Abstract
Allelic exclusion operates in B and T lymphocytes to ensure clonal expression of antigen receptors after V(D)J recombination. Germline transcription, which proceeds V(D)J recombination, has been postulated to provide an instructive signal for allelic exclusion. Here, we use a genetic marker to track germline transcription from a Vβ gene within the TCRβ locus. We find that developing thymocytes exhibit uniformed, bi-allelic activation of the Vβ gene before V-DJ recombination, a process subject to allelic exclusion. We further show that V-DJ rearrangement promotes activation rather than silencing of germline transcription from the remaining Vβ genes on either the functionally or non-functionally rearranged chromosome. Results presented here suggest that germline transcription, although necessary for V(D)J recombination, is not sufficient to instruct allelic exclusion.
Keywords: enhancer, promoter, thymus, Vβ8.2
Introduction
A diversified antigen (Ag) receptor repertoire is generated in developing lymphocytes through somatic recombination of variable (V), diversity (D) and joining (J) gene segments within each receptor gene locus, a process named V(D)J recombination (Fugmann et al, 2000; Grawunder and Harfst, 2001). Rearrangement of the TCRβ gene occurs exclusively in CD4−CD8− double-negative (DN)-stage thymocytes following the sequence of Dβ to Jβ and then Vβ to DJβ recombination. In spite of the autosomal nature of the TCRβ locus, each mature T cell only expresses TCRβ protein from one successfully rearranged allele, a phenomenon known as allelic exclusion (Khor and Sleckman, 2002). TCRβ allelic exclusion is primarily regulated at the Vβ genes since Dβ to Jβ rearrangement occurs on both alleles in all developing T cells, whereas Vβ rearranges to DJβ only one allele at a time (Uematsu et al, 1988; Bergman and Cedar, 2004).
TCRβ gene allelic exclusion is regulated at two separate steps as follows: first, V-DJ rearrangement of the TCRβ locus proceeds in a monoallelic manner. Second, an in-frame rearrangement of a TCRβ allele triggers a feedback signal to suppress further rearrangement of the remaining Vβ genes. The feedback mechanism, which requires the expression of a functional TCRβ polypeptide, is supported by the observation that forced expression of a TCRβ transgene in developing thymocytes can effectively block V-DJ rearrangement at the endogenous TCRβ locus (Fenton et al, 1988; Pircher et al, 1990).
The lymphoid-specific V(D)J recombination is mediated by the lymphoid-restricted RAG1 and RAG2 recombinase complex, which recognizes and cleaves the conserved recombination signal sequences (RSSs) flanking V, D and J gene segments. Locus- and stage-specific V(D)J recombination in B and T cells is regulated, at least in part, by the accessibility of substrate gene segments to the RAG recombinase. Although the accessibility model was proposed two decades ago, the molecular mechanism of RSS accessibility only began to emerge from more recent studies of chromatin structure of antigen receptor gene loci (Alt et al, 1986; Yancopoulos and Alt, 1986; Bergman et al, 2003; Krangel, 2003; Schlissel, 2003). Active germline transcription, increased nuclease sensitivity and epigenetic modification of histones, including H3 acetylation and H3 K4 methylation, are characteristics of rearrangement permissive chromatin. The importance of promoters and the Eβ enhancer within the TCRβ locus was also demonstrated in gene targeting analysis. Disruption of promoters associated with individual Dβ or Vβ gene segments specifically blocks local DJ or V-DJ rearrangement, respectively (Whitehurst et al, 1999; Ryu et al, 2004). In addition to the promoters, the enhancer Eβ located downstream of the constant regions plays an essential role for germline transcription of the Dβ genes and DJ rearrangement (Capone et al, 1993; Bories et al, 1996). The impact of Eβ on Vβ promoter is less defined, although the initial germline transcription from Vβ genes in DN cells seems independent of Eβ (Bouvier et al, 1996). In vitro studies using bulk population of thymocytes have shown that transition from DN to DP thymocytes was accompanied by loss of chromatin accessibility and diminished germline transcription of Vβ region of the unrearranged TCRβ locus (Chattopadhyay et al, 1998; Senoo and Shinkai, 1998; Tripathi et al, 2002). Thus, it has been postulated that changes in chromatin structure from accessible to non-accessible configuration provide a barrier to prevent TCRβ locus from further rearrangement in DP thymocytes when RAG is re-expressed.
Under certain circumstances, the allelically excluded chromosome has been shown to be transcriptionally active in DP thymocytes. For example, Vβ14 is strictly subject to allelic exclusion despite its unique close proximity to Eβ. In contrast to the main Vβ gene cluster, germline transcription and H3 acetylation of Vβ14 were upregulated during DN to DP transition (Senoo and Shinkai, 1998; Wang et al, 2002). In addition, induction of a DNase hypersensitive site (HS) and DNA demethylation in the Vβ14 flanking region were also observed in the transition from DN to DP (Chattopadhyay et al, 1998). Another example of allelic exclusion uncoupled from germline transcription came from the study in which Vβ10 was placed within the Eβ regulatory region through a large deletion (475 kb) of TCRβ locus. Surprisingly, allelic exclusion of Vβ10 was well maintained even though germline transcription of Vβ10 was greatly enhanced in DN and further increased in DP (Senoo et al, 2003). Finally, a more recent study showed that introduction of TCRα enhancer (Eα) into TCR Vβ region was able to maintain an open, accessible chromatin structure at TCR Vβ locus in DP stage, but was not sufficient to induce further Vβ rearrangement in DP thymocytes (Jackson et al, 2005). Taken together, these studies suggest that maintenance of allelic exclusion in DP stage does not always require shutting down germline transcription.
Recent study of the Igκ locus showed that low-frequency locus activation and germline transcription are linked to monoallelic recombination (Liang et al, 2004). However, it is not known whether the same mechanism operates for TCRβ locus. Although low level of germline transcription before recombination has been observed for many TCR Vβ genes (Chen et al, 2001), there is no direct evidence that germline transcription in TCR Vβ genes is linked to or responsible for monoallelic recombination. To test whether transcriptional activation of TCR Vβ locus contributes to the initiation and/or maintenance of TCRβ gene allelic exclusion, we generated a mouse strain carrying a visible marker in a single TCR Vβ gene. This genetic marker allowed us to examine germline Vβ transcripts in single cells in the context of normal T-cell development. Our study indicates that Vβ germline transcription is uncoupled from allelic exclusion.
Results
Generation of TCR Vβ8.2CD2 knock-in allele
The entire TCRβ locus spans approximately 700 kb and contains 20 functional Vβ genes. All Vβ genes except Vβ14 are clustered at the 5′ end of the TCRβ locus. Two D-J-Cβ clusters are located at the 3′ end of the locus and are separated from the Vβ gene cluster by a 250 kb region containing trypsinogen genes, which are silent in T cells (Figure 1A). Each Vβ gene is known to produce germline transcripts before V-DJ recombination (Chen et al, 2001). We designed a gene targeting strategy to follow germline transcription of the Vβ8.2 gene, which is located in the center of the main Vβ gene cluster (Figure 1A).
Figure 1.
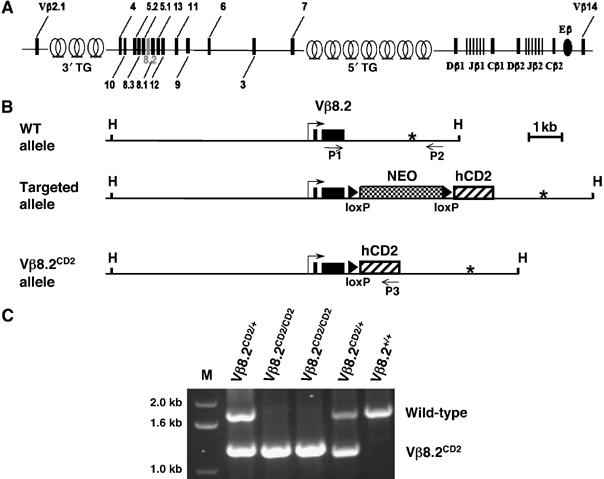
TCRβ locus and Vβ8.2CD2 gene targeting. (A) Structure of the murine TCRβ locus. Genomic organization of TCRβ gene is diagrammed to show the positions of Vβ segments relative to D, J, and C gene segments and β enhancer (Eβ). Locations of trypsinogen gene (TG) clusters in TCR Vβ region are also shown as intercalated circles. (B) Schematic of TCR Vβ8.2 wild-type allele, the targeted allele with integrated loxP-flanked PGK-Neo cassette and IRES-human CD2 cDNA (hCD2) and the Vβ8.2CD2 allele, with hCD2 immediately following Vβ8.2 RSS after a single loxP site. Vβ8.2 exons are indicated as solid boxes. Vβ8.2 transcription start site and putative poly(A) signal sequence are highlighted with bent arrow and asterisk, respectively. The positions of genotyping primers P1, P2 and P3, which recognize the common, wild-type-specific, mutant-specific sequences, respectively are indicated as arrows. HpaI (H) site is also indicated. (C) PCR analysis of toe genomic DNA samples from an intercross of Vβ8.2CD2/+ heterozygous mice. DNA ladder (1 kb) is indicated as ‘M'. Wild-type band and Vβ8.2CD2 band are labeled.
A tail-less human CD2 cDNA driven by an internal ribosome entry site (IRES) was placed 1.5 kbp upstream of the putative poly(A) site and 104 bp downstream of Vβ8.2 RSS so that the hCD2 marker will be expressed exclusively from germline transcription and will be deleted after recombination involving Vβ8.2 or any upstream Vβ gene (Figure 1B). The phosphoglycerate kinase (PGK)-diphtheria toxin (DT) and PGK-neomycin (Neo) cassette were included in the targeting construct for negative and positive selection of homologous recombinants, respectively. The floxed Neo gene was subsequently removed by transient expression of Cre recombinase in ES cells. The targeting strategy ensures that the expression of the inserted hCD2 cDNA is under the control of the endogenous Vβ8.2 promoter or any promoter upstream of Vβ8.2, which can drive transcription through the Vβ8.2 promoter (Chen et al, 2001). Mice heterozygous for the TCR Vβ8.2CD2 allele (designated as Vβ8.2CD2 hereafter) were produced and intercrossed to generate Vβ8.2CD2/CD2, Vβ8.2CD2/+ and wild-type littermates (Figure 1C).
Normal thymocyte development in Vβ8.2CD2 knock-in mice
Thymocyte development in Vβ8.2CD2 knock-in mice was found to be comparable to that of wild-type littermates with regards to total cell number and relative ratios of cells progressing through each developmental stage, including DN, DP and SP (Figure 2A). Normal numbers of T and B cells were also detected in the peripheral lymphoid organs of Vβ8.2CD2/CD2 mice in comparison with the wild-type and heterozygous controls. Vβ utilization was evaluated by surface staining of splenocytes (data not shown) or lymph node cells (Figure 2B) from Vβ8.2CD2/CD2, Vβ8.2CD2/+ and wild-type mice. No significant changes in Vβ repertoire were detected between Vβ8.2CD2 knock-in mice and wild-type littermates.
Figure 2a.
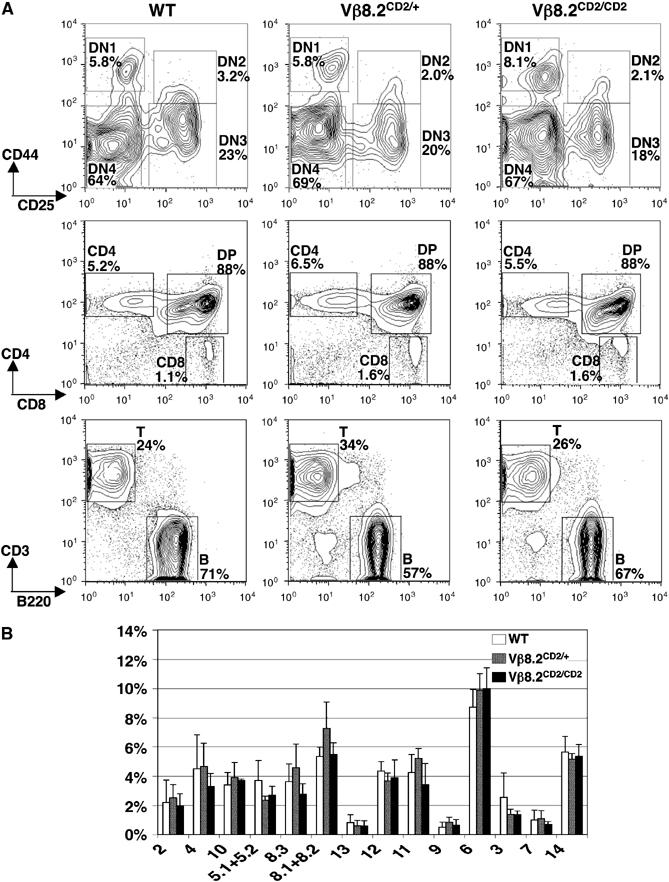
T-cell development and peripheral Vβ repertoire in Vβ8.2CD2 knock-in mice. (A) T-cell development in Vβ8.2CD2/CD2, Vβ8.2CD2/+ and wild-type (WT) mice. DN thymocytes (top panel) were analyzed with CD44 and CD25 staining after gating out DP and SP cells and non-T lineage cells as described in Materials and methods. CD4 and CD8 staining of thymocytes (middle panel) allowed separation of DP, CD4SP and CD8SP cells. Splenocytes (bottom panel) were analyzed with CD3 and B220 for T and B cells, respectively. The relative percentage of each gated population is given in the plots. Results are representative of at least five separate experiments. (B) Percentage of a specific CD4+ TCR Vβ subpopulation in total CD4+ lymph node T cells from Vβ8.2CD2/CD2, Vβ8.2CD2/+ and WT mice. Numbers under column clusters indicate TCR Vβ subtypes. Results are the mean±s.e.m. of five independent experiments.
Reduced Vβ8.2 germline transcription and rearrangement from the knock-in allele
This analysis does not distinguish the usage of Vβ8.2 from Vβ8.1 since the antibody recognizing Vβ8.2 also reacts with Vβ8.1. In order to specifically determine whether hCD2 insertion affects the usage of Vβ8.2, we designed a PCR strategy for unbiased and simultaneous amplification of Vβ8.2 and Vβ8.1 cDNA resulting from V-DJ rearrangement. Vβ8.2 and Vβ8.1 are two closely located Vβ genes and share high degree of sequence homology. The PCR product was then subject to digestion by the restriction enzyme AlfIII, which specifically cuts the Vβ8.2 sequence but not the Vβ8.1 sequence. Analysis of DN and total thymocytes showed that Vβ8.2 cDNA from the knock-in cells is less represented than from the wild-type cells (Figure 2C). We also noticed that Vβ8.2 cDNA is underrepresented in the peripheral T-cell pool for all genotypes including the wild-type mice. This result indicates that the T cells identified by the antibody specific for Vβ8.2 and Vβ8.1 represent mostly Vβ8.1 usage in the Vβ repertoire analysis shown in Figure 2B.
Figure 2b.
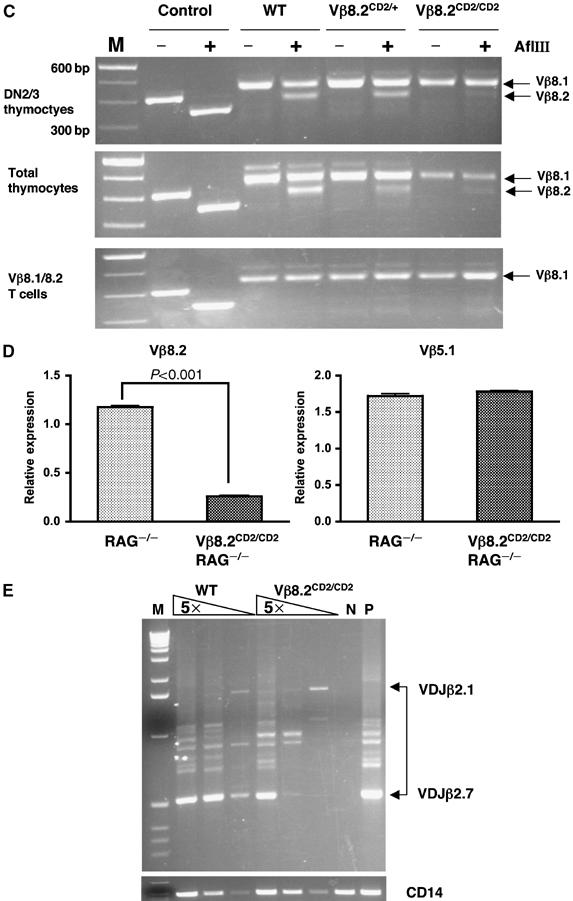
(C) Vβ8.1 and Vβ8.2 expression in sorted DN2/3 thymocytes, total thymocytes and sorted Vβ8.1/8.2 peripheral T cells from Vβ8.2CD2/CD2, Vβ8.2CD2/+ and wild-type mice. mRNA were extracted from each cell population and RT–PCR-amplified before subject to AflIII digestion as described in Materials and methods. Vβ8.1 cDNA remained as 450 bp full-length PCR product and Vβ8.2 cDNA was converted to a 390 bp fragment after AflIII digestion. Control reaction was carried out with a 400 bp Vβ8.2 genomic sequence containing the same AflIII site. Complete digestion of control DNA produced a 340 bp fragment. Size markers (M) are 100 bp DNA ladder. (D) Vβ8.2 and Vβ5.1 germline transcription in total thymocytes from Vβ8.2CD2/CD2RAG2−/− mice and RAG2−/− mice. Germline transcription of Vβ8.2 and Vβ5.1 gene was determined by quantitative PCR and normalized to GAPDH expression. Normalized expression of each Vβ gene is plotted in relative to its expression in total thymocytes from RAG2−/− mice. Results are the mean±s.e.m. of triplicates from one PCR as a representative of three independent PCR reactions. (E) Vβ8.2 rearrangement in sorted DN2/3 thymocytes from Vβ8.2CD2/CD2 and wild-type mice. PCR analysis of genomic DNA in five-fold serial dilution was carried out with the Jβ2.7 reverse primer and the Vβ8.2 forward primer. Rearrangement products from Jβ2.1 and Jβ2.7 are indicated by the upper and lower boundaries of the bracket. DNA samples from thymocytes of RAG2−/− and LAT−/− mice were included as negative (N) and positive (P) controls, respectively. PCR amplification of the CD14 gene is used as a loading control shown at the bottom panel.
To allow direct comparison of levels of Vβ8.2 transcripts between the knock-in allele and wild-type allele, real-time RT–PCR analysis was used to analyze the 5′ region of Vβ8.2 transcripts common to both alleles. We carried out this analysis with thymocytes isolated from the Vβ8.2CD2/CD2 mice, which have been backcrossed to the RAG2-deficient background; as such, all thymocytes under analysis retain germline TCRβ DNA. We found that the level of Vβ8.2 germline transcription from the knock-in allele is significantly lower in comparison with that from the wild-type allele (Figure 2D). The effect of knock-in sequence on germline transcription seems to be highly localized to Vβ8.2, since the germline transcription of Vβ5.1, a Vβ gene located immediately upstream of Vβ8.2 is comparable between Vβ8.2CD2/CD2 RAG2−/− thymocytes and RAG2−/− thymocytes (Figure 2D). Thus, the knock-in allele results in a specific reduction of levels of Vβ8.2 transcripts. We then directly evaluated the effect of knock-in on Vβ8.2 rearrangement. A PCR strategy was used to assay rearrangements involving Vβ8.2 and DJβ2 in DN2/3 thymocytes. Similar to the reduction in germline transcription, rearrangement is also decreased at the knock-in allele (Figure 2E). Thus, the inserted IRES human CD2 cassette appears to lower the efficiency of germline transcription and rearrangement involving Vβ8.2.
Lineage- and stage-specific expression of hCD2 from the knock-in allele.
A broad survey by fluorescence-activated cell sorting (FACS) analysis showed that hCD2 expression is absent in non-T lineage hematopoietic cells including B cells and NK cells (Supplementary Figure 1). In contrast, hCD2 expression from the Vβ8.2CD2 allele is detected among T cells undergoing various stages of development in the thymus (Figure 3A–C). A fraction of γ/δ T cells in thymus, spleen and gut epithelium was also found to express hCD2 (Supplementary Figure 2). The earliest stage of T-cell development in thymus is defined as CD44+CD25− DN1 thymocytes. hCD2 expression was detected in a small fraction of DN1 thymocytes (Figure 3A). Among DN1 cells, the earliest T-cell progenitors for both α/β and γ/δ lineages have been defined by expression of c-kit (DN1a) followed by CD24 (DN1b) (Laurent et al, 2004; Porritt et al, 2004). A recent study showed that TCRδ locus is first activated at DN1b stage (Prinz et al, 2006). We find that both DN1a and DN1b cells start to express low level of hCD2 (Figure 2B). DN1d and DN1e cells, which have limited T-cell progenitor activities, also contain hCD2-positive cells. Together, these studies indicate that knock-in allele expression is highly restricted to the T-cell lineage and is activated in the earliest stage in T-cell development.
Figure 3a.
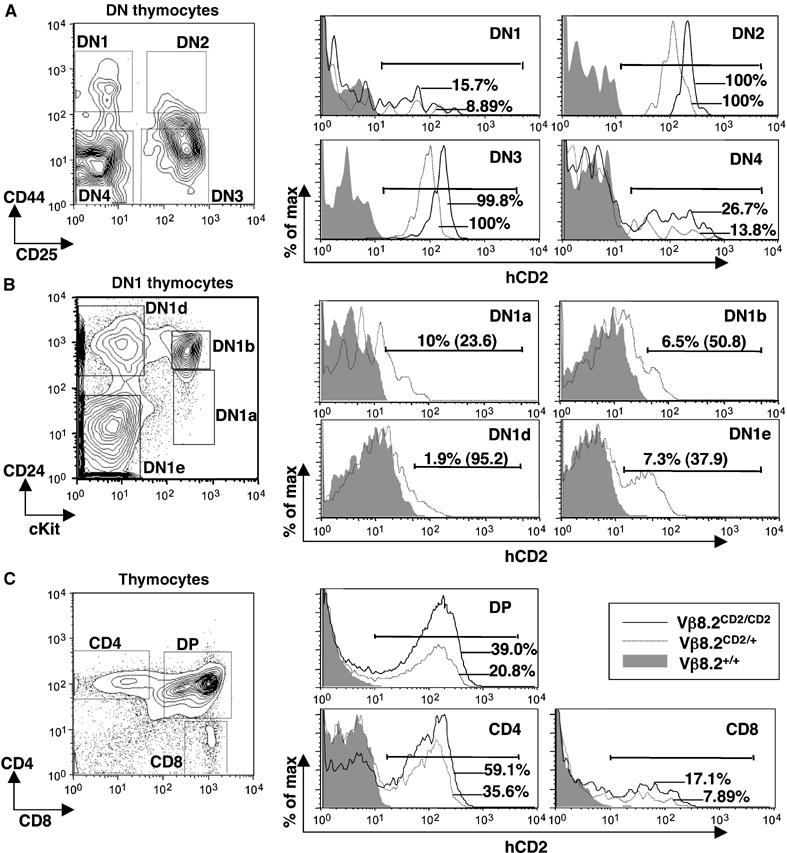
hCD2 expression in thymocytes from Vβ8.2CD2 knock-in mice. (A) hCD2 expression in DN thymocytes of Vβ8.2CD2/CD2, Vβ8.2CD2/+ and wild-type mice. The gating of DN1–4 thymocytes is indicated in the contour plots. In each gated compartment, hCD2 staining of thymocytes from Vβ8.2CD2/CD2 (black line), Vβ8.2CD2/+ (grey line) and wild-type (shade) mice is presented by overlayed histograms. Percentages of hCD2-positive cells (defined by bracketed area) are also shown in the histograms. Results are representative of 5–12 separate experiments. (B) DN1 cells from (A) were further separated by CD24 and c-kit markers to allow identification of early progenitors. hCD2 expression in each gated DN1 subset was displayed in histogram. Percentage of hCD2-positive cells (defined by bracketed area) and mean fluorescence intensity (number in parentheses) are shown. (C) hCD2 expression in DP and SP thymocytes of Vβ8.2CD2/CD2, Vβ8.2CD2/+ and wild-type mice. The gating of DP and SP thymocytes is indicated in the contour plots. Histogram analysis was as in (A). Results are representative of 5–12 separate experiments.
Bi-allelic activation of Vβ8.2CD2 allele before allelic exclusion
TCRβ gene rearrangement primarily occurs at DN2 and DN3 stage. Strikingly, the entire DN2 and DN3 thymocyte compartments express the hCD2 marker (Figure 3A and D), suggesting a biallelic nature of hCD2 expression. The level of hCD2 expression in DN2/3 thymocytes from Vβ8.2CD2/CD2 mice was approximately twice as high as that from Vβ8.2CD2/+ mice as indicated by mean fluorescence intensity of hCD2 staining (Figure 3E). This result was independently verified by real-time PCR analysis, which shows two-fold difference in the levels of hCD2 transcript in sorted DN2/3 thymocytes between Vβ8.2CD2/CD2 mice and Vβ8.2CD2/+ mice (Figure 3F). These results demonstrate that Vβ8.2 is activated on both chromosomes in all committed T cells undergoing TCRβ gene rearrangement. The percentage of hCD2-positive cells in DN4 thymocytes, which have completed TCRβ gene rearrangement but are still undergoing differentiation to DP, decreased to 10–20% (Figure 3D). This decrease could be due to either deletion of hCD2 sequence or active repression of the non-rearranged allele after recombination.
Figure 3b.
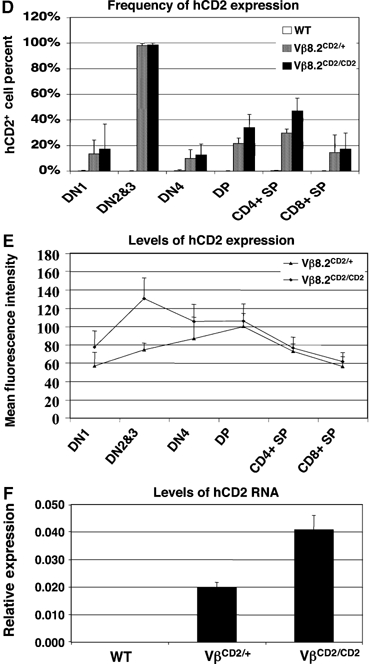
(D) Percentage of hCD2-positive cells in each thymocyte compartment. Results are the mean±s.e.m. of 5–12 independent experiments. (E) Mean fluorescence intensity of hCD2 staining in each thymocyte compartment. Results are the mean±s.e.m. of 5–12 independent experiments. (F) Relative expression level of germline transcripts from Vβ8.2CD2 knock-in allele in sorted DN2/3 thymocytes was determined by quantitative PCR. Results from two independent PCRs were analyzed and plotted in relative to GAPDH expression.
The completion of TCRβ gene rearrangement is coupled with developmental transition from DN to DP stage where TCRα gene rearrangement occurs. DP cells expressing functional TCRα and TCRβ chains are selected to become either CD4 helper or CD8 cytotoxic single positive (SP) cells. We consistently observed about 20 and 35% DP thymocytes expressing hCD2 in Vβ8.2CD2/+ and Vβ8.2CD2/CD2 mice, respectively (Figure 3C and D). hCD2 expression was also detected in a fraction of SP cells with slightly higher frequency in CD4 SP cells than in CD8 SP cells. In contrast to DN2/3 cells, the level of hCD2 expression in the hCD2-positive DP or SP cells remains constant between Vβ8.2CD2 heterozygous and homozygous mice (Figure 3E). These results indicate that only a fraction of DP and SP cells express hCD2 and that the expression is mostly monoallelic.
Ubiquitous hCD2 expression in all DN2/3 thymocytes before Dβ to Jβ rearrangement
The activation of Vβ germline transcription is coincident with TCRβ D-J rearrangement, which also begins at DN2 stage. To further delineate the relationship between D-Jβ rearrangement and Vβ gene germline transcription, we crossed the Vβ8.2CD2 allele into RAG-deficient (RAG2−/−) mice. In the absence of RAG recombinase, T-cell development is blocked at the DN3 stage and TCR Vβ genes remain in germline configuration. Ubiquitous hCD2 expression in all DN2/3 cells was detected in Vβ8.2CD2/+RAG2−/− and Vβ8.2CD2/CD2 RAG2−/− mice (Figure 4). The level of hCD2 expression is in proportion with the copy numbers of the Vβ8.2CD2 allele. This result demonstrates that bi-allelic Vβ8.2 germline transcription occurs independent of D-J and V-DJ rearrangement.
Figure 4.
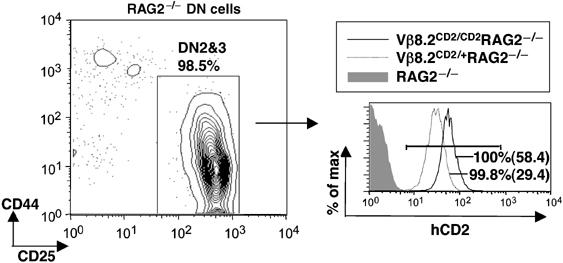
hCD2 expression in thymocytes on RAG-deficient background. DN cells on RAG−/− background were analyzed in CD44 and CD25 plots and the DN2/3 fractions were gated for histogram analysis. Percentages of hCD2-positive cells (defined by bracketed area) from Vβ8.2CD2/CD2RAG2−/− (black line) and Vβ8.2CD2/+RAG2−/− (dotted line) mice are shown in overlayed histogram. Mean fluorescence intensity of hCD2-positive cells are given in parentheses. Results are representative of three separate experiments.
Levels of Vβ germline transcription from endogenous locus are proportional to gene copies
To extend our investigation of Vβ8.2CD2 knock-in allele to the endogenous allele and also to other TCR Vβ genes, we compared germline transcription between B6 inbred and B6/SJL F1 mice. The B6 allele possesses the full-length TCR locus, whereas the SJL allele contains a 80 kb deletion from Vβ5.2 to Vβ9 within the TCR Vβ locus (Figure 5A). We used real-time PCR to compare levels of germline transcription from Vβ5.1, Vβ8.2 and Vβ12 genes (all absent in the SJL allele) between B6 and F1 mice. If germline transcription is biallelic, we expect to observe 50% reduction of germline transcript from these genes in F1 mice. However, if every T cell produces germline transcription only from one allele, we should observe the same level of germline transcription from these Vβ genes between F1 and B6 mice. DN2 thymocytes purified by FACS sorting (Supplementary Figure 4) were used in this assay to ensure germline configuration for every Vβ gene. In agreement with previous publications, we find that Vβ8.2 germline transcripts are much easily detected than germline transcripts from other Vβ genes (Figure 5B). The result shows that the ratio of germline transcripts between B6 and F1 mice was 2:1 for Vβ5.1, Vβ8.2 and Vβ12 genes and 1:1 for Vβ4, a gene outside the deleted region (Figure 5B). This result indicates that the level of Vβ germline transcription is correlated with the number of alleles present in DN2 thymoyctes.
Figure 5.
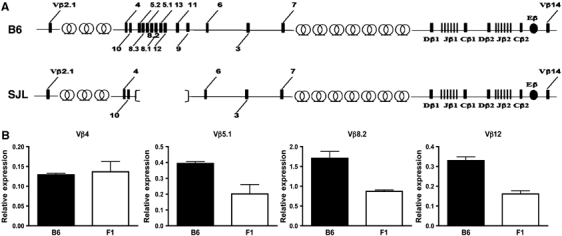
Vβ germline transcription in B6 vs B6/SJL F1 mice. (A) Schematic diagrams of the B6 and SJL TCR β locus showing V, D, J and C gene segments (boxes), Eβ (black oval) and trypsinogen gene clusters (circles). The SJL TCR β locus (lower panel) lacks an 80 kb region spanning Vβ5.2 to Vβ9. (B) Vβ germline transcription in sorted DN2 thymocytes from B6 mice and B6/SJL F1 mice. Germline transcription of each Vβ gene was determined by quantitative PCR and normalized to GAPDH expression. Normalized expression of each Vβ gene is plotted relative to its expression in sorted DN3 thymocytes from B6 mice. Results are the mean±s.e.m. of triplicates from one PCR as a representative of two independent PCR reactions.
Active Vβ germline transcription in mature T cells
hCD2 is also found to be expressed in a fraction of peripheral T cells (Figure 6A). FACS analysis with Vβ-specific antibodies showed that hCD2-positive T cells in periphery are enriched in T cells specific for Vβ genes downstream of Vβ8.2, such as Vβ12, while very few T cells using Vβ genes upstream of Vβ8.2, such as Vβ4, are hCD2 positive (Figure 6B). Further analysis using more complete collection of Vβ antibodies revealed that the frequency of hCD2 expression, as indicated by the percentage of hCD2-positive cells in each Vβ subpopulation, increases as Vβ gene usage moves from the 3′ region towards Vβ8.2 and drops immediately after rearrangement passing Vβ8.2 (Figure 6C). Since recombination using TCR Vβ8.2 or any of the Vβ gene segments upstream of it results in the deletion of the hCD2 marker, the residual hCD2 expression in T cells using Vβ genes upstream of Vβ8.2 (between Vβ2 and Vβ8.3) must come from the allelically excluded chromosome. Rearrangement using any of the Vβ genes upstream of Vβ8.2 results in 10 or 20% frequency of hCD2 expression in Vβ8.2CD2 heterozygous mice or homozygous mice, respectively. Thus, approximately 20% of post rearranging cells maintains Vβ8.2 germline transcription on the allelically excluded chromosome.
Figure 6.
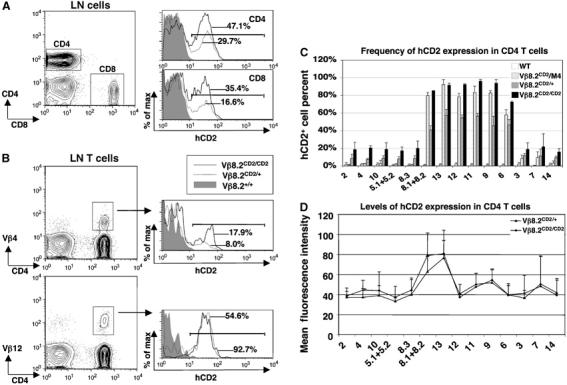
hCD2 expression in peripheral T cells from Vβ8.2CD2 knock-in mice. (A) hCD2 expression in peripheral lymph node (LN) T cells of Vβ8.2CD2/CD2, Vβ8.2CD2/+ and wild-type mice. The gating of CD4+ and CD8+ LN T cells is indicated in the contour plot. hCD2 staining of CD4+ and CD8+ T cells from Vβ8.2CD2/CD2 (black line), Vβ8.2CD2/+ (dotted line), and wild-type (shade) mice is shown in overlayed histograms. Percentages of hCD2-positive cells (defined by bracketed area) are indicated in the histograms. Results are representative of 5–12 separate experiments. (B) hCD2 expression in Vβ4- and Vβ12-specific LN CD4+T cells of Vβ8.2CD2/CD2, Vβ8.2CD2/+ and wild-type mice. The gating of Vβ+ LN CD4+T cells in the contour plots and histogram analysis are as in (A). Results are representative of five separate experiments. (C) Percentage of hCD2-positive cells in each Vβ subtype specific population of CD4+ LN T cells. Data from Vβ8.2CD2/M4 transheterozygous mice are also included. Numbers under column clusters indicate Vβ subtypes. They are arranged in the same order as the relative locations of each Vβ segment in the TCRβ locus. Results are the mean±s.e.m. of 5–12 independent experiments. (D) Mean fluorescence intensity (MFI) of hCD2 staining in each Vβ subtype-specific population of CD4+ LN T cells. The MFI of hCD2 staining in Vβ13-specific CD4+ T cells is significantly higher (P<0.01) than that of other Vβ-specific T cells. Results are the mean±s.e.m. of 5–12 independent experiments.
In order to evaluate hCD2 expression on a single rearranging chromosome, we generated mice trans-heterozygous for the Vβ8.2CD2 allele and a non-recombinant TCR allele M4, which carries targeted mutations in the DJ region (Bassing et al, 2000). In these Vβ8.2CD2/M4 mice, hCD2 expression and V-DJ recombination can only occur on the single Vβ8.2CD2 allele. As predicted, no hCD2 expression is detected from T cells using V genes upstream of Vβ8.2 (Figure 6C). Downstream of Vβ8.2, the frequency of hCD2 expression in Vβ8.2CD2/M4 mice increases as Vβ gene recombination moves from the 3′ region toward Vβ8.2. The frequency of Vβ8.2 transcriptional activation is about 10% when rearrangement uses Vβ7, which is 150 kb away from the Vβ8.2 promoter and increases to approximately 80% in cells that express Vβ9, which is 42 kb away from Vβ8.2. The level of hCD2 expression was also analyzed by examining the mean fluorescence intensity of hCD2 staining among hCD2-positive cells (Figure 6D). In each Vβ subpopulation, the level of hCD2 expression in hCD2-positive cells is the same between Vβ8.2CD2/+ and Vβ8.2CD2/CD2 mice, suggesting that Vβ8.2 germline transcript is mostly expressed from a single allele in mature T cells. hCD2-positive cells in Vβ13 and Vβ8.1 (which is immediately downstream of Vβ8.2) subpopulations show significantly higher hCD2 expression level than those in the rest of Vβ groups in this analysis. This result implies that the rate of germline transcription from the Vβ8.2 promoter is higher in cells expressing Vβ13 and Vβ8.1 than those expressing other Vβ genes.
Inhibition of Vβ germline transcription in T cells expressing a TCRβ transgene
The above study suggested that Vβ germline transcription in mature T cells is dependent on Eβ, which is brought closer the Vβ genes due to V-DJ rearrangement. It is not clear whether the Vβ promoter on its own is sufficient to maintain Vβ gene germline transcription in the absence of V-DJ rearrangement. To evaluate Vβ promoter activity in the absence of rearrangement, we introduced an active TCRβ transgene (Fenton et al, 1988) into our Vβ8.2CD2 knock-in mice. This TCRβ transgene has been shown to be capable of enforcing allelic exclusion by suppressing V-DJ rearrangement of the endogenous TCRβ genes (Shinkai et al, 1993; Jackson et al, 2005). We found that introduction of the TCRβ transgene results in reduced frequency of hCD2+ DN1 thymocytes. However, the percentage of hCD2+ cells in DN2/3 thymocytes remains largely unchanged, although the expression level of hCD2 is slightly decreased in the presence of the TCRβ transgene (Figure 7A). In contrast, hCD2 expression is inhibited in DN4, DP and SP thymocytes (Figure 7B). Most remaining hCD2-positive cells in CD4 SP and CD8 SP cells are found to express low levels of CD5, suggesting that these cells are developmentally immature. Most peripheral CD4 and CD8 cells are also negative for hCD2 expression in the presence of the TCR transgene (Figure 7D). Thus, the result supports the notion that V-DJ recombination is required for germline transcription in DP, SP and peripheral T cells.
Figure 7.
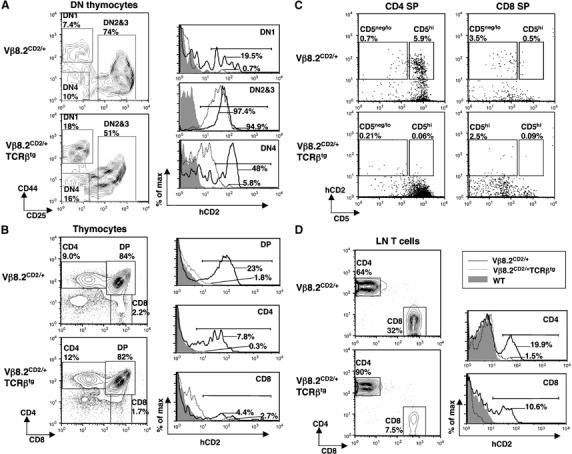
hCD2 expression from Vβ8.2CD2 on TCRβ transgenic background. hCD2 expression was evaluated in thymocytes (A–C) and peripheral T cells (D) of 3-week-old Vβ8.2CD2 TCRβ transgene-positive (Vβ8.2CD2/+TCRβtg) mice, Vβ8.2CD2/+ mice and wild-type mice. The relative percentage of each gated population is given in the contour plot. Contour lots in (C) were gated from CD4SP and CD8SP shown in (B). Percentages of hCD2-positive cells (defined by bracketed area) are shown in histograms. Results are representative of three separate experiments.
Discussion
The use of genetic marker allowed us to evaluate germline transcription at the TCR Vβ locus for the first time at the single-cell level. Any genetic manipulation may result in some perturbation of the endogenous locus. To avoid any artificial effect on the locus, we designed the Vβ8.2CD2 knock-in allele by inserting a marker away from any known transcriptional regulatory elements. The marker cassette is composed of the minimal IRES sequence and the coding sequence for hCD2 without adding any exogenous splicing or transcriptional stop sites. However, we find an inadvertent downregulation of Vβ8.2 germline transcription and rearrangement from the knock-in allele. The negative effect on levels of germline transcript could be due to either a change in transcription efficiency or in stability of the chimeric Vβ8.2-hCD2 message. We cannot distinguish between these two possibilities in our assay system. Similarly, the cause of reduction in Vβ8.2 rearrangement may be linked to germline transcription or structural change around the Vβ8.2 RSS region. Although the knock-in allele is not completely neutral, several compelling evidence suggest that the hCD2 marker represents the transcriptional activity of the endogenous Vβ8.2 locus. First, hCD2 expression from the knock-in allele is highly restricted to the T-cell lineage. Second, the hCD2 marker begins to express at the developmental stage when T-cell lineage commitment occurs. Third, the hCD2 expression is appropriately downregulated at the population level as development move from DN3 to DN4 stage. All three observations fit to the hallmarks of Vβ germline transcription defined in previous studies (Senoo and Shinkai, 1998; Chen et al, 2001).
Our study of hCD2 expression in DN2/3 cells indicates that TCRβ genes are activated in a biallelic manner. Two possible mechanisms have been proposed to account for allelic exclusion. The first model suggests that instructive signals such as epigenetic modification may allow differential accessibility of the two alleles. The second model suggests that monoallelic rearrangement is due to low frequency and stochastic usage of the two alleles from the homologous chromosomes. Both instructive and stochastic models have gained support in the study of Igκ rearrangement in pre-B cells. It has been shown that Igκ locus is subject to monoallelic regulation such as asynchronous DNA replication (Mostoslavsky et al, 2001), monoallelic demethylation (Mostoslavsky et al, 1998; Goldmit et al, 2002) and monoallelic association with repressive heterochromatin (Goldmit et al, 2005) during pre-B-cell development. These epigenetic changes may provide signals for monoallelic rearrangement (Bergman and Cedar, 2004). Noticeably, biallelic germline transcription from the J gene segment within the Igκ locus was observed in a study using single-cell RT–PCR and RNA fluorescence in situ hybridization (Singh et al, 2003). This study suggested that germline transcription, at least around the J gene, is not linked to allelic exclusion. However, this study did not rule out the possibility that germline transcription in the Vκ genes may still occur in a monoallelic manner and such contribute to allelic exclusion. Support for the stochastic model came from a recent gene targeting approach, in which Igκ germline transcription is genetically labeled with a GFP marker. This study showed that monoallelic Igκ rearrangement is linked to low frequent and stochastic germline transcription within the J gene segments (Liang et al, 2004). In contrast to the Igκ gene, the results from our Vβ8.2CD2 knock-in mouse model show that Vβ germline transcription at the TCR gene locus is biallelic before rearrangement. Our study suggests that germline transcription, while may be essential for efficient rearrangement involving the transcribed region, is unlikely to provide sufficient instructive signals for allelic exclusion at the Vβ locus.
As an indicator of TCR Vβ8.2 germline transcription, hCD2 was found to be continuously expressed in a fraction of DP and SP thymocytes as well as peripheral mature T cells. It is possible that some hCD2 expression could come from extrachromosomal excision circles (TRECs) generated from the upstream Vβ genes recombining to DJβ. However, we did not detect any hCD2 expression from T cells using Vβ genes upstream of Vβ8.2 in Vβ8.2CD2/M4 trans-heterozygous mice, suggesting the Vβ8.2CD2 containing TRECs are either diluted out or silent in these cells. Furthermore, a recent report has shown that TREC is quickly diluted out and become undetectable even by PCR as thymocytes develop from DN to DP stage (Jackson et al, 2005).
Our study also showed that maintenance of allelic exclusion at the TCRβ locus is also uncoupled from Vβ gene germline transcription. It has been reported that germline transcription of Vβ genes (Senoo and Shinkai, 1998) and histone H3 and H4 acetylation (Tripathi et al, 2002) are downregulated upon transition from DN to DP cells. Therefore, it is believed that a suppressive chromatin environment is important in the maintenance of allelic exclusion. However, these analyses were carried out on the RAG-deficient background, which do not account for the effect of V-DJ rearrangement on the remaining germline Vβ genes. Under physiological conditions, after V-DJ rearrangement, the distal Vβ region is brought into close proximity of Eβ, which will cooperate with Vβ promoters to activate Vβ gene transcription in mature T cells (McDougall et al, 1988; Khor and Sleckman, 2002). A recent study showed that the effect of Eβ can go beyond the functionally rearranged Vβ gene and keep the nearby upstream germline Vβ genes in active chromatin structure, as characterized by H3 acetylation, H3 K4 methylation and germline transcription (Jackson and Krangel, 2005). Our study suggests that the effect of Eβ on germline transcription is dependent on V-DJ rearrangement since inhibition of V-DJ rearrangement by a TCRβ transgene can effectively eliminate most hCD2 expression in DP, SP and peripheral T cells. This result is in contrast to the studies on IgH gene, which showed rearrangement to the proximal V genes in the presence of a functional μ transgene (Iacomini et al, 1991; Costa et al, 1992). It is possible that regulation of TCR Vβ gene transcriptional activation resembles distal VH genes rather than to proximal VH genes, which have been documented as differentially regulated from the distal ones (Corcoran, 2005).
The TCRβ knock-in system provides a unique way to evaluate the effect of an enhancer on a promoter separated by varying distance in the context of an endogenous locus. It has been suggested that enhancers regulate the probability of transcription rather than the rate of transcription (Walters et al, 1995). Study of Vβ8.2CD2 germline transcription in mature T cells suggested that Eβ might be affecting both the probability and the rate of Vβ8.2 germline transcription after Vβ to DJβ rearrangement. Our data revealed that the probability of transcriptional activation of Vβ8.2 can be more than 90% among Vβ13+ T cells. As Vβ usage moves further downstream, the distance between Eβ and Vβ8.2 promoter increases, and the probability of Vβ8.2 transcription gradually reduces to 10%. Noticeably, it seems that Eβ is still able to actively influence Vβ8.2 promoter even when it is more than 150 Kb away from Vβ8.2 promoter in the case of Vβ7-positive T cells. The decrease of transcription activation could be due to the presence of an unidentified inhibitory cis-element within the region between Vβ6 and Vβ3. Alternatively, it can be explained by the possibility that Eβ becomes less potent simply because it is physically further separated away from the Vβ8.2 promoter. Our study also shows that the level of germline transcription also varies with changing distance between Vβ8.2 promoter and the productively rearranged Vβ gene. A significant enhancement of the rate of Vβ8.2 transcription is observed for Vβ13 and Vβ8.1T cells when the Vβ8.2 promoter is located within 20–30 kb from Eβ. Although this result is consistent with the distance effect of Eβ function, we cannot rule out the possibility that increased germline transcription is due to reduced promoter competition after elimination of most Vβ promoters between Vβ8.2 and Eβ.
The method of marking germline transcription from an individual chromosome also allowed us to examine germline transcription on the allelically excluded chromosome. In the case of productive rearrangement using Vβ genes upstream of Vβ8.2, we can evaluate germline transcription from Vβ8.2 on the allelically excluded chromosome. Our study showed that T cells using these upstream Vβ genes have 20% of chance expressing hCD2 on the allelically excluded chromosome. Because these hCD2-positive cells are absent in hCD2/M4 mice and absent in hCD2 TCRβ transgenic mice, we conclude that these hCD2-positive T cells must come from non-functional V-DJ rearrangement using Vβ genes downstream Vβ8.2 on the allelically excluded chromosome. Because Vβ8.2 is located in the middle of Vβ gene cluster, we can estimate that these hCD2-positive cells only represent approximately half of non-functional V-DJ rearrangement on the allelically excluded chromosome. This number is consistent with the fact that the total percentage of non-functional V-DJ rearrangement in the allelically excluded chromosome has been mathematically calculated and experimentally verified as 40% (Yancopoulos and Alt, 1986; Khor and Sleckman, 2005). Thus, similar to the functional allele, Vβ germline transcription on the allelically excluded chromosome is most likely due to non-functional rearrangement, which brings Vβ promoter into close proximity to Eβ.
Our data on Vβ8.2 germline transcription are a representative of the 20 Vβ genes in the locus. Vβ8.2 is known to produce relatively high level of germline transcripts in comparison with most other Vβ genes (Jolly and O'Neill, 1997; Chen et al, 2001). We provide evidence to show that germline transcription from several other Vβ genes, like Vβ8.2, is strictly correlated with gene copies regardless of levels of expression. However, the population-based RT–PCR analysis cannot distinguish germline transcription in a mosaic versus a uniformed pattern within the population of DN2 cells. Thus, the experimental result is also compatible with the monoallelic activation if only a small fraction of cells is involved in germline transcription. Future analysis with single-cell resolution is clearly needed to determine whether bi-allelic activation of Vβ genes occurs across the entire Vβ gene locus.
Materials and methods
Generation of targeted ES cells and mutant mice
TCR Vβ and homologous arms were generated using 129 genomic DNA with the FailSafeTM PCR system (Epicentre, Madison, WI). Sequencing of TCR Vβ8.2 promoter and entire Vβ8.2 gene segment was performed to confirm PCR accuracy. The fragments were cloned into the pLoxR vector containing PGK promoter-driven Neo and DT selection markers. The YZ-C22 129 ES cell line (established in Zhuang laboratory) was transfected with linearized targeting vector. Homologous recombination was verified by Southern blot using both HpaI-digested genomic DNA analyzed with a 3′ probe and XbaI-digested genomic DNA analyzed with a 5′ probe (data not shown). The loxP-flanked PGKNeo gene was deleted from a targeted ES clone after transient expression of Cre recombinase. Chimeric mice were crossed with C57Bl/6 mice to detect germline transmission and progeny were backcrossed to C57Bl/6 once before used in intercross.
PCR genotyping of the Vβ8.2CD2 allele
PCR genotyping was conducted by amplifying toe DNA with a sense primer, P1 (5′-ATG GTG CTG GCA GCA CTG AG-3′) and two antisense primers, P2 (5′-CCG GAA TTC AGG GAT GTT GTG TCA TAT TAT GAT GC-3′) and P3 (5′-CCT GAT CAT CGG TCT TCA GAT GC-3′). The PCR products were then separated on agarose gels. The wild-type allele and the Vβ8.2CD2 allele were detected as 1.8- and 1.1-kb products, respectively.
Flow cytometry
Bone marrow, spleen, lymph node and thymus cells were isolated from 4- to 6-week-old (except noted otherwise in figure legend) mice in phosphate-buffered saline supplemented with 5% bovine calf serum and were used immediately for FACS analysis. Cell suspensions were stained with a combination of an FITC-conjugated antibody, a PE-conjugated antibody and an APC-conjugated antibody plus 7-aminoactinomycin D (7AAD; Molecular Probes) and analyzed on a FACS Caliber (Becton Dickinson). PE-Cy5-conjugated anti-CD3, -CD4, -CD8, -B220, -Gr-1, and -Mac-1 antibodies were also used together with 7AAD as the dump channel in the analysis of DN thymocytes. Two-dimensional contour plot and histogram analyses were performed with FlowJo software. For DN2 and DN2/3 thymocyte purification, total DN thymocytes were enriched by AUTO MACS through CD25-positive selection or CD4/CD8 depletion, respectively. Enriched DN thymocytes were then subject to sorting by FACS-Diva (Beckman Coulter). APC-conjugated anti-c-kit antibody (eBioscience) staining is also used for DN2 thymocyte sorting.
Real-time RT–PCR
Total RNA were extracted using TRI Reagent (Sigma) from purified DN2 or DN2/3 thymoyctes and reverse transcribed into cDNA using M-MLV reverse transcriptase (Invitrogen). Germline transciption was determined by quantitative PCR analysis using Roche LightCycler and a FastStart DNA master SYBR green I kit (Roche) and the data were normalized to GAPDH expression. Serial dilutions of cDNA extracted from RAG2−/− thymocytes were used as the source of standard curve for germline transcription from endogenous TCR Vβ genes shown in Figure 2D. Serial dilutions of total thymocyte genomic DNA were used as the source of standard curve for Vβ8.2CD2 knock-in allele germline transcription quantification shown in Figure 3F. Serial dilutions of cDNA extracted from sorted DN3 thymocytes were used as the source of standard curve for germline transcription from endogenous TCR Vβ genes shown in Figure 5. All results are confirmed by PCR reactions run in separate batches. The primers used for quantitative PCR are listed and described in Supplementary Table 1.
TCR Vβ8.1. and TCR Vβ8.2 usage analyses
Total RNA were extracted from total thymocytes, sorted DN2/3 thymocytes or sorted Vβ8.1/8.2-positive mature T cells. Vβ8.1/8.2-positive cells were sorted from peripheral LN cells stained with FITC-conjugated anti-Vβ8.1/8.2, PE-conjugated anti-CD3, APC-conjugated anti-B220 and 7AAD as described. Vβ8.1- and Vβ8.2-specific cDNA were amplified by RT–PCR using Vβ8.1/8.2 consensus primer (Vβ8.1/8.2 e for 5′-GGT GGC AGT AAC AGG AGG AAA G-3′) and TCR Cβ1/2 consensus primer (Cβ1/2 e rev 5′-GCT CAG CTC CAC GTG GTC AGG G-3′). Vβ8.2 cDNA contains a single unique AflIII site, which is not present in Vβ8.1. As a positive control for complete AflIII digestion, genomic DNA containing the same AflIII was amplified by primer Vβ8.1/8.2 e for and Vβ8.2 rev (5′-TAC GCC TGC AGG CTG AGA CCT ATG TTA TAA GGT TCC TGG-3′), using Vβ8.2CD2 targeting construct as template. PCR product was then purified by PCR purification kit (QIAGEN). One half of the purified PCR product was subject to AflIII digestion while the other half used as undigested.
Vβ8.2 rearrangement analysis
CD4/CD8-depleted thymocytes from Vβ8.2CD2/CD2 and wild-type mice were stained with FITC-conjugated anti-CD44 antibody, PE-conjugated anti-hCD2 antibody and APC-conjugated anti-CD25 antibody plus PE-Cy5-conjugated anti-CD3, -CD4, -CD8, -B220, -Gr-1, -Mac-1 antibodies and anti-7AAD. Genomic DNA was extracted from sorted DN2/3 thymocytes and amplified with Jβ2.7 rev (5′-TTG GGT GGA AGC GAG AGA TGT GAA -3′) and Vβ8.2 LF (5′-ATG GGC TCC AGG CTC TTC TTC GTG -3′) primers using a touch down PCR program.
Preparation of intraepithelial lymphocytes
Peyer's patches and contents were removed from the small intestines. Clean small intestines were then cut into pieces of <5 mm in length and stirred at 37°C for 20 min in CMF/FBS/DTT buffer (10% FBS in PBS with 20 mM HEPES and 0.154 mg/ml DTT). Supernatant were collected twice with fresh CMF/FBS/DTT buffer added between the collections. Supernatant was centrifuged through a 44–67% discontinuous Percoll (Amersham Biosciences) gradient that had been adjusted by 10 × PBS at 1700 r.p.m. for 30 min at room temperature. Cells at the interface were collected and washed with CMF buffer (2% FBS in PBS with 20 mM HEPES) before FACS analysis.
Supplementary Material
Supplementary Information
Acknowledgments
We thank members of Zhuang laboratory and Drs Michael Krangel and Annette Jackson for comments and suggestions, Dr Yuowen He for the gift of human CD2 construct, Dr Krangel for providing TCRβ transgenic and M4 mice and Duke transgenic facility for microinjection of ES cells into mouse embryos. This work was supported by funding from NIH R01GM059638 to YZ.
References
- Alt FW, Blackwell TK, DePinho RA, Reth MG, Yancopoulos GD (1986) Regulation of genome rearrangement events during lymphocyte differentiation. Immunol Rev 89: 5–30 [DOI] [PubMed] [Google Scholar]
- Bassing CH, Alt FW, Hughes MM, D'Auteuil M, Wehrly TD, Woodman BB, Gartner F, White JM, Davidson L, Sleckman BP (2000) Recombination signal sequences restrict chromosomal V (D)J recombination beyond the 12/23 rule. Nature 405: 583–586 [DOI] [PubMed] [Google Scholar]
- Bergman Y, Cedar H (2004) A stepwise epigenetic process controls immunoglobulin allelic exclusion. Nat Rev Immunol 4: 753–761 [DOI] [PubMed] [Google Scholar]
- Bergman Y, Fisher A, Cedar H (2003) Epigenetic mechanisms that regulate antigen receptor gene expression. Curr Opin Immunol 15: 176–181 [DOI] [PubMed] [Google Scholar]
- Bories JC, Demengeot J, Davidson L, Alt FW (1996) Gene-targeted deletion and replacement mutations of the T-cell receptor beta-chain enhancer: the role of enhancer elements in controlling V (D)J recombination accessibility. Proc Natl Acad Sci USA 93: 7871–7876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier G, Watrin F, Naspetti M, Verthuy C, Naquet P, Ferrier P (1996) Deletion of the mouse T-cell receptor beta gene enhancer blocks alphabeta T-cell development. Proc Natl Acad Sci USA 93: 7877–7881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone M, Watrin F, Fernex C, Horvat B, Krippl B, Wu L, Scollay R, Ferrier P (1993) TCR beta and TCR alpha gene enhancers confer tissue- and stage-specificity on V (D)J recombination events. EMBO J 12: 4335–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay S, Whitehurst CE, Schwenk F, Chen J (1998) Biochemical and functional analyses of chromatin changes at the TCR-beta gene locus during CD4−CD8− to CD4+CD8+ thymocyte differentiation. J Immunol 160: 1256–1267 [PubMed] [Google Scholar]
- Chen F, Rowen L, Hood L, Rothenberg EV (2001) Differential transcriptional regulation of individual TCR V beta segments before gene rearrangement. J Immunol 166: 1771–1780 [DOI] [PubMed] [Google Scholar]
- Corcoran AE (2005) Immunoglobulin locus silencing and allelic exclusion. Semin Immunol 17: 141–154 [DOI] [PubMed] [Google Scholar]
- Costa TE, Suh H, Nussenzweig MC (1992) Chromosomal position of rearranging gene segments influences allelic exclusion in transgenic mice. Proc Natl Acad Sci USA 89: 2205–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton RG, Marrack P, Kappler JW, Kanagawa O, Seidman JG (1988) Isotypic exclusion of gamma delta T cell receptors in transgenic mice bearing a rearranged beta-chain gene. Science 241: 1089–1092 [DOI] [PubMed] [Google Scholar]
- Fugmann SD, Lee AI, Shockett PE, Villey IJ, Schatz DG (2000) The RAG proteins and V (D)J recombination: complexes, ends, and transposition. Annu Rev Immunol 18: 495–527 [DOI] [PubMed] [Google Scholar]
- Goldmit M, Ji Y, Skok J, Roldan E, Jung S, Cedar H, Bergman Y (2005) Epigenetic ontogeny of the Igk locus during B cell development. Nat Immunol 6: 198–203 [DOI] [PubMed] [Google Scholar]
- Goldmit M, Schlissel M, Cedar H, Bergman Y (2002) Differential accessibility at the kappa chain locus plays a role in allelic exclusion. EMBO J 21: 5255–5261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grawunder U, Harfst E (2001) How to make ends meet in V (D)J recombination. Curr Opin Immunol 13: 186–194 [DOI] [PubMed] [Google Scholar]
- Iacomini J, Yannoutsos N, Bandyopadhay S, Imanishi-Kari T (1991) Endogenous immunoglobulin expression in mu transgenic mice. Int Immunol 3: 185–196 [DOI] [PubMed] [Google Scholar]
- Jackson A, Kondilis HD, Khor B, Sleckman BP, Krangel MS (2005) Regulation of T cell receptor beta allelic exclusion at a level beyond accessibility. Nat Immunol 6: 189–197 [DOI] [PubMed] [Google Scholar]
- Jackson AM, Krangel MS (2005) Allele-specific regulation of TCR beta variable gene segment chromatin structure. J Immunol 175: 5186–5191 [DOI] [PubMed] [Google Scholar]
- Jolly CJ, O'Neill HC (1997) Specific transcription of the unrearranged TCR V beta 8.2 gene in lymphoid tissues occurs independently of V (D)J rearrangement. Immunol Cell Biol 75: 13–20 [DOI] [PubMed] [Google Scholar]
- Khor B, Sleckman BP (2002) Allelic exclusion at the TCRbeta locus. Curr Opin Immunol 14: 230–234 [DOI] [PubMed] [Google Scholar]
- Khor B, Sleckman BP (2005) Intra- and inter-allelic ordering of T cell receptor beta chain gene assembly. Eur J Immunol 35: 964–970 [DOI] [PubMed] [Google Scholar]
- Krangel MS (2003) Gene segment selection in V (D)J recombination: accessibility and beyond. Nat Immunol 4: 624–630 [DOI] [PubMed] [Google Scholar]
- Laurent J, Bosco N, Marche PN, Ceredig R (2004) New insights into the proliferation and differentiation of early mouse thymocytes. Int Immunol 16: 1069–1080 [DOI] [PubMed] [Google Scholar]
- Liang HE, Hsu LY, Cado D, Schlissel MS (2004) Variegated transcriptional activation of the immunoglobulin kappa locus in pre-b cells contributes to the allelic exclusion of light-chain expression. Cell 118: 19–29 [DOI] [PubMed] [Google Scholar]
- McDougall S, Peterson CL, Calame K (1988) A transcriptional enhancer 3′ of C beta 2 in the T cell receptor beta locus. Science 241: 205–208 [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Singh N, Kirillov A, Pelanda R, Cedar H, Chess A, Bergman Y (1998) Kappa chain monoallelic demethylation and the establishment of allelic exclusion. Genes Dev 12: 1801–1811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostoslavsky R, Singh N, Tenzen T, Goldmit M, Gabay C, Elizur S, Qi P, Reubinoff BE, Chess A, Cedar H, Bergman Y (2001) Asynchronous replication and allelic exclusion in the immune system. Nature 414: 221–225 [DOI] [PubMed] [Google Scholar]
- Pircher H, Ohashi P, Miescher G, Lang R, Zikopoulos A, Burki K, Mak TW, MacDonald HR, Hengartner H (1990) T cell receptor (TcR) beta chain transgenic mice: studies on allelic exclusion and on the TcR+ gamma/delta population. Eur J Immunol 20: 417–424 [DOI] [PubMed] [Google Scholar]
- Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT (2004) Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity 20: 735–745 [DOI] [PubMed] [Google Scholar]
- Prinz I, Sansoni A, Kissenpfennig A, Ardouin L, Malissen M, Malissen B (2006) Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nat Immunol 7: 995–1003 [DOI] [PubMed] [Google Scholar]
- Ryu CJ, Haines BB, Lee HR, Kang YH, Draganov DD, Lee M, Whitehurst CE, Hong HJ, Chen J (2004) The T-cell receptor beta variable gene promoter is required for efficient V beta rearrangement but not allelic exclusion. Mol Cell Biol 24: 7015–7023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlissel MS (2003) Regulating antigen-receptor gene assembly. Nat Rev Immunol 3: 890–899 [DOI] [PubMed] [Google Scholar]
- Senoo M, Shinkai Y (1998) Regulation of Vbeta germline transcription in RAG-deficient mice by the CD3epsilon-mediated signals: implication of Vbeta transcriptional regulation in TCR beta allelic exclusion. Int Immunol 10: 553–560 [DOI] [PubMed] [Google Scholar]
- Senoo M, Wang L, Suzuki D, Takeda N, Shinkai Y, Habu S (2003) Increase of TCR V beta accessibility within E beta regulatory region influences its recombination frequency but not allelic exclusion. J Immunol 171: 829–835 [DOI] [PubMed] [Google Scholar]
- Shinkai Y, Koyasu S, Nakayama K, Murphy KM, Loh DY, Reinherz EL, Alt FW (1993) Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science 259: 822–825 [DOI] [PubMed] [Google Scholar]
- Singh N, Bergman Y, Cedar H, Chess A (2003) Biallelic germline transcription at the kappa immunoglobulin locus. J Exp Med 197: 743–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi R, Jackson A, Krangel MS (2002) A change in the structure of Vbeta chromatin associated with TCR beta allelic exclusion. J Immunol 168: 2316–2324 [DOI] [PubMed] [Google Scholar]
- Uematsu Y, Ryser S, Dembic Z, Borgulya P, Krimpenfort P, Berns A, von Boehmer H, Steinmetz M (1988) In transgenic mice the introduced functional T cell receptor beta gene prevents expression of endogenous beta genes. Cell 52: 831–841 [DOI] [PubMed] [Google Scholar]
- Walters MC, Fiering S, Eidemiller J, Magis W, Groudine M, Martin DI (1995) Enhancers increase the probability but not the level of gene expression. Proc Natl Acad Sci USA 92: 7125–7129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Senoo M, Habu S (2002) Differential regulation between gene expression and histone H3 acetylation in the variable regions of the TCRbeta locus. Biochem Biophys Res Commun 298: 420–426 [DOI] [PubMed] [Google Scholar]
- Whitehurst CE, Chattopadhyay S, Chen J (1999) Control of V (D)J recombinational accessibility of the D beta 1 gene segment at the TCR beta locus by a germline promoter. Immunity 10: 313–322 [DOI] [PubMed] [Google Scholar]
- Yancopoulos GD, Alt FW (1986) Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol 4: 339–368 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


