Abstract
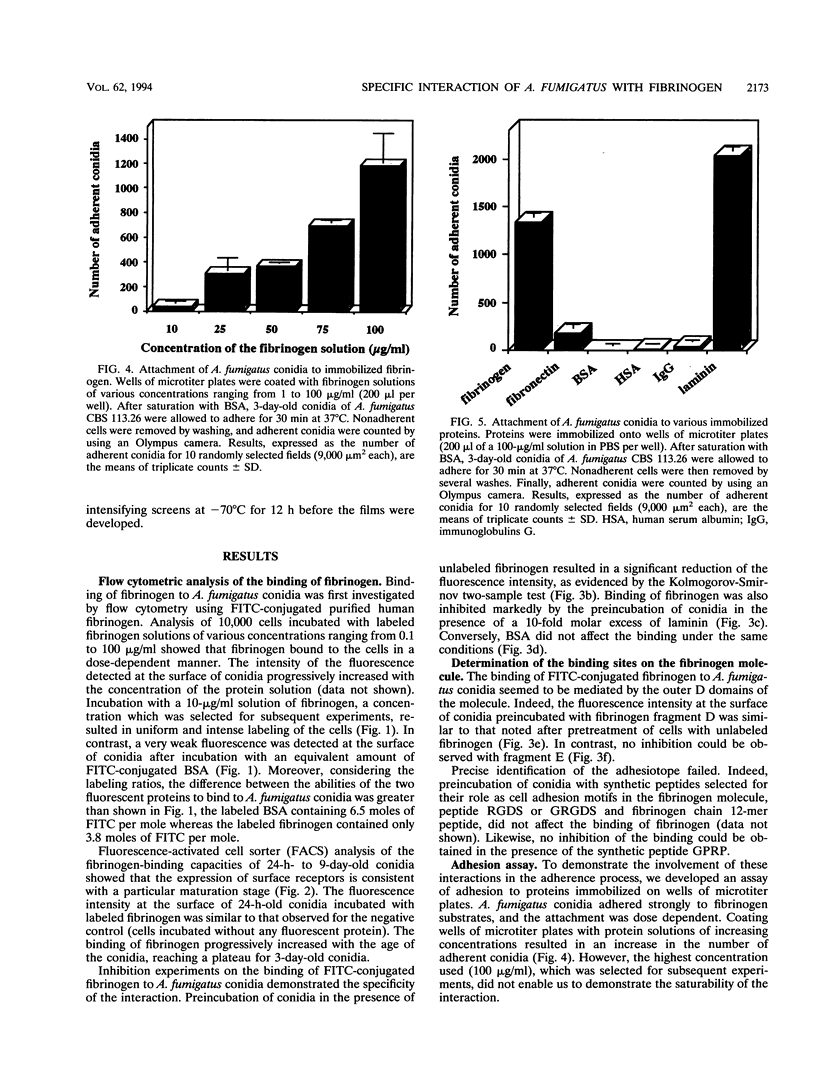
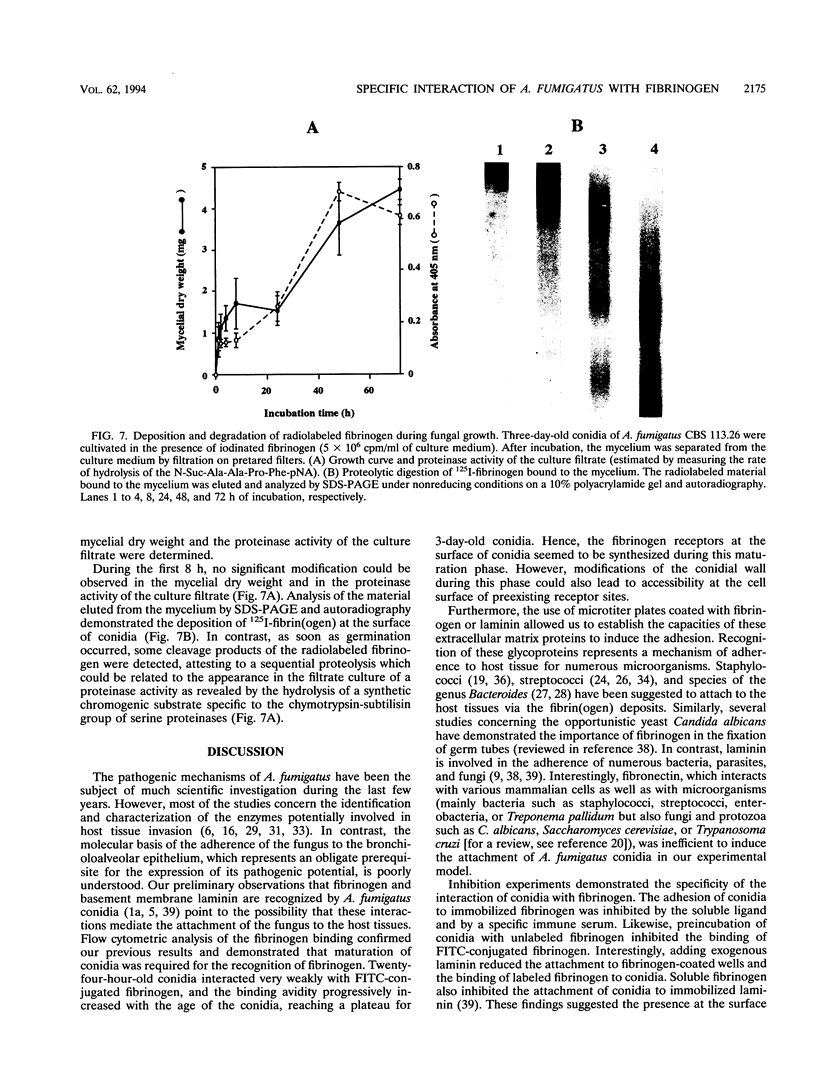
Interaction between Aspergillus fumigatus conidia and different proteins known to mediate the attachment of malignant tumor cells or microorganisms to the host tissues was studied in vitro. Flow cytometry using fluorescein isothiocyanate-conjugated fibrinogen confirmed that binding of human fibrinogen to the conidia was dose dependent and specific. Binding was inhibited by unlabeled fibrinogen and by basement membrane laminin. Moreover, the expression of fibrinogen receptors at the surfaces of conidia seemed to be related to the maturation of the conidia. Binding sites appeared to be located in the D domains of the fibrinogen molecule. However, the peptide sequence recognized by the fungus could not be identified but was different from the classical adhesive recognition sequences, RGDS and fibrinogen gamma-chain dodecapeptide. In addition, an assay of adherence to proteins immobilized onto microtiter plates allowed us to establish the role of these interactions in fungal adhesion. Conidia strongly adhered to human fibrinogen and to laminin but not to fibronectin. Adhesion to fibrinogen substrates was specific, since it was inhibited by soluble fibrinogen and by specific antibodies, and seemed to be mediated by the D domains of the molecule. Study of the adhesion of numerous strains or clinical isolates to various mammalian fibrinogens did not reveal any particular affinity of strains for some animal species. Finally, by cultivation of the fungus in the presence of 125I-human fibrinogen and analysis of the radiolabeled material bound to the surface of the fungus, we were able to specify the sequence of events allowing its installation within the host. The interactions identified here may play an important role in governing fungal adherence and host tissue invasion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Annaix V., Bouchara J. P., Larcher G., Chabasse D., Tronchin G. Specific binding of human fibrinogen fragment D to Aspergillus fumigatus conidia. Infect Immun. 1992 May;60(5):1747–1755. doi: 10.1128/iai.60.5.1747-1755.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M., Gerl M., Sonnenberg A., Deutzmann R., Timpl R. Identification of the Arg-Gly-Asp sequence in laminin A chain as a latent cell-binding site being exposed in fragment P1. FEBS Lett. 1990 Mar 12;262(1):82–86. doi: 10.1016/0014-5793(90)80159-g. [DOI] [PubMed] [Google Scholar]
- Bodey G. P. The emergence of fungi as major hospital pathogens. J Hosp Infect. 1988 Feb;11 (Suppl A):411–426. doi: 10.1016/0195-6701(88)90220-4. [DOI] [PubMed] [Google Scholar]
- Bodey G. P., Vartivarian S. Aspergillosis. Eur J Clin Microbiol Infect Dis. 1989 May;8(5):413–437. doi: 10.1007/BF01964057. [DOI] [PubMed] [Google Scholar]
- Bouchara J. P., Bouali A., Tronchin G., Robert R., Chabasse D., Senet J. M. Binding of fibrinogen to the pathogenic Aspergillus species. J Med Vet Mycol. 1988;26(6):327–334. [PubMed] [Google Scholar]
- Bouchara J. P., Larcher G., Joubaud F., Penn P., Tronchin G., Chabasse D. Extracellular fibrinogenolytic enzyme of Aspergillus fumigatus: substrate-dependent variations in the proteinase synthesis and characterization of the enzyme. FEMS Immunol Med Microbiol. 1993 Jun;7(1):81–91. doi: 10.1111/j.1574-695X.1993.tb00385.x. [DOI] [PubMed] [Google Scholar]
- Burke C., Mayo K. H., Skubitz A. P., Furcht L. T. 1H NMR and CD secondary structure analysis of cell adhesion promoting peptide F-9 from laminin. J Biol Chem. 1991 Oct 15;266(29):19407–19412. [PubMed] [Google Scholar]
- CLARK H. F., SHEPARD C. C. A DIALYSIS TECHNIQUE FOR PREPARING FLUORESCENT ANTIBODY. Virology. 1963 Aug;20:642–644. doi: 10.1016/0042-6822(63)90292-7. [DOI] [PubMed] [Google Scholar]
- Campbell J. H., Terranova V. P. Laminin: molecular organization and biological function. J Oral Pathol. 1988 Aug;17(7):309–323. doi: 10.1111/j.1600-0714.1988.tb01543.x. [DOI] [PubMed] [Google Scholar]
- Cartwright T., Kekwick R. G. A comparative study of human, cow, pig and sheep fibrinogen. Biochim Biophys Acta. 1971 Jun 29;236(3):550–562. doi: 10.1016/0005-2795(71)90239-x. [DOI] [PubMed] [Google Scholar]
- Chhatwal G. S., Dutra I. S., Blobel H. Fibrinogen binding inhibits the fixation of the third component of human complement on surface of groups A, B, C, and G streptococci. Microbiol Immunol. 1985;29(10):973–980. doi: 10.1111/j.1348-0421.1985.tb02961.x. [DOI] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Plow E. F. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci. 1991 Jul;16(7):246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Denning D. W., Follansbee S. E., Scolaro M., Norris S., Edelstein H., Stevens D. A. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med. 1991 Mar 7;324(10):654–662. doi: 10.1056/NEJM199103073241003. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem. 1984;53:195–229. doi: 10.1146/annurev.bi.53.070184.001211. [DOI] [PubMed] [Google Scholar]
- Frosco M., Chase T., Jr, Macmillan J. D. Purification and properties of the elastase from Aspergillus fumigatus. Infect Immun. 1992 Mar;60(3):728–734. doi: 10.1128/iai.60.3.728-734.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold J. W. Opportunistic fungal infections in patients with neoplastic disease. Am J Med. 1984 Mar;76(3):458–463. doi: 10.1016/0002-9343(84)90665-x. [DOI] [PubMed] [Google Scholar]
- Gustafson T. L., Schaffner W., Lavely G. B., Stratton C. W., Johnson H. K., Hutcheson R. H., Jr Invasive aspergillosis in renal transplant recipients: correlation with corticosteroid therapy. J Infect Dis. 1983 Aug;148(2):230–238. doi: 10.1093/infdis/148.2.230. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hawiger J., Timmons S., Strong D. D., Cottrell B. A., Riley M., Doolittle R. F. Identification of a region of human fibrinogen interacting with staphylococcal clumping factor. Biochemistry. 1982 Mar 16;21(6):1407–1413. doi: 10.1021/bi00535a047. [DOI] [PubMed] [Google Scholar]
- Kirschbaum N. E., Mosesson M. W., Amrani D. L. Characterization of the gamma chain platelet binding site on fibrinogen fragment D. Blood. 1992 May 15;79(10):2643–2648. [PubMed] [Google Scholar]
- Klimowski L. L., Rotstein C., Cummings K. M. Incidence of nosocomial aspergillosis in patients with leukemia over a twenty-year period. Infect Control Hosp Epidemiol. 1989 Jul;10(7):299–305. doi: 10.1086/646032. [DOI] [PubMed] [Google Scholar]
- Kronvall G., Schönbeck C., Myhre E. Fibrinogen binding structures in beta-hemolytic streptococci group A, C, and G. Comparisons with receptors for IgG and aggregated beta 2-microglobulin. Acta Pathol Microbiol Scand B. 1979 Oct;87(5):303–310. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lantz M. S., Allen R. D., Bounelis P., Switalski L. M., Hook M. Bacteroides gingivalis and Bacteroides intermedius recognize different sites on human fibrinogen. J Bacteriol. 1990 Feb;172(2):716–726. doi: 10.1128/jb.172.2.716-726.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz M. S., Allen R. D., Vail T. A., Switalski L. M., Hook M. Specific cell components of Bacteroides gingivalis mediate binding and degradation of human fibrinogen. J Bacteriol. 1991 Jan;173(2):495–504. doi: 10.1128/jb.173.2.495-504.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larcher G., Bouchara J. P., Annaix V., Symoens F., Chabasse D., Tronchin G. Purification and characterization of a fibrinogenolytic serine proteinase from Aspergillus fumigatus culture filtrate. FEBS Lett. 1992 Aug 10;308(1):65–69. doi: 10.1016/0014-5793(92)81052-n. [DOI] [PubMed] [Google Scholar]
- Lämmler C., Chhatwal G. S., Blobel H. Variations in the binding of mammalian fibrinogens to streptococci of different animal origin. Med Microbiol Immunol. 1983;172(3):191–196. doi: 10.1007/BF02123805. [DOI] [PubMed] [Google Scholar]
- Monod M., Togni G., Rahalison L., Frenk E. Isolation and characterisation of an extracellular alkaline protease of Aspergillus fumigatus. J Med Microbiol. 1991 Jul;35(1):23–28. doi: 10.1099/00222615-35-1-23. [DOI] [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard U., Büttner S., Eiffert H., Staib F., Rüchel R. Purification and characterisation of an extracellular serine proteinase from Aspergillus fumigatus and its detection in tissue. J Med Microbiol. 1990 Dec;33(4):243–251. doi: 10.1099/00222615-33-4-243. [DOI] [PubMed] [Google Scholar]
- Reuterswärd A., Miörner H., Wagner M., Kronvall G. Variations in binding of mammalian fibrinogens to streptococci groups A, B, C, E, G and to Staphylococcus aureus. Acta Pathol Microbiol Immunol Scand B. 1985 Apr;93(2):77–82. doi: 10.1111/j.1699-0463.1985.tb02855.x. [DOI] [PubMed] [Google Scholar]
- Rinaldi M. G. Invasive aspergillosis. Rev Infect Dis. 1983 Nov-Dec;5(6):1061–1077. doi: 10.1093/clinids/5.6.1061. [DOI] [PubMed] [Google Scholar]
- Strong D. D., Laudano A. P., Hawiger J., Doolittle R. F. Isolation, characterization, and synthesis of peptides from human fibrinogen that block the staphylococcal clumping reaction and construction of a synthetic clumping particle. Biochemistry. 1982 Mar 16;21(6):1414–1420. doi: 10.1021/bi00535a048. [DOI] [PubMed] [Google Scholar]
- Sturtevant J. E., Latgé J. P. Interactions between conidia of Aspergillus fumigatus and human complement component C3. Infect Immun. 1992 May;60(5):1913–1918. doi: 10.1128/iai.60.5.1913-1918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Annaix V., Robert R., Senet J. M. Fungal cell adhesion molecules in Candida albicans. Eur J Epidemiol. 1991 Jan;7(1):23–33. doi: 10.1007/BF00221338. [DOI] [PubMed] [Google Scholar]
- Tronchin G., Bouchara J. P., Larcher G., Lissitzky J. C., Chabasse D. Interaction between Aspergillus fumigatus and basement membrane laminin: binding and substrate degradation. Biol Cell. 1993;77(2):201–208. doi: 10.1016/s0248-4900(05)80189-3. [DOI] [PubMed] [Google Scholar]
- Weinberger M., Elattar I., Marshall D., Steinberg S. M., Redner R. L., Young N. S., Pizzo P. A. Patterns of infection in patients with aplastic anemia and the emergence of Aspergillus as a major cause of death. Medicine (Baltimore) 1992 Jan;71(1):24–43. doi: 10.1097/00005792-199201000-00003. [DOI] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Inhibition of complement-mediated opsonization and phagocytosis of Streptococcus pyogenes by D fragments of fibrinogen and fibrin bound to cell surface M protein. J Exp Med. 1985 Dec 1;162(6):1983–1997. doi: 10.1084/jem.162.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingard J. R., Beals S. U., Santos G. W., Merz W. G., Saral R. Aspergillus infections in bone marrow transplant recipients. Bone Marrow Transplant. 1987 Aug;2(2):175–181. [PubMed] [Google Scholar]
- Yamada K. M. Adhesive recognition sequences. J Biol Chem. 1991 Jul 15;266(20):12809–12812. [PubMed] [Google Scholar]



