Abstract
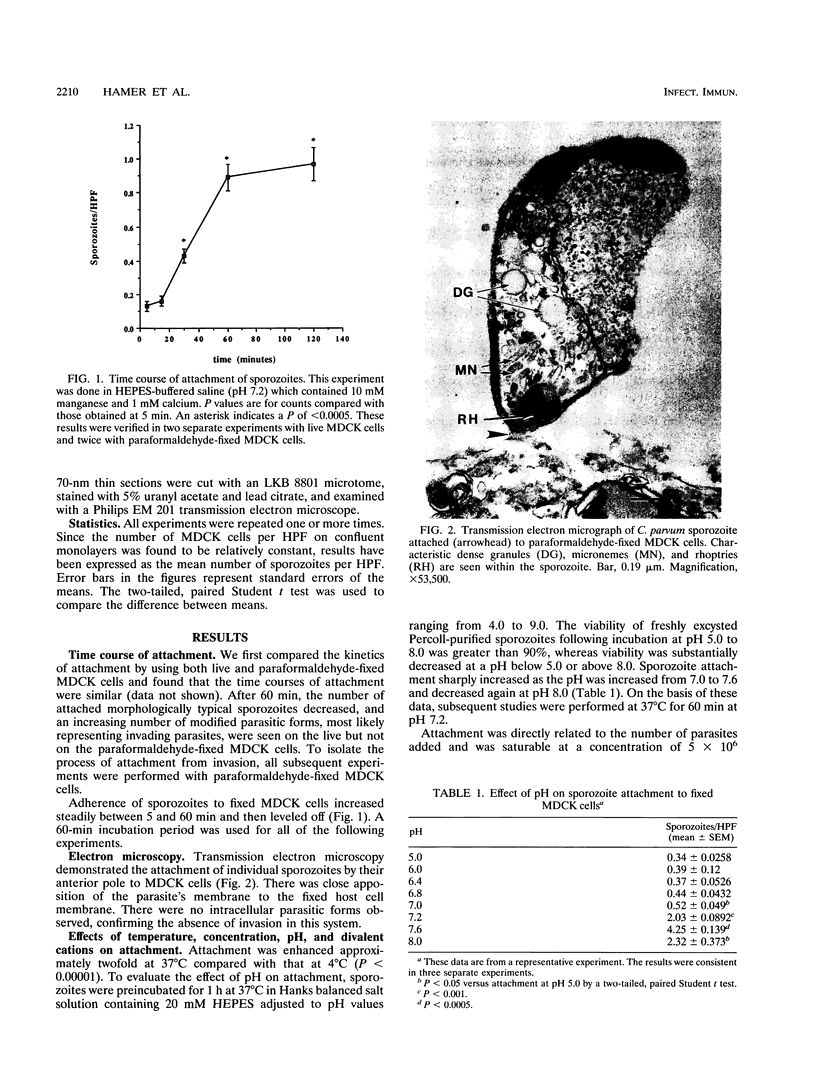
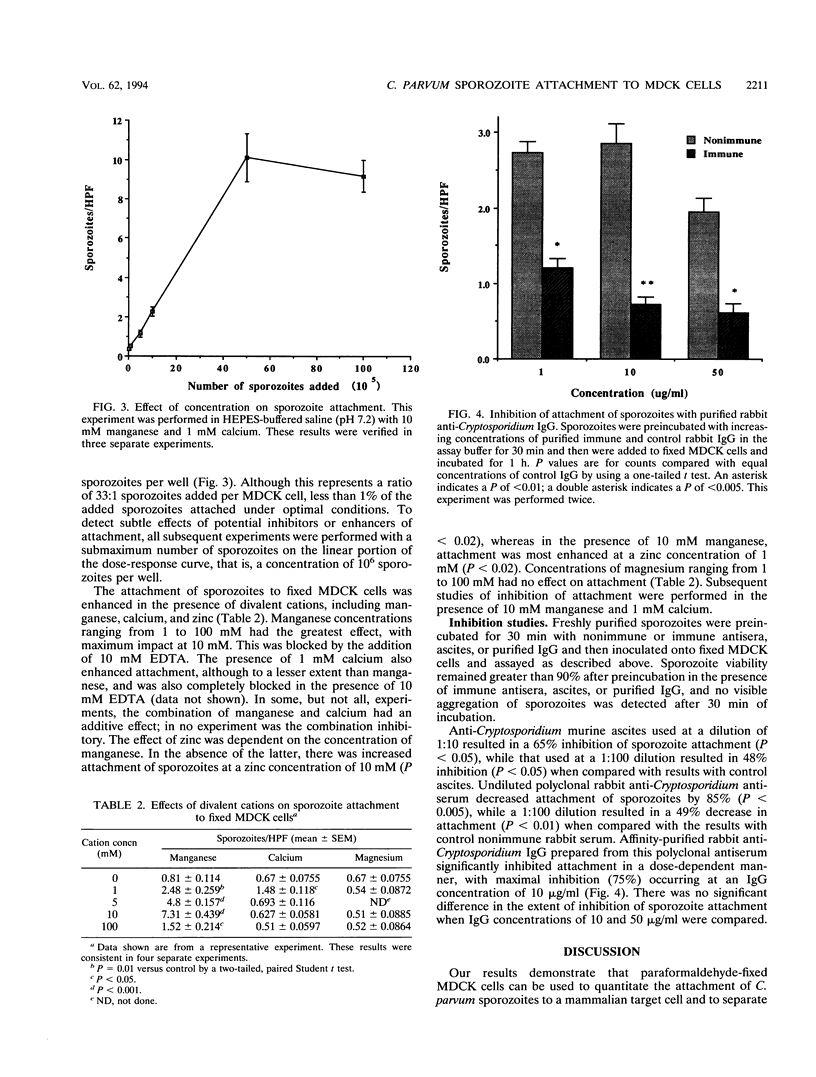
The initial attachment of Cryptosporidium parvum sporozoites to host cells in vivo may be a critical event in the pathogenesis of this infection. The molecular basis of attachment and the conditions influencing this host-parasite interaction have not been studied systematically. Therefore, we have developed a sporozoite attachment model by using paraformaldehyde-fixed Madin-Darby canine kidney (MDCK) cells. Attachment of sporozoites to fixed MDCK cells was quantitated by indirect immunofluorescence and confirmed by transmission electron microscopy. Attachment in this system was time, temperature (37 degrees C), and pH (7.2 to 7.6) dependent. Dose-response studies demonstrated that the attachment of sporozoites to fixed MDCK cells was a saturable process. Attachment was enhanced in the presence of 10 mM manganese, 1 mM calcium, and 1 to 10 mM zinc. Attachment of sporozoites to MDCK cells was inhibited in a dose-dependent manner by polyclonal anti-Cryptosporidium antisera and by purified immunoglobulin G (IgG). This model will be useful for the study of parasite and host cell molecules involved in the initial interaction of C. parvum sporozoites with their target cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aji T., Flanigan T., Marshall R., Kaetzel C., Aikawa M. Ultrastructural study of asexual development of Cryptosporidium parvum in a human intestinal cell line. J Protozool. 1991 Nov-Dec;38(6):82S–84S. [PubMed] [Google Scholar]
- Akerström B., Brodin T., Reis K., Björck L. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J Immunol. 1985 Oct;135(4):2589–2592. [PubMed] [Google Scholar]
- Arrowood M. J., Sterling C. R. Isolation of Cryptosporidium oocysts and sporozoites using discontinuous sucrose and isopycnic Percoll gradients. J Parasitol. 1987 Apr;73(2):314–319. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buraud M., Forget E., Favennec L., Bizet J., Gobert J. G., Deluol A. M. Sexual stage development of cryptosporidia in the Caco-2 cell line. Infect Immun. 1991 Dec;59(12):4610–4613. doi: 10.1128/iai.59.12.4610-4613.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Current W. L., Garcia L. S. Cryptosporidiosis. Clin Microbiol Rev. 1991 Jul;4(3):325–358. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Current W. L., Haynes T. B. Complete development of Cryptosporidium in cell culture. Science. 1984 May 11;224(4649):603–605. doi: 10.1126/science.6710159. [DOI] [PubMed] [Google Scholar]
- Flanigan T. P., Aji T., Marshall R., Soave R., Aikawa M., Kaetzel C. Asexual development of Cryptosporidium parvum within a differentiated human enterocyte cell line. Infect Immun. 1991 Jan;59(1):234–239. doi: 10.1128/iai.59.1.234-239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S., Locklear T. W. Ionic constituents and osmolality of gastric and small-intestinal fluids after eating. Am J Dig Dis. 1966 Jul;11(7):503–521. doi: 10.1007/BF02233563. [DOI] [PubMed] [Google Scholar]
- Gut J., Petersen C., Nelson R., Leech J. Cryptosporidium parvum: in vitro cultivation in Madin-Darby canine kidney cells. J Protozool. 1991 Nov-Dec;38(6):72S–73S. [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Jones K. H., Senft J. A. An improved method to determine cell viability by simultaneous staining with fluorescein diacetate-propidium iodide. J Histochem Cytochem. 1985 Jan;33(1):77–79. doi: 10.1177/33.1.2578146. [DOI] [PubMed] [Google Scholar]
- Kobiler D., Mirelman D. Adhesion of Entamoeba histolytica trophozoites to monolayers of human cells. J Infect Dis. 1981 Dec;144(6):539–546. doi: 10.1093/infdis/144.6.539. [DOI] [PubMed] [Google Scholar]
- Leighton J., Brada Z., Estes L. W., Justh G. Secretory activity and oncogenicity of a cell line (MDCK) derived from canine kidney. Science. 1969 Jan 31;163(3866):472–473. doi: 10.1126/science.163.3866.472. [DOI] [PubMed] [Google Scholar]
- Lumb R., Smith K., O'Donoghue P. J., Lanser J. A. Ultrastructure of the attachment of Cryptosporidium sporozoites to tissue culture cells. Parasitol Res. 1988;74(6):531–536. doi: 10.1007/BF00531630. [DOI] [PubMed] [Google Scholar]
- Marcial M. A., Madara J. L. Cryptosporidium: cellular localization, structural analysis of absorptive cell-parasite membrane-membrane interactions in guinea pigs, and suggestion of protozoan transport by M cells. Gastroenterology. 1986 Mar;90(3):583–594. doi: 10.1016/0016-5085(86)91112-1. [DOI] [PubMed] [Google Scholar]
- McCabe R. E., Yu G. S., Conteas C., Morrill P. R., McMorrow B. In vitro model of attachment of Giardia intestinalis trophozoites to IEC-6 cells, an intestinal cell line. Antimicrob Agents Chemother. 1991 Jan;35(1):29–35. doi: 10.1128/aac.35.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Barria E., Pereira M. E. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell. 1991 Oct 18;67(2):411–421. doi: 10.1016/0092-8674(91)90192-2. [DOI] [PubMed] [Google Scholar]
- Rhodes J., Prestwich C. J. Acidity at different sites in the proximal duodenum of normal subjects and patients with duodenal ulcer. Gut. 1966 Oct;7(5):509–514. doi: 10.1136/gut.7.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs M. W., Perryman L. E. Infectivity and neutralization of Cryptosporidium parvum sporozoites. Infect Immun. 1987 Sep;55(9):2081–2087. doi: 10.1128/iai.55.9.2081-2087.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosales M. J., Cifuentes J., Mascaró C. Cryptosporidium parvum: culture in MDCK cells. Exp Parasitol. 1993 Mar;76(2):209–212. doi: 10.1006/expr.1993.1024. [DOI] [PubMed] [Google Scholar]
- Schenkman S., Diaz C., Nussenzweig V. Attachment of Trypanosoma cruzi trypomastigotes to receptors at restricted cell surface domains. Exp Parasitol. 1991 Jan;72(1):76–86. doi: 10.1016/0014-4894(91)90123-e. [DOI] [PubMed] [Google Scholar]
- Thea D. M., Pereira M. E., Kotler D., Sterling C. R., Keusch G. T. Identification and partial purification of a lectin on the surface of the sporozoite of Cryptosporidium parvum. J Parasitol. 1992 Oct;78(5):886–893. [PubMed] [Google Scholar]
- Tzipori S. Cryptosporidiosis in perspective. Adv Parasitol. 1988;27:63–129. doi: 10.1016/S0065-308X(08)60353-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward H. D., Kane A. V., Ortega-Barria E., Keusch G. T., Pereira M. E. Identification of developmentally regulated Giardia lamblia cyst antigens using GCSA-1, a cyst-specific monoclonal antibody. Mol Microbiol. 1990 Dec;4(12):2095–2102. doi: 10.1111/j.1365-2958.1990.tb00570.x. [DOI] [PubMed] [Google Scholar]
- Ward H. D., Lev B. I., Kane A. V., Keusch G. T., Pereira M. E. Identification and characterization of taglin, a mannose 6-phosphate binding, trypsin-activated lectin from Giardia lamblia. Biochemistry. 1987 Dec 29;26(26):8669–8675. doi: 10.1021/bi00400a027. [DOI] [PubMed] [Google Scholar]