Abstract
The activity within the autonomic nervous system may be altered following sustained exposure to hypoxia, and it is possible that this increase in activity underlies the early acclimatization of both ventilation and the pulmonary vasculature to hypoxia. To test this hypothesis, seven individuals were infused with the ganglionic blocker trimetaphan before and after an 8 h exposure to hypoxia. The short half-life of trimetaphan should ensure that the initial infusion does not affect acclimatization to the 8 h hypoxia exposure, and the use of a ganglion blocking agent should inhibit activity within all branches of the autonomic nervous system. During the infusions of trimetaphan, measurements of ventilation and echocardiographic assessments of pulmonary vascular tone (ΔPmax) were made during euoxia and during a short period of isocapnic hypoxia. Subjects were also studied on two control days, when a saline infusion was substituted for trimetaphan. Trimetaphan had no effect on either euoxic ventilation or the sensitivity of ventilation to acute hypoxia. Trimetaphan significantly reduced ΔPmax in euoxia (P < 0.05), but had no significant effect on the sensitivity of ΔPmax to acute hypoxia once changes in cardiac output had been controlled for. The 8 h period of hypoxia elevated euoxic ventilation (P < 0.001) and ΔPmax (P < 0.001) and increased their sensitivities to acute hypoxia (P < 0.001 for both), indicating that significant acclimatization had occurred. Trimetaphan had no effect on the acclimatization response of any of these variables. We conclude that altered autonomic activity following 8 h of hypoxia does not underlie the acclimatization observed in ventilation or pulmonary vascular tone.
Exposure of humans to a steady degree of hypoxia over a period of several hours leads to gradual increases in ventilation (V̇E), pulmonary artery pressure (PAP), heart rate (HR) and cardiac output (CO) (Howard & Robbins, 1995; Dorrington et al. 1997; Clar et al. 2001). In addition, the sensitivity of some or all of these variables to brief exposures to acute hypoxia is increased following such a period of sustained hypoxia. Both of these kinds of responses are commonly subsumed under the heading ‘early acclimatization to hypoxia’, and the mechanisms underlying them remain obscure.
The autonomic nervous system plays a major role in regulating HR and CO, and a perhaps more subtle role in modulating V̇E and PAP. Both sympathetic and parasympathetic activities have been shown to change during exposure to hypoxia in humans (Petersen et al. 1974; Saito et al. 1988; Rowell et al. 1989). Furthermore, the elevation in sympathetic nervous system activity persists for a considerable period following relief from the hypoxic stimulus (Morgan et al. 1995). Chronic hypoxia causes a still more marked activation of both the parasympathetic and the sympathetic nervous system in healthy humans, despite a return towards normal of the arterial O2 content with acclimatization (Boushel et al. 2001; Calbet, 2003). In direct neurophysiological recording of sympathetic nerve activity, Hansen & Sander, (2003) found that acclimatization to high altitude is accompanied by marked elevations in resting levels of sympathetic neural discharge to the skeletal muscle vasculature. Furthermore, the high altitude-induced sympathetic activation persisted for days after return to sea level, demonstrating a long-lasting sympathetic neural overactivity following relief from hypoxia.
It has long been recognised that stimulation of the sympathetic nervous supply to the carotid body increases the frequency of sinus nerve chemosensory discharges (Floyd & Neil, 1952; Eyzaguirre & Lewin, 1961). More recently, two types of excitatory response have been distinguished, and it has been suggested that these relate to two distinct components of sympathetic innervation, one arising from the innervation of the vasculature and the other arising from direct sympathetic innervation of the type I cells of the carotid body (O'Regan, 1981). Using circulating and urinary noradrenaline concentrations or spillover as indirect measures of sympathetic nerve activity, a progressive increase in sympatho-excitation in humans during exposure to high altitude has been found, and this increase has been shown to correlate with the increase in V̇E (Asano et al. 1997).
An autonomic modulation of pulmonary vascular tone appears possible given that both sympathetic and parasympathetic branches of the autonomic nervous system innervate pulmonary vessels (Hebb, 1966; Downing & Lee, 1980). Levitzky et al. (1978) have shown in the dog that systemic arterial hypoxaemia induces a chemoreflex-mediated pulmonary vascular dilatation. A later study, employing experimental vagotomy in dogs, has suggested that the autonomic pulmonary vasodilator effect of systemic hypoxaemia has a parasympathetic origin (Wilson & Levitzky, 1989). However, other literature suggests that these effects are mediated through the pulmonary sympathetic supply (Kazemi et al. 1972; Murray et al. 1986; Brimioulle et al. 1997).
Based on the observations described above, in particular the persistence of an elevated sympathetic activity following the relief of hypoxia and the ability of the autonomic nervous system to influence V̇E and pulmonary vascular tone, we wished to test the hypothesis that persistently altered autonomic activity underlies part or all of early acclimatization to hypoxia. We studied the effects of a short period of exposure to isocapnic hypoxia on ventilation and the pulmonary circulation, both before and after conditioning with 8 h of hypoxia to induce acclimatization to hypoxia (first control). On a subsequent day, we repeated this sequence but we administered the short acting ganglion-blocking agent trimetaphan to abolish peripheral autonomic activity while we were testing the effects of acute exposure to isocapnic hypoxia on ventilation and the pulmonary circulation, before and after 8 h of hypoxia. On the third and final day, we undertook a second control sequence, the same as the first.
On the test day, because of its short duration of action, trimetaphan should not have been active during the period of sustained hypoxia, and therefore should not have prevented the acclimatization process from occurring. If it is the case that a persistent elevation in autonomic nervous activity is responsible for the acclimatization-induced increases in ventilatory and pulmonary vascular sensitivities to acute hypoxia, then the infusion of trimetaphan following sustained hypoxia would be expected to remove the effects of acclimatization. In this case, under the conditions of trimetaphan infusion, the ventilatory and pulmonary vascular responses to acute hypoxia should be the same before and after exposure to sustained hypoxia.
Methods
Participants
Seven healthy volunteers (5 men, 2 women) with an age of 28 ± 4 years, height of 177.3 ± 8.9 cm, and weight of 71.1 ± 12.7 kg (means ± s.d.) participated in the study. Each gave informed consent before participating. The study was approved by the Oxfordshire Clinical Research Ethics Committee and performed in accordance with the Declaration of Helsinki. Each volunteer visited the laboratory twice before undertaking any of the main experimental protocols to become familiar with the laboratory and its procedures. On these visits we measured the normal end-tidal PCO2 (PET,CO2) of the participants and confirmed that they were suitable for echocardiographic measurement of tricuspid regurgitation.
Protocols
Each volunteer attended the laboratory for three main protocols. In the test protocol, trimetaphan was administered (Protocol T), which acts as a competitive inhibitor at the cholinergic binding sites in autonomic ganglia (Gilman et al. 1990). Trimetaphan also induces a degree of histamine release through an independent mechanism (McCubbin & Page, 1952), although this does not appear to play an important role in the haemodynamic responses to trimetaphan (Fahmy & Soter, 1985). The two other protocols were control protocols in which placebo was administered (Protocol C1 and Protocol C2). Each protocol lasted 1 day and the protocols were separated from each other by at least 1 week. The order of these protocols was fixed as C1, T and C2.
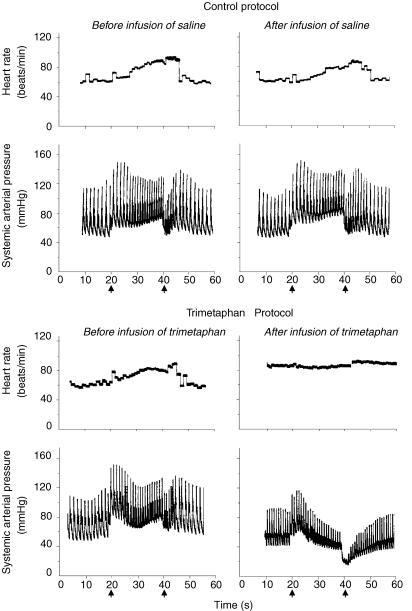
Participants reported to the laboratory at 08.00 h. After 30 min of supine rest, the participants performed a Valsalva manoeuvre with an expiratory strain of 40 mmHg for 20 s. The strain pressure during the Valsalva manoeuvre was monitored by a manometer. Typical changes in systemic arterial pressure (AP) and HR during the Valsalva manoeuvre were observed in all subjects before ganglion blockade (Fig. 1). In Protocol T, intravenous trimetaphan (trimetaphan camsylate, Cambridge Laboratories, Wallsend, UK) was administered at an initial rate of infusion of 2 mg min−1 after performance of the initial Valsalva manoeuvre. Three minutes after starting the infusion, the Valsalva manoeuvre was performed again to evaluate the HR and AP responses to changes in intrathoracic pressure. The infusion rate was increased incrementally until the tachycardia and AP recovery during phase II of the Valsalva manoeuvre, and overshoot in AP during phase IV of the Valsalva manoeuvre, were all abolished (Fig. 1). Once complete blockade was achieved, the infusion of trimetaphan was maintained at this peak dose throughout the time of exposure to acute hypoxia. The maximum rate of infusion used was 4 mg min−1. In Protocol C1 and C2, saline was infused instead. During the exposure to acute hypoxia, end-tidal PO2 (PET,O2) was held at 100 Torr in the first 10 min, then at 50 Torr for 20 min, and at 100 Torr again for the final 10 min. PET,CO2 was held constant at 2 Torr above each volunteer's normal baseline value throughout the exposures to acute hypoxia.
Figure 1. Typical changes in arterial pressure and heart rate during the Valsalva manoeuvre in control and trimetaphan protocols.
The efficacy of ganglion blockade by trimetaphan was demonstrated not only by the absence of heart rate response, but also by the absence of BP recovery during phase II or BP overshoot during phase IV of the Valsalva manoeuvre. Arrows show the time points when Valsalva manoeuvre was started or stopped.
After the acute hypoxic exposure the infusion was terminated and participants took a 30 min break. They then entered a purpose-built chamber to undergo an 8 h period of sustained hypoxic exposure under isocapnic conditions. During this period, the composition of the chamber gas was adjusted so as to hold the subject's PET,O2 at 50 Torr and to hold the subject's PET,CO2 at his or her initial control air-breathing value.
At the end of the 8 h period of sustained hypoxia, the subject left the chamber and was left to breathe room air for 30 min. Following this, a second set of measurements was made of the responses to acute hypoxia. The procedure that was followed was identical to that used before the 8 h exposure to sustained hypoxia.
Control of end-tidal gases during the acute hypoxic exposure and respiratory measurements
During the acute hypoxic exposures, volunteers lay on their left side on a couch and breathed through a mouthpiece with the nose occluded. Ventilatory volumes were measured by a turbine volume-measuring device and flows by a pneumotachograph, both in series with the mouthpiece. Respired gases were sampled via a fine catheter close to the mouth and analysed continuously for PCO2 and PO2 by mass spectrometry. A pulse oximeter was used to monitor arterial O2 saturation. Inspired and expired volumes, end-inspiratory and end-expiratory PCO2 and PO2, and saturation were detected in real time by a computer and logged breath by breath. A dynamic end-tidal forcing system (Robbins et al. 1982) was used to control the end-tidal gases in the manner specified for the determination of the acute hypoxic ventilatory response. Before the start of each experiment, a cardiorespiratory model was used to construct a forcing function that contained the breath-by-breath values for inspiratory PCO2 and PO2 predicted to produce the desired end-tidal sequences. During the experiment, a computer-controlled gas-mixing system was used to generate this sequence in a modified manner. The modifications resulted from feedback control based on the deviations of the measured values for PET,CO2 and PET,O2 from their desired values.
Measurements of pulmonary blood pressure, cardiac output and systemic arterial pressure
The majority of people have detectable regurgitation during systole through their tricuspid valves. Doppler echocardiography can be used to detect the presence of this jet of blood and measure the velocity with which it moves back into the right atrium. On the assumption that the flow within the jet may be regarded as steady, Bernoulli's equation can be used to calculate the maximum pressure difference between the right ventricle and right atrium (ΔPmax) from the density of blood (ρ) and the peak velocity of the jet (v). This gives the relationship ΔPmax = ρv2/2. Assuming right atrial pressure remains constant, changes in ΔPmax will be equal to changes in the peak systolic PAP.
Echocardiographic measurements were performed using a Hewlett-Packard Sonos 5500 ultrasound machine with an S4 two-dimensional transducer (2–4 MHz). HR and respiratory waveform were also recorded.
A standard technique was used for the measurement of ΔPmax (Balanos et al. 2003). The tricuspid valve was visualized in an apical four-chamber view, and then colour Doppler format allowed the regurgitant jet to be detected. After proper alignment of the probe, continuous wave spectral analysis at a sweep speed of 50 mm s−1 was used to record the velocity profile of the jet. During analysis, the maximal velocity of the jet was measured using an electronic caliper tool. ΔPmax values were then computed automatically.
In order to measure CO, the flow through the aortic valve during systole was identified using an apical five-chamber view. The velocity profile was then obtained in pulsed wave spectral mode at a display screen sweep speed of 100 mm s−1. Doppler sampling of the flow was made just below the orifice of the aortic valve. An automated procedure was then used to calculate the velocity time integral (VTI) of the flow for each ventricular contraction. The diameter (D) of the aortic valve was measured from a parasternal long axis view. From these data, CO was calculated as HR × VTI × π × (D/2)2.
Systemic arterial pressure was measured continuously using finger plethysmography (Portapres, TNO-TPD Biomedical Instrumentation, the Netherlands) during Valsalva manoeuvres and the exposures to acute hypoxia.
Eight hour sustained exposure to hypoxia
The sustained hypoxic exposure was conducted using a purpose-built chamber, which was large enough to allow subjects to sit or stand comfortably. Subjects were studied individually in the chamber so that the inspiratory gas composition could be tailored to regulate their end-tidal gas composition accurately. Respired gas was sampled via a fine nasal catheter at the opening of the subject's nostril. The samples were continually analysed for PCO2 and PO2. Arterial O2 saturation was monitored using a pulse oximeter. A computer sampled the PCO2, PO2 and saturation signals at a frequency of 100 Hz. It detected end-inspiratory and end-expiratory values for PCO2 and PO2 and recorded these breath by breath along with values for saturation. The desired values for PET,CO2 and PET,O2 were entered into the computer. The computer then automatically adjusted the composition of the gas in the chamber every 5 min, or at manually overridden intervals, to minimize the error between desired and actual values for the end-tidal gases. This system has been described in detail elsewhere (Howard et al. 1995). The subject was kept under continuous observation throughout the 8 h exposure. In addition, an environmental oxygen monitor was employed within the chamber as a further safety precaution.
Modelling of hypoxic ventilatory responses
To obtain numerical values for the sensitivity of V̇E to the acute hypoxic exposure, a respiratory model was fitted to the data. The particular respiratory model employed was Model I of those described by Liang et al. (1997). This model separates the total ventilation, V̇E, into a central, hypoxia-independent component, V̇c, and a peripheral, hypoxia-dependant component, V̇p, and may be written:
where Tp is the time constant for development of the peripheral response, Kp is the peripheral drive in the absence of hypoxia, Gp is the peripheral chemoreflex sensitivity, and S is the saturation function (Severinghaus, 1979) calculated at time t delayed by the peripheral time delay, Dp. Th is the time constant for the development of hypoxic ventilatory decline, G100 is the steady-state chemoreflex sensitivity in the absence of hypoxic ventilatory decline (i.e. following conditioning at 100% arterial oxygen saturation), and Gh defines the magnitude of hypoxic ventilatory decline as the ratio of the decrease in peripheral chemoreflex sensitivity to the decrease in S.
In order to allow for the autocorrelation that exists between measurements of ventilation on successive breaths, a model of the noise processes was fitted to the data in parallel with the fitting of the model of the ventilatory response to hypoxia (Liang et al. 1996). The model parameters were estimated by fitting the models to the data using a standard subroutine to minimize the sum of squares of the residuals (subroutine E04FDF, Numerical Algorithms Group, Oxford).
Modelling of pulmonary vascular responses
To quantify fully the pulmonary vascular responses to acute hypoxia observed in this study, a mathematical model was fitted to the data. In this model, it was supposed that the dynamics of the response could be represented by a simple, linear, first order process and that the changes in ΔPmax could be related in a linear manner to changes in saturation, at least of the range for PO2 values studied. Given these assumptions, a simple difference equation may be written of the form:
where the difference between successive two time points (t) and (t − 1) is Δt, S is the saturation (Severinghaus, 1979), ΔP100 is the steady-state value for ΔPmax in the absence of hypoxia (i.e. when saturation is 100%), GΔP is the sensitivity of ΔPmax to a decrease in saturation and TΔP is the time constant for the response. The parameters of this model (ΔP100, GΔP and TΔP) were estimated using the function LSQNONLIN in Matlab (version 7.0, The MathWorks, Inc., Natick, MA, USA) to minimize the sum of squares of the residuals.
Quantification of systemic cardiovascular responses
The responses of the systemic circulation to acute hypoxia were not sufficiently well defined to quantify through a model fitting process. Accordingly simple averages of these responses were calculated under the conditions of euoxia and hypoxia.
Statistical analysis
A full factorial ANOVA was used to assess the statistical significance of the results, with fixed factors of Protocol (control protocols (Protocols C1, C2) versus Protocol T) and Time (before the sustained hypoxic exposure (AM measurements) versus after the sustained hypoxic exposure (PM measurements)) together with a random factor of Subject. The null hypothesis of the experiment was that infusion of trimetaphan would not abrogate the acclimatization observed after sustained hypoxia. The acceptance or rejection of this null hypothesis was assessed through the significance or otherwise of the interactive term between Protocol and Time.
Results
Control over the end-tidal gases during the acute exposure to hypoxia
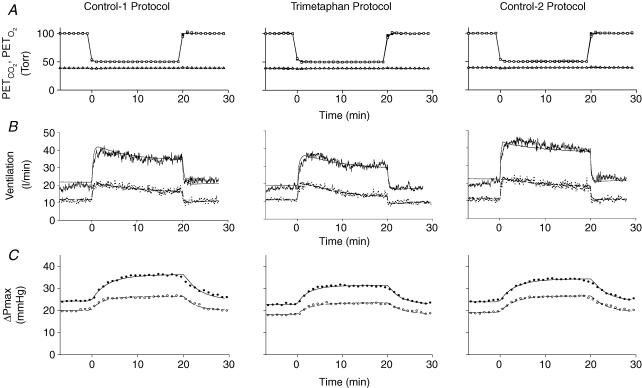
Figure 2A illustrates the average time course for PET,O2 and PET,CO2 across all volunteers for both exposures to acute hypoxia in each protocol. The acute hypoxic stimulus was generated accurately in all cases. The background level of PET,CO2 was held very constant throughout each exposure. Overall, no discernable differences among the three protocols could be detected.
Figure 2. Mean values for end-tidalPO2(PET,O2) (squares) and end-tidalPCO2(PET,CO2) (triangles) (A), ventilation (B) and maximum systolic pressure difference across the tricuspid valve (ΔPmax) (C) during acute hypoxic exposures.
Open symbols and dashed lines indicate responses before sustained hypoxic exposure and filled symbols or continuous lines indicate responses after sustained hypoxic exposure. Lines through data for ventilation indicate fit of respiratory model to data. Lines through data for ΔPmax indicate fit of pulmonary vascular response model to data.
Ventilatory responses to hypoxia
Figure 2B illustrates the average responses for V̇E to acute hypoxia before (AM) and after (PM) exposure to the 8 h period of sustained hypoxia. Table 1 lists the parameters obtained from the model fitting. From these results, it is clear that trimetaphan had no discernable effect on the ventilatory response to acute hypoxia. Significant acclimatization to sustained hypoxia was evident in all three protocols, both through a significant increase in V̇E in euoxia (corresponding to parameter V̇c of the model) and through a significant increase in the acute ventilatory sensitivity to hypoxia (corresponding to parameter G100 of the model) in the PM measurements as compared with the AM measurements. The presence of trimetaphan in Protocol T caused no discernable reduction in the degree of acclimatization observed.
Table 1.
Ventilatory responses to hypoxia: parameter values (mean ±s.e.m) for the model of the ventilatory response to acute hypoxia for the trimetaphan and control protocols before and after an 8 h period of sustained hypoxic exposure
| Protocol/factor | G100 (l min−1%−1) | V̇c(l min−1) | Tp (s) | Dp (s) | Gh (l min−1%−2) | Th (s) | Kp (%) |
|---|---|---|---|---|---|---|---|
| AM measurements – before sustained hypoxia | |||||||
| Control 1 | 0.85 ± 0.20 | 7.58 ± 1.51 | 11.77 ± 4.40 | 5.83 ± 147 | 0.028 ± 0.011 | 537 ± 135 | 3.48 ± 1.41 |
| Trimetaphan | 0.78 ± 0.23 | 4.17 ± 1.28 | 9.09 ± 5.40 | 5.69 ± 1.17 | 0.022 ± 0.008 | 820 ± 136 | 10.64 ± 7.49 |
| Control 2 | 1.01 ± 0.20 | 7.45 ± 1.39 | 11.88 ± 4.21 | 6.12 ± 1.71 | 0.030 ± 0.010 | 643 ± 162 | 1.99 ± 1.62 |
| PM measurements – after sustained hypoxia | |||||||
| Control 1 | 1.82 ± 0.29 | 15.58 ± 1.28 | 20.97 ± 3.47 | 6.10 ± 2.19 | 0.054 ± 0.023 | 471 ± 115 | 1.54 ± 0.89 |
| Trimetaphan | 1.40 ± 0.23 | 12.03 ± 2.95 | 12.17 ± 3.60 | 4.63 ± 0.91 | 0.045 ± 0.021 | 880 ± 114 | 2.77 ± 1.57 |
| Control 2 | 1.75 ± 0.15 | 16.61 ± 0.99 | 12.83 ± 3.96 | 2.74 ± 0.63 | 0.030 ± 0.020 | 594 ± 134 | 0.83 ± 0.77 |
| Statistical analysis – ANOVA | |||||||
| Time (before/after sustained hypoxia) | < 0.001 | < 0.001 | — | — | — | — | — |
| Protocol (C1, C2 versus T) | — | — | — | — | — | — | — |
| Interactive term | — | — | — | — | — | — | — |
G100, steady-state chemoreflex sensitivity to hypoxia in absence of any hypoxic ventilatory depression (arterial oxygen saturation is 100%); V̇E, hypoxia independent (central chemoreflex) contribution to V̇c; Tp, time constant for the peripheral chemoreflex responses to hypoxia; Dp, time delay for the peripheral chemoreflex; Gh, sensitivity to hypoxic ventilatory decline, expressed as the ratio of the decrease in the sensitivity of the peripheral chemoreflex to the decrease in conditioning arterial oxygen saturation; Th, time constant associated with the development of hypoxic ventilatory decline; Kp, bias term, representing peripheral drive in absence of hypoxia; AM, morning; PM, afternoon. ANOVA conducted on the parameter values with fixed factors Time (before/after sustained hypoxia) and Protocol (control protocols (Protocols C1, C2) versus Protocol T). Statistical significance was accepted at P < 0.05.
Pulmonary vascular responses to hypoxia
Figure 2C illustrates the average responses for ΔPmax to acute hypoxia before (AM) and after (PM) exposure to 8 h period of sustained hypoxia. Table 2 lists the parameters obtained from the model fitting process. Trimetaphan significantly reduced ΔPmax under conditions of euoxia (corresponding to parameter P100 of the model) and significantly attenuated the sensitivity of ΔPmax to acute hypoxia (corresponding to parameter GΔP of the model). A significant acclimatization following the 8 h period of sustained hypoxia was observed through the increase in the baseline value for ΔPmax in the absence of hypoxia (corresponding to parameter P100 of the model) and through the increase in sensitivity of ΔPmax to acute hypoxia (corresponding to parameter GΔP of the model) in the PM values as compared with the AM values. No significant abrogation of these effects of sustained hypoxia was observed through the administration of trimetaphan in Protocol T.
Table 2.
Pulmonary vascular responses to hypoxia: parameter values (mean ±s.e.m.) for the model of the pulmonary vascular response to acute hypoxia for the trimetaphan and control protocols before and after an 8 h period of sustained hypoxic exposure
| Protocol/factor | GΔP (mmHg %−1) | ΔP100 (mmHg) | TΔP (min) |
|---|---|---|---|
| AM measurements – before sustained hypoxia | |||
| Control 1 | 0.50 ± 0.06 | 18.9 ± 1.5 | 2.41 ± 0.41 |
| Trimetaphan | 0.41 ± 0.04 | 17.4 ± 1.2 | 3.06 ± 0.32 |
| Control 2 | 0.58 ± 0.06 | 17.9 ± 1.2 | 3.24 ± 0.20 |
| PM measurements – after sustained hypoxia | |||
| Control 1 | 0.95 ± 0.10 | 22.2 ± 1.8 | 3.90 ± 0.64 |
| Trimetaphan | 0.68 ± 0.07 | 21.2 ± 1.6 | 2.91 ± 0.35 |
| Control 2 | 0.82 ± 0.04 | 22.1 ± 1.4 | 3.61 ± 0.40 |
| Statistical analysis – ANOVA | |||
| Time (before/after sustained hypoxia) | < 0.001 | < 0.001 | — |
| Protocol (C1, C2 versus T) | < 0.02 | < 0.05 | — |
| Interactive term | — | — | < 0.05 |
GΔP is the sensitivity of maximum pressure difference across tricuspid valve during systole (ΔPmax) to a decrease in saturation; ΔP100 is the steady-state value for ΔPmax in the absence of hypoxia (i.e. when saturation is 100%); TΔP is the time constant for the response; AM, morning; PM, afternoon. ANOVA conducted on the parameter values with fixed factors Time (before/after sustained hypoxia) and Protocol (control protocols (Protocols C1, C2) versus Protocol T). Statistical significance was accepted at P < 0.05.
Cardiovascular responses to hypoxia
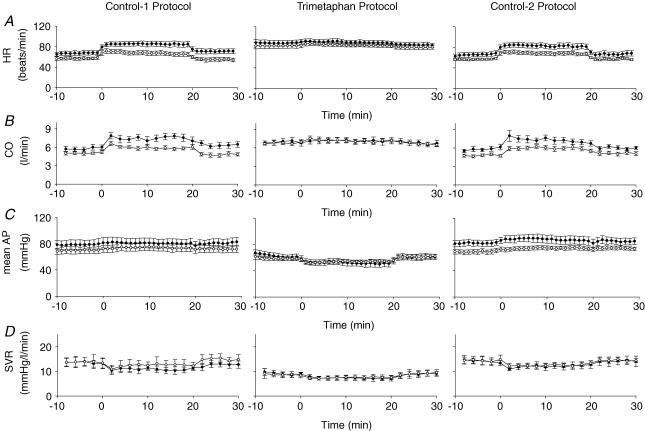
The systemic cardiovascular responses are illustrated in Fig. 3 and the average values for the various variables under both euoxic and hypoxic conditions are listed in Table 3. As expected, autonomic blockade with trimetaphan: (i) elevated HR in euoxia; (ii) abolished the normal increase in HR observed with acute hypoxia and (iii) reduced systemic vascular resistance (SVR), calculated as (mean AP)/CO. Trimetaphan did not block the reduction observed in SVR with hypoxia. In euoxia, these effects led to the expected reduction in mean AP and increase in CO. At the induction of hypoxia, the effects were associated with a loss of the normal increase in CO that occurs with acute hypoxia and also with a fall in mean AP distinct from the slight elevation in mean AP that was observed with acute hypoxia in the absence of trimetaphan.
Figure 3.
Mean values (±s.e.m.) for heart rate (HR) (A), cardiac output (CO) (B), mean systemic arterial pressure (AP) (C) and systemic vascular resistance (SVR) (D) during acute hypoxia before (○) and after (•) sustained hypoxic exposure.
Table 3.
Cardiovascular responses to hypoxia: values (mean ±s.e.m.) for heart rate (HR), cardiac output (CO), mean systemic arterial pressure (mean AP) and systemic vascular resistance (SVR) for the trimetaphan and control protocols before and after an 8 h period of sustained hypoxic exposure
| Protocol/Factor | HR (beats min−1) | CO (l min−1) | Mean AP (mmHg) | SVR (mmHg l−1 min−2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Euoxia | Hypoxia | Increase | Euoxia | Hypoxia | Increase | Euoxia | Hypoxia | Increase | Euoxia | Hypoxia | Increase | |
| AM measurements – before sustained hypoxia | ||||||||||||
| Control 1 | 57 ± 3 | 67 ± 4 | 10 ± 2** | 5.1 ± 0.2 | 6.0 ± 0.3 | 0.9 ± 0.1** | 70 ± 5 | 74 ± 6 | 4 ± 2 | 13.3 ± 1.1 | 12.5 ± 0.9 | −0.8 ± 0.6 |
| Trimetaphan | 83 ± 5 | 85 ± 3 | 2 ± 2 | 6.8 ± 0.3 | 7.2 ± 0.3 | 0.4 ± 0.3 | 59 ± 3 | 54 ± 2 | − 5 ± 2* | 8.7 ± 0.5 | 7.6 ± 0.4 | −1.1 ± 0.2** |
| Control 2 | 57 ± 2 | 69 ± 3 | 12 ± 2** | 4.8 ± 0.2 | 5.9 ± 0.3 | 1.1 ± 0.2** | 69 ± 3 | 74 ± 3 | 5 ± 2 | 14.5 ± 1.1 | 12.7 ± 0.8 | −1.8 ± 0.6* |
| PM measurements – after sustained hypoxia | ||||||||||||
| Control 1 | 67 ± 4 | 85 ± 5 | 18 ± 1** | 5.8 ± 0.4 | 7.5 ± 0.5 | 1.7 ± 0.2** | 79 ± 7 | 81 ± 7 | 2 ± 2 | 12.8 ± 0.7 | 11.0 ± 0.9 | −1.8 ± 0.4* |
| Trimetaphan | 88 ± 5 | 90 ± 4 | 2 ± 1 | 6.9 ± 0.5 | 7.2 ± 0.5 | 0.2 ± 0.2 | 59 ± 5 | 50 ± 4 | − 9 ± 3* | 8.9 ± 1.1 | 7.2 ± 0.9 | −1.7 ± 0.3* |
| Control 2 | 66 ± 4 | 83 ± 5 | 17 ± 2** | 5.8 ± 0.4 | 7.3 ± 0.5 | 1.5 ± 0.4** | 88 ± 7 | 92 ± 7 | 5 ± 2 | 15.4 ± 1.7 | 13.1 ± 1.4 | −2.3 ± 0.7* |
| Statistical analysis – ANOVA | ||||||||||||
| Time (before/after sustained hypoxia) | < 0.002 | < 0.01 | < 0.05 | < 0.05 | < 0.05 | — | — | — | — | — | — | — |
| Protocol (C1, C2 versus T) | < 0.001 | < 0.01 | < 0.01 | < 0.001 | < 0.05 | < 0.01 | < 0.01 | < 0.001 | < 0.01 | < 0.001 | < 0.001 | — |
| Interactive term | — | < 0.01 | 0.050 | < 0.05 | < 0.01 | < 0.05 | — | — | — | — | — | — |
AM, morning; PM, afternoon. A one-sample t test was conducted on the increase from euoxia to hypoxia, with results indicated as follows
P < 0.05
P < 0.01. ANOVA conducted on the parameter values with fixed factors Time (before/after sustained hypoxia) and Protocol (control protocols (Protocols C1, C2) versus Protocol T). Statistical significance was accepted at P < 0.05.
Following the 8 h period of hypoxia, a significant increase in HR could be detected. This effect was greater in hypoxia compared with euoxia, indicating that there was also a significant increase in the sensitivity of HR to acute hypoxia. In contrast, no significant effects of the 8 h period of hypoxia were detected on SVR. Infusion of trimetaphan clearly abrogated much of the acclimatization effects of sustained hypoxia on HR in hypoxia, as evidenced by the significant interactive term between Protocol (C1, C2 versus T) and Time (AM versus PM) for HR. This interactive effect was not, however, significant for HR in acute euoxia, and only of marginal significance (P = 0.05) for the increase in HR with acute hypoxia.
Discussion
The main finding of this study was that administration of trimetaphan was unable to reverse the acclimatization-induced changes in both ventilatory and pulmonary vascular control. From these observations we infer that changes in autonomic function over an 8 h period of hypoxia are unlikely to underlie at least the early stages of acclimatization to hypoxia in these systems. In contrast, administration of trimetaphan abrogated most, if not all, of the effect on HR of conditioning with an 8 h exposure to hypoxia.
Study design
Our study used control hypoxia protocols both before and after the trimetaphan hypoxia protocol. As there was no euoxia control protocol in the study design, in theory it is possible that there is a significant ‘time of day’ effect incorporated within the acclimatization effect. In practice we have conducted a considerable number of other studies involving 8 h exposures to hypoxia, which have included euoxia control protocols, and in none of these controls have we found a significant time of day effect (Howard & Robbins, 1995; Tansley et al. 1997; Fatemian & Robbins, 1998; Clar et al. 1999, 2000; Ren et al. 2000).
The effect of trimetaphan on the ventilatory response to hypoxia
The present study found no effect of ganglion blockade on either the ventilatory sensitivity to acute hypoxia or the acclimatization-induced changes in respiratory control. We are unaware of any other studies in humans of the effects of either complete ganglionic blockade, or indeed α-receptor blockade, on these ventilatory responses to hypoxia. However, our results are consistent with a number of previous studies examining blockade of other specific components of the autonomic system. In particular, Clar et al. (1999) found no effect of β-blockade with propranolol on ventilatory acclimatization to 8 h of hypoxia and, similarly, Clar et al. (2001) found no effect of parasympathetic blockade with glycopyrrolate. In neither of these studies was any effect detected on the acute ventilatory response to hypoxia – a finding consistent with the earlier results of Koller et al. (1988).
In anaesthetized cats, carotid-body sympathectomy did not affect carotid-chemoreceptor afferent activity during the first 10 min of hypoxia, but thereafter chemoreceptor discharge was augmented compared with controls (Prabhakar & Kou, 1994). However, in awake goats, ventilation and the hypoxic-ventilatory response were found to be attenuated at 1 and 4 week after carotid-body sympathectomy (Ryan et al. 1995). In that study, carotid-body sympathectomy was found to have no effect on ventilatory acclimatization to hypoxia.
Overall, whilst it is recognized that alterations in autonomic activity can undoubtedly modify respiratory control (Floyd & Neil, 1952; Eyzaguirre & Lewin, 1961), there is little evidence to suggest that either a normal level of autonomic activity, or an altered level of autonomic activity, such as can be induced by the level and duration of hypoxia in our experiments, affects respiratory control in humans.
The effect of trimetaphan on pulmonary vascular responses to hypoxia
The pulmonary circulation is innervated by both adrenergic and cholinergic nerves (Hebb, 1966; Downing & Lee, 1980). The cholinergic system appears to be vasodilatory (Nandiwada et al. 1983). Within the adrenergic system, stimulation of the α receptors causes vasoconstriction and stimulation of the β receptors causes vasodilatation (Silove & Grover, 1968; Howard et al. 1975; Rubin & Lazar, 1983). Numerically and functionally, the α receptors tend to dominate such that sympathetic stimulation causes vasoconstriction and sympathetic blockade causes vasodilatation (Porcelli & Bergofsky, 1973). Overall, ganglionic blockade would be expected to exert both vasodilatory and vasoconstrictive influences within the lung, consistent with the variable nature of overall response observed in experimental animals (Murray et al. 1986; Lodato et al. 1988). In the present human study, the net effect of blockade was vasodilatory.
In the present study, trimetaphan caused a modest reduction in the pulmonary vascular sensitivity to acute hypoxia. One possibility is that this finding is an artifact because trimetaphan also removed the normal rise in CO that occurs with the onset of acute hypoxia. Using a value from the literature relating the increase in ΔPmax to the increase in CO (Balanos et al. 2005), the values obtained for the increase in ΔPmax with acute hypoxia may be corrected for the effect of any concomitant change in CO. Once this correction is made, the significance of the effect of trimetaphan on the sensitivity of the pulmonary vasculature to hypoxia is lost. This finding is consistent with that of Lodato et al. (1988) in conscious dogs, who concluded that, once the confounding effects of changes in CO had been controlled for, neither sympathetic blockade nor total autonomic blockade (with hexamethonium) affected the pulmonary vascular sensitivity to acute hypoxia.
In the present studies, ganglionic blockade did not abrogate the increase in basal tone and sensitivity to hypoxia induced by acclimatization with 8 h of hypoxia. We are unaware of any previous studies with which this may be reasonably compared.
The effect of trimetaphan on cardiovascular responses to hypoxia
As anticipated, autonomic blockade with trimetaphan reduced SVR and increased HR, with a consequential rise in CO and fall in mean AP. Trimetaphan blocked the increase in HR that normally occurs with the induction of acute hypoxia. This effect of trimetaphan is very similar to that observed with blockade of just the parasympathetic component using muscarinic antagonists (Petersen et al. 1974; Clar et al. 2001). Trimetaphan did not, however, block the reduction in SVR with acute hypoxia consistent with hypoxia acting as a local vasodilator (Weisbrod et al. 2001). As a result, a further rise in CO and fall in AP occurred.
Sustained hypoxia of 8 h duration induced a rise in HR under subsequent euoxic conditions and a rise in the sensitivity of HR to acute hypoxia that was similar to that reported in previous studies (Clar et al. 2000; Clar et al. 2001). Much of this was abrogated with the acute infusion of trimetaphan in Protocol T, consistent with the previous report that much of the change in HR following sustained hypoxia arises through alterations in parasympathetic function (Clar et al. 2001). Possible causes of any small elevation in HR following 8 h of hypoxia that persists during trimetaphan infusion include both alterations in humoral factors and alterations in intrinsic factors induced by hypoxia, as well as the possibility that it somehow reflects an increase in the work of breathing caused by the more vigorous ventilatory response. Longer-term studies of hypoxia report increases in both parasympathetic and sympathetic activities in regulating HR, with the result that HR in chronic hypoxia is only mildly elevated (Boushel et al. 2001; Calbet, 2003; Hansen & Sander, 2003).
Of note in this study was the absence of any conditioning effect of sustained hypoxia on SVR. This appears to be somewhat at variance with the results of Dorrington et al. (1997) where SVR appeared to remain below the control euoxic value for at least 2 h following the relief of the sustained hypoxia. In the absence of any conditioning effect of the sustained hypoxia on SVR, the hypothesis that trimetaphan might modify such conditioning immediately fails.
Acknowledgments
We thank David O'Connor for skilled technical assistance and the volunteers for enthusiastic participation. We thank Dr Shou Yan Wang for assistance with modelling the pulmonary vascular responses to hypoxia. This study was supported by the Wellcome Trust.
References
- Asano K, Mazzeo RS, McCullough RE, Wolfel EE, Reeves JT. Relation of sympathetic activation to ventilation in man at 4300 m altitude. Aviat Space Environ Med. 1997;68:104–110. [PubMed] [Google Scholar]
- Balanos GM, Talbot NP, Dorrington KL, Robbins PA. Human pulmonary vascular response to 4 h of hypercapnia and hypocapnia measured using Doppler echocardiography. J Appl Physiol. 2003;94:1543–1551. doi: 10.1152/japplphysiol.00890.2002. [DOI] [PubMed] [Google Scholar]
- Balanos GM, Talbot NP, Robbins PA, Dorrington KL. Separating the direct effect of hypoxia from the indirect effect of changes in cardiac output on the maximum pressure difference across the tricuspid valve in healthy humans. Pflugers Arch. 2005;450:372–380. doi: 10.1007/s00424-005-1422-6. [DOI] [PubMed] [Google Scholar]
- Boushel R, Calbet JA, Radegran G, Sondergaard H, Wagner PD, Saltin B. Parasympathetic neural activity accounts for the lowering of exercise heart rate at high altitude. Circulation. 2001;104:1785–1791. doi: 10.1161/hc4001.097040. [DOI] [PubMed] [Google Scholar]
- Brimioulle S, Vachiery JL, Brichant JF, Delcroix M, Lejeune P, Naeije R. Sympathetic modulation of hypoxic pulmonary vasoconstriction in intact dogs. Cardiovasc Res. 1997;34:384–392. doi: 10.1016/s0008-6363(97)00028-x. [DOI] [PubMed] [Google Scholar]
- Calbet JA. Chronic hypoxia increases blood pressure and noradrenaline spillover in healthy humans. J Physiol. 2003;551:379–386. doi: 10.1113/jphysiol.2003.045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clar C, Dorrington KL, Fatemian M, Robbins PA. Cardiovascular effects of 8 h of isocapnic hypoxia with and without β-blockade in humans. Exp Physiol. 2000;85:557–565. [PubMed] [Google Scholar]
- Clar C, Dorrington KL, Fatemian M, Robbins PA. Effects of 8 h of isocapnic hypoxia with and without muscarinic blockade on ventilation and heart rate in humans. Exp Physiol. 2001;86:529–538. doi: 10.1113/eph8602174. [DOI] [PubMed] [Google Scholar]
- Clar C, Dorrington KL, Robbins PA. Ventilatory effects of 8 hours of isocapnic hypoxia with and without β-blockade in humans. J Appl Physiol. 1999;86:1897–1904. doi: 10.1152/jappl.1999.86.6.1897. [DOI] [PubMed] [Google Scholar]
- Dorrington KL, Clar C, Young JD, Jonas M, Tansley JG, Robbins PA. Time course of the human pulmonary vascular response to 8 hours of isocapnic hypoxia. Am J Physiol Heart Circ Physiol. 1997;273:H1126–H1134. doi: 10.1152/ajpheart.1997.273.3.H1126. [DOI] [PubMed] [Google Scholar]
- Downing SE, Lee JC. Nervous control of the pulmonary circulation. Annu Rev Physiol. 1980;42:199–210. doi: 10.1146/annurev.ph.42.030180.001215. [DOI] [PubMed] [Google Scholar]
- Eyzaguirre C, Lewin J. The effect of sympathetic stimulation on carotid nerve activity. J Physiol. 1961;159:251–267. doi: 10.1113/jphysiol.1961.sp006806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahmy NR, Soter NA. Effects of trimethaphan on arterial blood histamine and systemic hemodynamics in humans. Anesthesiology. 1985;62:562–566. doi: 10.1097/00000542-198505000-00003. [DOI] [PubMed] [Google Scholar]
- Fatemian M, Robbins PA. Human ventilatory response to CO2 after 8 h of isocapnic or poikilocapnic hypoxia. J Appl Physiol. 1998;85:1922–1928. doi: 10.1152/jappl.1998.85.5.1922. [DOI] [PubMed] [Google Scholar]
- Floyd WF, Neil E. The influence of the sympathetic innervation of the carotid bifurcation on chemoceptor and baroceptor activity in the cat. Arch Int Pharmacodyn Ther. 1952;91:230–239. [PubMed] [Google Scholar]
- Gilman AG, Rall TW, Nies AS, Taylor P. Goodman and Gilman's the Pharmacological Basis of Therapeutics. Oxford: Pergamon Press; 1990. pp. 183–184. [Google Scholar]
- Hansen J, Sander M. Sympathetic neural overactivity in healthy humans after prolonged exposure to hypobaric hypoxia. J Physiol. 2003;546:921–929. doi: 10.1113/jphysiol.2002.031765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebb C. Motor innervation of the pulmonary blood vessels of mammals. In: Fishman AP, Hecht HH, editors. Pulmonary Circulation and Interstitial Space. Chicago: University of Chicago Press; 1966. pp. 195–222. [Google Scholar]
- Howard P, Barer GR, Thompson B, Warren PM, Abbott CJ, Mungall IP. Factors causing and reversing vasoconstriction in unventilated lung. Respir Physiol. 1975;24:325–345. doi: 10.1016/0034-5687(75)90022-5. [DOI] [PubMed] [Google Scholar]
- Howard LSGE, Barson RA, Howse BPA, McGill TR, McIntyre ME, O'Connor DF, Robbins PA. Chamber for controlling the end-tidal gas tensions over sustained periods in humans. J Appl Physiol. 1995;78:1088–1091. doi: 10.1152/jappl.1995.78.3.1088. [DOI] [PubMed] [Google Scholar]
- Howard LSGE, Robbins PA. Ventilatory response to 8 h of isocapnic and poikilocapnic hypoxia in humans. J Appl Physiol. 1995;78:1092–1097. doi: 10.1152/jappl.1995.78.3.1092. [DOI] [PubMed] [Google Scholar]
- Kazemi H, Bruecke PE, Parsons EF. Role of the autonomic nervous system in the hypoxic response of the pulmonary vascular bed. Respir Physiol. 1972;15:245–254. doi: 10.1016/0034-5687(72)90101-6. [DOI] [PubMed] [Google Scholar]
- Koller EA, Drechsel S, Hess T, Macherel P, Boutellier U. Effects of atropine and propranolol on the respiratory, circulatory, and ECG responses to high altitude in man. Eur J Appl Physiol Occup Physiol. 1988;57:163–172. doi: 10.1007/BF00640657. [DOI] [PubMed] [Google Scholar]
- Levitzky MG, Newell JC, Dutton RE. Effect of chemoreceptor denervation on the pulmonary vascular response to atelectasis. Respir Physiol. 1978;35:43–51. doi: 10.1016/0034-5687(78)90039-7. [DOI] [PubMed] [Google Scholar]
- Liang PJ, Bascom DA, Robbins PA. Extended models of the ventilatory response to sustained isocapnic hypoxia in humans. J Appl Physiol. 1997;82:667–677. doi: 10.1152/jappl.1997.82.2.667. [DOI] [PubMed] [Google Scholar]
- Liang P-J, Pandit JJ, Robbins PA. Statistical properties of breath-to-breath variations in ventilation at constant end-tidal PetCO2 and PetO2 in humans. J Appl Physiol. 1996;81:2274–2286. doi: 10.1152/jappl.1996.81.5.2274. [DOI] [PubMed] [Google Scholar]
- Lodato RF, Michael JR, Murray PA. Absence of neural modulation of hypoxic pulmonary vasoconstriction in conscious dogs. J Appl Physiol. 1988;65:1481–1487. doi: 10.1152/jappl.1988.65.4.1481. [DOI] [PubMed] [Google Scholar]
- McCubbin J, Page IH. Nature of the hypotensive action of a thiophanium derivative (Ro 2-2222) in dogs. J Pharmacol Exp Ther. 1952;105:437–442. [PubMed] [Google Scholar]
- Morgan BJ, Crabtree DC, Palta M, Skatrud JB. Combined hypoxia and hypercapnia evokes long-lasting sympathetic activation in humans. J Appl Physiol. 1995;79:205–213. doi: 10.1152/jappl.1995.79.1.205. [DOI] [PubMed] [Google Scholar]
- Murray PA, Lodato RF, Michael JR. Neural antagonists modulate pulmonary vascular pressure-flow plots in conscious dogs. J Appl Physiol. 1986;60:1900–1907. doi: 10.1152/jappl.1986.60.6.1900. [DOI] [PubMed] [Google Scholar]
- Nandiwada PA, Hyman AL, Kadowitz PJ. Pulmonary vasodilator responses to vagal stimulation and acetylcholine in the cat. Circ Res. 1983;53:86–95. doi: 10.1161/01.res.53.1.86. [DOI] [PubMed] [Google Scholar]
- O'Regan RG. Responses of carotid body chemosensory activity and blood flow to stimulation of sympathetic nerves in the cat. J Physiol. 1981;315:81–98. doi: 10.1113/jphysiol.1981.sp013734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen ES, Vejby-Christensen H, Nielsen TM. Effect of autonomic blockade on the transient changes in cardiac frequency on relief of hypoxia in man. Clin Sci Mol Med. 1974;47:521–530. doi: 10.1042/cs0470521. [DOI] [PubMed] [Google Scholar]
- Porcelli RJ, Bergofsky EH. Adrenergic receptors in pulmonary vasoconstrictor responses to gaseous and humoral agents. J Appl Physiol. 1973;34:483–488. doi: 10.1152/jappl.1973.34.4.483. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Kou YR. Inhibitory sympathetic action on the carotid body responses to sustained hypoxia. Respir Physiol. 1994;95:67–79. doi: 10.1016/0034-5687(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Ren X, Fatemian M, Robbins PA. Changes in respiratory control in humans induced by 8 h of hyperoxia. J Appl Physiol. 2000;89:655–662. doi: 10.1152/jappl.2000.89.2.655. [DOI] [PubMed] [Google Scholar]
- Robbins PA, Swanson GD, Howson MG. A prediction-correction scheme for forcing alveolar gases along certain time courses. J Appl Physiol. 1982;52:1353–1357. doi: 10.1152/jappl.1982.52.5.1353. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Johnson DG, Chase PB, Comess KA, Seals DR. Hypoxemia raises muscle sympathetic activity but not norepinephrine in resting humans. J Appl Physiol. 1989;66:1736–1743. doi: 10.1152/jappl.1989.66.4.1736. [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Lazar JD. Nonadrenergic effects of isoproterenol in dogs with hypoxic pulmonary vasoconstriction. Possible role of prostaglandins. J Clin Invest. 1983;71:1366–1374. doi: 10.1172/JCI110889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan ML, Hedrick MS, Pizarro J, Bisgard GE. Effects of carotid body sympathetic denervation on ventilatory acclimatization to hypoxia in the goat. Respir Physiol. 1995;99:215–224. doi: 10.1016/0034-5687(94)00096-i. [DOI] [PubMed] [Google Scholar]
- Saito M, Mano T, Iwase S, Koga K, Abe H, Yamazaki Y. Responses in muscle sympathetic activity to acute hypoxia in humans. J Appl Physiol. 1988;65:1548–1552. doi: 10.1152/jappl.1988.65.4.1548. [DOI] [PubMed] [Google Scholar]
- Severinghaus JW. Simple, accurate equations for human blood O2 dissociation computations. J Appl Physiol. 1979;46:599–602. doi: 10.1152/jappl.1979.46.3.599. [DOI] [PubMed] [Google Scholar]
- Silove ED, Grover RF. Effects of α adrenergic blockade and tissue catecholamine depletion on pulmonary vascular response to hypoxia. J Clin Invest. 1968;47:274–285. doi: 10.1172/JCI105724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tansley JG, Clar C, Pedersen MEF, Robbins PA. Human ventilatory response to acute hyperoxia during and after 8 h of both isocapnic and poikilocapnic hypoxia. J Appl Physiol. 1997;82:513–519. doi: 10.1152/jappl.1997.82.2.513. [DOI] [PubMed] [Google Scholar]
- Weisbrod CJ, Minson CT, Joyner MJ, Halliwill JR. Effects of regional phentolamine on hypoxic vasodilatation in healthy humans. J Physiol. 2001;537:613–621. doi: 10.1111/j.1469-7793.2001.00613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson LB, Levitzky MG. Chemoreflex blunting of hypoxic pulmonary vasoconstriction is vagally mediated. J Appl Physiol. 1989;66:782–791. doi: 10.1152/jappl.1989.66.2.782. [DOI] [PubMed] [Google Scholar]