Abstract
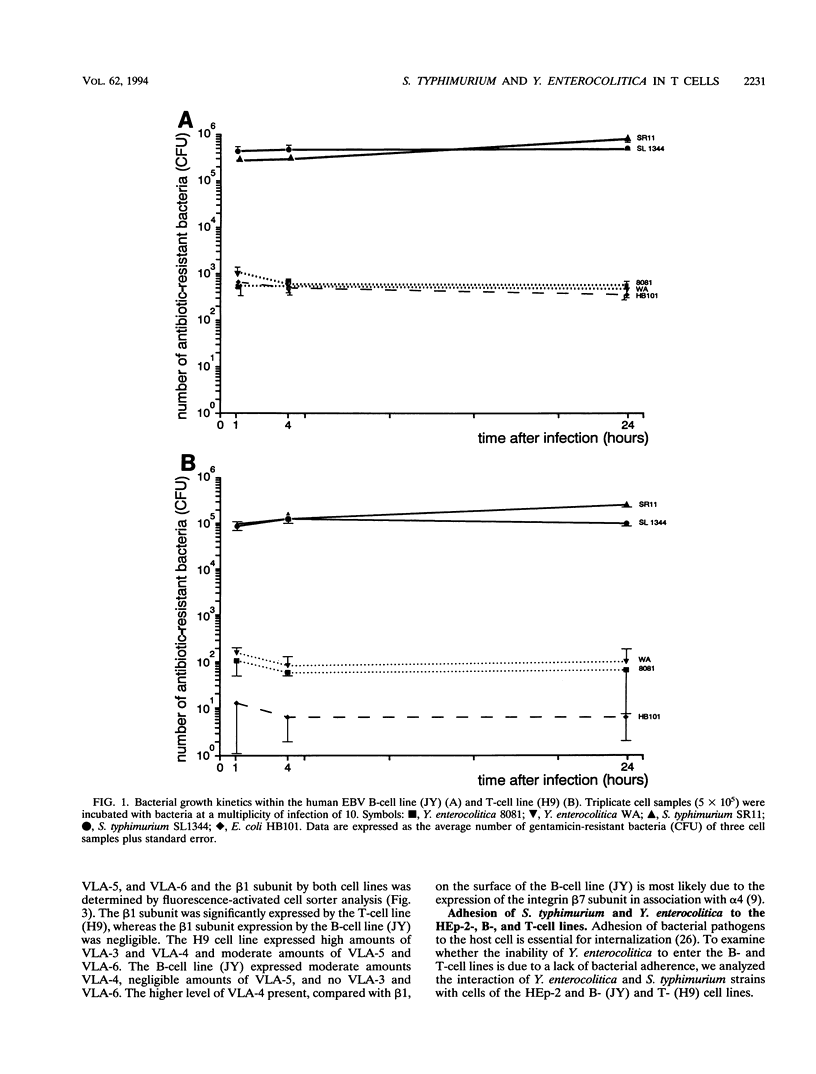
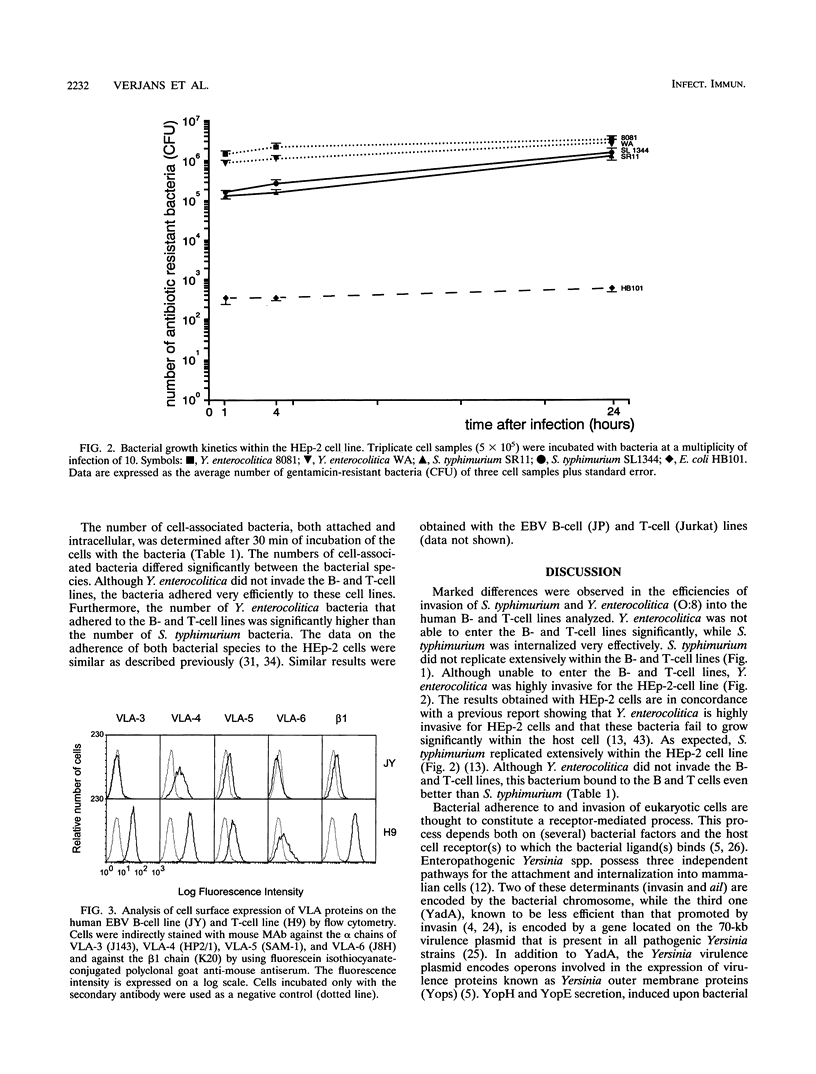
Lymphocytes, located within the Peyer's patches, might be involved in the dissemination of enteropathogenic Salmonella typhimurium and Yersinia enterocolitica bacteria. To test this hypothesis, we have investigated the susceptibility of human B- and T-cell lines to bacterial adhesion and invasion. The two S. typhimurium strains analyzed were highly invasive, while the two Y. enterocolitica (O:8) strains adhered to the B- and T-cell lines but did not enter the cell lines in significant amounts. We hypothesize that the incapability of the Y. enterocolitica (O:8) strains to enter the human B- and T-cell lines is most probably due to the bacterial inability to induce the internalization process upon adhesion to both cell lines. Although immortalized B- and T-cell lines were used in this study, the results presented suggest the possibility that both cell types could play a role in the dissemination of intracellularly residing S. typhimurium in vivo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amiot M., Bernard A., Tran H. C., Leca G., Kanellopoulos J. M., Boumsell L. The human cell surface glycoprotein complex (gp 120,200) recognized by monoclonal antibody K20 is a component binding to phytohaemagglutinin on T cells. Scand J Immunol. 1986 Jan;23(1):109–118. doi: 10.1111/j.1365-3083.1986.tb01948.x. [DOI] [PubMed] [Google Scholar]
- Asai S., Namikawa I., Ito Y. Invasion of Burkitt's lymphoma cell lines by Yersinia enterocolitica. Proc Soc Exp Biol Med. 1983 Feb;172(2):243–249. doi: 10.3181/00379727-172-41553. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J. Pedigrees of some mutant strains of Escherichia coli K-12. Bacteriol Rev. 1972 Dec;36(4):525–557. doi: 10.1128/br.36.4.525-557.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Copass M. C., Falkow S. The Yersinia pseudotuberculosis adhesin YadA mediates intimate bacterial attachment to and entry into HEp-2 cells. Infect Immun. 1993 Sep;61(9):3914–3921. doi: 10.1128/iai.61.9.3914-3921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliska J. B., Galán J. E., Falkow S. Signal transduction in the mammalian cell during bacterial attachment and entry. Cell. 1993 Jun 4;73(5):903–920. doi: 10.1016/0092-8674(93)90270-z. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Inhibition of macrophage phagosome-lysosome fusion by Salmonella typhimurium. Infect Immun. 1991 Jul;59(7):2232–2238. doi: 10.1128/iai.59.7.2232-2238.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bye W. A., Allan C. H., Trier J. S. Structure, distribution, and origin of M cells in Peyer's patches of mouse ileum. Gastroenterology. 1984 May;86(5 Pt 1):789–801. [PubMed] [Google Scholar]
- Chan B. M., Elices M. J., Murphy E., Hemler M. E. Adhesion to vascular cell adhesion molecule 1 and fibronectin. Comparison of alpha 4 beta 1 (VLA-4) and alpha 4 beta 7 on the human B cell line JY. J Biol Chem. 1992 Apr 25;267(12):8366–8370. [PubMed] [Google Scholar]
- Dunlap N. E., Benjamin W. H., Jr, McCall R. D., Jr, Tilden A. B., Briles D. E. A 'safe-site' for Salmonella typhimurium is within splenic cells during the early phase of infection in mice. Microb Pathog. 1991 Apr;10(4):297–310. doi: 10.1016/0882-4010(91)90013-z. [DOI] [PubMed] [Google Scholar]
- Ennis E., Isberg R. R., Shimizu Y. Very late antigen 4-dependent adhesion and costimulation of resting human T cells by the bacterial beta 1 integrin ligand invasin. J Exp Med. 1993 Jan 1;177(1):207–212. doi: 10.1084/jem.177.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Isberg R. R., Portnoy D. A. The interaction of bacteria with mammalian cells. Annu Rev Cell Biol. 1992;8:333–363. doi: 10.1146/annurev.cb.08.110192.002001. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Common themes in microbial pathogenicity. Microbiol Rev. 1989 Jun;53(2):210–230. doi: 10.1128/mr.53.2.210-230.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Comparison of the invasion strategies used by Salmonella cholerae-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988 Aug;70(8):1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- Finlay B. B., Falkow S. Salmonella as an intracellular parasite. Mol Microbiol. 1989 Dec;3(12):1833–1841. doi: 10.1111/j.1365-2958.1989.tb00170.x. [DOI] [PubMed] [Google Scholar]
- Francis C. L., Ryan T. A., Jones B. D., Smith S. J., Falkow S. Ruffles induced by Salmonella and other stimuli direct macropinocytosis of bacteria. Nature. 1993 Aug 12;364(6438):639–642. doi: 10.1038/364639a0. [DOI] [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Cloning and molecular characterization of genes whose products allow Salmonella typhimurium to penetrate tissue culture cells. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6383–6387. doi: 10.1073/pnas.86.16.6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Pace J., Hayman M. J. Involvement of the epidermal growth factor receptor in the invasion of cultured mammalian cells by Salmonella typhimurium. Nature. 1992 Jun 18;357(6379):588–589. doi: 10.1038/357588a0. [DOI] [PubMed] [Google Scholar]
- Heesemann J., Laufs R. Construction of a mobilizable Yersinia enterocolitica virulence plasmid. J Bacteriol. 1983 Aug;155(2):761–767. doi: 10.1128/jb.155.2.761-767.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Schwarz L. The VLA protein family. Characterization of five distinct cell surface heterodimers each with a common 130,000 molecular weight beta subunit. J Biol Chem. 1987 Mar 5;262(7):3300–3309. [PubMed] [Google Scholar]
- Hogervorst F., Kuikman I., Noteboom E., Sonnenberg A. The role of phosphorylation in activation of the alpha 6A beta 1 laminin receptor. J Biol Chem. 1993 Sep 5;268(25):18427–18430. [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hsu H. S. Pathogenesis and immunity in murine salmonellosis. Microbiol Rev. 1989 Dec;53(4):390–409. doi: 10.1128/mr.53.4.390-409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R. Determinants for thermoinducible cell binding and plasmid-encoded cellular penetration detected in the absence of the Yersinia pseudotuberculosis invasin protein. Infect Immun. 1989 Jul;57(7):1998–2005. doi: 10.1128/iai.57.7.1998-2005.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R. Discrimination between intracellular uptake and surface adhesion of bacterial pathogens. Science. 1991 May 17;252(5008):934–938. doi: 10.1126/science.1674624. [DOI] [PubMed] [Google Scholar]
- Isberg R. R., Leong J. M. Multiple beta 1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990 Mar 9;60(5):861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- Isberg R. R. Pathways for the penetration of enteroinvasive Yersinia into mammalian cells. Mol Biol Med. 1990 Feb;7(1):73–82. [PubMed] [Google Scholar]
- Ishibashi Y., Arai T. Specific inhibition of phagosome-lysosome fusion in murine macrophages mediated by Salmonella typhimurium infection. FEMS Microbiol Immunol. 1990 May;2(1):35–43. doi: 10.1111/j.1574-6968.1990.tb03476.x. [DOI] [PubMed] [Google Scholar]
- Keizer G. D., Te Velde A. A., Schwarting R., Figdor C. G., De Vries J. E. Role of p150,95 in adhesion, migration, chemotaxis and phagocytosis of human monocytes. Eur J Immunol. 1987 Sep;17(9):1317–1322. doi: 10.1002/eji.1830170915. [DOI] [PubMed] [Google Scholar]
- Kingsley G., Sieper J. Current perspectives in reactive arthritis. Immunol Today. 1993 Aug;14(8):387–391. doi: 10.1016/0167-5699(93)90139-C. [DOI] [PubMed] [Google Scholar]
- Kusters J. G., Mulders-Kremers G. A., van Doornik C. E., van der Zeijst B. A. Effects of multiplicity of infection, bacterial protein synthesis, and growth phase on adhesion to and invasion of human cell lines by Salmonella typhimurium. Infect Immun. 1993 Dec;61(12):5013–5020. doi: 10.1128/iai.61.12.5013-5020.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lian C. J., Hwang W. S., Pai C. H. Plasmid-mediated resistance to phagocytosis in Yersinia enterocolitica. Infect Immun. 1987 May;55(5):1176–1183. doi: 10.1128/iai.55.5.1176-1183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., van Putten J. P., Meyer T. F. Phase variation of the opacity outer membrane protein controls invasion by Neisseria gonorrhoeae into human epithelial cells. EMBO J. 1991 Jun;10(6):1307–1315. doi: 10.1002/j.1460-2075.1991.tb07649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Falkow S. Evidence for two genetic loci in Yersinia enterocolitica that can promote invasion of epithelial cells. Infect Immun. 1988 May;56(5):1242–1248. doi: 10.1128/iai.56.5.1242-1248.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Farmer J. J., 3rd, Hill W. E., Falkow S. The ail locus is found uniquely in Yersinia enterocolitica serotypes commonly associated with disease. Infect Immun. 1989 Jan;57(1):121–131. doi: 10.1128/iai.57.1.121-131.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller V. L., Finlay B. B., Falkow S. Factors essential for the penetration of mammalian cells by Yersinia. Curr Top Microbiol Immunol. 1988;138:15–39. [PubMed] [Google Scholar]
- Peters P. J., Neefjes J. J., Oorschot V., Ploegh H. L., Geuze H. J. Segregation of MHC class II molecules from MHC class I molecules in the Golgi complex for transport to lysosomal compartments. Nature. 1991 Feb 21;349(6311):669–676. doi: 10.1038/349669a0. [DOI] [PubMed] [Google Scholar]
- Pierson D. E., Falkow S. Nonpathogenic isolates of Yersinia enterocolitica do not contain functional inv-homologous sequences. Infect Immun. 1990 Apr;58(4):1059–1064. doi: 10.1128/iai.58.4.1059-1064.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulido R., Elices M. J., Campanero M. R., Osborn L., Schiffer S., García-Pardo A., Lobb R., Hemler M. E., Sánchez-Madrid F. Functional evidence for three distinct and independently inhibitable adhesion activities mediated by the human integrin VLA-4. Correlation with distinct alpha 4 epitopes. J Biol Chem. 1991 Jun 5;266(16):10241–10245. [PubMed] [Google Scholar]
- Small P. L., Isberg R. R., Falkow S. Comparison of the ability of enteroinvasive Escherichia coli, Salmonella typhimurium, Yersinia pseudotuberculosis, and Yersinia enterocolitica to enter and replicate within HEp-2 cells. Infect Immun. 1987 Jul;55(7):1674–1679. doi: 10.1128/iai.55.7.1674-1679.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaudaux P., Waldvogel F. A. Gentamicin antibacterial activity in the presence of human polymorphonuclear leukocytes. Antimicrob Agents Chemother. 1979 Dec;16(6):743–749. doi: 10.1128/aac.16.6.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. B., Falkow S., Schoolnik G. K. The invasin protein of Yersinia enterocolitica: internalization of invasin-bearing bacteria by eukaryotic cells is associated with reorganization of the cytoskeleton. J Cell Biol. 1992 Jan;116(1):197–207. doi: 10.1083/jcb.116.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Binnendijk R. S., van Baalen C. A., Poelen M. C., de Vries P., Boes J., Cerundolo V., Osterhaus A. D., UytdeHaag F. G. Measles virus transmembrane fusion protein synthesized de novo or presented in immunostimulating complexes is endogenously processed for HLA class I- and class II-restricted cytotoxic T cell recognition. J Exp Med. 1992 Jul 1;176(1):119–128. doi: 10.1084/jem.176.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]



