S, Gruman CA, Acosta RD, Prestwood KM. Effects of transdermal testosterone on cognitive function and health perception in older men with low bioavailable testosterone levels. J Gerontol A Biol Sci Med Sci. 2002;57(5):M321–5. doi: 10.1093/gerona/57.5.m321. [DOI] [PubMed] [Google Scholar]
- Kenny AM, Fabregas G, Song C, Biskup B, Bellantonio S. Effects of testosterone on behavior, depression, and cognitive function in older men with mild cognitive loss. J Gerontol A Biol Sci Med Sci. 2004;59(1):75–8. doi: 10.1093/gerona/59.1.m75. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychologcial Assessment. Third ed. Oxford University Press; 1995. [Google Scholar]
- Liverman CT, Blazer DG. Committee on Assessing the Need for Clinical Trials of Testosterone Replacement Therapy. National Academies Press; 2004. Testosterone and Aging: Clinical Research Directions. [PubMed] [Google Scholar]
- Matsumoto AM. Andropause: clinical implications of the decline in serum testosterone levels with aging in men. J Gerontol A Biol Sci Med Sci. 2002;57(2):M76–99. doi: 10.1093/gerona/57.2.m76. [DOI] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale. Psychological Assessment Resources, Inc.; 1988. [Google Scholar]
- Moffat SD, Hampson E. A curvilinear relationship between testosterone and spatial cognition in humans: possible influence of hand preference. Psychoneuroendocrinology. 1996;21(3):323–337. doi: 10.1016/0306-4530(95)00051-8. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Metter EJ, Blackman MR, Harman SM, Resnick SM. Longitudinal assessment of serum free testosterone concentration predicts memory performance and cognitive status in elderly men. J Clin Endocrinol Metab. 2002;87(11):5001–7. doi: 10.1210/jc.2002-020419. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Cognitive resources and dual-task interference effects on retrieval in normal people: The role of the frontal lobes and medial temporal cortex. Neuropsychology. 1994;8:524–534. [Google Scholar]
- Muller M, Aleman A, Grobbee DE, de Haan EH, van der Schouw YT. Endogenous sex hormone levels and cognitive function in aging men. Neurology. 2005;64:866–871. doi: 10.1212/01.WNL.0000153072.54068.E3. [DOI] [PubMed] [Google Scholar]
- O'Connor DB, Archer J, Hair WM, Wu FC. Activational effects of testosterone on cognitive function in men. Neuropsychologia. 2001;39(13):1385–94. doi: 10.1016/s0028-3932(01)00067-7. [DOI] [PubMed] [Google Scholar]
- Shute VJ, Pellegrino JW, Hubert L, Reynolds RW. The relationship between androgen levels and human spatial abilities. Bulletin of the psychonomic society. 1983;21:465–468. [Google Scholar]
- Sih R, Morley JE, Kaiser FE, Perry HM, Patrick P, Ross C. Testosterone replacement in older hypogonadal men: A 12 month randomized controlled trial. Journal of Clinical Endocrinology and Metabolism. 1997;82(6):1661–1667. doi: 10.1210/jcem.82.6.3988. [DOI] [PubMed] [Google Scholar]
- Swerdloff RS, Wang C. Androgens and aging men. Experimental Gerontology. 1993;28:435–446. doi: 10.1016/0531-5565(93)90069-p. [DOI] [PubMed] [Google Scholar]
- Tiernan K, Schenk K, Shimonova M, Schollaert D, Swadberg D, Boorkman P, Cherrier MM. An Examination of the Validity and Reliability of a Novel Route Test in Healthy Older Adults and AD Patients. The Clinical Neuropsychologist. 2004;8(1):39–42. [Google Scholar]
- Wolf O, Pruet R, Hellhammer DH, Kudielka BM, Schurmeyer TH, Kirschbaum C. Testosterone and Cognition in Elderly Men: A single testosterone injection blocks the practice effect in verbal fluency, but has no effect on spatial or verbal memory. Biological Psychiatry. 2000;47:650–654. doi: 10.1016/s0006-3223(99)00145-6. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Lui LY, Zmuda J, Cauley J. Sex hormones and cognitive function in older men. J Am Geriatr Soc. 2002;50(4):707–12. doi: 10.1046/j.1532-5415.2002.50166.x. [DOI] [PubMed] [Google Scholar]