Abstract
In Xenopus, an asymmetric distribution of Wnt activity that follows cortical rotation in the fertilized egg leads to the dorsal–ventral (DV) axis establishment. However, how a clear DV polarity develops from the initial difference in Wnt activity still remains elusive. We report here that the Teashirt-class Zn-finger factor XTsh3 plays an essential role in dorsal determination by enhancing canonical Wnt signaling. Knockdown of the XTsh3 function causes ventralization in the Xenopus embryo. Both in vivo and in vitro studies show that XTsh3 substantially enhances Wnt signaling activity in a β-catenin-dependent manner. XTsh3 cooperatively promotes the formation of a secondary axis on the ventral side when combined with weak Wnt activity, whereas XTsh3 alone has little axis-inducing ability. Furthermore, Wnt1 requires XTsh3 for its dorsalizing activity in vivo. Immunostaining and protein analyses indicate that XTsh3 is a nuclear protein that physically associates with β-catenin and efficiently increases the level of β-catenin in the nucleus. We discuss the role of XTsh3 as an essential amplifying factor of canonical Wnt signaling in embryonic dorsal determination.
Keywords: β-catenin, axis development, Tsh3, Wnt, Xenopus
Introduction
Dorsal–ventral (DV) axis specification is a major event in the establishment of embryonic polarity during early vertebrate embryogenesis. A large body of molecular embryological studies (particularly in Xenopus) has shown a central role for canonical Wnt signaling in the early phase of the DV determination (Moon et al, 2002; Heasman, 2006). In Xenopus, sperm entry triggers an oriented microtubule rearrangement, called cortical rotation, which causes movement of the vegetal cortical cytoplasm containing the maternally derived dorsal determinants (reviewed by Weaver and Kimelman, 2004). A prevailing idea for the molecular mechanism of DV axis specification has been that cortical rotation leads to the unequal distribution of certain intracellular molecules that regulate the canonical Wnt pathway such as GSK3-binding protein and Dishevelled (Dsh), either by an active or passive transport process (Weaver and Kimelman, 2004). A recent report provides compelling evidence that the initial Wnt pathway is also asymmetrically activated by an extracellular ligand, maternal Wnt11 (Tao et al, 2005; Heasman, 2006).
Besides the arguments about the molecular nature of the Wnt regulators involved in this initial step, some questions remain unanswered with respect to the downstream events that lead to DV specification (Heasman, 2006). One major question, which we addressed in this study concerns the mechanism of how the asymmetrical patterning signal is enhanced along the DV axis. Although previous studies have demonstrated a biased localization of several RNAs and proteins involved in Wnt signaling, their initial difference along the DV axis is relatively small; for instance, although a preferential localization of Wnt11 RNA on the dorsal side is detected at the 8–16-cell stage by quantitative PCR, the quantitative difference in RNA amounts is only about two-fold (Tao et al, 2005; a similar observation was reported for Wnt11 proteins at the 64-cell stage; Schroeder et al, 1999). Therefore, it is likely that certain reinforcing mechanisms are necessary for the establishment of the clear DV polarity found in the early gastrula embryo.
In this study, we introduce the zinc-finger (Zn finger) nuclear protein XTsh3 as a key factor that enhances the Wnt signal in the dorsal axis determination of the Xenopus embryo. XTsh3 belongs to the protein family related to Drosophila Teashirt (Tsh), which was first identified as a homeotic selector for a trunk (prothoracic) segment identity versus an anterior (labial) identity (Fasano et al, 1991; Röder et al, 1992; Andrew et al, 1994). In addition, fly tsh plays crucial roles in segment polarity (Gallet et al, 1998) and in tissue development (Mathies et al, 1994; Wu and Cohen, 2000; Bessa et al, 2002) by interacting (directly or indirectly) with several transcriptional regulators such as Homothorax (Bessa et al, 2002), Brinker (Saller et al, 2002), Eyeless (Bessa et al, 2002) and Armadillo (fly β-catenin; Gallet et al, 1998, 1999) in a context-dependent manner.
A clue of Tsh's biological activity can be seen with its relationship with Wnt signaling. In the segment polarity phenotype of tsh mutant larvae, the denticle pattern of the trunk (abdominal) segment resembles that of the wingless (fly Wnt) or armadillo mutation (Gallet et al, 1998). Epistatic analyses have shown that Tsh and Wingless/Armadillo are mutually required for their activities (Gallet et al, 1998). Positive gene interactions between tsh and wingless pathway genes have been recently reported also for eye development (Bessa and Casares, 2005). Interestingly, Tsh has been shown to bind to Armadillo (Gallet et al, 1999), although its biological mechanism in the control of Wingless signaling still remains uncertain.
At least, three Tsh-related genes are found in the mouse and frog genomes (Caubit et al, 2000; Koebernick et al, 2006; our unpublished observations). Although vertebrate and fly Tsh-related factors have relatively diverse domain structures except for the first three Zn fingers (see Supplementary Figure S1A), mTsh1-3 can rescue the phenotypes of tsh mutants when overexpressed in the fly (Manfroid et al, 2004), suggesting that Tsh-related factors have some conserved activities. However, the exact in vivo roles of the vertebrate Tsh-class factors in embryogenesis are so far unknown.
Among the Xenopus tsh-related genes, we became interested in XTsh3, which is strongly expressed in the early frog embryo (see below). Here, we show that XTsh3 plays an indispensable role in Wnt-mediated dorsal axis determination. We discuss the function of XTsh3 as an essential promoting factor for Wnt-mediated dorsalizing signals and propose augmentation of the nuclear β-catenin level as a possible mechanism for its function.
Results
Expression pattern of XTsh3
XTsh3 encodes a protein with five Zn-finger motifs (C2H2-type) and a Hox domain (Figure 1A). The amino-terminal domain (particularly within the first three Zn-finger motifs) is well conserved between XTsh3 and Drosophila Tsh, while the carboxyl-terminal domains (including the Hox domain and two Zn-finger motifs) are conserved only among vertebrate Tsh-related factors (Supplementary Figure S1A), and not in Tsh or Tiptop (a fly paralog of Tsh; Laugier et al, 2005), suggesting that the carboxyl-terminal regions are diverse within this protein group. In addition, XTsh3 has a basic motif between the first and second Zn fingers that is conserved among vertebrate Tsh-related factors but not in the fly ones (Figure 1A and Supplementary Figure S1A). Thus, in terms of structural diversity, the fly and vertebrate Tsh-related factors are better thought of as family proteins (Tsh family factors, hereafter) rather than as mutual orthologs.
Figure 1.
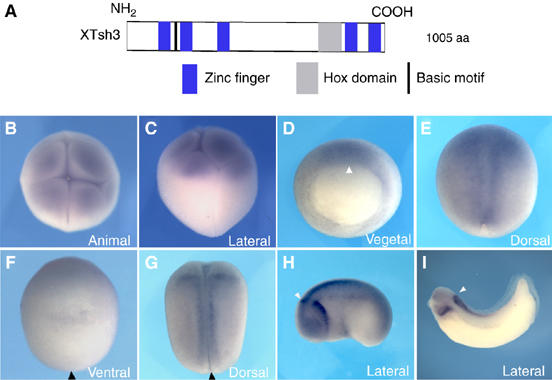
Deduced protein structure and expression profile of XTsh3. (A) Deduced domain structure of the XTsh3 protein. Blue, Zn-finger motif; gray, Hox domain; and black, basic motif. (B–I) Whole-mount in situ hybridization analysis of XTsh3 expression. (B, C) Maternal expression in the animal region at the eight-cell stage. Animal (B) and lateral (C) views. (D) Dorsally dominant expression at stage 11. Vegetal view. Arrowhead, dorsal lip. (E, F) stage 12.5, dorsal (E) and ventral (F) views. Arrowhead, closing blastopore. Section analysis also showed strong XTsh3 expression in the dorsal mesoderm and ectoderm (not shown). (G) Stage 18, dorsal view. (H, I) Tailbud (stage 24) and early larval (stage 31) stages, lateral views. Arrowhead, anterior expression border at the hindbrain. Strong XTsh3 expression gradually became limited to the caudal CNS.
We first examined the in vivo expression pattern of XTsh3 by whole-mount in situ hybridization analysis. Maternal XTsh3 transcripts were detected widely in the animal region of early cleavage-stage embryos (Figure 1B and C; at the eight-cell stage, XTsh3 RNA was found in the animal blastomeres and also in the equatorial parts of the vegetal blastomeres). At early gastrulation, zygotic XTsh3 expression was observed strongly in the dorsal marginal zone (MZ) and dorsal ectoderm, and relatively weakly on the ventral side (Figure 1D). The ventral expression continued to fade in both the mesoderm and ectoderm as gastrulation proceeded, whereas the dorsal expression (both ectoderm and mesoderm) persisted (Figure 1E and F). The wide dorsal expression gradually decreased during the late neurula stages (Figure 1G), leaving strong XTsh3 expression mainly in the caudal central nervous system (CNS; the hindbrain and spinal cord) and in the caudal branchial arch at the tailbud (Figure 1H) and larval (Figure 1I) stages. Quantitaitve PCR analysis showed that total amounts of XTsh3 RNA (maternal and zygotic) were relatively constant during early embryogenesis and substantially increased after the mid-neurula stage (Supplementary Figure S2A).
XTsh3 is an indispensable factor for dorsal axis determination
To elucidate the in vivo function of XTsh3 during early Xenopus development, we first performed loss-of-function experiments using XTsh3-MO. Injection of XTsh3-MO (20 ng, XTsh3-MO-A; Supplementary Figure S2B and D) into the two dorsal blastomeres of the four-cell embryo caused ventralization phenotypes (lacking an axis or having a strongly reduced dorsal axis; 80%, n=35; Figure 2A and B). In contrast, ventral injection of XTsh3-MO did not cause obvious ventralization (n=29; Figure 2C). Control MOs (5-base mismatched; Supplementary Figure S2B and D) did not affect axial patterning regardless of the injection side (data not shown).
Figure 2.
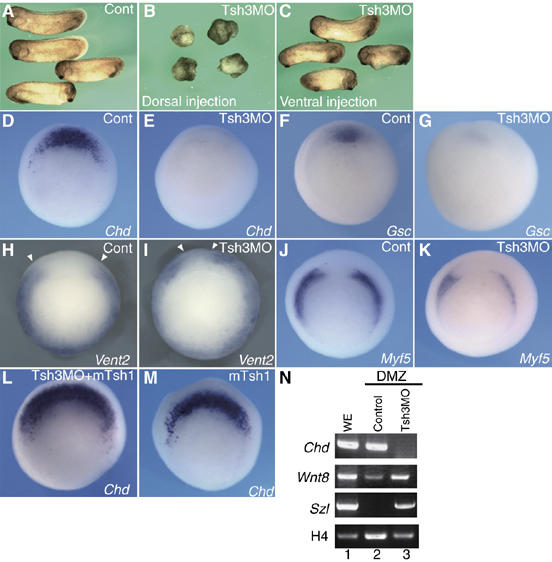
XTsh3 is essential for dorsal axial development in Xenopus. (A–C) Ventralization by XTsh3-MO. Injection of XTsh3-MO (MO-A, 20 ng/cell; B) into two dorsal blastomeres of four-cell embryos depleted the dorsal axes, while such ventralization was not seen with ventral XTsh3-MO injection (C). (D–K) Effects of radial XTsh3-MO injection on DV marker gene expression (D, E, Chd; F, G, Gsc; H, I, Vent2; J, K, Myf5) at the gastrula stage shown by in situ hybridization. Cont, control injection. Open arrowheads, dorsal borders of Vent2 expression. (L, M) Chd expression suppressed with XTsh3-MO (E) was rescued by coinjection of mTsh1 (L). (M) mTsh1 injection alone laterally expanded Chd expression (D, M). (N) RT–PCR of DMZ explants (stage 13 equivalent). Lane 1, whole embryo; lane 2, control DMZ; and lane 3, XTsh3-MO-injected DMZ.
Consistent with the morphological defects, XTsh3-MO injection suppressed the dorsal mesodermal markers Chordin (Chd) and goosecoid (Gsc) in the early gastrula embryo (strong suppression in 92%, n=13 and 47%, n=17, respectively, when injected into all four blastomeres; Figure 2D–G). Conversely, the expression of the ventrolateral marker Xvent2 was dorsally expanded (65%, n=12; Figure 2H and I), while the dorsal-lateral marker Myf5 was moderately reduced (60%, n=15; Figure 2J and K). Similar effects on DV markers (suppression of Chd and Gsc and expansion of the ventral markers Xvent1 and Sizzled) were seen with another XTsh3-MO (XTsh3-MO-C; Supplementary Figures S2C and D and S3) but not with its control MO (not shown). Quantitative PCR analysis showed consistent decrease and increase in expression of dorsal and ventral gene markers, respectively (Supplementary Figure S2E–G). The suppression phenotype of Chd by XTsh3-MO was reversed by coinjection of mouse Tsh1 (not overlapping with the MO; suppression of Chd expression in 92%, n=13 and 42%, n=24 without and with RNA coinjection, respectively; Figure 2E and L), suggesting that the suppression by the MO was specific to the loss of Tsh-related activity. Similar rescue was also seen for Myf5 expression (Supplementary Figure S2I and J).
In addition, ventralizing effects of XTsh3-MO were observed in dorsal MZ explants (Figure 2N), where injection of XTsh3-MO caused the suppression of Chd expression (dorsal) and induction of Sizzled expression (ventral). These in vivo and in vitro observations indicate that the XTsh3 function is necessary for the dorsal determination of the early Xenopus embryo.
We next examined the role of XTsh3 in gain-of-function studies. Injection of XTsh3 RNA into all the blastomeres at the four-cell stage (400 pg/cell) expanded the expression of Chd and Gsc at the gastrula stage (65%, n=23 and 52%, n=21, respectively; Figure 3A–D). In contrast, the ventral genes Xvent1 and Sizzled were suppressed (24%, n=17 and 43%, n=21, respectively; Figure 3E–H). Taken together with the MO studies (Figure 2), these findings demonstrate that XTsh3 acts as an essential factor for dorsal development.
Figure 3.
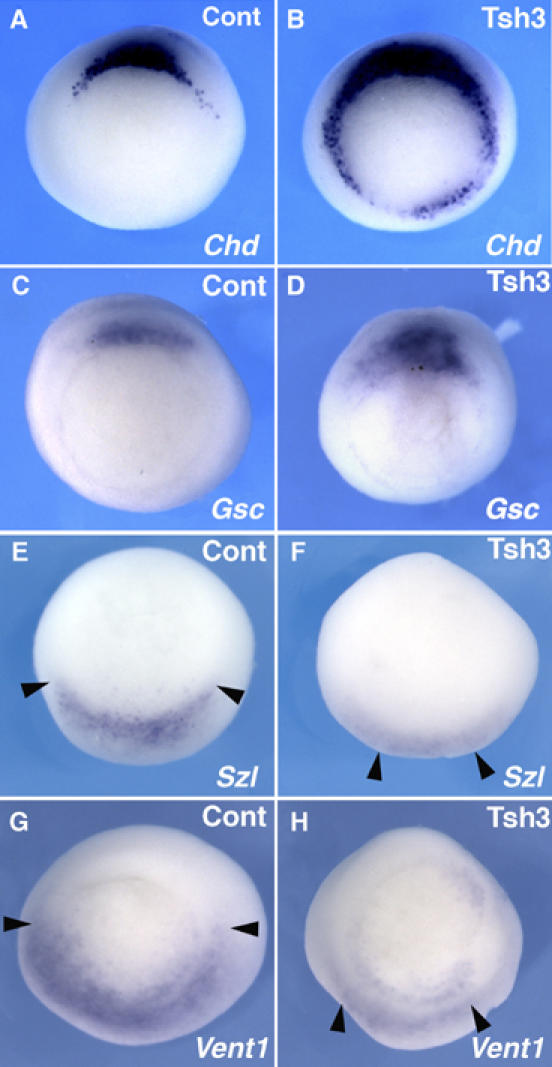
Dorsalizing activity of XTsh3. Whole-mount in situ hybridization analysis of DV marker gene expression (A, B, Chd; C, D, Gsc; E, F, Szl; G, H, Vent1) in control (A, C, E, G) and XTsh3-injected (400 pg/cell, four-cell stage; B, D, F, H) gastrula embryos. Vegetal view with the dorsal side up. The expression of the ventral markers was reduced and narrowed (indicated by arrowheads) by XTsh3 injection.
XTsh3 is an essential enhancer of canonical Wnt signaling
We next investigated the molecular mechanism of the activity of XTsh3 in DV patterning. Three major signaling pathways are known to play key roles in the dorsal specification of the early gastrula embryo (Heasman, 2006): the canonical Wnt pathway (Sokol et al, 1991; Weaver and Kimelman, 2004), the inhibition of BMP signaling (by antagonists; De Robertis et al, 2000; De Robertis and Kuroda, 2004; Khokha et al, 2005) and the Nodal/Activin signal (Smith, 1995; Osada and Wright, 1999).
We first examined the canonical Wnt pathway. An in vivo analysis using the luciferase reporter plasmid for Wnt signaling (TOPflash; conveying three copies of Tcf/LEF-binding sites) showed that XTsh3 strongly elevated the Wnt-responsive promoter activity in the animal cap (Figure 4A, lanes 1 and 2). Forced expression of XTsh3 also augmented the TOPflash activity in HEK293T cells (Figure 4B, lanes 1 and 2), suggesting that the increased TOPflash activity was not just secondary to the change in the differentiation state of the animal cap. Enhancement of luciferase activity by XTsh3 was not seen with the control FOPflash reporter in animal caps or 293T cells (Supplementary Figure S4A and B). In both animal caps and HEK293 cells, the enhanced reporter activity by XTsh3 was reduced when GSK3β and dominant-negative Tcf3 (dnTcf3; lacking the amino-terminal region), both of which negatively regulate Wnt signaling (He et al, 1995; Molenaar et al, 1996; Moon and Kimelman, 1998), were introduced (Figure 4A and B, lanes 3 and 4). XTsh3-MO injection attenuated the Wnt1-induced TOPflash activity in the animal cap (Figure 4C, lanes 3 and 4), indicating that XTsh3 is required for proper signaling of the canonical Wnt pathway.
Figure 4.
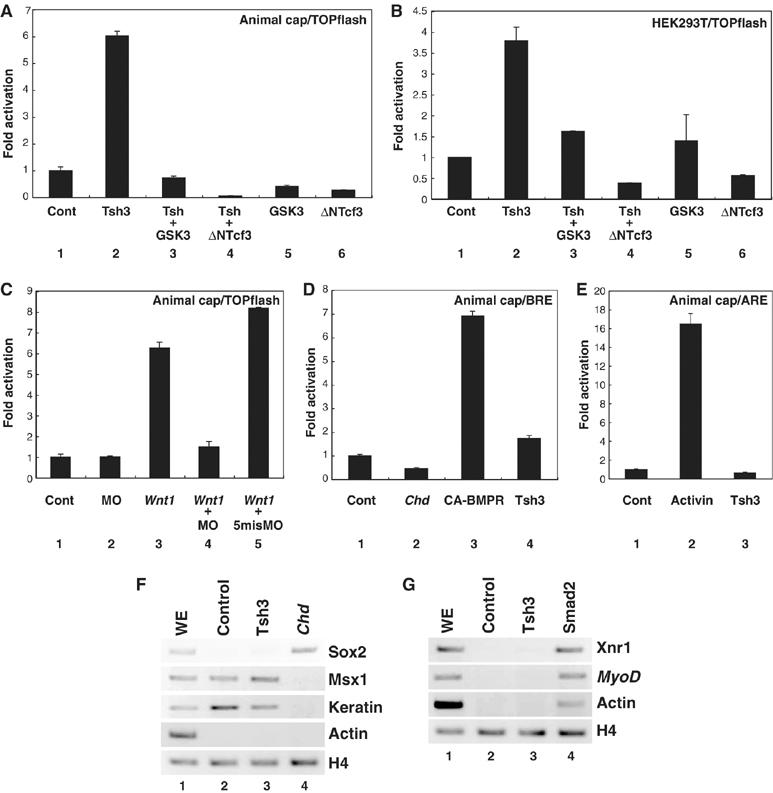
XTsh3 is required for efficeint canonical Wnt signaling. (A–C) Topflash-luciferase assay using animal caps (A, C) and 293T cells (B). (A, B) RNAs (A) or expression plasmids (B) used: lane 1, control; lane 2, XTsh3; lane 3, XTsh3 and GSK3β; lane 4, XTsh3 and dnTcf3, lane 5, and GSK3β; lane 6, dnTcf3. (C) RNAs used: lane 1, control; lane 2, XTsh3-MO; lane 3, Wnt1; lane 4, Wnt1 and XTsh3-MO, and lane 5, Wnt1 and control MO. (D) BRE (BMP response element)-luciferase assay using animal caps. RNAs used: lane 1, control; lane 2, Chd; lane 3, CA-BMPR (constitutive active); and lane 4, XTsh3. (E) ARE (Activin response element) luciferase assay using animal caps. Lane 1, control; lane 2, Activin treatment; and lane 3, XTsh3. (F) RT–PCR analysis of neural and non-neural ectodermal marker genes in RNA-injected animal caps (stage 12 equivalent). Unlike Chd injection (lane 4), XTsh3 injection (lane 3) did not induce strong expression of the neural marker Sox2 or suppress the non-neural genes Msx1 and Keratin. (G) RT–PCR analysis of mesodermal marker genes in injected animal caps (stage 12 equivalent). Unlike Smad2 injection (lane 4; mimicking Activin signaling), XTsh3 injection (lane 3) did not induce strong expression of Xnr1, MyoD or cardiac actin.
In contrast, XTsh3 neither suppressed the vent2-promoter activity for BMP signaling (BRE; Figure 4D, lanes 1 and 4) nor enhanced the Mix2-promoter activity for Nodal/Activin signaling (ARE; Figure 4E, lanes 1 and 3). Consistent with our findings, XTsh3 induced neither strong neural differentiation nor mesodermal differentiation in the animal cap (Figure 4F and G), unlike BMP inhibitors or activin (also see Supplementary Figure S4C and D for the lack of induction of the activin target genes Xbra and Mix2).
In vivo analysis showed that Chd expression that was increased by XTsh3 overexpression was strongly suppressed by coinjecting GSK3β, β-catenin-MO or dnTcf3 (suppression in 76%, n=21, 85%, n=13 and 100%, n=20, respectively; Figure 5B–D and data not shown). These findings suggest that the dorsalizing effect of XTsh3 depends on the Wnt/β-catenin pathway.
Figure 5.
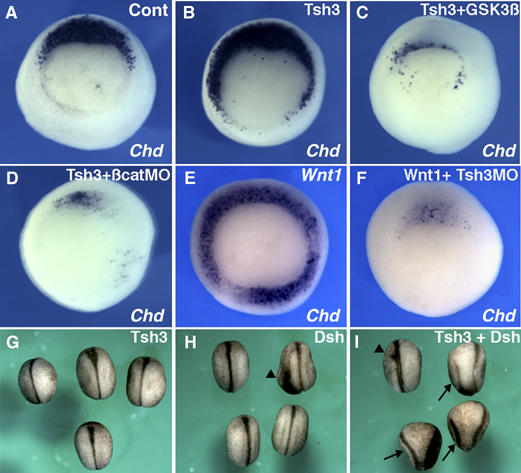
XTsh3 enhances and requires Wnt/β-catenin signaling in dorsal determination. (A–F) Effects on Chd expression at the gastrula stages. (B–D) Expansion of Chd expression by radial XTsh3 injection (B) was reversed by coinjecting GSK3β RNA (100 pg/cell; C) and β-catenin-MO (20 ng/cell; D). (E and F) Strong induction of Chd by radial Wnt1 injection (25 pg/cell; E) was inhibited by coinjecting XTsh3-MO (20 ng/cell; F). (G–I) Double axis formation by Dsh injection into a ventral-vegetal blastomere at the eight-cell stage (analyzed at the late neurula stage). (G) XTsh3 (100 pg/cell), (H) Dsh (100 pg/cell), and (I) Dsh and XTsh3. Arrows, secondary axes and arrowheads, weak axes.
We next examined whether XTsh3 is required for Wnt signaling. Ectopic Chd expression caused by radial Wnt1 injection (25 pg RNA/cell at the four-cell stage; 100%, n=25; Figure 5E) was suppressed by XTsh3-MO (suppressed in 100%, n=27; Figure 5F), suggesting that XTsh3 is required for the dorsalizing effect of the Wnt signal.
Conversely, coinjection of XTsh3 with Dsh (a relatively weak axis inducer) increased the frequency of double axis formation (24%, n=42 for Dsh alone, and 64%, n=56 for Dsh coinjected with XTsh3; Figure 5H and I), while single ventral injection of XTsh3 alone caused no axis duplication (n=30; Figure 5G). A similar enhancement of axis-inducing activity was observed when XTsh3 was coinjected with a low dose (1 pg) of β-catenin (in 38%, n=18 for β-catenin alone; 61%, n=46 for β-catenin coinjected with XTsh3; not shown).
Collectively, these observations indicate that XTsh3 enhances Wnt signaling in a β-catenin/Tcf-dependent manner and is required for proper Wnt-mediated dorsal determination of the Xenopus embryo.
Physical interaction of XTsh3 with β-catenin
Since the dorsalizing activity of XTsh3 was strongly suppressed by β-catenin-MO (Figure 5D), we next studied the interaction of XTsh3 with β-catenin at the protein level (Figure 6). When the extracts of RNA-injected animal cap cells were subjected to immunoprecipitation, XTsh3 (tagged with HA) was co-precipitated with β-catenin (tagged with myc; Figure 6A, lane 3). Co-precipitation of XTsh3 and β-catenin was also observed when their interaction was examined using in vitro translation products (Figure 6B, lane 3). To further test the specificity, when an antibody against endogenous β-catenin protein was used, XTsh3 (tagged) was co-immunoprecipitated with endogenous β-catenin from the whole embryo extract (Figure 6C, lane 5).
Figure 6.
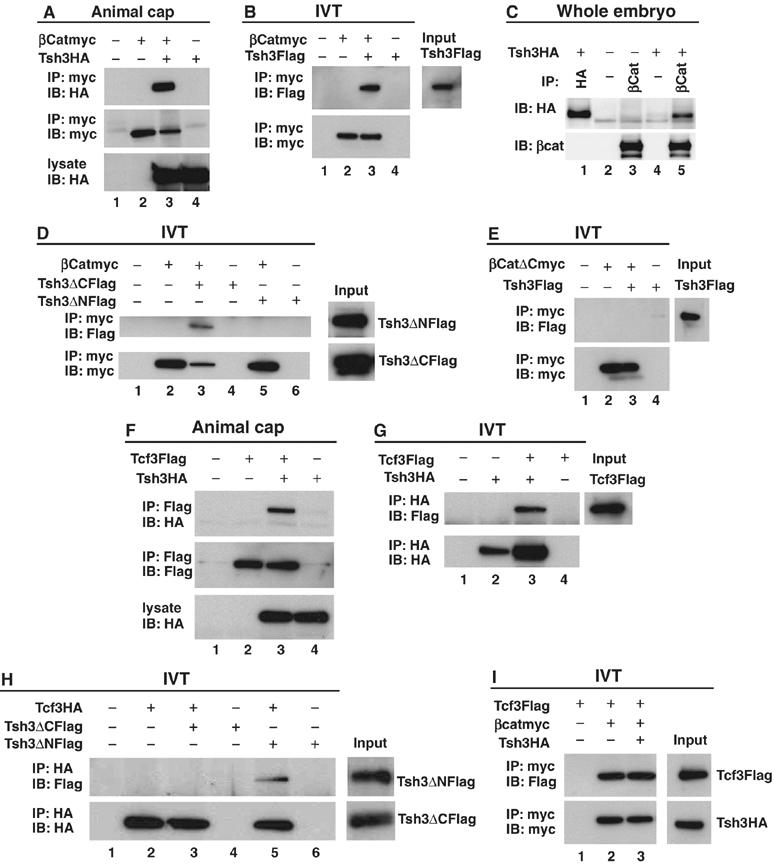
Protein interaction of XTsh3 with β-catenin. (A–D) Immunoprecipitation assay of XTsh3 and β-catenin. (A) Immunoprecipitation from animal cap extracts injected with XTsh3-HA (lanes 3 and 4) and/or β-catenin-myc (lanes 2 and 3). (B) Immunoprecipitation with in vitro translation products of XTsh3-Flag (lanes 3 and 4) and/or β-catenin-myc (lanes 2 and 3). (C) Immunoprecipitation of XTsh3 and endogenous β-catenin from the whole-embryo extract. Anti-β-catenin antibody (which binds to endogenous β-catenin) co-precipitated XTsh3-HA (lane 5; negative control in lane 3) while the control antibody (that does not bind to β-catenin) did not (lane 4). (D) Co-precipitation of in vitro translation products was seen with β-catenin-myc and XTsh3-ΔC-Flag (lane 3) but not with β-catenin-myc and XTsh3-ΔN-Flag (lane 5). (E) Co-precipitation of in vitro translation products was not seen with β-catenin-ΔC-myc and XTsh3-Flag (lane 3). (F–H) Immunoprecipitation assay of XTsh3 and Tcf3. (F) Immunoprecipitation from animal cap extracts injected with XTsh3-HA (lanes 3 and 4) and/or Tcf3-Flag (lanes 2 and 3). (G) Immunoprecipitation with in vitro translation products of XTsh3-HA (lanes 2 and 3) and/or Tcf3-Flag (lanes 3 and 4). (H) Co-precipitation of in vitro translation products was seen with Tcf3-HA and XTsh3-ΔN-Flag (lane 5) but not with Tcf3-HA and XTsh3-ΔC-Flag (lane 3). (I) Co-precipitation of Tcf3 and β-catenin was not affected by the presence of XTsh3 (lane 3).
Co-precipitation of β-catenin was seen with XTsh3-ΔC (lacking the carboxyl-terminal region; see Supplementary Figure 1B) but not with XTsh3-ΔN (lacking the amino-terminal region; Figure 6D, lanes 3 and 5), showing that the amino-terminal portion of XTsh3 is essential for the physical interaction with β-catenin. Conversely, the carboxyl-terminal deletion form of β-catenin (1–664) did not bind to XTsh3 (Figure 6E, lane 3). These findings show that XTsh3 physically interacts with β-catenin in a domain-specific manner.
The DNA-binding Fox factors Tcf/LEF, which activate transcription when they form a complex with β-catenin in the nucleus, directly regulate the transcription of target genes downstream of Wnt signaling (Brannon et al, 1997). Interestingly, XTsh3 was co-precipitated with Tcf3 both in the animal cap assay (Figure 6F, lane 3) and the in vitro translation system (Figure 6G, lane 3), showing that XTsh3 not only interacts with β-catenin but also binds to Tcf3. However, unlike that of β-catenin, Tcf3 co-precipitated with the carboxyl-terminal portion of XTsh3 (Figure 6H, lane 5) and not with the amino-terminal portion (lane 3). This finding indicates that XTsh3 interacts with the two factors using distinct domains, and suggests that the association of XTsh3 and β-catenin is independent of the Tcf3-interacting domain. The co-precipitation of β-catenin and Tcf3 was largely unaffected in the presence of XTsh3 in vitro (Figure 6I; lanes 2 and 3).
Increase of nuclear β-catenin by XTsh3
The nuclear accumulation of β-catenin protein, which lacks a typical nuclear localization signal, is an essential event in canonical Wnt signaling for the activation of downstream genes (Peifer and Polakis, 2000; Weaver and Kimelman, 2004). However, the exact molecular mechanism by which β-catenin is transported and stabilized in the nucleus remains rather nebulous (Städeli et al, 2006). For instance, the nuclear transport of β-catenin does not require the common transport protein Importin-β (Fagotto et al, 1998) and the stabilization/degradation and export of β-catenin appear more intricate than previously thought (Henderson and Fagotto, 2002; Hendriksen et al, 2005). With this in mind, we next examined the effects of XTsh3 on the level of β-catenin proteins in the nucleus using the animal cap assay.
We first examined the subcellular localization of XTsh3 protein by immunostaining. XTsh3 protein (tagged with HA) was detected preferentially in the nucleus (Supplementary Figure S5A). Deletion analysis showed that the amino-terminal portion was required for the nuclear localization of XTsh3. An XTsh3 protein lacking the carboxyl-terminal region (XTsh3-ΔC) accumulated in the nuclei of animal cap cells (Supplementary Figure S5C), whereas an amino-terminal deletion mutant (XTsh3-ΔN) did not show clear nuclear localization (Supplementary Figure S5E). Importantly, the nuclear localization of XTsh3 was not affected by coinjection of Wnt1 or β-catenin-MO (Supplementary Figure S6G and I), even at sufficient doses for strong dorsalization (the former) or ventralization (the latter), indicating that its localization is largely independent of Wnt signaling.
We next analyzed the effects of increasing and decreasing XTsh3 on the level of β-catenin localized in the nucleus. Injection of XTsh3 substantially promoted the nuclear level of endogenous β-catenin proteins in animal cap cells (cells with strong nuclear β-catenin immunostaining were found in 7.7%, n=183 nuclei and 50.6% n=146 without and with XTsh3, respectively; Figure 7A and D; note that the strong nuclear β-catenin staining are lit up in yellow in the images merged with DAPI signals in the right column). Conversely, the increased level of nuclear β-catenin induced by Wnt1 was reversed by coinjecting XTsh3-MO (75.8%, n=149 and 4%, n=250 without and with XTsh3-MO; Figure 7G and J). Taken together with the embryonic phenotypes in Figures 3 and 5, these findings show that XTsh3 is required for canonical Wnt signaling as a promoting factor that increases nuclear β-catenin.
Figure 7.
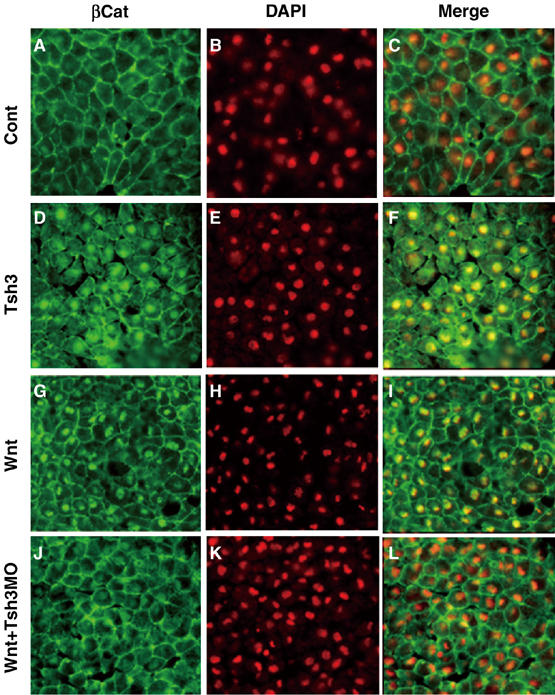
XTsh3 is essential for the nuclear localization of β-catenin induced by Wnt. Immunostaining study of the nuclear accumulation of β-catenin protein in animal cap cells. Animal caps (excised at stage 9 and fixed at stage 11) were prepared from embryos injected with control (A–C), XTsh3-HA (D–F), Wnt1 (G–I), Wnt1 and XTsh3-MO (J–L). (A, D, G, J) Fluorescent immunostaining of endogenous β-catenin. (B, E, H, K) Nuclear DAPI staining. (C, F, I, L) Merged pictures of the β-catenin and DAPI signals.
We then examined the effects of XTsh3-MO on the level of nuclear β-catenin in the embryo. Previous reports have shown that a high accumulation of endogenous nuclear β-catenin is observed on the dorsal side of the late blastula and early gastrula embryos (Schneider et al, 1996; Supplementary Figure 7G and not shown). Injection of XTsh3-MO reduced the level of nuclear β-catenin in the mesoendodermal cell of the dorsal marginal zone of the early gastrula embryo (Supplementary Figure S7G–L). This observation is consistent with the idea that XTsh3 plays an essential role in the augmentation of nuclear β-catenin levels during DV axis establishment.
Discussion
XTsh3 as an essential promoting factor of Wnt/β-catenin signaling
In this study, we introduced a vertebrate Tsh family nuclear protein, XTsh3, as an essential promoting factor for the dorsal axis determination in the Xenopus embryo. This dorsal-promoting activity of XTsh3, at least its major part, is exerted by facilitation of canonical Wnt signaling. XTsh3 significantly augments TOPflash reporter activity in both animal cap and HEK293 cells (Figure 4). XTsh3 itself is required for the activity of the Wnt signal (Figures 4 and 5). The TOPflash reporter mainly contains the Tcf/LEF-binding sites, and XTsh3 does not bind to the TOPflash sequence in the gel-shift assay (our unpublished observations). Therefore, the event by which XTsh3 enhances Wnt signaling is likely to occur somewhere upstream of the transcriptional activation of Wnt target genes.
Unlike Wnt1 injection, however, XTsh3 injection alone into a ventral–vegetal blastomere of the eight-cell embryo does not efficiently induce a secondary axis (Figure 5G). In addition, the ventral expansion for dorsal marker expression into the ventral region is relatively weak even in the embryo injected with XTsh3 radially (Figure 3). Consistent with these findings, although XTsh3 dorsalizes the ventral marginal zone (ventral-most part of the marginal zone) in the explant assay, the extent of dorsalization (induction of Chd and suppression of Wnt8 and Vent2) is rather modest (Supplementary Figure S7A). These observations suggest that the dorsalizing function of XTsh3 depends, at least in part, on certain dorsally supplied signals. This idea is in good accordance with the observation that XTsh3 increases the frequency of double axis formation when coinjected ventrally with Dsh or a low dose of β-catenin, which causes weak dorsalization. Moreover, XTsh3 activity is inhibited by GSK3β, dnTcf3 and β-catenin-MO (Figure 5), indicating that XTsh3 does not function independent of the canonical pathway but rather requires certain levels of active β-catenin. Taken together, these findings demonstrate that XTsh3 is a context-dependent enhancing factor (rather than a direct activator) that is essential for canonical Wnt signaling in dorsal development.
XTsh3 is a nuclear protein (Supplementary Figure S5A), and overproduced XTsh3 protein physically interacts with β-catenin and Tcf3 via two distinct domains, that is, its amino- and carboxyl-terminal portions, respectively (Figure 6). Importantly, XTsh3 plays a crucial role in increasing the nuclear level of β-catenin (Figure 7). The increased level of nuclear β-catenin by Wnt1 is suppressed by XTsh3-MO, suggesting that XTsh3 is essential for the enhanced nuclear localization of β-catenin that occurs downstream of the Wnt signal. In vivo analysis also indicates that XTsh3 is required for the nuclear accumulation of β-catenin in the dorsal marginal zone of the embryo (Supplementary Figure S7G–L). Neither the inhibition nor the augmentation of Wnt signaling affects the nuclear localization of XTsh3, indicating that XTsh3 does not require Wnt signals (at least, strong ones) for its localization (Supplementary Figure S6).
The amino-terminal domain is required for both the nuclear localization and β-catenin interaction of XTsh3 (Figure 6 and Supplementary Figure S5). Our preliminary study showed that injection of XTsh3-ΔN did not affect the expression of DV markers in the gastrula embryo (Supplementary Figure S8A) or increase the level of nuclear β-catenin (not shown). In contrast, injection of XTsh3-ΔC (its gene product interacts with β-catenin and is capable of nuclear localization; Figure 6 and Supplementary Figure S5) causes ventralization in the embryo (Supplementary Figure S8A). Furthermore, XTsh3-ΔC antagonizes Wnt activity in both the TOPflash assay and the immunostaining assay of nuclear β-catenin of the injected animal caps (Supplementary Figure S4E and S8B). These observations indicate that besides the β-catenin-interacting amino-terminal region, the carboxyl-terminal domain (which interacts with Tcf3) has an active role in the Wnt signaling enhancement by XTsh3. In future investigation, it is intriguing to know whether and how XTsh3 forms a complex with the large regulatory machinery involving β-catenin and Tcf3 (e.g., Mosimann et al, 2006).
Collectively, the observations in this study lead to the hypothesis that the nuclear protein XTsh3 enhances canonical Wnt signaling by increasing the level of nuclear β-catenin when Wnt signaling maintains a sufficient level of functional β-catenin in the cell; this enhancement of Wnt signaling by XTsh3 is essential for the dorsal determination in early Xenopus embryos (Figure 2A and B).
Our preliminary experiments suggested that the XTsh3-driven enhancement of Wnt signaling is required in a quantitative (rather than qualitative) manner. XTsh3-MO injection inhibited the dorsalizing effect of Wnt1 (Figure 5F) and also antagonized the secondary axis-inducing activity of β-catenin (20 pg injected into a single blastomere), at least in part (38%, n=18 and 13%, n=54 without and with XTsh3-MO, respectively; Supplementary Figure 7B–D). However, when a higher dose of β-catenin (100 pg/cell) was radially injected at the four-cell stage, XTsh3-MO, at a sufficient dose for Wnt1 inhibition, did not suppress the ectopic Chd expression (Supplementary Figure 7E and F), suggesting that excessive β-catenin can somehow compensate for the loss of XTsh3. Therefore, XTsh3 could be a context-dependent quantitative enhancer of Wnt signaling, which plays an indispensable role in the case of DV axis determination.
Recently, Wnt11 was highlighted as a maternally supplied extracellular ligand. We have so far failed to see strong enhancement of Wnt11 activity by XTsh3 coinjection in the double-axis assay (our unpublished observations), unlike that seen by FRL1 coinjection previously (Tao et al, 2005). In addition, unlike Wnt11, dorsally biased distribution of maternal XTsh3 RNA was not observed in quantitative PCR analysis (Supplementary Figure S7M). The relationship between these factors remains to be clarified in the future.
Biological roles of XTsh3 during embryogenesis
Fly studies have demonstrated genetic interactions between tsh and Wingless-related signaling in segment polarity phenotypes (Röder et al, 1992; Gallet et al, 1998). Moreover, like XTsh3 and β-catenin, fly Tsh and Armadillo interact physically (Gallet et al, 1998), suggesting that interaction with the Wnt signaling mediator is common to the Tsh-family factors. However, fly tsh and frog XTsh3 may differ in some crucial aspects. First, fly tsh, unlike frog XTsh3, has not been implicated in the establishment of the embryonic DV axis, which is primarily controlled by a nuclear gradient of the maternal transcription factor Dorsal (Morisato and Anderson, 1995; Van Eeden and St Johnston, 1999). Thus, the roles of Tsh family genes in DV patterning appear to be species specific. From a mechanistic point of view, genetic studies indicate that, unlike XTsh3, fly tsh functions downstream of armadillo/β-catenin (in the ventral denticle phenotype; Gallet et al, 1998). Furthermore, the nuclear localization of fly Tsh proteins is reported to be dependent on Wg signaling (Gallet et al, 1999). This is in contrast to the nuclear localization of XTsh3, which is persistently observed regardless of the level of Wnt signaling (Supplementary Figure S6). One interpretation of this discrepancy could be that the functional regulation of invertebrate and vertebrate Tsh-family factors involves diverse mechanisms in details, even though both interact positively with β-catenin/Armadillo and promote canonical Wnt signaling. The structural divergence of the fly and vertebrate Tsh family proteins (Supplementary Figure S1A) might be relevant to this possibility.
The present study has shed light on the biological role of XTsh3 in Wnt-mediated dorsal development, but opens up several questions as well, particularly with regards to the cellular mechanisms of XTsh3's action. For instance, the effect (direct or indirect) of XTsh3 on β-catenin stabilization is an intriguing topic for future investigation. Importantly, the present study showed that XTsh3 enhances β-catenin signaling not only in the embryo but also in the animal cap and 293T cells (Figure 4A and B), both in which the basal Wnt signaling is considerably low. Since this enhancement is inhibited by coinjection of GSK3β, it remains to be clarified whether XTsh3 promotes β-catenin signaling partly by antagonizing the GSK3β-mediated β-catenin degradation that keeps basal β-catenin activity low. Alternatively, since some β-catenin/Tcf-dependent activity is detectable in these cells (Figure 4A and B, lanes 6; Supplementary Figure S4F), it is equally possible that XTsh3 elevates the nuclear level of β-catenin by strongly facilitating its import (or inhibiting its export) even if the cytoplasmic β-catenin level is low. In the case of 293T cells, the low basal β-catenin activity may also reflect the recently reported Wnt-independent activation of β-catenin (Abe and Takeichi, 2007).
Since β-catenin and Tcf3 forms a complex with multiple factors in the nucleus (Mosimann et al, 2006), the relationship of XTsh3 with such a transcription factor assembly should also be further investigated to elucidate the exact point of XTsh3's action. For instance, in this study, the protein–protein interaction was analyzed using overexpressed XTsh3 protein (epitope tagged), because we have so far failed to raise good antisera against the endogenous XTsh3 protein. In the future, proteomic studies using an anti-XTsh3 antibody (if available) may allow us to systematically analyze the relationship of XTsh3 with the known complicated transcriptional machinery.
Another future topic to pursue is detailed temporal analysis of XTsh3's action in DV specification during early embryogenesis, including a study on possible differential roles of maternal and zygotic XTsh3. This direction is important since the exact timing of Wnt activation required for DV axis establishment still remains largely elusive (Heasman, 2006). In addition, the role of the carboxyl-terminal homeodomain remains unknown. It is possible that its potential DNA-binding domain may enable XTsh3 to act more than just as a β-catenin signal modulator.
Finally, besides its role in DV patterning, the in vivo expression pattern of XTsh3 after gastrulation suggests that XTsh3 also plays some roles in Wnt-regulated embryological processes, particularly the caudal specification of the CNS. From mid-neurulation on, strong XTsh3 expression is found in the caudal CNS (hindbrain and spinal cord), whose development is promoted by Wnt signals (Sasai and De Robertis, 1997; Kazanskaya et al, 2000). Consistent with this idea, XTsh3 injection suppressed rostral CNS markers (e.g. Six3) in the embryo, while XTsh3-MO injection into the animal blastomeres (which does not cause general ventralization) expanded forebrain marker expression and suppressed caudal CNS marker expression (our unpublished observations), suggesting that XTsh3 plays a role in AP patterning. A recent report on XTsh1 has also suggested a role of the Tsh family in caudal brain development (Koebernick et al, 2006). To elucidate XTsh3's role in AP patterning in the future, we are particularly interested in examining possible interactions with a frog Sal homolog, XSalF, which acts as an essential factor for the forebrain specification by attenuating the cellular response to Wnt signals (Onai et al, 2004; Drosophila sal and tsh act antagonistically in the AP specification of segmental identity; Röder et al, 1992). Such an investigation might further help clarify the mechanisms of XTsh3 in enhancing/reinforcing the embryonic polarities.
Materials and methods
Plasmid construction and in vitro transcription
XTsh3 cDNA (database #DQ834387) was amplified by RT–PCR and subcloned into the pCS2 vector at BamHI/XhoI sites (pCS2-XTsh3). For mRNA injection, the plasmids were linearized with NotI and transcribed with SP6 polymerase (mMessage mMachine, Ambion). XTsh3-ΔC (amino-acid residues 1–399) and XTsh3-ΔN cDNA (amino-acid residues 400–1005) were generated by PCR and subcloned into the pCS2 vector. The expression plasmids for Wnt1, β-catenin, GSK3β and Dsh were generated and used as described previously (Hamilton et al, 2001; Onai et al, 2004; Sasai et al, 2004).
Embryonic manipulation and microinjection of RNA and MO
Embryos were staged according to the normal table of Nieukoop and Faber. The embryos were then transferred into 0.1 × Barth's solution until further manipulation or harvesting. For animal cap assays, the tissues were excised at stage 10 and cultured in 1 × LCMR supplemented with 0.2% BSA until the indicated stage. Injection of synthetic RNAs and MOs was carried out with a fine glass capillary and a pneumatic pressure injector (Narishige) in 1 × Barth's solution. The following MOs used here were designed as shown in Supplementary Figure 2; XTsh3-MO-A: CGCTGACATGTGAATCACTGTCCAT, 5 mis-XTsh3-MO-A: CGGTGAGATGTCAATCACTCTCGAT, XTsh3-MO-C: TCCTGCAAAAAGAACAAGTAGCTTG, 5 mis-XTsh3-MO-C: TGCTCCAAATAGAAGAAGTACCTTG. β-cateinin-MO was purchased from Gene Tools (OR, USA). MO-A and MO-C caused similar phenotypes when injected (20 ng/cell) at the four-cell stage. All experiments were performed at least twice to confirm reproducibility. The side of injection of MO or RNA was confirmed by coinjection of EGFP or LacZ RNA as a tracer (see examples in Supplementary Figure S2M–P).
RT–PCR, whole-mount in situ hybridization and immunostaining
RT–PCR and whole-mount in situ hybridization analyses were performed as described previously (Mizuseki et al, 1998; Sasai et al, 2001; Onai et al, 2004). Quantitative PCR was performed using the 7500 Fast Real-Time PCR System (Applied Biosystems) according to the manufacturer's instruction, and data were normalized by ornithine decarboxylase expression. Immunostaining of animal cap cells was performed using cryostat sections as described previously (Schohl and Fagotto, 2003), except for the use of MEMFA in the fixation of samples. The β-catenin antibody (rabbit polyclonal, 1:1000 dilution) was purchased from Sigma.
Luciferase assay
For luciferase assays using the animal cap, the TOPflash or control Fopflash reporter vector (50 pg/cell; Upstate, NY) for Wnt signaling was injected into the animal blastomeres of eight-cell embryos together with a loading control plasmid (phRL-null) and experimental mRNAs. The amount of DNA was made constant by adding an empty expression plasmid. Animal caps were excised at stage 10, cultured until the siblings reached stage 11.5 and subjected to luciferase assays using the dual luciferase reporter assay system (Promega). For the experiment using HEK293T cells, the cells (90% confluent in a well of a 96-well plate) were transfected with the reporter vectors (10 ng/well), expression plasmids and a loading control plasmid using Fugene 6 (Roche). The cells were harvested for the luciferase assay 24 h after transfection.
In vitro translation and co-precipitation assays
In vitro translation was performed with the TNT SP6 Quick Coupled Transcription/Translation System (Promega) using pCS2XTsh3-Flag, pCS2XTsh3-HA, pCS2Xβcatenin-myc, pCS2ΔNXTsh3-Flag, pCS2ΔCXTsh3-Flag, pCS2ΔCβcatenin-myc, pCS2xTcf3-Flag and pCS2xTcf3-HA. The total amount of protein input was made constant by using pCS2-venus. The translation products were mixed and incubated at 4°C for 1 h with nutation. For immunoprecipitation, an anti-Myc (Roche), anti-HA (Roche), anti-Flag or anti-β-catenin antibody (Sigma) was added and the sample was rotated at 4°C for 2 h. Protein A–Sepharose beads (Amersham) were then added to samples followed by another 1-h incubation with nutation. The beads were spun down and washed three times in washing buffer (1% NP-40, 150 mM NaCl, 5 mM EDTA, 10 mM and Tris–HCl, pH 7.4). Animal cap extracts were prepared as previously described (Snider et al, 2001). Briefly, the animal caps were excised at stage 9, lysed on ice in a solution containing 100 mM Tris–HCl, pH 7.4, 150 mM NaCl, 2 mM EDTA, 0.5% NP-40 and 1 × protease inhibitor cocktail (Nacalai, Kyoto, Japan), and cleared by microcentrifugation. Immunoprecipitation was performed as described above, except for the use of 0.5% NP-40 in the washing buffer. Samples were separated by SDS–PAGE and subjected to Western blotting using the ECL Western blotting detection reagents (Amersham).
Supplementary Material
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Figure Legends
Acknowledgments
We are grateful to Mototsugu Eiraku and Nick Love for critical reading of this manuscript, Tetsuro Watabe for advice on luciferase reporter assay and Masako Suzuki for excellent assistance in the maintenance of the frog facility. This work was supported in part by grants-in-aid (YS) from MEXT, the Kobe Cluster Project and the Leading Project.
References
- Abe K, Takeichi M (2007) NMDA-receptor activation induces calpain-mediated beta-catenin cleavages for triggering gene expression. Neuron 53: 387–397 [DOI] [PubMed] [Google Scholar]
- Andrew JD, Horner MA, Petitt MG, Smolik SM, Scott MP (1994) Setting limits on homeotic gene function: restrint of Sex combs reduced activity by teashirt and other homeotic genes. EMBO J 13: 1132–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa J, Casares F (2005) Restricted teashirt expression confers eye-specific responsiveness to Dpp and Wg signals during eye specification in Drosophila. Development 132: 5011–5020 [DOI] [PubMed] [Google Scholar]
- Bessa J, Gebelein B, Pichaud F, Casares F, Mann RS (2002) Combinatorial control of Drosophila eye development by Eyeless, Homothorax, and Teashirt. Gene Dev 16: 2415–2427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannon M, Gomperts M, Sumoy L, Moon RT, Kimelman D (1997) A beta-catenin/XTcf-3 complex binds to the siamois promoter to regulate dorsal axis specification in Xenopus. Genes Dev 11: 2359–2370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caubit X, Coré N, Boned A, Keridge S, Djabali M, Fasano L (2000) Vertebrate orthologues of the Drosophila region-specific patterning gene teashirt. Mech Dev 91: 445–448 [DOI] [PubMed] [Google Scholar]
- De Robertis EM, Kuroda H (2004) Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol 20: 285–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM, Larrain J, Oelgeschlager M, Wessely O (2000) The establishment of Spemann's organizer and patterning of the vertebrate embryo. Nat Rev Genet 1: 171–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Gluck U, Gumbiner BM (1998) Nuclear localization signal-independent and importin/karyopherin-independent nuclear import of beta-catenin. Curr Biol 8: 181–190 [DOI] [PubMed] [Google Scholar]
- Fasano L, Röder L, Coré N, Alexandre E, Vola C, Jacq B, Kerridge S (1991) The gene teashirt is required for the development of Drosophila embryonic trunk segments and encodes a protein with widely spaced zinc finger motifs. Cell 64: 63–79 [DOI] [PubMed] [Google Scholar]
- Gallet A, Angelats C, Erkner A, Charroux B, Fasano L, Kerridge S (1999) The C-terminal domain of Armadillo binds to hypophosphorylated Teashirt to modulate Wingless signaling in Drosophila. EMBO J 18: 2208–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet A, Erkner A, Charroux B, Fasano L, Kerridge S (1998) Trunk-specific modulation of Wingless signaling in Drosophila by Teashirt binding to Armadillo. Curr Biol 8: 893–902 [DOI] [PubMed] [Google Scholar]
- Hamilton FS, Wheeler GN, Hoppler S (2001) Difference in XTcf-3 dependency accounts for change in response to beta-catenin-mediated Wnt signalling in Xenopus blastula. Development 128: 2063–2073 [DOI] [PubMed] [Google Scholar]
- He X, Saint-Janet JP, Woodgett JR, Varmus HE, Dawid IB (1995) Glycogen synthase kinase-3 and dorsoventral patterning in Xenopus embryos. Nature 376: 617–622 [DOI] [PubMed] [Google Scholar]
- Heasman J (2006) Patterning the early Xenopus embryo. Development 133: 1205–1217 [DOI] [PubMed] [Google Scholar]
- Henderson BR, Fagotto F (2002) The ins and outs of APC and beta-catenin nuclear transport. EMBO Rep 3: 834–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriksen J, Fagotto F, van der Velde H, van Schie M, Noordermeer J, Fornerod M (2005) RanBP3 enhances nuclear export of active (beta)-catenin independently of CRM1. J Cell Biol 171: 785–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazanskaya O, Glinka A, Niehrs C (2000) The role of Xenopus dickkopf1 in prechordal plate specification and neural patterning. Development 127: 4981–4992 [DOI] [PubMed] [Google Scholar]
- Khokha MK, Yeh J, Grammer TC, Harland RM (2005) Depletion of three BMP antagonists from Spemann's organizer leads to a catastrophic loss of dorsal structures. Dev Cell 8: 401–411 [DOI] [PubMed] [Google Scholar]
- Koebernick K, Kashef J, Pieler T, Wedlich D (2006) Xenopus Teashirt1 regulates posterior identity in brain and cranial neural crest. Dev Biol 298: 312–326 [DOI] [PubMed] [Google Scholar]
- Laugier E, Yang Z, Fasano L, Kerridge S, Vola C (2005) A critical role of teashirt for patterning the ventral epidermis is masked by ectopic expression of tiptop, a paralog of teashirt in Drosophila. Dev Biol 283: 446–458 [DOI] [PubMed] [Google Scholar]
- Manfroid I, Caubit X, Kerridge S, Fasano L (2004) Three putative murine Teashirt orthologues specify trunk structures in Drosophila in the same way as the Drosophila teashirt gene. Development 131: 1065–1073 [DOI] [PubMed] [Google Scholar]
- Mathies LD, Kerridge S, Scott MP (1994) Role of the teashirt gene Drosophila midgut morphogenesis: secreted proteins mediate the action of homeotic genes. Development 120: 2799–2809 [DOI] [PubMed] [Google Scholar]
- Mizuseki K, Kishi M, Shiota K, Nakanishi S, Sasai Y (1998) Sox-D is an essential mediator for induction of anterior neural tissues in Xenopus embryos. Neuron 21: 77–85 [DOI] [PubMed] [Google Scholar]
- Molenaar M, Von de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Detrée O, Clevers H (1996) Xtcf-3 transcription factor mediates β-catenin-induced axis formation in Xenopus embryos. Cell 86: 391–399 [DOI] [PubMed] [Google Scholar]
- Moon RT, Bowerman B, Boutros M, Perrimon N (2002) The promise and perils of Wnt signaling through β-catenin. Science 296: 1644–1646 [DOI] [PubMed] [Google Scholar]
- Moon RT, Kimelman D (1998) From cortical rotation to organizer gene expression: toward a molecular explanation of axis specification in Xenopus. Bioessays 20: 536–545 [DOI] [PubMed] [Google Scholar]
- Morisato D, Anderson KV (1995) Signaling pathways that establish the dorsal-ventral pattern of the Drosophila embryo. Annu Rev Genet 29: 371–399 [DOI] [PubMed] [Google Scholar]
- Mosimann C, Hausmann G, Basler K (2006) Parafibromin/Hyrax activates Wnt/Wg target gene transcription by direct association with beta-catenin/Armadillo. Cell 125: 327–341 [DOI] [PubMed] [Google Scholar]
- Onai T, Sasai N, Matsui M, Sasai Y (2004) Xenopus XsalF: anterior neuroectodermal specification by attenuating cellular responsiveness to Wnt signaling. Dev Cell 7: 95–106 [DOI] [PubMed] [Google Scholar]
- Osada SI, Wright CV (1999) Xenopus nodal-related signaling is essential for mesendodermal patterning during early embryogenesis. Development 126: 3229–3240 [DOI] [PubMed] [Google Scholar]
- Peifer M, Polakis P (2000) Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 287: 1606–1609 [DOI] [PubMed] [Google Scholar]
- Röder L, Vola C, Kerridge S (1992) The role of the teashirt gene in trunk segmental identity in Drosophila. Development 115: 1017–1033 [DOI] [PubMed] [Google Scholar]
- Saller E, Kelley A, Bienz M (2002) The transcriptional repressor Brinker antagonizes Wingless signaling. Gene Dev 16: 1828–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai N, Mizuseki K, Sasai Y (2001) Requirement of FoxD3-class signaling for neural crest determination in Xenopus. Development 128: 2525–2536 [DOI] [PubMed] [Google Scholar]
- Sasai N, Nakazawa Y, Haraguchi T, Sasai Y (2004) The neurotrophin receptor-related protein NRH1 is essential for convergent extension movements. Nat Cell Biol 6: 741–748 [DOI] [PubMed] [Google Scholar]
- Sasai Y, De Robertis EM (1997) Ectodermal patterning in vertebrate embryos. Dev Biol 182: 5–20 [DOI] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P (1996) Beta-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev 57: 191–198 [DOI] [PubMed] [Google Scholar]
- Schohl A, Fagotto F (2003) A role for maternal beta-catenin in early mesoderm induction in Xenopus. EMBO J 22: 3303–3313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder KE, Condic ML, Eisenberg LM, Yost HJ (1999) Spatially regulated translation in embryos: asymmetric expression of maternal Wnt-11 along the dorsal–ventral axis in Xenopus. Dev Biol 214: 288–297 [DOI] [PubMed] [Google Scholar]
- Smith JC (1995) Mesoderm-inducing factors and mesodermal patterning. Curr Opin Cell Biol 7: 856–861 [DOI] [PubMed] [Google Scholar]
- Snider L, Thirlwell H, Miller JR, Moon RT, Groudine M, Tapscott SJ (2001) Inhibition of Tcf3 binding by I-mfa domain proteins. Mol Cell Biol 21: 1866–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol S, Christian JL, Moon RT, Melton DA (1991) Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67: 741–752 [DOI] [PubMed] [Google Scholar]
- Städeli R, Hoffmans R, Basler K (2006) Transcription under the control of nuclear Arm/beta-catenin. Curr Biol 16: R378–R385 [DOI] [PubMed] [Google Scholar]
- Tao Q, Yokota C, Puck H, Kofron M, Birsoy B, Yan D, Asashima M, Wylie CC, Lin X, Heasman J (2005) Maternal Wnt11 activates the canonical Wnt signaling pathway required for axis formation in Xenopus embryos. Cell 120: 857–871 [DOI] [PubMed] [Google Scholar]
- Van Eeden F, St Johnston D (1999) The polarization of the anterior-posterior and dorsal–ventral axes during Drosophila oogenesis. Curr Opin Gene Dev 9: 396–404 [DOI] [PubMed] [Google Scholar]
- Weaver C, Kimelman D (2004) Move it or lose it: axis specification in Xenopus. Development 131: 3491–3499 [DOI] [PubMed] [Google Scholar]
- Wu J, Cohen SM (2000) Proximal distal axis formation in the Drosophila leg: distinct functions of Teashirt and Homeothorax in the proximal leg. Mech Dev 94: 47–56 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure S1
Supplementary Figure S2
Supplementary Figure S3
Supplementary Figure S4
Supplementary Figure S5
Supplementary Figure S6
Supplementary Figure S7
Supplementary Figure S8
Supplementary Figure Legends


