Abstract
Many Wnts influence cell behavior by a conserved signaling cascade that promotes the stabilization and nuclear accumulation of β-catenin (β-cat), which then associates with TCF family members to activate target genes. The histone acetyltransferase CREB binding protein (CBP) can bind to TCF and inhibit Wnt signaling in Drosophila. In contrast, studies in vertebrates indicate a positive role for CBP and the closely related protein p300 as β-cat binding transcriptional co-activators. We address this discrepancy by demonstrating that in addition to its negative role, CBP has an essential positive role in Wnt signaling in flies. CBP binds directly to the C-terminus of Armadillo (Arm, the fly β-cat) and is recruited to a Wnt-regulated enhancer (WRE) in a Wnt- and Arm-dependent manner. In a human colorectal cancer cell line, we show that CBP and p300 can inhibit Wnt signaling and demonstrate that human p300 can bind directly to TCF4 in vitro. Our results argue that CBP/p300 has an evolutionarily conserved role as a buffer regulating TCF-β-cat/Arm binding. Subsequent to this interaction, it also has an essential role in mediating the transactivation activity of β-cat/Arm.
Keywords: Armadillo, β-catenin, CBP, p300, Wnt
Introduction
The Wnt/β-catenin (β-cat) pathway is a signaling cascade that is highly conserved from cnidarians to humans (Cadigan and Nusse, 1997; Guder et al, 2006). During development, this pathway is used to control a variety of cell fate decisions (Cadigan and Nusse, 1997; Logan and Nusse, 2004). Misregulation of Wnt/β-cat signaling plays a causal role in several types of human cancers (Polakis, 2000), as well as in defects in bone density and vascular defects of the eye (Logan and Nusse, 2004).
The level of Wnt/β-cat signaling revolves around the stability and cellular location of β-cat/Armadillo (Arm; the fly β-cat). In the absence of Wnt stimulation, there is a small pool of cytosolic β-cat/Arm due to constitutive phosphorylation by a complex containing Axin, the adenomatous polyposis coli (APC) protein and glycogen synthase kinase 3 (GSK3; Ding and Dale, 2002). Phospho-β-cat is then targeted to the ubiquitin/proteosome degration pathway (Daniels et al, 2001). Upon Wnt stimulation, the Axin/APC/GSK3 complex is antagonized, causing the accumulation of hypophosphorylated β-cat/Arm, which translocates into the nucleus where it complexes with transcription factors, most notably members of the TCF family of DNA-binding proteins (Roose and Clevers, 1999).
Without β-cat/Arm, TCFs are thought to function as repressors of Wnt target gene expression, in part by interacting with transcriptional corepressors of the Groucho/TLE (Gro) family (Cavallo et al, 1998; Roose et al, 1998). β-cat is thought to displace Gro from TCF through competitive binding (Daniels and Weis, 2005). In addition to relieving TCF repression, β-cat/Arm is thought to activate Wnt target gene expression by recruiting additional proteins to TCF-bound chromatin. The N-terminal portion of β-cat/Arm binds Legless (Lgs, called BCL9 in vertebrates) and Lgs/BCL-9 acts as an adaptor between β-cat/Arm and Pygopus (Pygo), which promotes transcriptional activation (Kramps et al, 2002; Thompson, 2004; Hoffmans et al, 2005). The C-terminus of β-cat/Arm has been shown to bind to several transcriptional coactivators, including Hyrax/Parafibromin (Mosimann et al, 2006) and the chromatin remodeler Brg-1 (Barker et al, 2001). These interactions contribute to the ability of TCF/β-cat/Arm to activate Wnt target genes, supporting the model that β-cat/Arm converts TCFs from repressors into transcriptional activators (van Es et al, 2003; Parker et al, 2007).
Despite the strong conservation of the Wnt/β-cat signaling pathway between invertebrates and vertebrates, some important differences have been noted. For example, the histone acetyltransferase (HAT) Creb-binding protein (CBP) has been shown to be a negative regulator of Wnt signaling in flies (Waltzer and Bienz, 1998), but positively regulates the pathway in vertebrates (Hecht et al, 2000; Miyagishi et al, 2000; Sun et al, 2000; Takemaru and Moon, 2000).
Mutations in the nejire (nej) gene, which encodes Drosophila CBP (Akimaru et al, 1997), had elevated levels of Wingless (Wg, a fly Wnt) signaling in the embryo and suppressed loss of Wg signaling phenotypes in the developing wing (Waltzer and Bienz, 1998). CBP was found to bind directly to the HMG domain of TCF and acetylate it on a conserved lysine in its N-terminal domain, reducing TCF's ability to bind to Arm. The data support a model where CBP negatively regulates TCF-Arm interaction, and thus Wg signaling, by binding and modifying TCF (Waltzer and Bienz, 1998).
In contrast to flies, the data from vertebrate systems support a positive role for CBP and the closely related HAT, p300, in Wnt signaling. Expression of either gene augments β-cat activation of reporter genes and inhibition of these genes reduces TCF reporter gene activity (Hecht et al, 2000; Miyagishi et al, 2000; Sun et al, 2000; Takemaru and Moon, 2000). These HATs can bind β-cat in vitro (Hecht et al, 2000; Miyagishi et al, 2000; Sun et al, 2000; Takemaru and Moon, 2000), and are recruited to Wnt-responsive elements (WREs) in the Cyclin D2, c-Myc and survivin genes upon activation of Wnt/β-cat signaling (Kioussi et al, 2002; Ma et al, 2005; Sierra et al, 2006). Inhibition of CBP using siRNA or a chemical inhibitor that disrupts β-cat-CBP binding (Emami et al, 2004) was found to block Wnt activation of survivin gene expression (Ma et al, 2005). These data are consistent with the view that β-cat recruitment of CBP/p300 to WREs is required for transcriptional activation. In addition, p300 has also been shown to bind to a specific isoform of TCF4 and act synergistically to activate TCF reporters (Hecht and Stemmler, 2003).
In this study, we explore this controversy by re-examining the role of CBP in Wnt signaling in Drosophila. As previously reported (Waltzer and Bienz, 1998), we find evidence for CBP playing an inhibitory role in Wg signaling. However, we also demonstrate that CBP is required for activation of Wg targets, both in cell culture and wing imaginal discs. Fly CBP can bind directly to and interact functionally with the C-terminal half of Arm, and CBP is recruited to a WRE in a Wg- and Arm-dependent manner. In addition, we demonstrate that human p300 can bind to TCF4, and find that siRNA reduction of p300 and CBP leads to an elevation of Wnt signaling in a human colorectal cancer cell line. Our data support the view that the relationship between CBP/p300 and the Wnt pathway is evolutionarily conserved between flies and vertebrates. These HATs act as a buffer to regulate TCF-β-cat/Arm interaction, but have an additional role as β-cat/Arm binding transcriptional coactivators.
Results
Overexpression of CBP can repress or activate Wg signaling depending on the context
We identified CBP in a misexpression screen where the Rorth collection of EP insertions (Rorth et al, 1998) was crossed to P[GMR-Gal4]/P[UAS-wg] (GMR/wg) flies, which have a severe reduction in adult eye size (Parker et al, 2002). Two EP transposons inserted just 5′ to the nej gene, which encodes the only fly CBP (Akimaru et al, 1997), were found to be slight but significant suppressors of the GMR/wg phenotype (data not shown). A P[UAS-CBP] line also suppressed GMR/wg, indicating that CBP was the gene responsible for inhibiting the Wg pathway.
To extend these findings, the effect of CBP expression was examined in the developing wing. Wg is expressed in a narrow stripe along the dorsal/ventral (D/V) boundary of the wing imaginal disc, where it activates proneural genes such as senseless (sens) (Parker et al, 2002) and specifies wing margin in the adult wing (Couso et al, 1994). Expression of CBP at the D/V boundary of the wing disc causes notches in the adult wings that are characteristic of a loss of Wg signaling (Figure 1B). Expression of CBP in a stripe perpendicular to the D/V boundary, via a Decapentaplegic (Dpp)-Gal4 driver causes a loss of Sens (Figure 1G), consistent with a block in Wg signaling. However, Wg expression was also consistently reduced (Figure 1F), as was Cut expression (data not shown), suggesting a block in Notch signaling (Micchelli et al, 1997). When the animals were reared at 18°C, when Gal4 is less active (Rorth et al, 1998), loss of Sens without loss of Wg expression was observed at a low frequency (12%; n=74), with the remainder having a wild-type pattern (55%) or a loss of both Sens and Wg (33%; data not shown). These results suggest that at low levels of expression, CBP can inhibit the Wg target Sens without effecting Wg expression, but CBP also has an inhibitory affect on Wg expression, probably due to reduced Notch signaling.
Figure 1.
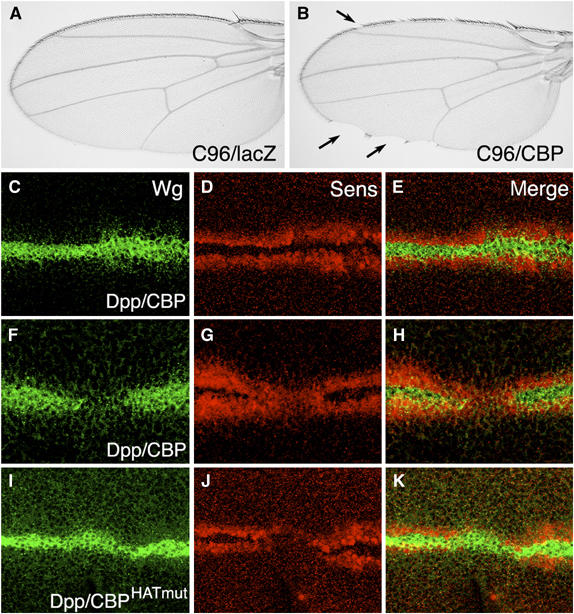
Misexpression of CBP and a CBPHATmut inhibit Wg target gene expression. (A, B) Micrographs of adult wings containing the P[C96-Gal4] driver and either P[UAS-lacZ] (A) or P[UAS-CBP] (B). Misexpression of CBP leads to loss of wing margin (arrows in (B)). Expression of CBPHatmut produced a similar phenotype (data not shown). (C–K) Confocal images of late third-instar wing imaginal discs stained for Wg (green) or the Wg target Sens (red). (C–E) P[Dpp-Gal4]/P[UAS-CBP] disc reared at 18°C. Mild defects in Wg and Sens expression are sometimes observed. (F–H) P[Dpp-Gal4]/P[UAS-CBP] disc reared at 25°C. Most of the discs have a loss of Wg and reduction of Sens in the Dpp expression domain. (I–K) P[Dpp-Gal4]/P[UAS-CBPHatmut] disc reared at 29°C. The majority of the discs have no effect on Wg expression and a strong reduction in Sens expression.
To examine further the relationship between CBP and Wg signaling, a mutant version of CBP containing point mutations in the HAT catalytic site (CBPHATmut; Ludlam et al, 2002) was expressed in the wing via Dpp-Gal4. The majority of these discs (56%; n=39) displayed a phenotype consistent with a loss of Wg signaling; a strong loss of Sens expression (Figure 1J) with no detectable reduction in Wg expression (Figure 1I). The remainder of the discs appeared normal (18%) or had loss of both Sens and Wg (26%). The Notch target Cut was largely unaffected in this background (data not shown). Expression of CBP and CBPHATmut had different effects on another readout of Wg signaling, Distal-less (Dll), which is activated by Wg in broad domain centered on the D/V stripe (Zecca et al, 1996; Neumann and Cohen, 1997). Wild-type CBP did not alter the Dll pattern, even in discs where Wg expression was inhibited (Figure 2A–C). In contrast, CBPHATmut caused a consistent (75%; n=20) reduction in Dll expression but had no effect or slightly expanded Wg expression (Figure 2D–F). These results suggest that CBP and CBPHATmut are interacting with the Wg pathway through different mechanisms, with the Hat mutant possibly acting like a dominant-negative, consistent with a positive role for CBP in Wg signaling.
Figure 2.
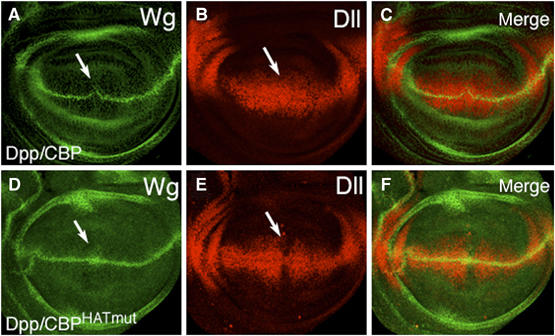
Misexpression of CBP and CBPHatmut have different effects on the Wg target Dll. Confocal images of late third-instar imaginal discs containing P[Dpp-Gal4] and either P[UAS-CBP] reared at 18°C (A–C) or P[UAS-CBPHatmut] reared at 29°C (D–F) stained for Wg (green) or Dll (red). Arrows indicate the location where the Dpp expression domain intersects the Wg D/V stripe. Although CBP expression causes a reduction of Wg expression in approximately half of the discs, the Dll pattern remains unchanged. In contrast, the large majority of discs expressing CBPHATmut had normal or slightly expanded Wg expression and reduction of Dll expression in the Dpp expression domain.
To explore the relationship between CBP and Wnt signaling in cell culture, we coexpressed a constitutively active, hypo-phosphorylated form of Arm (Arm*; Freeman and Bienz, 2001) with fly CBP in human embryonic kidney 293 (293) or Drosophila Kc167 (Kc) cells. The Topflash reporter, containing three TCF-binding sites upstream of the c-fos promoter (Korinek et al, 1997) was used in 293 cells and is activated by Arm* (Figure 3A). This construct is only expressed at low levels in Drosophila cells (M Fang and K Cadigan, unpublished observations), so the c-fos proximal promoter was replaced with that of the fly Hsp70 gene. This reporter, called Dropflash, is activated by Arm* in Kc cells (Figure 3B). In the human cells, fly CBP increased Arm-dependent activation of the reporter by 2–3-fold (Figure 3A), similar to the effects reported with β-cat and p300/CBP (Hecht et al, 2000; Miyagishi et al, 2000; Sun et al, 2000). However, no increase of Arm* activated Dropflash was observed in several experiments. Rather, there was a slight inhibition of reporter gene activation at higher CBP levels (Figure 3B). These data indicate that fly CBP can activate Arm transcriptional activity in some contexts but not others.
Figure 3.
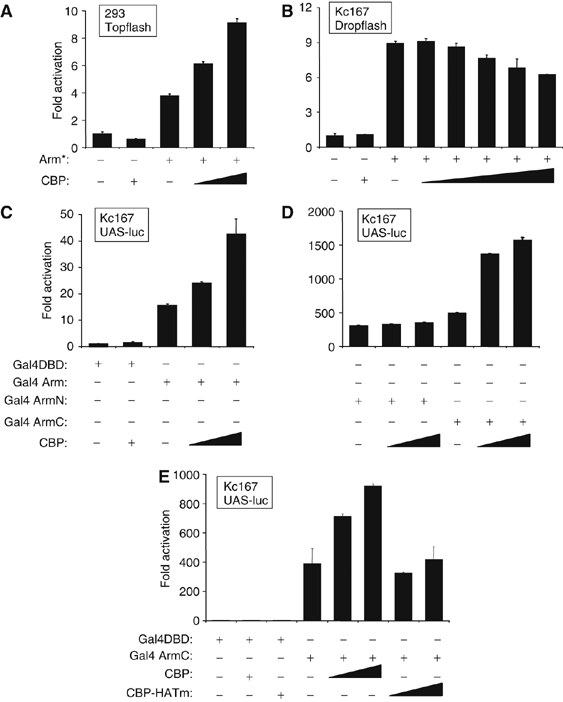
CBP augments Arm transcriptional activity in a context-dependent manner. (A) 293 HEK cells were transfected with plasmids expressing an activated form of Arm (Arm*; 20 ng) and fly CBP (200 or 400 ng) along with the Topflash luciferase reporter. CBP increases Arm activation of the Wnt reporter. (B) Fly Kc cells were transfected with Arm* (20 ng) and CBP (50, 100, 200 or 400 ng) expression constructs along with the Dropflash luciferase reporter gene. CBP had a slight inhibitory effect on the ability of Arm* to activate the Wg reporter. (C–E) Kc cells transfected with plasmids (20 ng) expressing the Gal4-DBD domain alone or Gal4-DBD fused to full-length Arm (Gal4-Arm), the N-terminal half of Arm (Gal4-ArmN; residues 1–428) or the C-terminal half of Arm (Gal4-ArmC; residues 429–815). CBP or CBPHatmut constructs were transfected as indicated at 20 or 50 ng. The Gal4 UAS-luciferase (UAS-luc) reporter gene was activated by Gal4-Arm, Gal4-ArmN and Gal4-ArmC, and CBP coexpression augmented this activation for Gal4-Arm and Gal4-ArmC but not Gal4-ArmN. CBPHATmut did not effect Gal4-ArmC activation of UAS-luc. All transfections contained a lacZ expression plasmid, and luciferase activities were determined 48 h post-transfection and normalized against β-galactosidase activity. Values are the mean of duplicate experiments (standard deviations are indicated) and expressed as relative activity compared with cells transfected with the reporter alone.
It is possible that in Kc cells, CBP's inhibitory activity masks the activation of Arm-mediated transcription. Since CBP inhibits the pathway by binding to TCF (Waltzer and Bienz, 1998), removing TCF from the system might uncover the positive effect of CBP on Arm. To do this, we utilized a construct expressing Arm fused to the DNA-binding domain of Gal4 (Gal4-DBD). Gal4-Arm can activate a UAS-luciferase (UAS-luc) reporter (Stadeli and Basler, 2005; Fang et al, 2006; Figure 3C). In contrast to Arm activation of Dropflash, coexpression of CBP consistently increased the transcriptional activity of Gal4-Arm (Figure 3C). Thus, the positive effect of CBP expression on Arm is only apparent in Kc cells when the requirement for TCF is bypassed.
CBP interacts functionally and physically with the C-terminus of Arm
There are at least two regions of Arm/β-cat that mediate transcriptional activation. The C-terminus is sufficient for transcriptional activation but the N-terminal half also has this ability (van de Wetering et al, 1997; Hsu et al, 1998; Cox et al, 1999; Natarajan et al, 2001; Stadeli and Basler, 2005; Fang et al, 2006). Consistent with this, both halves of Arm fused to Gal4 DBD (Gal4ArmN and Gal4ArmC) are potent activators of UAS-luc (Figure 3D). However, coexpression of CBP had no effect on Gal4ArmN but did augment the ability of Gal4ArmC to activate the reporter (Figure 3D). Interestingly, CBPHATmut had no effect on Gal4ArmC activity (Figure 3E), except an inhibitory one when CBPHATmut is expressed at high concentrations (data not shown). The results demonstrate a functional interaction between CBP and the C-terminal half of Arm that is dependent on CBP's HAT activity.
To determine whether the functional interaction between Arm and CBP reflects a physical association, we examined the ability of the two proteins to associate in several assays. A bacterially produced glutathione-S-transferase (GST)-Arm fusion protein was able to pull down CBP from an extract of human cells expressing fly CBP (Figure 4A). No pull down was observed from control extracts, or when a GST protein was used. Arm can be co-immunoprecipitated by CBP when both are coexpressed in Kc cells (Figure 4B). A co-immunoprecipitation of endogenous CBP and Arm was also observed when Kc cells are stimulated by conditioned media containing Wg protein (WCM; Figure 4C). These data demonstrate that CBP and Arm physically associate, although the interaction could be indirect.
Figure 4.
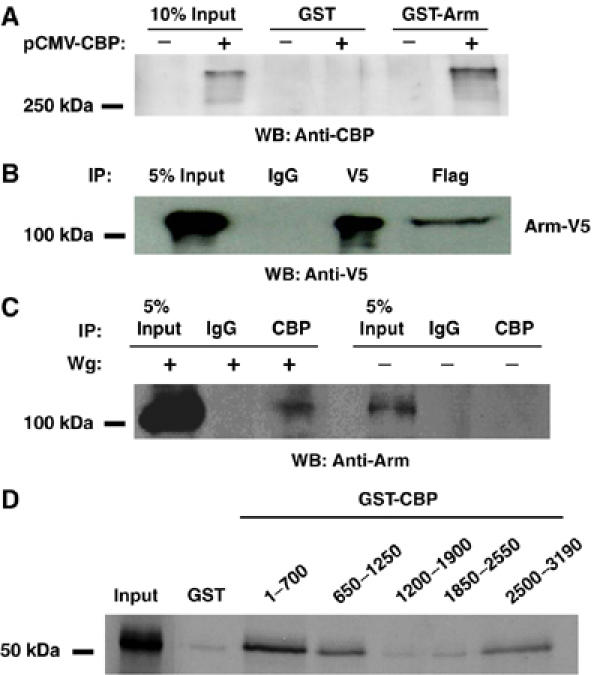
Arm interacts with CBP in vitro and in vivo. (A) Western blot with anti-CBP antisera demonstrating that GST-Arm, but not GST alone, is able to pull down fly CBP from extracts of 293 cells expressing CBP. (B) Arm and fly CBP interact when overexpressed in Kc cells. Cells were transfected with plasmids expressing V5-tagged Arm* and Flag-tagged CBP. Forty-eight hours post-transfection, cell extracts were prepared and immunoprecipitated with V5, Flag and control IgG antibodies and the precipitates were analyzed by Western blot with anti-V5 antibody. Flag precipitation pulled down a significant portion of Arm*-V5, compared with control IgG. (C) Endogenous Arm and CBP interact. Kc cells were treated with WCM or control media for 4 h before extract preparation. Proteins were immunoprecipitated with anti-CBP antisera or control IgG and Western blot were analyzed with anti-Arm antibody. CBP interacts with endogenous Arm in Wg-stimulated cells. (D) Arm and CBP interact directly in vitro. Bacterially expressed GST or GST–CBP fragments of the indicated residues were incubated with 35S-Met-labeled Arm C-terminal fragment (residues 429–815). Precipitated proteins were analyzed together with 5% of the input material by SDS–PAGE and autoradiography. Three CBP fragments bound to the C-terminal fragment of Arm, while fragments comprising residues 1200–2550 show similar binding as the GST-negative control. All experiments shown were performed multiple times, with similar results obtained.
As described above, CBP augments the activity of Gal4-ArmC but not Gal4-ArmN (Figure 3D). Consistent with this, GST-ArmC could pull down CBP from cell extracts, while GST-ArmN could not (data not shown). To determine whether the interaction between the C-terminal half of Arm and CBP is direct, fragments of CBP were fused to GST and incubated with ArmC produced by in vitro translation. Several CBP fragments at both the N- and C-termini could specifically interact with ArmC (Figure 4D). Thus, at least two domains of CBP can bind directly with the C-terminal portion of Arm, consistent with CBP directly acting with Arm to activate transcription.
CBP is required for activation of several endogenous Wg targets
To determine whether CBP is required for Wg activation of endogenous transcriptional targets, several genes activated by Wg signaling in Kc cells were examined. Naked cuticle (nkd) and notum/wingful (notum) are Wg antagonists whose expression is activated by Wg signaling in flies (Zeng et al, 2000; Gerlitz and Basler, 2002; Giraldez et al, 2002). Wg stimulation of Kc cells significantly induced the transcript levels of these genes (Figure 5A and B) (Fang et al, 2006). When CBP was depleted by RNA interference (RNAi), activation of both Wg targets was markedly reduced (Figure 5A and B). Similar results were obtained with CG6234 (data not shown, which is directly activated by Wg signaling in Kc cells; Fang et al, 2006). A more dramatic block in Wg activation of these genes could be observed by increasing the dose or time of the CBP RNAi treatment, but this alters the growth of the Kc cells and the expression of housekeeping genes is also reduced. Under the CBP depletion conditions used in Figure 5, the levels of β-tubulin, arm and TCF transcripts were unaffected (Figure 5C–E) and no decrease in TCF protein levels or the ability of Wg to stabilize Arm protein was observed (Figure 5F). Therefore, the defect in Wg activation of nkd and notum expression upon CBP knockdown is likely to be a conservative estimate of its requirement in the pathway.
Figure 5.
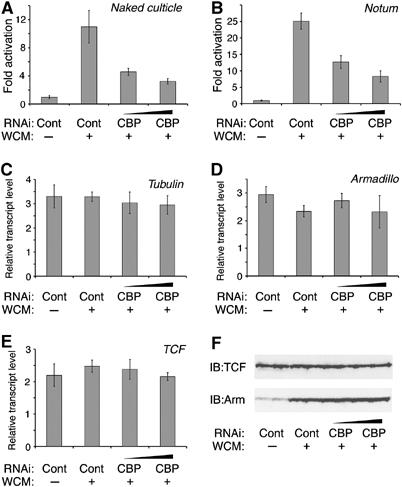
CBP is required for the transcriptional activation of Wg endogenous targets genes in Kc cells. (A–E) Cells were treated for 4 days with control dsRNA (10 μg/well) or different doses (7.5 and 10 μg/well) of dsRNA corresponding to CBP. Cells were then incubated for 5 h with control or WCM before transcript levels of nkd (A), notum (B), α-tubulin (C), arm (D) and TCF (E) were measured by quantitative RT–PCR as described in Materials and methods. Results for nkd and notum were normalized to the average of α-tubulin, arm and TCF expression, while the later three were normalized to total RNA. Wg activation of nkd and notum were reduced in CBP-depleted cells. (F) Western blot showing that induction of Arm protein and TCF protein levels were not affected by CBP depletion.
Fly embryos that are zygotically mutant for a strong allele of nej/CBP have no detectable loss in Wg signaling, rather there is a significant increase in the pathway (Waltzer and Bienz, 1998). However, these embryos have a significant amount of maternally provided CBP (Ludlam et al, 2002). This maternal contribution cannot be removed, because it is required for oogenesis (Waltzer and Bienz, 1998). Thus, it is possible that a positive role for CBP in Wg signaling in the embryo has remained undetected because it is technically impossible to remove most of CBP gene activity in embryos.
To examine the requirement of CBP in the wing imaginal discs, somatic clones of the strong (nej3) allele were induced in a Minute/+ background (see Materials and methods for details). Under these conditions, small CBP mutant clones were obtained at a low frequency but displayed phenotypes consistent with a loss in Wg signaling. Wg expression at the D/V stripe was unaltered in CBP mutant clones (e.g. Figure 6F), but in half the clones (n=8), ectopic Wg expression was observed near the D/V stripe (e.g. Figure 6B). Away from the D/V boundary, 68% of the clones (n=31) had a low level of Wg expression inside the clone (data not shown). Despite this variable increase in Wg expression, there was a consistent decrease in the expression of the two Wg targets examined. A total of 91% of the clones near the Wg stripe (n=11) had a strong reduction in Dll expression (e.g. Figure 6C). Further way from the D/V boundary, 76% of the clones (n=34) had reduced Dll, while 12% had no observable defect (data not shown). The remaining 12% consists of four clones removed far from the Wg stripe, where Dll is normally not expressed. These had a slight elevation of Dll expression (compared with surrounding tissue) and all four also displayed ectopic Wg expression. Seven clones were examined for Sens expression: one showed a partial loss of Sens, two clones a stronger loss (data not shown), while the remaining four had a complete loss of Sen expression (e.g. Figure 6G). Overall, these data support a strong requirement for CBP in Wg activation of Dll and Sen.
Figure 6.
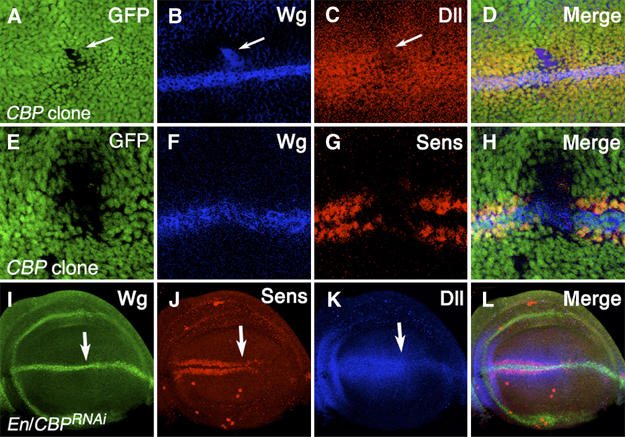
CBP is required in the wing imaginal discs for Wg signaling. Confocal images of late third-instar wing imaginal discs. (A–H) nej3 mutant clones stained for the clonal marker GFP (A, E), Wg (B, F), Dll (C) and Sens (G). (A–D) In this CBP mutant clone, Wg expression is upregulated but expression of the Wg target Dll is greatly reduced (white arrows). (E–H) In this CBP mutant clone, Wg expression is normal but the Wg target Sens in not expressed. (I–L) P[En-Gal4]/P[UAS-CBPRNAi] wing disc stained for Wg (I), Sens (J) and Dll (K). The En expression domain is to the right of the white arrows. Wg expression is unaffected by CBP depletion but Sens and Dll expression are greatly reduced.
Because it was so difficult to obtain nej3 clones and the ectopic expression of Wg within these clones raised the possibility that cells lacking CBP were undergoing programmed cell death (Huh et al, 2004; Perez-Garijo et al, 2004; Ryoo et al, 2004), an alternative method of reducing CBP activity was utilized. A UAS line expressing a CBP hairpin known to produce the RNAi effect (Kumar et al, 2004) was driven in the posterior half of the wing pouch using the Engrailed (En)-Gal4 driver. A total of 45% of the discs examined (n=20) had an intermediate reduction of Sens and Dll, while the remaining 55% exhibited a stronger loss of these Wg targets (e.g. Figure 6J and K). In all cases, Wg expression was unaffected or slightly expanded (e.g. Figure 6I). The RNAi experiments and clonal analysis strongly support a positive role for CBP in Wg signaling in the developing wing.
CBP is recruited to a WRE in a Wg and Arm-dependent manner
Since CBP is required for activation of Wg targets and can bind directly to Arm, it is possible that CBP is recruited to WREs by Arm. We examined a region of the nkd intron, approximately 5 kb downstream of the transcription start site, which we recently reported was bound by TCF, using chromatin immunoprecipitation (ChIP) (Fang et al, 2006). TCF binds to this region to a greater degree compared with other parts of the nkd locus, such as the nkd ORF (Figure 7A). In the absence of Wg stimulation, Arm is bound to this region at background levels (Figure 7B). However, addition of WCM for 4 h caused a marked increase in Arm binding (Figure 7B). As reported previously (Fang et al, 2006), a significant increase in TCF binding was also observed (Figure 7A). A 420 bp fragment encompassing this region and containing five putative TCF binding sites was fused upstream of the hsp70 core promoter driving luciferase (Nkd-luc). This construct was activated 30-fold by cotransfection with Arm* (Figure 7C). When all five TCF sites were mutated, this activation was abolished (Figure 7C). These data strongly support that the stretch of DNA identified by ChIP and reporter gene analysis is a bona fide WRE directly regulated by the Wg/Arm pathway.
Figure 7.
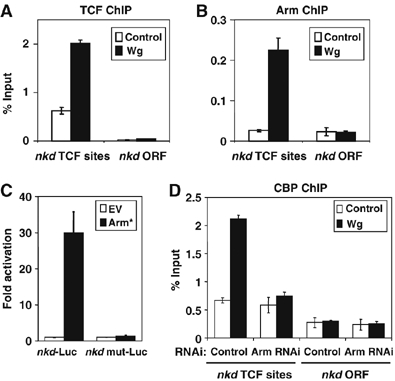
CBP is recruited to the WRE of the nkd gene in a Wg- and Arm-dependent manner. (A, B) ChIP using antibodies against TCF (A) and Arm (B) demonstrate enhanced binding to a cluster of TCF binding sites in the nkd intron (compared with the nkd ORF) in Kc cells stimulated with WCM for 4 h. (C) Kc cells were transfected with an Arm* expression plasmid and an hsp70 luciferase reporter containing a 420 bp fragment of the nkd intron (nkd-luc) or the same sequence with five predicted five TCF destroyed by site-directed mutagenesis (nkdmut-luc). Reporter gene activity was assayed as described in Figure 3 and Materials and methods. Arm* activated nkd-luc 30-fold but did not activate nkdmut-luc. (D) CBP is recruited to the nkd WRE in a Wg and arm-dependent manner. Cells were transfected with control or arm dsRNA and cultured for 4 days before treatment with control or Wg-CM for 4 h before lysis and ChIP analysis with anti-CBP antisera. Precipitated DNA were purified and detected by Q-PCR using primers specifically against the nkd WRE or ORF as described in Materials and methods. Values are the mean of duplicate precipitations (±standard deviations) and the data is expressed as percentage of input DNA.
To determine whether CBP was also recruited to the nkd intronic WRE, ChIP was performed using CBP antisera. This antisera specifically recognized CBP, as judged by Western blot of Kc cells with or without CBP RNAi and immunostaining on wild-type cells or nej3 embryos (data not shown). Binding of CBP was consistently enhanced 3–4-fold by Wg stimulation (Figure 7D). In the absence of Wg stimulation, the ChIP signal at the WRE was still reproducibly higher than at the ORF, suggesting it was present on the WRE. This signal was not reduced in cells depleted of Arm via RNAi. However, the Wg-dependent increase in CBP binding to the WRE was abolished by Arm RNAi (Figure 7D). CBP appears to occupy the nkd WRE in the absence of Wg signaling, but increases its occupancy upon Wg signaling in an Arm-dependent manner.
CBP and p300 repress Wnt signaling in a human colorectal cancer cell line
The data on Drosophila cells and imaginal discs clearly indicate a positive role for CBP in Wnt signaling, in addition to the inhibitory role previously described (Waltzer and Bienz, 1998). This raised the possibility that CBP/p300 also plays a negative role in vertebrate Wnt signaling. To test this, we examined whether the HMG domain of TCF4 could directly bind to a p300 fragment that contains the second cysteine/histidine-rich (CH2) domain. These portions of fly TCF and CBP had previously been shown to bind each other in vitro (Waltzer and Bienz, 1998). The TCF4 HMG domain can specifically precipitate the p300 fragment, compared to GST alone (Figure 8A).
Figure 8.
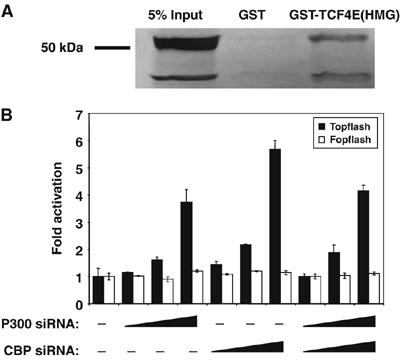
p300 binds to the HMG domain of TCF4E and CBP/p300 represses Wnt signaling in human cells. (A) The TCF4E HMG domain directly binds to p300. Bacterially expressed GST or GST-TCF4 (HMG domain; residues 326–396) fusion protein were incubated with a radiolabeled fragment of human p300 (residues 1254–1899). After pull down and washing, proteins retained by GST or GST-Arm were analyzed together with 5% of the input material by SDS–PAGE and autoradiography. (B) Human SW480 cells were transfected with the either the Topflash or Fopflash reporters, along with increasing amounts (100–500 ng) of pSuper-CBPRNAi or pSuper-p300RNAi constructs (empty pSUPER vector was used to normalize the amount of DNA transfected), which express short hairpins corresponding to each gene. CBP or p300 depletion increases TOPFLASH but not FOPFLASH reporter activity. Transfected cells were assayed for luciferase as described in Figure 3 legend and Materials and methods.
The interaction between p300 and TCF4 suggested that p300/CBP could functionally repress Wnt signaling in a manner similar to its fly counterparts. To test this, used SW480 cells, which contain mutations in the APC gene causing constitutive Wnt signaling (Polakis, 2000). The cells were transfected with increasing amounts of plasmids expressing short RNA hairpins corresponding to CBP and p300 and the Topflash and Fopflash reporters (these hairpins have distinct sequences; see Materials and methods). siRNA knockdown of either CBP or p300 caused a significant increase in Topflash reporter activity but had no effect on the control reporter Fopflash (Figure 8B). The increase in reporter gene expression in CBP siRNA-treated cells could not be rescued with expression of p300 or fly CBP. Rather expression of these genes usually caused an additional increase in Topflash activity (data not shown). While the activation of Topflash by CBP and p300 siRNA could be due to off-target effects, we suggest that the rescue experiment may be complicated due to the fact that CBP both represses and activates TCF-β-cat transcriptional activity.
Discussion
CBP/p300 are bimodal regulators of Wnt signaling in both flies and vertebrates
The controversy over CBP/p300 action in Wnt/β-cat signaling is complicated by the fact that the conflicting models are both supported by strong evidence. In Drosophila, loss-of-function genetics clearly supports a negative role for fly CBP in the pathway, which was buttressed by the finding that CBP can bind and acetylate TCF, reducing its ability to bind Arm (Waltzer and Bienz, 1998). In vertebrate systems, expression of CBP or p300 can augment the ability of β-cat to activate reporter gene expression, and β-cat and CBP/p300 have been shown to interact directly (Hecht et al, 2000; Miyagishi et al, 2000; Sun et al, 2000; Takemaru and Moon, 2000). Consistent with this, an increase in β-cat levels (by lithium treatment) results in CBP/p300 recruitment to WREs (Kioussi et al, 2002; Ma et al, 2005; Sierra et al, 2006) and inhibition of CBP-β-cat interaction reduces the ability of Wnt/β-cat signaling to activate transcriptional targets (Emami et al, 2004; Ma et al, 2005). The data support a view that CBP is a repressor of the pathway in flies and an activator in vertebrates.
This report resolves part of the discrepancy by providing strong support for a positive role for CBP in fly Wg signaling. Loss of CBP results in a dramatic reduction in the ability of Wg to activate transcriptional targets in fly cell culture (Figure 5) and the wing imaginal disc (Figure 6). This positive role for CBP was probably previously missed because technical reasons prevented the complete removal of CBP gene activity in the embryo. Our genetic results are complemented by our data showing that the C-terminus of Arm can bind to CBP both in vitro and in cells (Figure 4). Consistent with this, CBP is recruited to a WRE in a Wg- and Arm-dependent manner (Figure 7D). Thus, our data indicate a direct, essential requirement for CBP in Wg signaling in flies.
In addition to a positive role for CBP in Wg signaling, we also found evidence supporting the negative role previously described (Waltzer and Bienz, 1998). Expression of CBP inhibits Wg signaling in the Drosophila eye and wing (Figure 1 and data not shown), and a slight inhibition was also observed using a TCF reporter in fly cell culture (Figure 3B). In addition, CBP occupies a TCF-bound WRE in the nkd locus, even in the absence of Wg signaling (Figure 7D). These data are consistent with the model proposed by Waltzer and colleagues, stating that CBP negatively regulates Wg signaling through direct interaction with TCF (Waltzer and Bienz, 1998).
A negative role for p300 and CBP in mammalian Wnt signaling is suggested by our finding that human p300 can bind directly to human TCF4 (Figure 8A). This interaction was observed with the HMG domain of TCF4 and a fragment of p300 containing the CH2 domain, the same regions that interact with the fly counterparts (Waltzer and Bienz, 1998). In addition, siRNA knockdown of CBP and p300 causes a significant increase in activation of a Wnt reporter genes in human SW480 cells (Figure 8B). These results suggest that human p300 and CBP repress TCF-β-cat gene activation through interaction with TCF4 in a colon cancer cell line.
Taken together, our results suggest a model where CBP/p300 both represses and activates TCF-β-cat/Arm transcriptional activation. We envision that this bimodal regulation of Wnt signaling by CBP/p300 acts with other factors to set sharp thresholds of gene activation by nuclear β-cat/Arm. Depending on the cell type and level of nuclear β-cat/Arm, reduction of CBP/p300 will lead to either an increase, for example, the fly embryo (Waltzer and Bienz, 1998) and SW480 cells (Figure 8B), or a decrease, for example, Kc cells (Figure 5) or wing imaginal discs (Figure 6), in TCF transcriptional activity. This model can also explain why expression of CBP/p300 can lead to either activation (Hecht et al, 2000; Miyagishi et al, 2000; Sun et al, 2000; Takemaru and Moon, 2000) or repression (Figures 1A–H and 3B) of Wnt signaling.
Mechanism of CBP/p300 activation of Wnt signaling
Our working model is that Wnt signaling results in increased recruitment of CBP/p300 to WREs through direct interaction with β-cat/Arm. The N-terminal portion of CBP and p300 can bind β-cat (Labalette et al, 2004) and two domains, a transcriptional adaptor putative zinc finger (TAZ finger, sometimes called the CH1 domain) and a KIX domain, have been shown to be sufficient for the interaction (Sun et al, 2000; Takemaru and Moon, 2000). A TAZ finger in the C-terminal third of CBP/p300 (also called the CH3 domain) can also bind to β-cat (Daniels and Weis, 2002; Hecht et al, 2000; Miyagishi et al, 2000). In our studies with the fly proteins, fragments containing the N-terminal TAZ finger (residues 1–700) and the KIX domain (residues 650–1250) were positive for binding (Figure 4D). However, a fragment containing the C-terminal TAZ finger (residues 1850–2550) did not show binding in our assay. Rather, the extreme C-terminal bound Arm (Figure 4D). This region is not conserved with vertebrate CBP or p300. In agreement with the vertebrate studies (Hecht et al, 2000; Miyagishi et al, 2000; Sun et al, 2000; Takemaru and Moon, 2000; Daniels and Weis, 2002), we found that the C-terminal half of Arm was sufficient for binding to CBP (Figure 4D). Several groups also found binding between the N-terminal portion of β-cat and p300 (Sun et al, 2000) or CBP (Miyagishi et al, 2000). In our study, we did not observe either physical or functional interactions between the N-terminal half of Arm and CBP (Figure 3D and data not shown). Although all the data cannot be neatly reconciled, it appears that β-cat/Arm interacts with CBP/p300 at multiple sites on each binding partner.
What is the consequence of CBP/p300 recruitment to WREs? CBP and p300 possess intrinsic HAT activity and are thought to activate transcription by the acetylation of lysine residues in the N-terminal tails of H3 and H4 histone subunits (Grant and Berger, 1999). An increase in H3 acetylation was also correlated with CBP/p300 recruitment to the cyclin D2 WRE (Kioussi et al, 2002), but this correlation was not observed at the survivin WRE (Ma et al, 2005). In addition, CBP and p300 have also been shown to acetylate lysines on β-cat (Wolf et al, 2002; Labalette et al, 2004; Levy et al, 2004). Acetylation of β-cat increases its affinity for TCF, suggesting that this could account for its ability to augment TCF transcriptional activation (Levy et al, 2004). Casting doubt on the importance of these modifications is the finding that p300 lacking HAT activity can still augment β-cat activation of a TCF reporter gene (Hecht et al, 2000).
In this report, we observed that expression of CBPHATmut blocks Wg signaling in the wing imaginal disc (Figures 1J and 2E). HAT activity is thought to be required for the ability of CBP to repress the Wg pathway (Waltzer and Bienz, 1998). This suggests that the inhibition observed with CBPHATmut is due to a dominant-negative effect on CBP activation, that is, the mutant CBP outcompetes endogenous CBP for binding to Arm. In cell culture, expression of CBPHATmut failed to augment the transcriptional activity of a Gal4 fused to the C-terminal half of Arm (Figure 3E) and inhibited Gal4-ArmC activity when expressed at high levels (data not shown). These results support the notion that HAT activity is required for CBP promotion of Wg signaling. Further studies will be required to determine whether modification of H3/H4, TCF or other protein substrates by CBP contributes to activation of Wnt transcriptional targets.
Materials and methods
Plasmids
The constructs CMV-p300, and the Topflash/Fopflash reporters were a kind gift from A Hecht. pActin5.1-CBP was from S Smolik. cyclin D-luciferase (Tetsu and McCormick, 1999) was obtained from E Fearon. The Drosophila-specific TCF reporter Dropflash was made by replacing the c-fos promoter of Topflash with the fly hsp70 minimum promoter. Quick change site-directed mutagenesis (Stratagene) was used to engineer Arm* (T52A/S56A) and CBPHATmut (Y2160A/F2161A). The Gal4-Arm and UAS-luc constructs are as previously described (Fang et al, 2006). Expression vectors of Flag-CBP, CBPHAT mutant and Arm* were constructed in pActin5.1 vector by standard PCR cloning or subcloning. An Arm*-V5 vector using the V5 epitope present in pActin5.1 was also constructed. The pSuper-CBP and pSuper-p300 constructs for siRNA were constructed as according to the manufacturer's (Brummelkamp TR) instructions, using hairpins from the coding region of each gene (p300 is 5′-GCTTGATGAATGCAGCCAA-3′; CBP is 5′-TGCTGCAGGCGGTGCTGGA-3′). All prokaryotic vectors for GST fusions proteins were constructed in the pET42a vector (Novagene). Bacterial expression for 6his-Flag-Arm 428-C and 6his-Arm FL were constructed in PET28a vector (Novagene). A fragment of p300 (residues 1254–1899) was cloned into pBluescript for in vitro translation.
Cell culture, transfection and reporter gene assays
293 or SW480 cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) containing 10% FBS at 37°C in a 5% CO2/95% air atmosphere. For transient transfections, 1 million 293 or SW480 cells were seeded in to 12-well plates 12 h before transfection. For 293 cells, Lubrofactimine 2000 was used as the transfection reagent according to the protocol provided by the manufacturer (Invitrogen). SW480 were transfected using the FuGENE6 transfection reagent (Roche Molecular Biochemicals). Kc cells were cultured and transfected as previously described (Fang et al, 2006). Luciferase and β-galactosidase activities of total cell lysates were determined using Luc-ScreenTM and Galacto-StarTM kits (Tropix). The values reported are the means and standard deviations of the results from two independent experiments.
Immunoprecipitations, Western blotting and immunostains
For each immunoprecipitation reaction, 5 million Kc cells were lysed in 500 μl of lysis buffer (1% Chaps, 20 mM HEPES, pH 7.5 and 140 mM NaCl) with the protease inhibitor cocktail from Roche for 60 min on ice. After centrifugation at 10 000 r.p.m. at 4°C, the supernatant was transferred into a new tube and incubated with antibody at 4°C for 1 h, followed by incubation with a 20 μl bed volume of protein A or protein G–Sepharose (Amersham Pharmacia) for 1 h. A 5 μl volume of mouse monoclonal anti-Arm (Hybridoma Bank, University of Iowa), mouse anti-V5 (Invitrogen) and mouse anti-Flag (Sigma) was used for immunoprecipitations. For precipitating endogenous CBP, 5 μl of affinity-purified chicken anti-dCBP antibody followed by 5 μg of rabbit anti-chicken IgY secondary antibody were used. Immunoprecipitates were washed four times with lysis buffer at 4°C. Proteins bound to the beads were eluted with SDS–loading buffer at 98°C for 2 min, subject to SDS–PAGE and then transferred to membrane, followed by Western blotting analysis using ECL plus kit (Amersham Pharmacia). Immunostaining of wing imaginal discs with mouse anti-Wg, rabbit anti-Dll and guinea pig anti-Sen was performed as previously described (Fang et al, 2006).
RNAi knockdown and Wg conditioned media treatment
Double-stranded RNA (dsRNA) corresponding to CBP and arm was synthesized as described (Fang et al, 2006). Fragments of both genes were amplified with oligos containing the T7 promoter and the following gene-specific sequences (CBP:5′-GGGTACGCCTCCTTACATACCCGC and 5′-CCGCCACAGCTGTCATCCATAAACTCC and Arm: 5′-ATGAGTTACATGCCAGCCCAGAATCGAA and 5′-CGATGGTGTGATAAGTTGTGCAGTGTTCCTA).
One million Kc cells were seeded in 12-well plate in Drosophila SFM (Invitrogen) in the presence of 10 μg specific dsRNA or control dsRNA. After culture at room temperature for 2 h, 5% of FBS was added and the cells were cultured for 4 days. WCM was prepared using stable pTubwg S2 cells, kindly provided by Dr R Nusse from Stanford University, and was typically concentrated to approximately 50-fold using a Centricon tube (Millipore) and stored at −80°C. Kc cells were treated with Wg-CM (10 μl/1 × 106 cells) for 4–8 h before harvesting.
RT–PCR and Q-PCR assays
Total RNA of Kc cells was purified using RNAwiz RNA isolation reagent (Ambion) and cDNA was synthesized with oligo-dT primers using SuperScript (Invitrogen). Quantitative PCR (Q-PCR) was performed as previously described (Fang et al, 2006). Primer pairs for notum are 5′-GCTGCT CTGCGTGATCGTCTTC-3′ and 5′-TCTGGTGTTGGTGAACTCTCCTCC-3′; primer pairs for nkd are 5′-TAAAATTCTCGGCGGCTACAA-3′ and 5′-CGCACCTGGTGGTACATCAG-3′. β-Tubulin 56D levels are used as a loading control as previously described (Fang et al, 2006). The values reported are the means and standard deviations of the results from two independent experiments.
ChIP
Kc cells (five million) were treated for 20 min with 5 mM dimethyl 3,3′-dithiobispropionimidate–HCl (DTBP) (Pierce) in PBS at room temperature, rinsed with 100 mM Tris–HCl, 150 mM NaCl (pH 8.0) and crosslinked with 1% formaldehyde in PBS at 37°C from 20 min. Total cell lysates were sonicated to generated 200–1000 bp DNA fragments. Immunoprecipitation was performed with specific antibody or control IgG (Upstate) using ChIP assay kit (Upstate). Promoter regions were detected by Q-PCR with specific primers. Primers pairs for NKD TCF cluster are 5′-TCAATCAGACGTCAGAGGTACCG-3′ and 5′-CTGATGGAAGAACCGTGTTGG-3′; primer pairs for NKD ORF are 5′-CCAGCATCGCTATCGACCA-3′ and 5′-GCGTCCTTCTCCTTTTCGCT-3′.
Drosophila genetics
The Rorth collection of EP elements (Rorth et al, 1998) was screened and as described previously (Parker et al, 2002). The P[GMR-Gal4] P[UAS-wg] and P[GMR-Gal4] P[GMR-arm*] are as described previously (Parker et al, 2002). The C96 Gal4 drivers was provided by J Krupp and Dpp-Gal4 was obtained from the Bloomington Stock Center. The P[UAS-CBP] and P[UAS-CBPHatmut] (Ludlam et al, 2002) were generously provided by S Smolik, as were the nej1 and nej3 alleles. The P[UAS-CBPRNAi] transgenic stock (Kumar et al, 2004) was generously provided by J Duffy. The nej alleles were recombined onto a chromosome containing P[FRT]18A by recombination as described (Xu and Rubin, 1993). Clones of nej3 were generated by mitotic recombination using hsFLP and a P[FRT]18BP[Ubi-GFP] RpS52 chromosome carrying a Minute mutation via a 1 h 37°C heat shock at 24–48 h after egg laying. During these experiments, two types of GFP-negative clones were obtained: 52% (n=176) were small in size and showed a high penetrance of defects consistent with a block in Wg signaling; the remainder were extremely large in size (usually occupying half the disc) and were phenotypically wild type. We believe these large clones are not the result of mitotic recombination (and hence not mutant for CBP). Rather, they could have arisen from an intrachromosomal loss of both the P[Ubi-GFP] transgene and the RpS52m mutation. This could occur if there was an additional P[FRT] insertion on the chromosome. Because we consider it impossible that these large clones are homozygous for nej3, they were not included in the summary of the phenotypes described in the Results.
Acknowledgments
We thank Nathan Campbell for help with preparation of the CBP antisera. We also thank S Smolik, A Hecht, M Bienz, J Duffy and R Nusse for reagents. We thank the Bloomington Stock Center for fly cultures and the Hybridoma Bank for anti-Arm and especially thank E Fearon and Guido Bommer for SW480 cells. This work was supported by NIH grants RO1 GM59846 and CA95869 to KMC.
References
- Akimaru H, Hou DX, Ishii S (1997) Drosophila CBP is required for dorsal-dependent twist gene expression. Nat Genet 17: 211–214 [DOI] [PubMed] [Google Scholar]
- Barker N, Hurlstone A, Musisi H, Miles A, Bienz M, Clevers H (2001) The chromatin remodelling factor Brg-1 interacts with beta-catenin to promote target gene activation. EMBO J 20: 4935–4943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R (1997) Wnt signaling: a common theme in animal development. Genes Dev 11: 3286–3305 [DOI] [PubMed] [Google Scholar]
- Cavallo RA, Cox RT, Moline MM, Roose J, Polevoy GA, Clevers H, Peifer M, Bejsovec A (1998) Drosophila Tcf and Groucho interact to repress Wingless signalling activity. Nature 395: 604–608 [DOI] [PubMed] [Google Scholar]
- Couso JP, Bishop SA, Martinez Arias A (1994) The wingless signalling pathway and the patterning of the wing margin in Drosophila. Development 120: 621–636 [DOI] [PubMed] [Google Scholar]
- Cox RT, Pai LM, Kirkpatrick C, Stein J, Peifer M (1999) Roles of the C terminus of Armadillo in Wingless signaling in Drosophila. Genetics 153: 319–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels DL, Eklof Spink K, Weis WI (2001) beta-Catenin: molecular plasticity and drug design. Trends Biochem Sci 26: 672–678 [DOI] [PubMed] [Google Scholar]
- Daniels DL, Weis WI (2002) ICAT inhibits beta-catenin binding to Tcf/Lef-family transcription factors and the general coactivator p300 using independent structural modules. Mol Cell 10: 573–584 [DOI] [PubMed] [Google Scholar]
- Daniels DL, Weis WI (2005) Beta-catenin directly displaces Groucho/TLE repressors from Tcf/Lef in Wnt-mediated transcription activation. Nat Struct Mol Biol 12: 364–371 [DOI] [PubMed] [Google Scholar]
- Ding Y, Dale T (2002) Wnt signal transduction: kinase cogs in a nano-machine? Trends Biochem Sci 27: 327–329 [DOI] [PubMed] [Google Scholar]
- Emami KH, Nguyen C, Ma H, Kim DH, Jeong KW, Eguchi M, Moon RT, Teo JL, Kim HY, Moon SH, Ha JR, Kahn M (2004) A small molecule inhibitor of beta-catenin/CREB-binding protein transcription [corrected]. Proc Natl Acad Sci USA 101: 12682–12687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang M, Li J, Blauwkamp T, Bhambhani C, Campbell N, Cadigan KM (2006) C-terminal-binding protein directly activates and represses Wnt transcriptional targets in Drosophila. EMBO J 25: 2735–2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman M, Bienz M (2001) EGF receptor/Rolled MAP kinase signalling protects cells against activated Armadillo in the Drosophila eye. EMBO Rep 2: 157–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlitz O, Basler K (2002) Wingful, an extracellular feedback inhibitor of Wingless. Genes Dev 16: 1055–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez AJ, Copley RR, Cohen SM (2002) HSPG modification by the secreted enzyme Notum shapes the Wingless morphogen gradient. Dev Cell 2: 667–676 [DOI] [PubMed] [Google Scholar]
- Grant PA, Berger SL (1999) Histone acetyltransferase complexes. Semin Cell Dev Biol 10: 169–177 [DOI] [PubMed] [Google Scholar]
- Guder C, Philipp I, Lengfeld T, Watanabe H, Hobmayer B, Holstein TW (2006) The Wnt code: cnidarians signal the way. Oncogene 25: 7450–7460 [DOI] [PubMed] [Google Scholar]
- Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R (2000) The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J 19: 1839–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht A, Stemmler MP (2003) Identification of a promoter-specific transcriptional activation domain at the C terminus of the Wnt effector protein T-cell factor 4. J Biol Chem 278: 3776–3785 [DOI] [PubMed] [Google Scholar]
- Hoffmans R, Stadeli R, Basler K (2005) Pygopus and legless provide essential transcriptional coactivator functions to armadillo/beta-catenin. Curr Biol 15: 1207–1211 [DOI] [PubMed] [Google Scholar]
- Hsu SC, Galceran J, Grosschedl R (1998) Modulation of transcriptional regulation by LEF-1 in response to Wnt-1 signaling and association with beta-catenin. Mol Cell Biol 18: 4807–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Guo M, Hay BA (2004) Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol 14: 1262–1266 [DOI] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG (2002) Identification of a Wnt/Dvl/beta-catenin → Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 111: 673–685 [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Weger R, Kinzler KW, Vogelstein B, Clevers H (1997) Constitutive transcriptional activation by a beta-catenin–Tcf complex in APC−/− colon carcinoma. Science 275: 1784–1787 [DOI] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K (2002) Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109: 47–60 [DOI] [PubMed] [Google Scholar]
- Kumar JP, Jamal T, Doetsch A, Turner FR, Duffy JB (2004) CREB binding protein functions during successive stages of eye development in Drosophila. Genetics 168: 877–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labalette C, Renard CA, Neuveut C, Buendia MA, Wei Y (2004) Interaction and functional cooperation between the LIM protein FHL2, CBP/p300, and beta-catenin. Mol Cell Biol 24: 10689–10702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy L, Wei Y, Labalette C, Wu Y, Renard CA, Buendia MA, Neuveut C (2004) Acetylation of beta-catenin by p300 regulates beta-catenin–Tcf4 interaction. Mol Cell Biol 24: 3404–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R (2004) The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol 20: 781–810 [DOI] [PubMed] [Google Scholar]
- Ludlam WH, Taylor MH, Tanner KG, Denu JM, Goodman RH, Smolik SM (2002) The acetyltransferase activity of CBP is required for wingless activation and H4 acetylation in Drosophila melanogaster. Mol Cell Biol 22: 3832–3841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H, Nguyen C, Lee KS, Kahn M (2005) Differential roles for the coactivators CBP and p300 on TCF/beta-catenin-mediated survivin gene expression. Oncogene 24: 3619–3631 [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Rulifson EJ, Blair SS (1997) The function and regulation of cut expression on the wing margin of Drosophila: Notch, Wingless and a dominant negative role for Delta and Serrate. Development 124: 1485–1495 [DOI] [PubMed] [Google Scholar]
- Miyagishi M, Fujii R, Hatta M, Yoshida E, Araya N, Nagafuchi A, Ishihara S, Nakajima T, Fukamizu A (2000) Regulation of Lef-mediated transcription and p53-dependent pathway by associating beta-catenin with CBP/p300. J Biol Chem 275: 35170–35175 [DOI] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K (2006) Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/Armadillo. Cell 125: 327–341 [DOI] [PubMed] [Google Scholar]
- Natarajan L, Witwer NE, Eisenmann DM (2001) The divergent Caenorhabditis elegans beta-catenin proteins BAR-1, WRM-1 and HMP-2 make distinct protein interactions but retain functional redundancy in vivo. Genetics 159: 159–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann CJ, Cohen SM (1997) Long-range action of Wingless organizes the dorsal–ventral axis of the Drosophila wing. Development 124: 871–880 [DOI] [PubMed] [Google Scholar]
- Parker DS, Blauwkamp T, Cadigan KM (2007) Wnt-mediated transcriptional regulation. In Wnt Signaling in Embryonic Development, Sokol S (ed), Advances in Developmental Biology, Wassarman PM (ed) Vol 17, pp 1–61. San Diego: Elsevier [Google Scholar]
- Parker DS, Jemison J, Cadigan KM (2002) Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129: 2565–2576 [DOI] [PubMed] [Google Scholar]
- Perez-Garijo A, Martin FA, Morata G (2004) Caspase inhibition during apoptosis causes abnormal signalling and developmental aberrations in Drosophila. Development 131: 5591–5598 [DOI] [PubMed] [Google Scholar]
- Polakis P (2000) Wnt signaling and cancer. Genes Dev 14: 1837–1851 [PubMed] [Google Scholar]
- Roose J, Clevers H (1999) TCF transcription factors: molecular switches in carcinogenesis. Biochim Biophys Acta 1424: M23–M37 [DOI] [PubMed] [Google Scholar]
- Roose J, Molenaar M, Peterson J, Hurenkamp J, Brantjes H, Moerer P, van de Wetering M, Destree O, Clevers H (1998) The Xenopus Wnt effector XTcf-3 interacts with Groucho-related transcriptional repressors. Nature 395: 608–612 [DOI] [PubMed] [Google Scholar]
- Rorth P, Szabo K, Bailey A, Laverty T, Rehm J, Rubin GM, Weigmann K, Milan M, Benes V, Ansorge W, Cohen SM (1998) Systematic gain-of-function genetics in Drosophila. Development 125: 1049–1057 [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H (2004) Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Dev Cell 7: 491–501 [DOI] [PubMed] [Google Scholar]
- Sierra J, Yoshida T, Joazeiro CA, Jones KA (2006) The APC tumor suppressor counteracts beta-catenin activation and H3K4 methylation at Wnt target genes. Genes Dev 20: 586–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadeli R, Basler K (2005) Dissecting nuclear Wingless signalling: recruitment of the transcriptional co-activator Pygopus by a chain of adaptor proteins. Mech Dev 122: 1171–1182 [DOI] [PubMed] [Google Scholar]
- Sun Y, Kolligs FT, Hottiger MO, Mosavin R, Fearon ER, Nabel GJ (2000) Regulation of beta-catenin transformation by the p300 transcriptional coactivator. Proc Natl Acad Sci USA 97: 12613–12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemaru KI, Moon RT (2000) The transcriptional coactivator CBP interacts with beta-catenin to activate gene expression. J Cell Biol 149: 249–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F (1999) Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 398: 422–426 [DOI] [PubMed] [Google Scholar]
- Thompson BJ (2004) A complex of Armadillo, Legless, and Pygopus coactivates dTCF to activate wingless target genes. Curr Biol 14: 458–466 [DOI] [PubMed] [Google Scholar]
- van de Wetering M, Cavallo R, Dooijes D, van Beest M, van Es J, Loureiro J, Ypma A, Hursh D, Jones T, Bejsovec A, Peifer M, Mortin M, Clevers H (1997) Armadillo coactivates transcription driven by the product of the Drosophila segment polarity gene dTCF. Cell 88: 789–799 [DOI] [PubMed] [Google Scholar]
- van Es JH, Barker N, Clevers H (2003) You Wnt some, you lose some: oncogenes in the Wnt signaling pathway. Curr Opin Genet Dev 13: 28–33 [DOI] [PubMed] [Google Scholar]
- Waltzer L, Bienz M (1998) Drosophila CBP represses the transcription factor TCF to antagonize Wingless signalling. Nature 395: 521–525 [DOI] [PubMed] [Google Scholar]
- Wolf D, Rodova M, Miska EA, Calvet JP, Kouzarides T (2002) Acetylation of beta-catenin by CREB-binding protein (CBP). J Biol Chem 277: 25562–25567 [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM (1993) Analysis of genetic mosaics in developing and adult Drosophila tissues. Development 117: 1223–1237 [DOI] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G (1996) Direct and long-range action of a wingless morphogen gradient. Cell 87: 833–844 [DOI] [PubMed] [Google Scholar]
- Zeng W, Wharton KA Jr, Mack JA, Wang K, Gadbaw M, Suyama K, Klein PS, Scott MP (2000) Naked cuticle encodes an inducible antagonist of Wnt signalling. Nature 403: 789–795 [DOI] [PubMed] [Google Scholar]


