Abstract
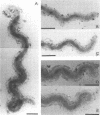
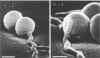
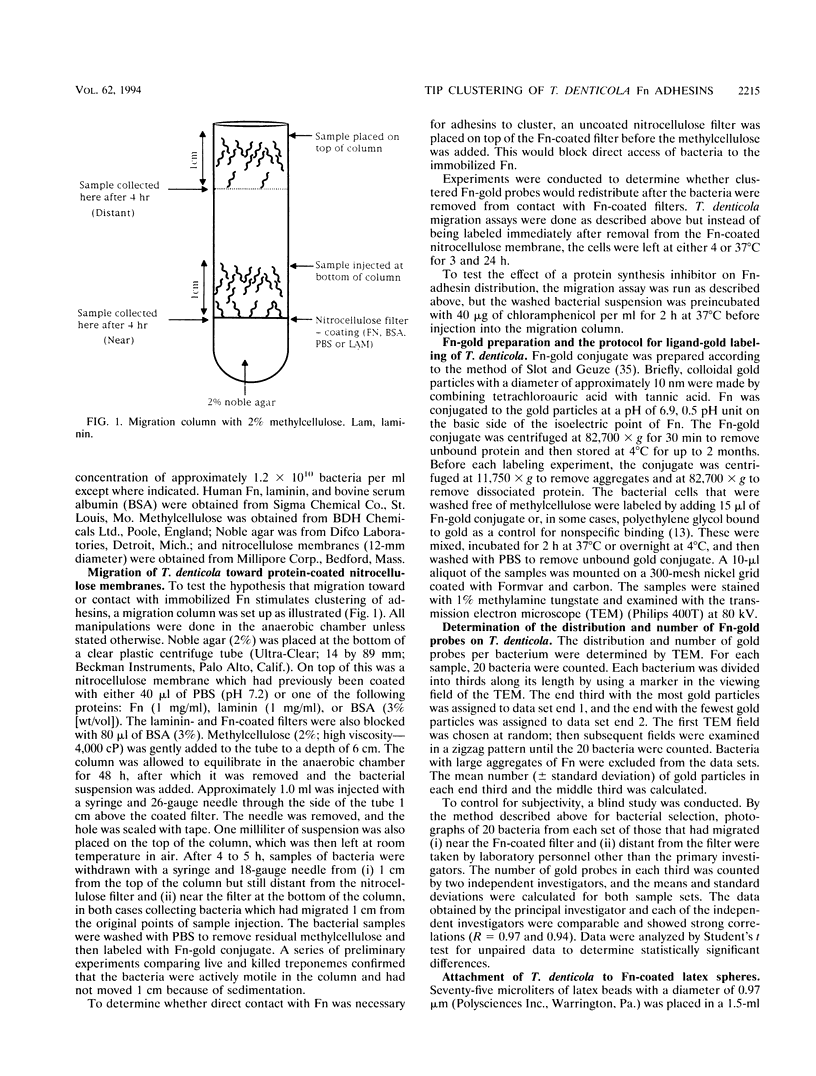
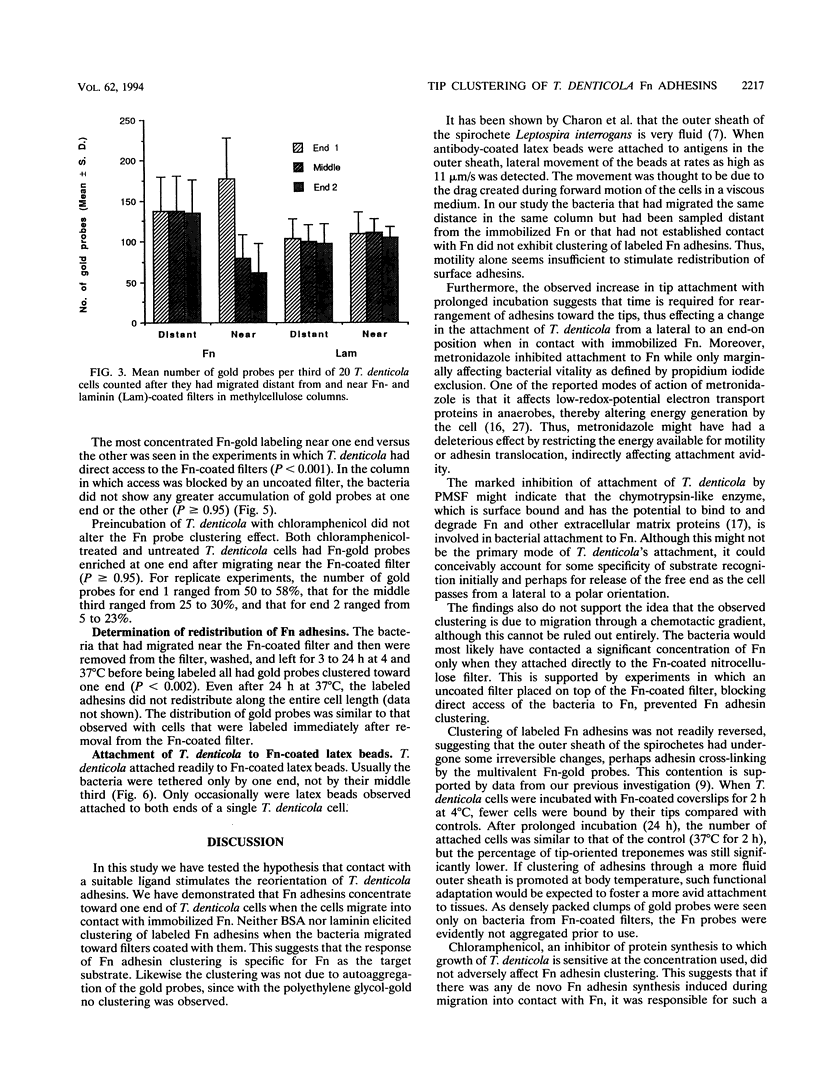
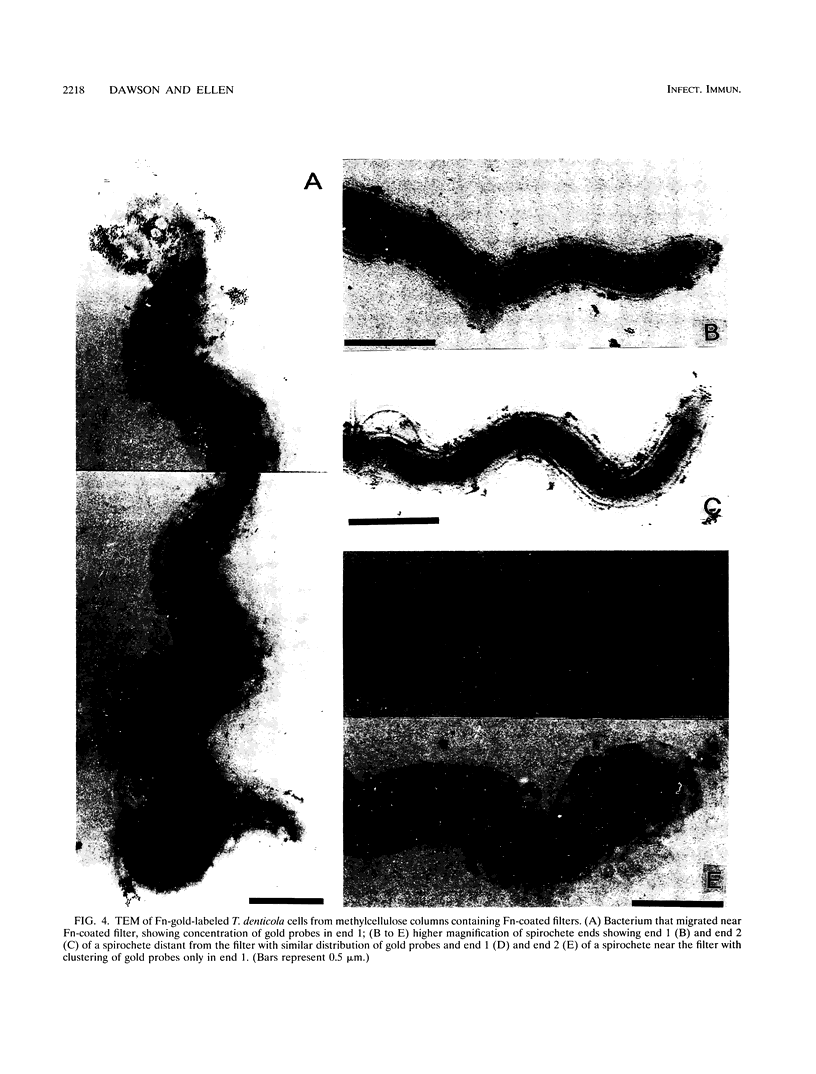
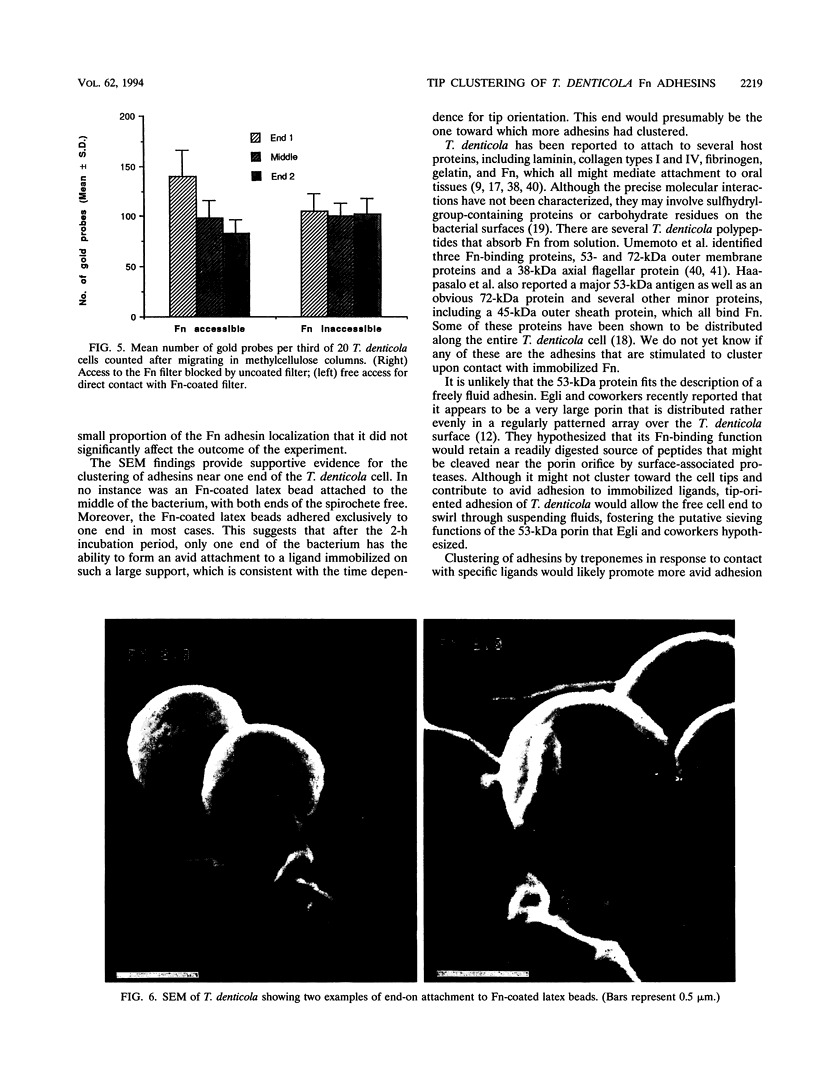
Treponema denticola has been shown to bind to immobilized fibronectin (Fn) by its tips. Yet labeling of cells in suspension with an Fn-gold conjugate to localize the Fn adhesins shows that they are distributed in patches along the entire cell length. Subsequent experiments have shown that the number and proportion of tip-oriented cells increase with time, suggesting that Fn contact stimulates T. denticola to rearrange adhesins toward its tips. To test this hypothesis, T. denticola cells were allowed to migrate in a 2% methylcellulose column toward nitrocellulose filters coated with Fn, laminin, bovine serum albumin, or phosphate-buffered saline. Cells close to and distant from the filters were collected, labeled with Fn-gold probes, and examined by transmission electron microscopy. The number of gold particles on each of 20 cells was counted, dividing each cell into thirds along its length: the end third with the most label (end 1), the middle third, and the end with the least label (end 2). The mean number (+/- standard deviation) of gold probes per third was calculated. Fn-gold probes clustered toward one end of T. denticola cells when in contact with Fn-coated nitrocellulose, with > 55% of probes in end 1. In contrast, no clustering toward T. denticola ends occurred with laminin-, bovine serum albumin, or phosphate-buffered saline-coated filters or in the absence of a filter. Blocking access of the T. denticola cells to the Fn-coated nitrocellulose filter by placing an uncoated filter between them prevented clustering of Fn-gold. Removal of T. denticola cells from direct contact with the Fn-coated filter did not promote redistribution of clustered probes. These data suggest that T. denticola is stimulated to cluster Fn adhesins irreversibly toward its tips when it migrates into contact with immobilized Fn. This might be significant for establishing multiple adhesive interactions with host cells and ligands.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alley M. R., Maddock J. R., Shapiro L. Requirement of the carboxyl terminus of a bacterial chemoreceptor for its targeted proteolysis. Science. 1993 Mar 19;259(5102):1754–1757. doi: 10.1126/science.8456303. [DOI] [PubMed] [Google Scholar]
- Baehni P. C., Song M., McCulloch C. A., Ellen R. P. Treponema denticola induces actin rearrangement and detachment of human gingival fibroblasts. Infect Immun. 1992 Aug;60(8):3360–3368. doi: 10.1128/iai.60.8.3360-3368.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi E. F., Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991 Nov 14;354(6349):161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- Boehringer H., Taichman N. S., Shenker B. J. Suppression of fibroblast proliferation by oral spirochetes. Infect Immun. 1984 Jul;45(1):155–159. doi: 10.1128/iai.45.1.155-159.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charon N. W., Lawrence C. W., O'Brien S. Movement of antibody-coated latex beads attached to the spirochete Leptospira interrogans. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7166–7170. doi: 10.1073/pnas.78.11.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallo S. F., Chavoya A., Baseman J. B. Characterization of the gene for a 30-kilodalton adhesion-related protein of Mycoplasma pneumoniae. Infect Immun. 1990 Dec;58(12):4163–4165. doi: 10.1128/iai.58.12.4163-4165.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson J. R., Ellen R. P. Tip-oriented adherence of Treponema denticola to fibronectin. Infect Immun. 1990 Dec;58(12):3924–3928. doi: 10.1128/iai.58.12.3924-3928.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli C., Leung W. K., Müller K. H., Hancock R. E., McBride B. C. Pore-forming properties of the major 53-kilodalton surface antigen from the outer sheath of Treponema denticola. Infect Immun. 1993 May;61(5):1694–1699. doi: 10.1128/iai.61.5.1694-1699.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson D., Ellen R. P., Buivids I. Labeling of binding sites for beta 2-microglobulin (beta 2m) on nonfibrillar surface structures of mutans streptococci by immunogold and beta 2m-gold electron microscopy. J Bacteriol. 1987 Jun;169(6):2507–2515. doi: 10.1128/jb.169.6.2507-2515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzoso G., Hu P. C., Meloni G. A., Barile M. F. The immunodominant 90-kilodalton protein is localized on the terminal tip structure of Mycoplasma pneumoniae. Infect Immun. 1993 Apr;61(4):1523–1530. doi: 10.1128/iai.61.4.1523-1530.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollop N., March P. E. Localization of the membrane binding sites of Era in Escherichia coli. Res Microbiol. 1991 Feb-Apr;142(2-3):301–307. doi: 10.1016/0923-2508(91)90045-c. [DOI] [PubMed] [Google Scholar]
- Greenstein G. The role of metronidazole in the treatment of periodontal diseases. J Periodontol. 1993 Jan;64(1):1–15. doi: 10.1902/jop.1993.64.1.1. [DOI] [PubMed] [Google Scholar]
- Grenier D., Uitto V. J., McBride B. C. Cellular location of a Treponema denticola chymotrypsinlike protease and importance of the protease in migration through the basement membrane. Infect Immun. 1990 Feb;58(2):347–351. doi: 10.1128/iai.58.2.347-351.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo M., Müller K. H., Uitto V. J., Leung W. K., McBride B. C. Characterization, cloning, and binding properties of the major 53-kilodalton Treponema denticola surface antigen. Infect Immun. 1992 May;60(5):2058–2065. doi: 10.1128/iai.60.5.2058-2065.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haapasalo M., Singh U., McBride B. C., Uitto V. J. Sulfhydryl-dependent attachment of Treponema denticola to laminin and other proteins. Infect Immun. 1991 Nov;59(11):4230–4237. doi: 10.1128/iai.59.11.4230-4237.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes N. S., Muse K. E., Collier A. M., Baseman J. B. Parasitism by virulent Treponema pallidum of host cell surfaces. Infect Immun. 1977 Jul;17(1):174–186. doi: 10.1128/iai.17.1.174-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keulers R. A., Maltha J. C., Mikx F. H., Wolters-Lutgerhorst J. M. Attachment of Treponema denticola strains to monolayers of epithelial cells of different origin. Oral Microbiol Immunol. 1993 Apr;8(2):84–88. doi: 10.1111/j.1399-302x.1993.tb00550.x. [DOI] [PubMed] [Google Scholar]
- Laughon B. E., Syed S. A., Loesche W. J. API ZYM system for identification of Bacteroides spp., Capnocytophaga spp., and spirochetes of oral origin. J Clin Microbiol. 1982 Jan;15(1):97–102. doi: 10.1128/jcm.15.1.97-102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J. The role of spirochetes in periodontal disease. Adv Dent Res. 1988 Nov;2(2):275–283. doi: 10.1177/08959374880020021201. [DOI] [PubMed] [Google Scholar]
- Maddock J. R., Shapiro L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science. 1993 Mar 19;259(5102):1717–1723. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V., Smibert R. M., Hash D. E., Burmeister J. A., Ranney R. R. Bacteriology of severe periodontitis in young adult humans. Infect Immun. 1982 Dec;38(3):1137–1148. doi: 10.1128/iai.38.3.1137-1148.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M. Mode of action of metronidazole on anaerobic bacteria and protozoa. Surgery. 1983 Jan;93(1 Pt 2):165–171. [PubMed] [Google Scholar]
- Ohta K., Makinen K. K., Loesche W. J. Purification and characterization of an enzyme produced by Treponema denticola capable of hydrolyzing synthetic trypsin substrates. Infect Immun. 1986 Jul;53(1):213–220. doi: 10.1128/iai.53.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson K., Baseman J. B., Alderete J. F. Molecular cloning of Treponema pallidum outer envelope fibronectin binding proteins, P1 and P2. Genitourin Med. 1987 Dec;63(6):355–360. doi: 10.1136/sti.63.6.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijntjens F. M., Mikx F. H., Wolters-Lutgerhorst J. M., Maltha J. C. Adherence of oral treponemes and their effect on morphological damage and detachment of epithelial cells in vitro. Infect Immun. 1986 Feb;51(2):642–647. doi: 10.1128/iai.51.2.642-647.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela M. N., Weinberg A., Borinsky R., Holt S. C., Dishon T. Inhibition of superoxide production in human polymorphonuclear leukocytes by oral treponemal factors. Infect Immun. 1988 Mar;56(3):589–594. doi: 10.1128/iai.56.3.589-594.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker B. J., Listgarten M. A., Taichman N. S. Suppression of human lymphocyte responses by oral spirochetes: a monocyte-dependent phenomenon. J Immunol. 1984 Apr;132(4):2039–2045. [PubMed] [Google Scholar]
- Simonson L. G., Goodman C. H., Bial J. J., Morton H. E. Quantitative relationship of Treponema denticola to severity of periodontal disease. Infect Immun. 1988 Apr;56(4):726–728. doi: 10.1128/iai.56.4.726-728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson L. G., Rouse R. F., Bockowski S. W. Monoclonal antibodies that recognize a specific surface antigen of Treponema denticola. Infect Immun. 1988 Jan;56(1):60–63. doi: 10.1128/iai.56.1.60-63.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slot J. W., Geuze H. J. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985 Jul;38(1):87–93. [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Fibronectin mediates Treponema pallidum cytadherence through recognition of fibronectin cell-binding domain. J Exp Med. 1985 Mar 1;161(3):514–525. doi: 10.1084/jem.161.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Baseman J. B., Alderete J. F. Putative Treponema pallidum cytadhesins share a common functional domain. Infect Immun. 1985 Sep;49(3):833–835. doi: 10.1128/iai.49.3.833-835.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto V. J., Grenier D., Chan E. C., McBride B. C. Isolation of a chymotrypsinlike enzyme from Treponema denticola. Infect Immun. 1988 Oct;56(10):2717–2722. doi: 10.1128/iai.56.10.2717-2722.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umemoto T., Nakatani Y., Nakamura Y., Namikawa I. Fibronectin-binding proteins of a human oral spirochete Treponema denticola. Microbiol Immunol. 1993;37(1):75–78. doi: 10.1111/j.1348-0421.1993.tb03182.x. [DOI] [PubMed] [Google Scholar]
- Umemoto T., Wadood A., Nakamura Y., Nakatani Y., Namikawa I. Antigenic behaviors of two axial flagellar proteins detected in Treponema denticola. Microbiol Immunol. 1993;37(2):159–163. doi: 10.1111/j.1348-0421.1993.tb03194.x. [DOI] [PubMed] [Google Scholar]
- Vinh T., Faine S., Adler B. Adhesion of leptospires to mouse fibroblasts (L929) and its enhancement by specific antibody. J Med Microbiol. 1984 Aug;18(1):73–85. doi: 10.1099/00222615-18-1-73. [DOI] [PubMed] [Google Scholar]
- Weinberg A., Holt S. C. Interaction of Treponema denticola TD-4, GM-1, and MS25 with human gingival fibroblasts. Infect Immun. 1990 Jun;58(6):1720–1729. doi: 10.1128/iai.58.6.1720-1729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]