Abstract
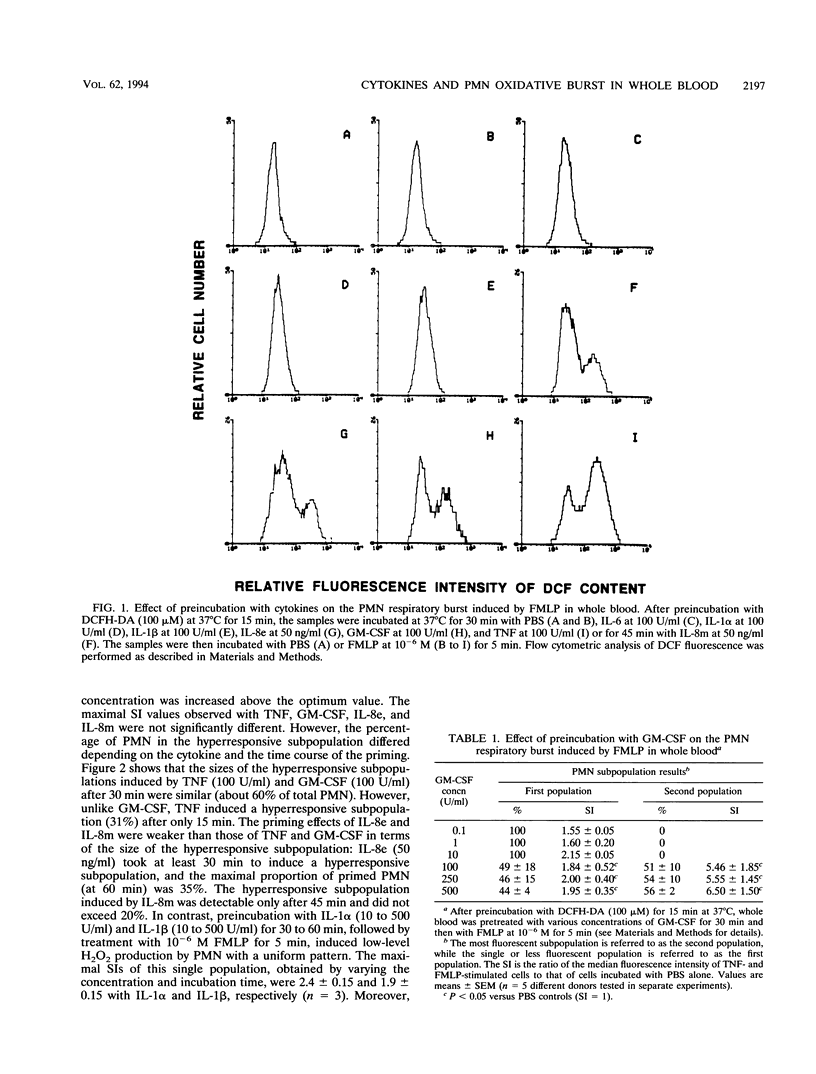
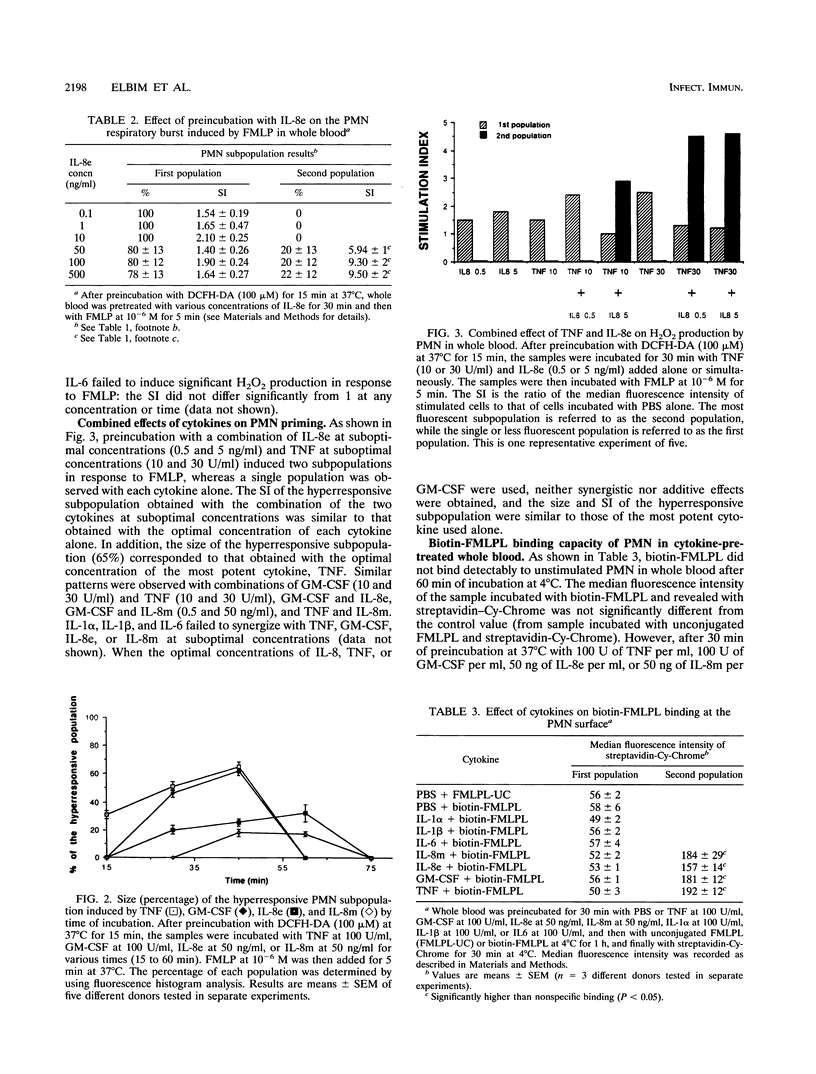
Cytokines such as tumor necrosis factor alpha (TNF-alpha), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin 8 (IL-8), IL-6, IL-1 alpha, and IL-1 beta produced during the immune and inflammatory responses to bacterial stimuli have been reported to interact with polymorphonuclear neutrophil (PMN) activities. However, contradictory findings on their direct and priming effects on the PMN oxidative burst, which is essential for bacterial killing, have been reported. We have used a flow cytometry method to study the effects of these cytokines on the oxidative burst of PMN in whole blood to avoid PMN activation related to isolation procedures. None of the cytokines tested directly activated the PMN oxidative burst, but they did have differential priming effects on the oxidative burst in response to bacterial N-formyl peptides. TNF, GM-CSF, and IL-8 strongly primed a subpopulation of PMN to produce H2O2 in response to N-formyl-methionyl-leucyl-phenylalanine (FMLP), while IL-1 alpha, IL-1 beta, and IL-6 failed to do so. Furthermore, the addition of TNF, GM-CSF, or IL-8 to whole blood increased the capacity of a subpopulation of PMN to bind N-formyl peptides, a phenomenon that could account, at least in part, for the strong H2O2 production in response to FMLP after priming by the cytokines. The size of the primed hyperresponsive subpopulation was greater after priming with TNF or GM-CSF than after priming with IL-8. However, GM-CSF, TNF, and IL-8 at suboptimal concentrations cooperated in the induction of a subpopulation hyperresponsive to FMLP. These results show that, of the various proinflammatory cytokines tested, TNF, GM-CSF, and IL-8 strongly prime the PMN oxidative burst in response to bacterial peptides in whole blood and suggest that these cytokines may play a critical role in bacterial killing in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson Y. H., Lopez A. F., Marasco W. A., Lucas C. M., Wong G. G., Burns G. F., Vadas M. A. Recombinant human granulocyte-macrophage colony-stimulating factor (rH GM-CSF) regulates f Met-Leu-Phe receptors on human neutrophils. Immunology. 1988 Jul;64(3):519–525. [PMC free article] [PubMed] [Google Scholar]
- Atkinson Y. H., Marasco W. A., Lopez A. F., Vadas M. A. Recombinant human tumor necrosis factor-alpha. Regulation of N-formylmethionylleucylphenylalanine receptor affinity and function on human neutrophils. J Clin Invest. 1988 Mar;81(3):759–765. doi: 10.1172/JCI113381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Bass D. A., Olbrantz P., Szejda P., Seeds M. C., McCall C. E. Subpopulations of neutrophils with increased oxidative product formation in blood of patients with infection. J Immunol. 1986 Feb 1;136(3):860–866. [PubMed] [Google Scholar]
- Bass D. A., Parce J. W., Dechatelet L. R., Szejda P., Seeds M. C., Thomas M. Flow cytometric studies of oxidative product formation by neutrophils: a graded response to membrane stimulation. J Immunol. 1983 Apr;130(4):1910–1917. [PubMed] [Google Scholar]
- Berkow R. L., Wang D., Larrick J. W., Dodson R. W., Howard T. H. Enhancement of neutrophil superoxide production by preincubation with recombinant human tumor necrosis factor. J Immunol. 1987 Dec 1;139(11):3783–3791. [PubMed] [Google Scholar]
- Borish L., Rosenbaum R., Albury L., Clark S. Activation of neutrophils by recombinant interleukin 6. Cell Immunol. 1989 Jul;121(2):280–289. doi: 10.1016/0008-8749(89)90026-9. [DOI] [PubMed] [Google Scholar]
- Chollet-Martin S., Montravers P., Gibert C., Elbim C., Desmonts J. M., Fagon J. Y., Gougerot-Pocidalo M. A. High levels of interleukin-8 in the blood and alveolar spaces of patients with pneumonia and adult respiratory distress syndrome. Infect Immun. 1993 Nov;61(11):4553–4559. doi: 10.1128/iai.61.11.4553-4559.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet-Martin S., Montravers P., Gibert C., Elbim C., Desmonts J. M., Fagon J. Y., Gougerot-Pocidalo M. A. Subpopulation of hyperresponsive polymorphonuclear neutrophils in patients with adult respiratory distress syndrome. Role of cytokine production. Am Rev Respir Dis. 1992 Oct;146(4):990–996. doi: 10.1164/ajrccm/146.4.990. [DOI] [PubMed] [Google Scholar]
- Darbonne W. C., Rice G. C., Mohler M. A., Apple T., Hébert C. A., Valente A. J., Baker J. B. Red blood cells are a sink for interleukin 8, a leukocyte chemotaxin. J Clin Invest. 1991 Oct;88(4):1362–1369. doi: 10.1172/JCI115442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeForge L. E., Fantone J. C., Kenney J. S., Remick D. G. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J Clin Invest. 1992 Nov;90(5):2123–2129. doi: 10.1172/JCI116097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djeu J. Y., Matsushima K., Oppenheim J. J., Shiotsuki K., Blanchard D. K. Functional activation of human neutrophils by recombinant monocyte-derived neutrophil chemotactic factor/IL-8. J Immunol. 1990 Mar 15;144(6):2205–2210. [PubMed] [Google Scholar]
- Dularay B., Elson C. J., Clements-Jewery S., Damais C., Lando D. Recombinant human interleukin-1 beta primes human polymorphonuclear leukocytes for stimulus-induced myeloperoxidase release. J Leukoc Biol. 1990 Feb;47(2):158–163. doi: 10.1002/jlb.47.2.158. [DOI] [PubMed] [Google Scholar]
- Elbim C., Chollet-Martin S., Bailly S., Hakim J., Gougerot-Pocidalo M. A. Priming of polymorphonuclear neutrophils by tumor necrosis factor alpha in whole blood: identification of two polymorphonuclear neutrophil subpopulations in response to formyl-peptides. Blood. 1993 Jul 15;82(2):633–640. [PubMed] [Google Scholar]
- Ferrante A., Nandoskar M., Walz A., Goh D. H., Kowanko I. C. Effects of tumour necrosis factor alpha and interleukin-1 alpha and beta on human neutrophil migration, respiratory burst and degranulation. Int Arch Allergy Appl Immunol. 1988;86(1):82–91. doi: 10.1159/000234610. [DOI] [PubMed] [Google Scholar]
- Figari I. S., Mori N. A., Palladino M. A., Jr Regulation of neutrophil migration and superoxide production by recombinant tumor necrosis factors-alpha and -beta: comparison to recombinant interferon-gamma and interleukin-1 alpha. Blood. 1987 Oct;70(4):979–984. [PubMed] [Google Scholar]
- Georgilis K., Schaefer C., Dinarello C. A., Klempner M. S. Human recombinant interleukin 1 beta has no effect on intracellular calcium or on functional responses of human neutrophils. J Immunol. 1987 May 15;138(10):3403–3407. [PubMed] [Google Scholar]
- Hack C. E., Hart M., van Schijndel R. J., Eerenberg A. J., Nuijens J. H., Thijs L. G., Aarden L. A. Interleukin-8 in sepsis: relation to shock and inflammatory mediators. Infect Immun. 1992 Jul;60(7):2835–2842. doi: 10.1128/iai.60.7.2835-2842.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlan J. M. Leukocyte-endothelial interactions. Blood. 1985 Mar;65(3):513–525. [PubMed] [Google Scholar]
- Kharazmi A., Nielsen H., Bendtzen K. Recombinant interleukin 1 alpha and beta prime human monocyte superoxide production but have no effect on chemotaxis and oxidative burst response of neutrophils. Immunobiology. 1988 Apr;177(1):32–39. doi: 10.1016/s0171-2985(88)80089-5. [DOI] [PubMed] [Google Scholar]
- Kharazmi A., Nielsen H., Rechnitzer C., Bendtzen K. Interleukin 6 primes human neutrophil and monocyte oxidative burst response. Immunol Lett. 1989 May;21(2):177–184. doi: 10.1016/0165-2478(89)90056-4. [DOI] [PubMed] [Google Scholar]
- Khwaja A., Carver J. E., Linch D. C. Interactions of granulocyte-macrophage colony-stimulating factor (CSF), granulocyte CSF, and tumor necrosis factor alpha in the priming of the neutrophil respiratory burst. Blood. 1992 Feb 1;79(3):745–753. [PubMed] [Google Scholar]
- Klebanoff S. J., Vadas M. A., Harlan J. M., Sparks L. H., Gamble J. R., Agosti J. M., Waltersdorph A. M. Stimulation of neutrophils by tumor necrosis factor. J Immunol. 1986 Jun 1;136(11):4220–4225. [PubMed] [Google Scholar]
- Kuijpers T. W., Tool A. T., van der Schoot C. E., Ginsel L. A., Onderwater J. J., Roos D., Verhoeven A. J. Membrane surface antigen expression on neutrophils: a reappraisal of the use of surface markers for neutrophil activation. Blood. 1991 Aug 15;78(4):1105–1111. [PubMed] [Google Scholar]
- Larrick J. W., Graham D., Toy K., Lin L. S., Senyk G., Fendly B. M. Recombinant tumor necrosis factor causes activation of human granulocytes. Blood. 1987 Feb;69(2):640–644. [PubMed] [Google Scholar]
- Marx J. L. Oxygen free radicals linked to many diseases. Science. 1987 Jan 30;235(4788):529–531. doi: 10.1126/science.3810154. [DOI] [PubMed] [Google Scholar]
- McColl S. R., Beauseigle D., Gilbert C., Naccache P. H. Priming of the human neutrophil respiratory burst by granulocyte-macrophage colony-stimulating factor and tumor necrosis factor-alpha involves regulation at a post-cell surface receptor level. Enhancement of the effect of agents which directly activate G proteins. J Immunol. 1990 Nov 1;145(9):3047–3053. [PubMed] [Google Scholar]
- Nathan C. F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987 Dec;80(6):1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Rossi A. G., Redman J. F., Jacobson D. P. Tumor necrosis factor-alpha regulates expression of receptors for formyl-methionyl-leucyl-phenylalanine, leukotriene B4, and platelet-activating factor. Dissociation from priming in human polymorphonuclear neutrophils. J Immunol. 1991 Dec 1;147(11):3842–3847. [PubMed] [Google Scholar]
- Offner F., Philippé J., Vogelaers D., Colardyn F., Baele G., Baudrihaye M., Vermeulen A., Leroux-Roels G. Serum tumor necrosis factor levels in patients with infectious disease and septic shock. J Lab Clin Med. 1990 Jul;116(1):100–105. [PubMed] [Google Scholar]
- Ozaki Y., Ohashi T., Kume S. Potentiation of neutrophil function by recombinant DNA-produced interleukin 1a. J Leukoc Biol. 1987 Dec;42(6):621–627. doi: 10.1002/jlb.42.6.621. [DOI] [PubMed] [Google Scholar]
- Peveri P., Walz A., Dewald B., Baggiolini M. A novel neutrophil-activating factor produced by human mononuclear phagocytes. J Exp Med. 1988 May 1;167(5):1547–1559. doi: 10.1084/jem.167.5.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta A. K., Oppenheim J. J., Matsushima K. Interleukin 8 (monocyte-derived neutrophil chemotactic factor) dynamically regulates its own receptor expression on human neutrophils. J Biol Chem. 1990 Jan 5;265(1):183–189. [PubMed] [Google Scholar]
- Sullivan G. W., Carper H. T., Novick W. J., Jr, Mandell G. L. Inhibition of the inflammatory action of interleukin-1 and tumor necrosis factor (alpha) on neutrophil function by pentoxifylline. Infect Immun. 1988 Jul;56(7):1722–1729. doi: 10.1128/iai.56.7.1722-1729.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G. W., Carper H. T., Sullivan J. A., Murata T., Mandell G. L. Both recombinant interleukin-1 (beta) and purified human monocyte interleukin-1 prime human neutrophils for increased oxidative activity and promote neutrophil spreading. J Leukoc Biol. 1989 May;45(5):389–395. doi: 10.1002/jlb.45.5.389. [DOI] [PubMed] [Google Scholar]
- Tennenberg S. D., Solomkin J. S. Activation of neutrophils by cachectin/tumor necrosis factor: priming of N-formyl-methionyl-leucyl-phenylalanine-induced oxidative responsiveness via receptor mobilization without degranulation. J Leukoc Biol. 1990 Mar;47(3):217–223. doi: 10.1002/jlb.47.3.217. [DOI] [PubMed] [Google Scholar]
- Thelen M., Peveri P., Kernen P., von Tscharner V., Walz A., Baggiolini M. Mechanism of neutrophil activation by NAF, a novel monocyte-derived peptide agonist. FASEB J. 1988 Aug;2(11):2702–2706. [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Golde D. W., Clark S. C., Wong G. G., Gasson J. C. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. 1985 Mar 28-Apr 3Nature. 314(6009):361–363. doi: 10.1038/314361a0. [DOI] [PubMed] [Google Scholar]
- Weisbart R. H., Kwan L., Golde D. W., Gasson J. C. Human GM-CSF primes neutrophils for enhanced oxidative metabolism in response to the major physiological chemoattractants. Blood. 1987 Jan;69(1):18–21. [PubMed] [Google Scholar]
- Yuo A., Kitagawa S., Kasahara T., Matsushima K., Saito M., Takaku F. Stimulation and priming of human neutrophils by interleukin-8: cooperation with tumor necrosis factor and colony-stimulating factors. Blood. 1991 Nov 15;78(10):2708–2714. [PubMed] [Google Scholar]



