Abstract
The histone chaperones CAF-1 and Rtt106p are required for heterochromatin silencing in the yeast Saccharomyces cerevisiae. Although it has been suggested that CAF-1 is involved in the maintenance of heterochromatin silencing, their exact functions during this process are not well understood. Here, we show that CAF-1 and Rtt106p are involved in the early stages of heterochromatin formation. The binding of Sir proteins to telomeric heterochromatin is significantly reduced and, additionally, Sir proteins are mislocalized in cells lacking CAF-1 and Rtt106p. At the HMR locus, CAF-1 and Rtt106p are required for the initial recruitment of Sir2p and Sir3p, but not Sir4p, to the HMR-E silencer, where silencing initiates, as well as the efficient spreading of all of these Sir proteins to the distal a1 gene. Moreover, silencing at the HMR locus is dramatically reduced in cells lacking CAF-1, Rtt106p, and Sir1p. Thus, these studies reveal a novel role for CAF-1 and Rtt106p in epigenetic silencing and indicate that the spreading of heterochromatin, a poorly understood process, requires histone chaperones.
Keywords: CAF-1, heterochromatin, histone chaperones, Rtt106, silencing
Introduction
In eukaryotic cells, heterochromatin structure governs a variety of cellular processes, including regulation of gene expression and maintenance of genomic integrity (Ahmad and Henikoff, 2002; Richards and Elgin, 2002). Accordingly, appropriate heterochromatin formation and maintenance are essential for normal cell functions, and disruption of these processes has been linked to developmental defects, aging, and cancer (Jones and Baylin, 2002). Thus, it is of great interest to determine the mechanisms by which proteins contribute to heterochromatin formation and maintenance.
A heterochromatin-like structure is formed at telomeres, the silent mating-type loci HMR and HML, and the rDNA locus in the budding yeast Saccharomyces cerevisiae (Grunstein, 1998; Rusche et al, 2003), leading to transcriptional silencing. Four Sir proteins, silent information regulators, serve as both structural and enzymatic components of yeast heterochromatin. Sir1p appears to be involved in silencing at the HM loci but not at telomeres (Aparicio et al, 1991), whereas the other three Sir proteins mediate heterochromatin formation at both telomers and the HM loci.
A stepwise model has been proposed to explain the formation of heterochromatin at telomeres and the HM loci in budding yeast (Triolo and Sternglanz, 1996; Hoppe et al, 2002; Luo et al, 2002; Rusche et al, 2002). In addition, this mechanism of heterochromatin formation seems to be conserved in higher eukaryotic cells (Moazed, 2001). First, Sir4p is recruited to a specific site such as telomere ends or silencers at the HM loci through interactions with sequence-specific DNA-binding proteins to initiate heterochromatin formation. Next, Sir4p recruits Sir2p, a nicotinamide adenine dinucleotide (NAD)-dependent histone deacetylase (Imai et al, 2000; Landry et al, 2000), which deacetylates histones of nearby nucleosomes. Subsequently, Sir3p and Sir4p are recruited to the adjacent nucleosome, because Sir3p and Sir4p bind to hypoacetylated forms of histones with higher affinity (Carmen et al, 2002; Liou et al, 2005). The sequential recruitment of Sir proteins and modification of histones is repeated, allowing for the spreading of Sir proteins throughout the silent chromatin domain (Moazed et al, 1997; Hoppe et al, 2002; Luo et al, 2002; Rusche et al, 2002). Finally, the spreading of heterochromatin must be terminated. At telomeres, acetylation of H4 lysine 16 (H4-K16) at adjacent euchromatin by the histone acetyltransferase Sas2p is important for restricting Sir proteins to the telomeric heterochromatin (Kimura et al, 2002; Suka et al, 2002), whereas at the HMR locus, boundary elements prevent the spreading of Sir proteins into euchromatin (Donze et al, 1999, 2001).
It has been suggested that formation of heterochromatin is a gradual process that consists of an early phase during which heterochromatin is partially assembled and histones are rapidly deacetylated, followed by a slow maturation phase when demethylation of histones gradually occurs (Katan-Khaykovich and Struhl, 2005). Analysis of silencing in single yeast cells indicates that silent chromatin formation is stochastic, with individual cells differentially acquiring the silenced state independent of progression through a set number of cell cycles (Xu et al, 2006). Despite significant progress in understanding the mechanisms of heterochromatin formation, it remains largely unknown how spreading, the second step of heterochromatin formation, occurs in yeast or other organisms.
CAF-1, Asf1p, Hir1p, and Rtt106p are histone H3 and H4 chaperones that are involved in either DNA replication-dependent (CAF-1, Asf1p, Rtt106p) (Tyler et al, 1999; Tagami et al, 2004; Huang et al, 2005) and/or DNA replication-independent (Hir1p and Asf1p) (Green et al, 2005) nucleosome assembly. Cells deficient in each histone chaperone are partially defective in heterochromatin silencing (Enomoto et al, 1997; Kaufman et al, 1997; Enomoto and Berman, 1998; Sutton et al, 2001; Huang et al, 2005). Whereas Rtt106p, Asf1p, and Hir1p appear to function in the same genetic pathway, CAF-1 may mediate a partially redundant, but genetically distinguishable, silencing pathway in yeast cells (Sharp et al, 2001; Huang et al, 2005). However, it remains largely unknown how these histone chaperones affect silencing. At least two models have been proposed to explain the function of these histone chaperones in silencing. First, it has been proposed that CAF-1 is involved in the maintenance but not re-establishment (i.e. formation) of heterochromatin in yeast (Enomoto and Berman, 1998). Second, CAF-1 and Asf1p physically interact with Sas2p (Meijsing and Ehrenhofer-Murray, 2001; Osada et al, 2001); thus, it has been proposed that the silencing defects observed in CAF-1 mutant cells are due to loss of H4-K16 acetylation. Here, we show that yeast cells containing mutations in histone chaperones CAF-1 and Rtt106 are defective in silencing not owing to altered acetylation of H4-K16, but instead because of the reduced association of Sir proteins with telomeric heterochromatin. Moreover, we present evidence showing for the first time that CAF-1 and Rtt106p mediate heterochromatin formation by contributing to the spreading of Sir proteins during the early stages of heterochromatin formation. Thus, this study reveals a novel role for CAF-1 and Rtt106p in heterochromatin formation.
Results
Sir4p binding to telomere ends or the HMR-E silencer does not depend on CAF-1 and Rtt106p
Although deletion of each histone chaperone CAF-1, Rtt106p, Asf1p, or Hir1p is known to affect heterochromatin silencing in yeast, their exact functions in this process are unknown. Therefore, to gain insights into the functions of these histone chaperones in silencing, we decided to perform chromatin immunoprecipitation (ChIP) assays and analyze the effects of mutations in histones chaperones on the binding of Sir proteins to different regions of silent chromatin, including telomeric heterochromatin and the HMR locus. As previous studies have shown that deletion of the CAC1 gene, the large subunit of CAF-1, alone has very minor, if any, effects on the localization of Sir proteins (Enomoto and Berman, 1998; Zhou et al, 2006), we decided to study the effects of double deletion mutants for CAC1 and RTT106, ASF1, or HIR1. Similar defects in the localization of Sir proteins at heterochromatin were observed for the double mutants (cac1Δ rtt106Δ, cac1Δ asf1Δ, cac1Δ hir1Δ), supporting the concept that Rtt106p, Asf1p, and Hir1p function in the same genetic pathway (Huang et al, 2005). Thus, we have focused on the role of CAF-1 in combination with Rtt106p in heterochromatin silencing, and the data supporting a role for Asf1p and Hir1p in this process are presented as Supplementary data.
Telomeric silent chromatin is initiated by the recruitment of Sir4p to telomere ends through Rap1–Sir4p interactions in a manner that does not require the presence of Sir2p and Sir3p. In contrast, the association of Sir4p to the more distal telomeric heterochromatin does require Sir2p and Sir3p, as well as yKu70 and yKu80 (Martin et al, 1999; Luo et al, 2002). Therefore, we first determined whether CAF-1 and Rtt106p are required for the initial recruitment of Sir4p to telomere ends using ChIP assays. In order to restrict our analysis to the binding of Sir4p to telomere ends as opposed to telomeric heterochromatin (see cartoon, Figure 1A), we deleted the SIR3 gene from the cac1Δ rtt106Δ double mutant cells, which should eliminate the binding of Sir4p to telomeric heterochromatin. The association of Sir4p with telomere ends was then compared between cac1Δ rtt106Δ sir3Δ triple mutant cells and sir3Δ mutant cells. In this way, we could differentiate the potential effects of CAF-1 and Rtt106p on the binding of Sir4p to telomere ends versus telomeric heterochromatin using ChIP assays, despite the fact that the DNA fragments used in the ChIP assays were on average 0.5–1 kb in length. Sir4p bound preferentially to the telomere end (0.07 kb from the telomere end) as compared with euchromatin (20 kb from the telomere end) in wild-type cells and this binding was abolished in sir4Δ mutant cells, demonstrating the specificity of the antibodies against Sir4p used to perform the ChIP assays (Figure 1B). In addition, significant amounts of Sir4p were still detected at telomere ends in the sir3Δ mutant cells, as previously reported (Luo et al, 2002). More importantly, similar amounts of Sir4p were detected at telomere ends in cac1Δ rtt106Δ sir3Δ triple mutant cells compared with sir3Δ mutant cells (Figure 1B). Thus, CAF-1 and Rtt106p are not required for the initial recruitment of Sir4p to telomere ends to initiate silencing.
Figure 1.
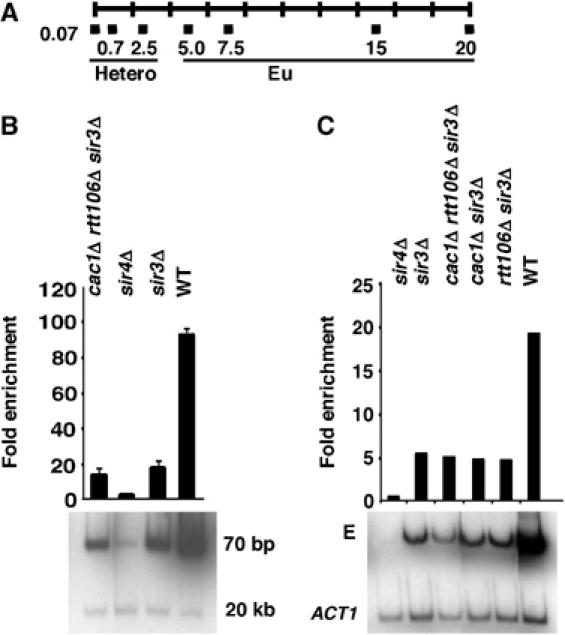
Sir4p binding to telomere ends and the HMR-E silencer is independent of the CAF-1 and Rtt106p. (A) A schematic representation of PCR primers used to analyze DNA immunoprecipitated with anti-Sir4p antibodies. The primers (black boxes) were targeted to areas representing telomeric heterochromatin (Hetero; 0.07, 0.77, and 2.5 kb from the telomere end) or the adjacent euchromatin (Eu; 5.0, 7.5, 15, and 20 kb from the telomere end) of chromosome VI-R. (B) CAF-1 and Rtt106p are not required for Sir4p binding to telomere ends. ChIP assays were performed using yeast strains of the indicated genotype and antibodies against Sir4p. The immunoprecipitated DNA was detected using either a PCR primer set amplifying DNA 70 bp to the right end of chromosome VI or another primer set amplifying DNA 20 kb away from the telomere end, as a negative control. The relative enrichment was calculated as described in Supplementary data. (C) CAF-1 and Rtt106p are not required for the association of Sir4p with the HMR-E silencer. ChIP assays were performed as described above and the immunoprecipitated DNA was analyzed using PCR primers amplifying the HMR-E silencer and ACT1 gene, as negative control.
Although heterochromatin formation occurs through slightly different mechanisms at HM loci and telomeres, initiation of silencing at the HMR-E silencer does involve the recruitment of Sir4p (Hoppe et al, 2002; Rusche et al, 2002). Therefore, we next tested whether CAF-1 and Rtt106p affect Sir4p binding to the HMR-E silencer. As shown in Figure 1C and in agreement with results published by others, recruitment of Sir4p to the HMR-E silencer was independent of the presence of Sir3p (Rusche et al, 2002). Moreover, similar amounts of Sir4p were detected at the HMR-E silencer in cac1Δ rtt106Δ sir3Δ triple mutant cells and sir3Δ mutant cells. Thus, like the initial recruitment of Sir4p to telomere ends, recruitment of Sir4p to the HMR-E silencer also does not require CAF-1 and Rtt106p.
Sir4p binding to telomeric heterochromatin is dramatically reduced in cells lacking histone chaperones
We next tested whether mutations in CAF-1 and Rtt106p affect the binding of Sir4p to telomeric heterochromatin. ChIP assays were performed on cac1Δ rtt106Δ double mutant cells as well as sir3Δ and sir4Δ single mutant cells as controls, using antibodies against Sir4p. Primer sets targeting DNA 0.77 and 2.5 kb away from the telomere end (telomeric heterochromatin) or 5, 7.5, and 15 kb away from the telomere end (euchromatin) were used to amplify the immunoprecipitated DNA (Figure 1A). As shown in Figure 2A and B, the association of Sir4p with telomeric heterochromatin in cac1Δ rtt106Δ double mutant cells was significantly reduced compared with wild-type cells. In addition to the reduced binding of Sir4p, we also observed a dramatic reduction in the amounts of Sir2p and Sir3p bound to telomeric heterochromatin in cac1Δ rtt106Δ double mutant cells (Supplementary Figure S1). Together, these results suggest that silencing defects observed in cac1Δ rtt106Δ double mutant cells, as well as cac1Δ asf1Δ and cac1Δ hir1Δ double mutant cells (Supplementary Figure S2), are due to a reduction of Sir proteins at telomeric heterochromatin.
Figure 2.
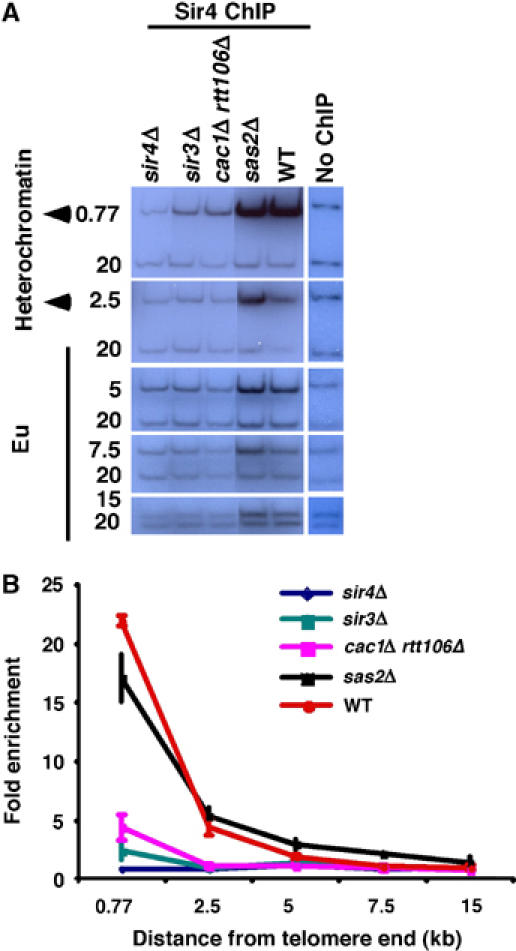
Sir4p binding to telomeric heterochromatin requires CAF-1 and Rtt106p. (A, B) The amount of Sir4p associated with telomeric heterochromatin is decreased in cac1Δ rtt106Δ double mutant cells as compared with wild-type cells. ChIP assays were performed using yeast strains of the indicated genetic backgrounds. The relative enrichment was calculated and plotted against the positions of primers from the telomere end (Figure 1A). As controls, binding of Sir4p in sir3Δ, sir4Δ, and sas2Δ mutant cells was also determined. In contrast to cac1Δ rtt106Δ double mutant cells, an increased association of Sir4p (twofold) with euchromatin was observed in sas2Δ mutant cells as compared to wild-type cells.
Deletion of CAC1 and RTT106 does not lead to aberrant spreading of Sir4p to telomeric euchromatin
Sir proteins are restricted to telomeric heterochromatin by modification of histones located at euchromatin, such as acetylation of H4-K16 by the histone acetyltransferase Sas2p (Kimura et al, 2002; Suka et al, 2002; Sutton et al, 2003). Mutations in Sas2p result in promiscuous spreading of Sir proteins to euchromatin (Kimura et al, 2002; Suka et al, 2002). Indeed, we observed a spreading of Sir4p into euchromatin in sas2Δ mutant cells (Figure 2A and B). CAF-1 physically interacts with Sas2p; thus, deletion of CAC1 and RTT106 could affect the levels of Sir4p at telomeric heterochromatin by altering the localization or activity of Sas2p. If this were the case, a similar phenotype, that is the spreading of Sir4p into euchromatin, would be expected in sas2Δ and cac1Δ rtt106Δ mutant cells. However, we did not detect any significant association of Sir4p with telomeric euchromatin in cac1Δ rtt106Δ double mutant cells (Figure 2A and B), indicating that these histone chaperones mediate heterochromatin silencing in a manner distinct from that of Sas2p.
Next we directly tested whether H4-K16 acetylation at the heterochromatin–euchromatin boundary is affected in cac1Δ rtt106Δ double mutant cells. Therefore, ChIP assays were performed in cac1Δ rtt106Δ double mutant cells as well as wild-type, sas2Δ, sir3Δ, and sir4Δ cells as controls, using antibodies against acetylated H4-K16 (Figure 3). As expected, in wild-type cells, acetylated H4-K16 was enriched at euchromatic as compared with heterochromatic regions, whereas acetylation of H4-K16 at euchromatin was significantly reduced in sas2Δ mutant cells. In contrast, H4-K16 acetylation was increased at telomeric heterochromatin in both sir3Δ and sir4Δ single mutant cells. Interestingly, acetylation of H4-K16 at heterochromatic region was altered in cac1Δ rtt106Δ double mutant cells in a manner similar to that observed for sir3Δ and sir4Δ single mutant cells, namely, H4-K16 acetylation at telomeric heterochromatin was increased, but different from that of sas2Δ mutant cells. Similar effects were also observed in cac1Δ hir1Δ and cac1Δ asf1Δ double mutant cells (Supplementary Figure S3). Thus, these results further support the concept that silencing defects in cac1Δ rtt106Δ double mutant cells are not due to alterations in H4-K16 acetylation as proposed (Rusche et al, 2003), but rather reduced association of Sir proteins with telomeric heterochromatin.
Figure 3.
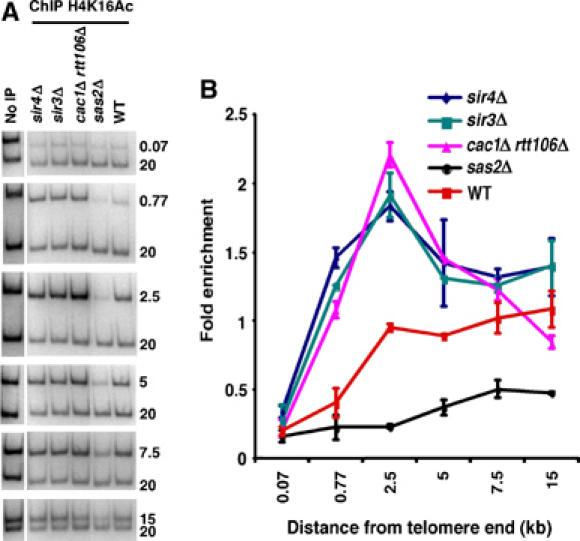
Deletion of CAC1 and RTT106 affects acetylation of histone H4 lysine 16 in a manner similar to that of sir3Δ and sir4Δ mutants. (A, B) ChIP assays were performed using yeast strains of the indicated genotype and antibodies that recognize H4 acetylated at lysine 16 (H4K16Ac). The immunoprecipitated DNA was analyzed using the primer sets depicted in Figure 1A and resolved on polyacrylamide gels (A). The relative enrichment was calculated and plotted against primer positions from the telomere end (B).
Localization of Sir3p and Sir4p to the nuclear periphery is altered in cac1Δ rtt106Δ double mutant cells
In yeast cells, Sir2p, Sir3p, and Sir4p localize to distinct foci at the nuclear periphery, which most likely represent clusters of telomeres (Palladino et al, 1993; Hoppe et al, 2002). Our above results suggest that histone chaperones are required for the proper association of Sir proteins with telomeric heterochromatin. Therefore, we next determined whether CAF-1 and Rtt106p play a role in the global localization of Sir proteins. To observe the localization of Sir3p and Sir4p in living cells, the proteins were tagged with green fluorescent protein (GFP) and expressed in either wild-type, cac1Δ, rtt106Δ, or cac1Δ rtt106Δ cells. As reported (Hoppe et al, 2002), Sir3p-GFP and Sir4p-GFP localized to telomeric foci in wild-type cells (Figure 4A and C). Although mutations in either CAF-1 or Rtt106p had little effect on the normal peripheral nuclear localization of Sir proteins (data not shown), Sir3p-GFP and Sir4p-GFP became dispersed in a majority of cac1Δ rtt106Δ double mutant cells (Figure 4B and D). Some Sir3p-GFP and Sir4p-GFP foci were detected in a small fraction of mutant cells (data not shown), possibly as a result of incomplete silencing or residual binding of Sir3p-GFP and Sir4p-GFP to telomere ends in these mutant cells. These results, combined with the observed association of Sir proteins with telomeric heterochromatin using ChIP assays, strongly support the idea that both CAF-1 and Rtt106p are required for the proper association of Sir proteins with telomeric heterochromatin.
Figure 4.
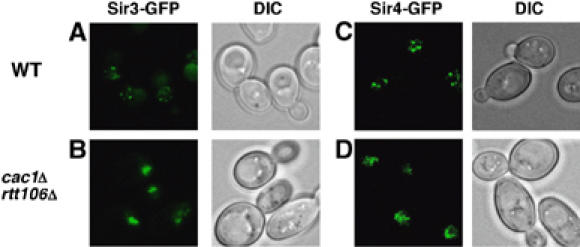
Localization of Sir3p and Sir4p to foci at the nuclear periphery is altered in cac1Δ rtt106Δ double mutant cells. (A–D) In wild-type (WT) cells, GFP-tagged Sir3p (Sir3p-GFP; A) and Sir4p (Sir4p-GFP; C) are detected as discrete foci at the nuclear periphery, presumably representing clustered telomeres. In contrast, in most cac1Δ rtt106Δ double mutant cells, both Sir3p-GFP (B) and Sir4p-GFP (D) exhibit a diffuse localization pattern. DIC images of the corresponding fluorescence micrographs are also shown.
CAF-1 and Rtt106p are required to silence the a1 gene at the HMR locus
In addition to telomeric silencing, cac1Δ rtt106Δ double mutant cells exhibited a significant reduction in silencing at the HMR locus when assayed using a GFP reporter gene (Huang et al, 2005). Therefore, to gain further insight into the function of CAF-1 and Rtt106p in silencing, we next determined whether CAF-1 and Rtt106p participate in the formation of heterochromatin at the HMR locus. For these experiments, we deleted the endogenous SIR3 gene from either wild-type or mutant strains (cac1Δ, rtt106Δ, cac1Δ rtt106Δ) in order to abolish silencing at the HMR locus and then monitored the ability of galactose-induced HA-tagged Sir3p (Sir3p-HA) expression to initiate silent chromatin formation in these various genetic backgrounds (Figure 5A and Supplementary data). Briefly, cells were first arrested in early S phase using hydroxyurea (HU); Sir3p-HA expression was then induced following release of synchronized cells from the cell cycle block; and finally, samples were analyzed at 1.5-h intervals for cell cycle progression (FACS analysis; data not shown), transcription of the a1 gene (Figure 5B), and Sir3p-HA expression (Figure 5C). Transcription of the a1 gene was monitored, because in normal wild-type MATα strains, the a1 gene is located at the HMR locus and is silenced owing to the formation of heterochromatin. In addition, the half-life of a1 mRNA is very short (about 3 min), making it possible to detect potential differences in heterochromatin formation at the HMR locus over a relatively short time frame. In wild-type cells, Sir3p-HA expression could be detected after 1.5 h of induction and did not increase significantly over the next 6 h (Figure 5C). Interestingly, silencing of the a1 gene was observed over a similar time frame and by 7.5 h post-induction, a1 mRNA was barely detectable (Figure 5B and D). The cell doubling time was about 3 h under these conditions; thus, our results are consistent with published results, indicating that it takes multiple cell divisions for cells to silence the a1 gene upon expression of Sir3p-HA (Katan-Khaykovich and Struhl, 2005). In the cac1Δ, rtt106Δ, and cac1Δ rtt106Δ mutant cells, significantly more a1 mRNA was detected as compared with wild-type cells during the first 3 h of Sir3p-HA expression (Figure 5B and D), with the double mutant cells exhibiting a much greater effect than either single mutant alone. This reduction in silencing of a1 transcription at early time points in the cac1Δ, rtt106Δ, and cac1Δ rtt106Δ mutant cells suggests that the formation of heterochromatin at the HMR locus is impaired by depletion of either Cac1p or Rtt106p, and further that depletion of both proteins has a synergistic effect. In support of this potential synergism between Cac1p and Rtt106p in heterochromatin formation, whereas a1 mRNA was barely detectable 7.5 h after expression of Sir3p-HA in cac1Δ and rtt106Δ single mutant cells, significant amounts of a1 mRNA could still be detected in the cac1Δ rtt106Δ double mutant cells at this time point (Figure 5B and D). The defects in the silencing of a1 transcription observed in cac1Δ, rtt106Δ, and cac1Δ rtt106Δ mutant cells did not appear to be due to differences in cell cycle progression (data not shown) or expression levels of Sir3p-HA (Figure 5C). Thus, these results indicate that CAF-1 and Rtt106p play a direct role in the early stages of heterochromatin formation at the HMR locus.
Figure 5.
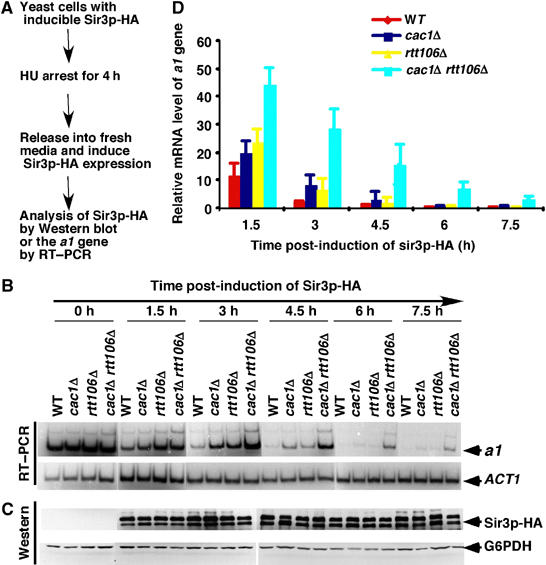
Silencing of the a1 gene at the HMR locus is altered in cac1Δ and rtt106Δ single and double mutant cells. (A) A schematic diagram showing the experimental design for analyzing silencing of the a1gene; see Supplementary data for details. (B) Single and double mutants of cac1Δ and rtt106Δ exhibit a reduced ability to silence the a1 gene. RT–PCR was performed to monitor expression of the a1 gene and ACT1 mRNA was used as a loading control for each time point. (C) Similar levels of Sir3p-HA expression are induced over the same time frame in wild-type and different deletion mutant cells of the indicated genotype. Sir3p-HA levels and G6PDHp levels, as a loading control, were detected by Western blot analysis using anti-HA and anti-G6PDHp antibodies, respectively. (D) Quantification of expression of the a1 gene. PCR products shown in (B) were quantified using a phosphorimager and plotted against time following induction of Sir3p-HA expression.
Under certain conditions, HU inhibits silent chromatin formation and subsequent repression of a1 transcription when the gene is present within the chromosome (Kirchmaier and Rine, 2006). In the above experiments, we synchronized cells first by arresting them in early S phase using HU; thus, this could result in indirect effects of cac1Δ and rtt106Δ on transcription of the a1 gene. As shown in Supplementary Figure S4, silencing of the a1 gene was also significantly compromised in unsynchronized cac1Δ rtt106Δ double mutant cells not exposed to HU. Therefore, HU treatment does not appear to contribute to the impaired ability of cac1Δ rtt106Δ double mutant cells to form silent chromatin. Thus, we conclude that CAF-1 and Rtt106p are directly involved in heterochromatin formation at the HMR locus.
CAF-1 and Rtt106p are involved in the binding of Sir2p and Sir3p to the HMR-E silencer and subsequent spreading of all Sir proteins to the a1 gene
The histone chaperones CAF-1 and Rtt106p could mediate silent chromatin formation at the HMR locus through their effects on Sir protein recruitment to the HMR-E silencer, where silencing initiates, and/or Sir protein spreading to the distal a1 gene. Therefore, we next tested whether the recruitment of Sir proteins to the HMR-E silencer and/or spreading of Sir proteins to the distal a1 gene was altered in cac1Δ rtt106Δ double mutant cells by performing ChIP assays using antibodies against Sir2p, Sir3p, and Sir4p. As described above, wild-type and cac1Δ rtt106Δ double mutant cells were first arrested using HU and then ChIP assays were performed after release of the cell cycle block and subsequent induction of Sir3p-HA expression. The immunoprecipitated DNA was amplified using primer sets targeting either the HMR-E silencer or a1 gene (Figure 6A). In wild-type cells, recruitment of Sir2p, Sir3p-HA, and Sir4p to both the HMR-E silencer (Figure 6B–D) and the a1 gene (Figure 6E–G) could be detected 1.5 h after induction of Sir3p-HA expression, and the amounts of Sir proteins at the sites tested did not increase significantly after 3 h of Sir3p-HA expression. Sir4p was recruited to the HMR-E silencer in the cac1Δ rtt106Δ double mutant cells over a time frame similar to that observed in wild-type cells (Figure 6D), consistent with our observation that Sir4p binding to the HMR-E silencer is independent of CAF-1 and Rtt106p (Figure 1C). In contrast, the association of Sir4p with the distal a1 gene was significantly delayed in cac1Δ rtt106Δ double mutant cells compared with wild-type cells during the first 3 h of Sir3p-HA expression (Figure 6G), suggesting that the spreading of Sir4p from the HMR-E silencer was affected. Similar amounts of Sir4p were, however, detected at the a1 gene at later time points in wild-type and cac1Δ rtt106Δ double mutant cells (Figure 6G). Thus, these results indicate that although recruitment of Sir4p to the HMR-E silencer does not require CAF-1 and Rtt106p, they are necessary for efficient spreading of Sir4p to the distal a1 gene.
Figure 6.
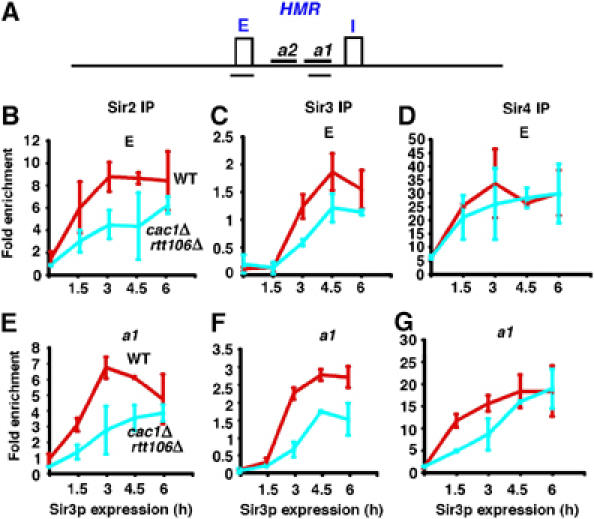
CAF-1 and Rtt106p are required for the spreading of Sir proteins during heterochromatin formation. (A) A schematic representation of the HMR locus. Two silencers, HMR-E (E) and HMR-I (I), and two genes, a2 and a1, as well as the locations of the two PCR primer sets (black underlines) used to amplify the HMR-E silencer and a fragment of the a1 gene are shown. (B–D) The binding of Sir2p (B) and Sir3p-HA (C) to the HMR-E silencer is reduced in cac1Δ rtt106Δ double mutant cells during the formation of silent chromatin as compared with wild-type cells, whereas Sir4p binding (D) to this site is not affected. (E–G) At the distal a1 gene, all three Sir proteins exhibit a delayed association with this site in the cac1Δ rtt106Δ double mutant cells following expression of Sir3p-HA. ChIP assays were performed using antibodies against Sir2p, Sir3p, or Sir4p following release of cells from an HU block and induction of Sir3p-HA expression for the indicated amounts of time. The immunoprecipitated DNA was analyzed using primers as depicted in (A) and PCR products were quantified as described in Supplementary data.
In yeast cells, Sir4p forms a complex with Sir2p (Hoppe et al, 2002). Thus, CAF-1 and Rtt106p may also mediate the spreading of other Sir proteins to the a1 gene. Indeed, spreading of Sir2p and Sir3p-HA to the distal a1 site was delayed in cac1Δ rtt106Δ double mutant cells as compared with wild-type cells (Figure 6E and F). Surprisingly, even though deletion of CAC1 and RTT106 had no effect on the recruitment of Sir4p to the HMR-E silencer (Figure 6D), the recruitment of Sir2p and Sir3p-HA to this site was affected in cac1Δ rtt106Δ double mutant cells (Figure 6B and C). Therefore, CAF-1 and Rtt106p are not required for the initial recruitment of Sir4p to the HMR-E silencer, but they are necessary for the subsequent recruitment of Sir2p and Sir3p to this site as well as for the efficient spreading of all three Sir proteins to the distal a1 gene.
Silencing at the HMR locus is dramatically reduced in cells lacking CAF-1, Rtt106p, and Sir1p
Cells lacking CAF-1 and Rtt106p exhibit a significant delay in the recruitment of Sir proteins to the HMR locus as compared to wild-type cells during the early stages of silent chromatin formation. At later time points, however, the amount of Sir proteins bound to the HMR locus in cac1Δ rtt106Δ double mutant cells was near that of wild-type cells (Figure 6), suggesting that mechanisms in addition to those involving Cac1p and Rtt106p exist to promote silent chromatin formation at the HMR locus. Sir1p is known to be involved in the formation of silent chromatin at the HMR locus, but not telomeres. Moreover, double mutant cells lacking Cac1p and Sir1p or Asf1p and Sir1p exhibit a synergistic reduction in silencing at the HMR locus (Enomoto and Berman, 1998; Meijsing and Ehrenhofer-Murray, 2001; Osada et al, 2001). Thus, it is possible that Sir1p is involved in the formation of silent chromatin at the HMR locus in the absence of CAF-1 and Rtt106p. Therefore, we tested the effects of cac1Δ rtt106Δ sir1Δ triple mutant cells on silencing at the HMR locus using a semiquantitative GFP silencing assay (Laney and Hochstrasser, 2003; Huang et al, 2005). In this assay, a gene encoding for GFP is integrated at the HMR locus and is silenced in wild-type cells (0.02% GFP positive). In sir3Δ mutant cells, silencing of the GFP gene is abolished, resulting in the expression of GFP in all cells (99.2% GFP positive), as determined by FACS. Consistent with published results, cells double mutant for cac1Δ sir1Δ, rtt106Δ sir1Δ, or cac1Δ rtt106Δ exhibited a synergistic reduction in silencing at the HMR locus when compared with any of the single mutant strains. More importantly, silencing of the GFP gene at the HMR locus was more completely abolished in cac1Δ rtt106Δ sir1Δ triple mutant cells (97.87% GFP positive) than in either sir1Δ (13.26% GFP positive) or cac1Δ rtt106Δ (71.91% GFP positive) mutant cells (Table I). Thus, these results suggest that CAF-1, Rtt106p, and Sir1p function independently to promote silencing at the HMR locus.
Table 1.
Silencing at the HMR locus is dramatically reduced in cells lacking Cac1p, Rtt106p, and Sir1p
| Genotype | %GFP positive |
|---|---|
| Wild type | 0.02 |
| sir3Δ | 99.20 |
| sir1Δ | 13.26 |
| cac1Δ | 0.09 |
| rtt106Δ | 0.08 |
| cac1Δ sir1Δ | 56.06 |
| cac1Δ rtt106Δ | 71.91 |
| rtt106Δ sir1Δ | 45.54 |
| cac1Δ rtt106Δ sir1Δ | 97.87 |
| Expression of the GFP gene at the HMR locus in each strain of the indicated genotype was measured by FACS and the percentage GFP expressing cells was calculated. | |
Rtt106p interacts with Sir4p
CAF-1 and Rtt106p are required for the efficient spreading of Sir proteins at the HMR locus, suggesting that CAF-1 and Rtt106p may interact directly with Sir proteins. To test this idea, we determined whether Rtt106p interacts with Sir3p and Sir4p. As shown in Figure 7A, GST-Rtt106p pulled down in vitro-translated Sir4p but not Sir3p. Under the same conditions, a GST-tagged control protein not involved in heterochromatin formation, GST-REGα, did not bind Sir3p or Sir4p. These results demonstrate that Rtt106p binds to Sir4p in vitro. To determine whether Rtt106p also binds to Sir4p in vivo, we expressed Flag-tagged Rtt106p in a yeast strain containing TAP-tagged Sir4p. Subsequently, Sir4p was purified in the presence of a crosslinker using the TAP method and the absence or presence of Rtt106p was detected by Western blot analysis. Indeed, Rtt106p was co-immunoprecipitated with Sir4p (Figure 7B). Thus, Rtt106p binds to Sir4p in vitro and in vivo.
Figure 7.
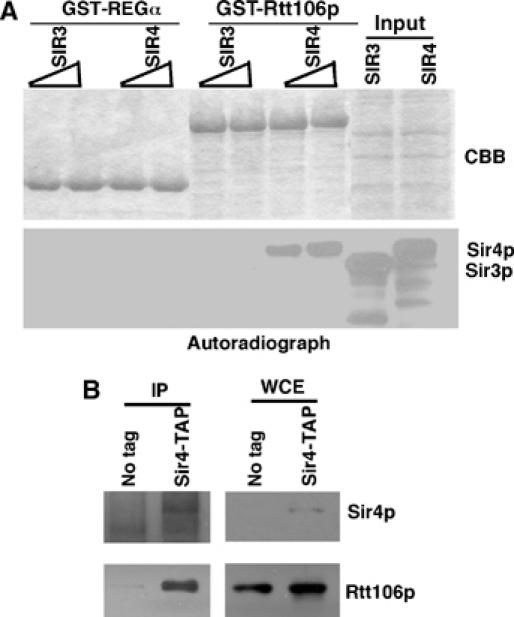
Rtt106p interacts with Sir4p. (A) GST-Rtt106p binds to Sir4p in vitro. GST-Rtt106p was incubated with in vitro-translated [35S]methionine-labeled Sir3p or Sir4p. After washing away unbound proteins, proteins that bound to Rtt106p were eluted with SDS sample buffer, resolved on SDS–PAGE gel, and detected by autoradiography. (B) Rtt106p binds to Sir4p in yeast cells. A plasmid encoding for the expression of Rtt106p-Flag was transformed into a yeast strain containing Sir4p-TAP or wild-type Sir4p, and Sir4p was purified using the TAP method. Copurified Rtt106p-Flag and Sir4p were detected using anti-Flag and anti-Sir4p antibodies, respectively. Whole-cell extracts (WCE) were probed with anti-IgG and anti-Flag antibodies.
Discussion
Histone chaperones are involved in heterochromatin silencing by unknown mechanisms. Here, we show that CAF-1 and Rtt106p together are involved in early stages of heterochromatin formation. CAF-1 and Rtt106p are required for the association of Sir2p, Sir3p, and Sir4p with telomeric heterochromatin as well as the localization of Sir3p and Sir4p to distinct foci at the nuclear periphery. In addition, the effects of CAC1 and RTT106 deletion on the localization of Sir proteins are not due to alterations in H4-K16 acetylation at the telomeric heterochromatin–euchromatin boundary. We did, however, detect an interaction between Rtt106p and Sir4p, suggesting that histone chaperones may directly affect Sir protein localization. At the HMR locus, CAF-1 and Rtt106p are required for the formation of silent chromatin by mediating the recruitment of Sir2p and Sir3p to the HMR-E silencer and the efficient spreading of Sir2p, Sir3p, and Sir4p from the HMR-E silencer to the distal a1 gene. Further, CAF-1, Rtt106p, and Sir1p function independently in the formation of silent chromatin at the HMR locus. Thus, our results suggest that CAF-1 and Rtt106p mediate early stages of heterochromatin formation through their effects on the recruitment and spreading of Sir proteins, providing further insights into the roles of these proteins in epigenetic silencing.
The histone chaperones Rtt106p, Asf1p, and Hir1p function in the same genetic pathway in silencing, whereas CAF-1 appears to function in an overlapping, but genetically distinct, pathway (Tyler et al, 1999; Sharp et al, 2001; Sutton et al, 2001; Huang et al, 2005). Here, we did not detect any differences in the effects of cac1Δ rtt106Δ, cac1Δ asf1Δ, and cac1Δ hir1Δ double mutant cells on the association of Sir proteins at telomeres or H4-K16 acetylation at the telomeric heterochromatin–euchromatin boundary, supporting this idea. Further, the apparent similar roles of Rtt106p, Asf1p, and Hir1p in silencing indicates that results from our analysis of the effects of CAF-1 and Rtt106p on heterochromatin formation may also aid in understanding the functions of Asf1p and Hir1p in this process in budding yeast as well as in higher eukaryotic cells.
CAF-1 and Rtt106p mediate telomeric silencing at telomeric heterochromatin but not telomere ends
Our studies on CAF-1 and Rtt106p have revealed surprising insights into how histone chaperones participate in telomeric silencing. First, the association of Sir4p with telomere ends was not affected in cac1Δ rtt106Δ double mutant cells. This result is somewhat surprising, as Sir4p binding to telomere ends depends on an interaction between Sir4p and Rap1p (Luo et al, 2002), and it has been reported that Rap1p is mislocalized in cac1Δ mutant cells (Enomoto and Berman, 1998). Possibly, the effect of Rap1p mislocalization on the binding of Sir4p to telomere ends is too small to be detected using ChIP assays. Nonetheless, combined deletion of CAC1 and RTT106 did not reduce the binding of Sir4p to telomere ends to a further extent than deletion of the SIR3 gene. A similar phenotype was also observed in cells lacking yKu70/yK80, proteins required for the localization of Sir4p to telomeric heterochromatin, but not telomere ends (Luo et al, 2002). These results suggest that CAF-1 and Rtt106p are not required for the initiation of telomeric silent chromatin formation through the recruitment of Sir4p to telomere ends but rather the subsequent spreading of heterochromatin.
CAF-1 and Asf1p are known to interact with Sas2p (Meijsing and Ehrenhofer-Murray, 2001; Osada et al, 2001), which acetylates H4-K16 at euchromatin and thereby prevents promiscuous association of Sir proteins with euchromatin (Kimura et al, 2002; Suka et al, 2002; Sutton et al, 2003). Mutations in CAF-1 and Rtt106p might, therefore, be expected to affect heterochromatin silencing by altering H4-K16 acetylation in a manner similar to sas2Δ mutant cells (Rusche et al, 2003). Remarkably, the effect of cac1Δ rtt106Δ double mutant cells on H4-K16 acetylation was similar to that of sir3Δ and sir4Δ single mutant cells, but distinct from that of sas2Δ mutant cells. Moreover, in contrast to the promiscuous association of Sir4p with telomeric euchromatin in sas2Δ mutant cells, Sir4p was not detectable at telomeric euchromatin in cac1Δ rtt106Δ double mutant cells. Thus, the silencing defects observed in cac1Δ rtt106Δ double mutant cells are not due to altered H4-K16 acetylation, but rather the reduced association of Sir proteins with telomeric heterochromatin.
CAF-1 and Rtt106p are involved in the spreading of Sir proteins at early stages of heterochromatin formation
The analysis of telomeric heterochromatin did not allow us to definitively determine whether CAF-1 and Rtt106p function in silent chromatin formation, maintenance, or both. However, analysis of the HMR locus indicated that CAF-1 and Rtt106p are required for efficient heterochromatin formation. Indeed, we have shown for the first time that silencing of the a1 gene is compromised in cac1Δ and rtt106Δ single and double mutant cells. Moreover, although CAF-1 and Rtt106p were not required for the initial recruitment of Sir4p to the HMR-E silencer, they were required for the spreading of Sir4p to the distal a1 gene. In contrast, CAF-1 and Rtt106p were required for both the recruitment of Sir2p and Sir3p to the HMR-E silencer and spreading of these two Sir proteins to the a1 gene. Interestingly, CAF-1 and Rtt106p appear to mediate silencing at the HMR locus using a pathway genetically distinct from that of Sir1p. Together, our results indicate that CAF-1 and Rtt106p are involved in heterochromatin formation at the HMR locus downstream of the initial recruitment of Sir4p to the HMR-E silencer.
Our conclusion seems to contradict an earlier study suggesting that CAF-1 is not required for the re-establishment of silencing, but instead is involved in the maintenance of silencing (Enomoto and Berman, 1998). This conclusion was partly based on results from a mating assay indicating that silencing could be established in cac1Δ mutant cells as efficiently as in wild-type cells after re-expression of Sir3p-HA. However, our results show that at short time periods following the induction of Sir3p-HA expression, silencing of the a1 gene is compromised in cac1Δ mutant cells. The discrepancy between these two results could be due to the different assays used. Using the mating assay, silencing would have been analyzed in cells that had undergone several cycles of cell division following re-expression of Sir3p-HA, whereas in our assay the defect in silencing of the a1 gene in cac1Δ mutant cells was observed after short time periods of Sir3p-HA expression. Although our results provide evidence that CAF-1 and Rtt106p function in the early steps of heterochromatin formation, they by no means exclude the possibility that CAF-1 and Rtt106p also play a role in the maintenance of silent chromatin.
Mechanisms for histone chaperone-mediated spreading of Sir proteins during heterochromatin formation
Silencing appears to be a stochastic event requiring progression through the cell cycle: not all cells acquire the silenced ‘off' state following the same number of cell divisions (Xu et al, 2006). Our studies here employing ChIP assays using populations of cells showed that CAF-1 and Rtt106p significantly affect the spreading of Sir proteins to the a1 gene during early time points following Sir3p-HA expression (before 3 h). However, at later time points (after 4.5 h), Sir protein levels at the a1 gene in cac1Δ rtt106Δ double mutant cells were near those of wild-type cells. This could reflect either a reduced amount of Sir proteins at the a1 gene in all cells early on or the presence of distinct cell populations with only some cells having sufficient amounts of Sir proteins at the a1 gene to mediate silencing. Because the amounts of Sir4p at the HMR-E silencer in cac1Δ rtt106Δ double mutant cells were similar to those of wild-type cells after Sir3p-HA expression at all time points, we favor the first possibility. However, it will be interesting to analyze the effects of cac1Δ rtt106Δ double mutant cells, as well as other histone chaperone mutant cells, on silencing in single cells at the HM loci.
We envision two non-exclusive models whereby CAF-1 and Rtt106p function in spreading during the early step of heterochromatin formation. First, it is possible that reduced spreading of Sir proteins at the HMR locus in CAF-1 and Rtt106p mutant cells is due to reduced nucleosome density. Alternatively, histone chaperones may mediate the spreading of Sir proteins through direct physical interactions with Sir proteins. In support of the first model, it has been shown that nucleosome density at telomeric heterochromatin is reduced in CAF-1 mutant cells (Tamburini et al, 2006). However, two observations argue against this model. First, it is not known whether the reduced nucleosome density at telomeres observed in cac1Δ mutant cells is the cause or a consequence of reduced silencing. It has been shown that nucleosomes are regularly spaced at the HMR locus, and in sir3 mutant cells, no specific nucleosome structure could be detected at the HMR locus (Ravindra et al, 1999). This suggests that nucleosome density at the HMR locus is most likely reduced in sir3 mutant cells. As Sir3p is not involved in nucleosome deposition, loss of nucleosome structure in the sir3 mutant is most likely the consequence, not the cause, of the silencing defect. Second, the ability of histone chaperones to promote nucleosome formation is not linked to their functions in silencing. For instance, Asf1p is involved in both DNA replication-dependent and DNA replication-independent nucleosome assembly pathways, whereas Hir1p is involved only in DNA replication-independent nucleosome assembly pathways (Tyler et al, 1999; Sharp et al, 2001; Green et al, 2005). However, the silencing defects observed in asf1Δ mutants are linked to loss of interactions with Hir1p (Daganzo et al, 2003). Thus, there is no correlation between the nucleosome assembly ability of Asf1p and its function in silencing.
In the second model, which we favor, CAF-1 and Rtt106p would be involved in the spreading of Sir proteins through their physical interactions with Sir proteins. Supporting this idea, we have shown that Rtt106p interacts with Sir4p. In addition, it has previously been shown that CAF-1 interacts with Sir1p and that this interaction is required for centromere integrity (Sharp et al, 2003). Further, in mammalian cells, CAF-1 interacts with HP-1 (Murzina et al, 1999; Quivy et al, 2004), the functional homolog of yeast Sir proteins, and HIRA, the human ortholog of yeast Hir1p, and Asf1a are required for the formation of senescence-induced heterochromatin (Zhang et al, 2005). Thus, it is possible that CAF-1 and Rtt106p, as well as other histone chaperones, directly interact with Sir proteins to mediate the formation and spreading of heterochromatin.
Materials and methods
Yeast strains and plasmids
All yeast strains used were of the W303-1A (leu2-3, 112 ura3-1 his3-11, trp1-1, ade2-1 can 1-100) genetic background. Standard yeast media and manipulations were used.
ChIP and silencing assays
ChIP was performed as described (Aparicio et al, 1997; Zhang et al, 2002). Quantification of immunoprecipitated DNA, silencing assays, and assays to detect interactions between Rtt106p and Sir4p are described in Supplementary data.
Fluorescence microscopy
Yeast cells expressing either Sir3p-GFP or Sir4p-GFP in wild-type or mutant backgrounds were grown at 26°C and harvested at log phase. After washing cells three times using SCM-TRP medium, cells were resuspended in small amounts of SCM-TRP medium and plated on slides for imaging. The GFP-expressing cells were then imaged using an LSM10 confocal microscope with a Plan-Apochromat × 100/1.4 oil lens.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Dr Heather M Thompson for help in editing the manuscript and Drs Moazed and Struhl for yeast strains and plasmids. This work was supported by a grant from the NIH.
References
- Ahmad K, Henikoff S (2002) Epigenetic consequences of nucleosome dynamics. Cell 111: 281–284 [DOI] [PubMed] [Google Scholar]
- Aparicio OM, Billington BL, Gottschling DE (1991) Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell 66: 1279–1287 [DOI] [PubMed] [Google Scholar]
- Aparicio OM, Weinstein DM, Bell SP (1997) Components and dynamics of DNA replication complexes in S. cerevisiae: redistribution of MCM proteins and Cdc45p during S phase. Cell 91: 59–69 [DOI] [PubMed] [Google Scholar]
- Carmen AA, Milne L, Grunstein M (2002) Acetylation of the yeast histone H4 N terminus regulates its binding to heterochromatin protein SIR3. J Biol Chem 277: 4778–4781 [DOI] [PubMed] [Google Scholar]
- Daganzo SM, Erzberger JP, Lam WM, Skordalakes E, Zhang R, Franco AA, Brill SJ, Adams PD, Berger JM, Kaufman PD (2003) Structure and function of the conserved core of histone deposition protein Asf1. Curr Biol 13: 2148–2158 [DOI] [PubMed] [Google Scholar]
- Donze D, Adams CR, Rine J, Kamakaka RT (1999) The boundaries of the silenced HMR domain in Saccharomyces cerevisiae. Genes Dev 13: 698–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donze D, Dhillon N, Kamakaka RT (2001) RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. EMBO J 20: 520–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto S, Berman J (1998) Chromatin assembly factor I contributes to the maintenance, but not the re-establishment, of silencing at the yeast silent mating loci. Genes Dev 12: 219–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto S, McCune-Zierath PD, Gerami-Nejad M, Sanders MA, Berman J (1997) RLF2, a subunit of yeast chromatin assembly factor-I, is required for telomeric chromatin function in vivo. Genes Dev 11: 358–370 [DOI] [PubMed] [Google Scholar]
- Green EM, Antczak AJ, Bailey AO, Franco AA, Wu KJ, Yates JR III, Kaufman PD (2005) Replication-independent histone deposition by the HIR complex and Asf1. Curr Biol 15: 2044–2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M (1998) Yeast heterochromatin: regulation of its assembly and inheritance by histones. Cell 93: 325–328 [DOI] [PubMed] [Google Scholar]
- Hoppe GJ, Tanny JC, Rudner AD, Gerber SA, Danaie S, Gygi SP, Moazed D (2002) Steps in assembly of silent chromatin in yeast: Sir3-independent binding of a Sir2/Sir4 complex to silencers and role for Sir2-dependent deacetylation. Mol Cell Biol 22: 4167–4180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Zhou H, Katzmann D, Hochstrasser M, Atanasova E, Zhang Z (2005) Rtt106p is a histone chaperone involved in heterochromatin-mediated silencing. Proc Natl Acad Sci USA 102: 13410–13415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403: 795–800 [DOI] [PubMed] [Google Scholar]
- Jones PA, Baylin SB (2002) The fundamental role of epigenetic events in cancer. Nat Rev Genet 3: 415–428 [DOI] [PubMed] [Google Scholar]
- Katan-Khaykovich Y, Struhl K (2005) Heterochromatin formation involves changes in histone modifications over multiple cell generations. EMBO J 24: 2138–2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PD, Kobayashi R, Stillman B (1997) Ultraviolet radiation sensitivity and reduction of telomeric silencing in Saccharomyces cerevisiae cells lacking chromatin assembly factor-I. Genes Dev 11: 345–357 [DOI] [PubMed] [Google Scholar]
- Kimura A, Umehara T, Horikoshi M (2002) Chromosomal gradient of histone acetylation established by Sas2p and Sir2p functions as a shield against gene silencing. Nat Genet 32: 370–377 [DOI] [PubMed] [Google Scholar]
- Kirchmaier AL, Rine J (2006) Cell cycle requirements in assembling silent chromatin in Saccharomyces cerevisiae. Mol Cell Biol 26: 852–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R (2000) The silencing protein SIR2 and its homologs are NAD-dependent protein deacetylases. Proc Natl Acad Sci USA 97: 5807–5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laney JD, Hochstrasser M (2003) Ubiquitin-dependent degradation of the yeast Mat(alpha)2 repressor enables a switch in developmental state. Genes Dev 17: 2259–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou GG, Tanny JC, Kruger RG, Walz T, Moazed D (2005) Assembly of the SIR complex and its regulation by O-acetyl-ADP-ribose, a product of NAD-dependent histone deacetylation. Cell 121: 515–527 [DOI] [PubMed] [Google Scholar]
- Luo K, Vega-Palas MA, Grunstein M (2002) Rap1-Sir4 binding independent of other Sir, yKu, or histone interactions initiates the assembly of telomeric heterochromatin in yeast. Genes Dev 16: 1528–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SG, Laroche T, Suka N, Grunstein M, Gasser SM (1999) Relocalization of telomeric Ku and SIR proteins in response to DNA strand breaks in yeast. Cell 97: 621–633 [DOI] [PubMed] [Google Scholar]
- Meijsing SH, Ehrenhofer-Murray AE (2001) The silencing complex SAS-I links histone acetylation to the assembly of repressed chromatin by CAF-I and Asf1 in Saccharomyces cerevisiae. Genes Dev 15: 3169–3182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moazed D (2001) Common themes in mechanisms of gene silencing. Mol Cell 8: 489–498 [DOI] [PubMed] [Google Scholar]
- Moazed D, Kistler A, Axelrod A, Rine J, Johnson AD (1997) Silent information regulator protein complexes in Saccharomyces cerevisiae: a SIR2/SIR4 complex and evidence for a regulatory domain in SIR4 that inhibits its interaction with SIR3. Proc Natl Acad Sci USA 94: 2186–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzina N, Verreault A, Laue E, Stillman B (1999) Heterochromatin dynamics in mouse cells: interaction between chromatin assembly factor 1 and HP1 proteins. Mol Cell 4: 529–540 [DOI] [PubMed] [Google Scholar]
- Osada S, Sutton A, Muster N, Brown CE, Yates JR III, Sternglanz R, Workman JL (2001) The yeast SAS (something about silencing) protein complex contains a MYST-type putative acetyltransferase and functions with chromatin assembly factor ASF1. Genes Dev 15: 3155–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino F, Laroche T, Gilson E, Axelrod A, Pillus L, Gasser SM (1993) SIR3 and SIR4 proteins are required for the positioning and integrity of yeast telomeres. Cell 75: 543–555 [DOI] [PubMed] [Google Scholar]
- Quivy JP, Roche D, Kirschner D, Tagami H, Nakatani Y, Almouzni G (2004) A CAF-1 dependent pool of HP1 during heterochromatin duplication. EMBO J 23: 3516–3526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravindra A, Weiss K, Simpson RT (1999) High-resolution structural analysis of chromatin at specific loci: Saccharomyces cerevisiae silent mating-type locus HMRa. Mol Cell Biol 19: 7944–7950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards EJ, Elgin SC (2002) Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell 108: 489–500 [DOI] [PubMed] [Google Scholar]
- Rusche LN, Kirchmaier AL, Rine J (2002) Ordered nucleation and spreading of silenced chromatin in Saccharomyces cerevisiae. Mol Biol Cell 13: 2207–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusche LN, Kirchmaier AL, Rine J (2003) The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu Rev Biochem 72: 481–516 [DOI] [PubMed] [Google Scholar]
- Sharp JA, Fouts ET, Krawitz DC, Kaufman PD (2001) Yeast histone deposition protein Asf1p requires Hir proteins and PCNA for heterochromatic silencing. Curr Biol 11: 463–473 [DOI] [PubMed] [Google Scholar]
- Sharp JA, Krawitz DC, Gardner KA, Fox CA, Kaufman PD (2003) The budding yeast silencing protein Sir1 is a functional component of centromeric chromatin. Genes Dev 17: 2356–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suka N, Luo K, Grunstein M (2002) Sir2p and Sas2p opposingly regulate acetylation of yeast histone H4 lysine16 and spreading of heterochromatin. Nat Genet 32: 378–383 [DOI] [PubMed] [Google Scholar]
- Sutton A, Bucaria J, Osley MA, Sternglanz R (2001) Yeast ASF1 protein is required for cell cycle regulation of histone gene transcription. Genetics 158: 587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A, Shia W-J, Band D, Kaufman PD, Osada S, Workman JL, Sternglanz R (2003) Sas4 and Sas5 are required for the histone acetyltransferase activity of Sas2 in the SAS complex. J Biol Chem 278: 16887–16892 [DOI] [PubMed] [Google Scholar]
- Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y (2004) Histone h3.1 and h3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 116: 51–61 [DOI] [PubMed] [Google Scholar]
- Tamburini BA, Carson JJ, Linger JG, Tyler JK (2006) Dominant mutants of the Saccharomyces cerevisiae ASF1 histone chaperone bypass the need for CAF-1 in transcriptional silencing by altering histone and Sir protein recruitment. Genetics 173: 599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triolo T, Sternglanz R (1996) Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature 381: 251–253 [DOI] [PubMed] [Google Scholar]
- Tyler JK, Adams CR, Chen SR, Kobayashi R, Kamakaka RT, Kadonaga JT (1999) The RCAF complex mediates chromatin assembly during DNA replication and repair. Nature 402: 555–560 [DOI] [PubMed] [Google Scholar]
- Xu EY, Zawadzki KA, Broach JR (2006) Single-cell observations reveal intermediate transcriptional silencing states. Mol Cell 23: 219–229 [DOI] [PubMed] [Google Scholar]
- Zhang R, Poustovoitov MV, Ye X, Santos HA, Chen W, Daganzo SM, Erzberger JP, Serebriiskii IG, Canutescu AA, Dunbrack RL, Pehrson JR, Berger JM, Kaufman PD, Adams PD (2005) Formation of MacroH2A-containing senescence-associated heterochromatin foci and senescence driven by ASF1a and HIRA. Dev Cell 8: 19–30 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hayashi MK, Merkel O, Stillman B, Xu RM (2002) Structure and function of the BAH-containing domain of Orc1p in epigenetic silencing. EMBO J 21: 4600–4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Madden BJ, Muddiman DC, Zhang Z (2006) Chromatin assembly factor 1 interacts with histone H3 methylated at lysine 79 in the processes of epigenetic silencing and DNA repair. Biochemistry 45: 2852–2861 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


