Abstract
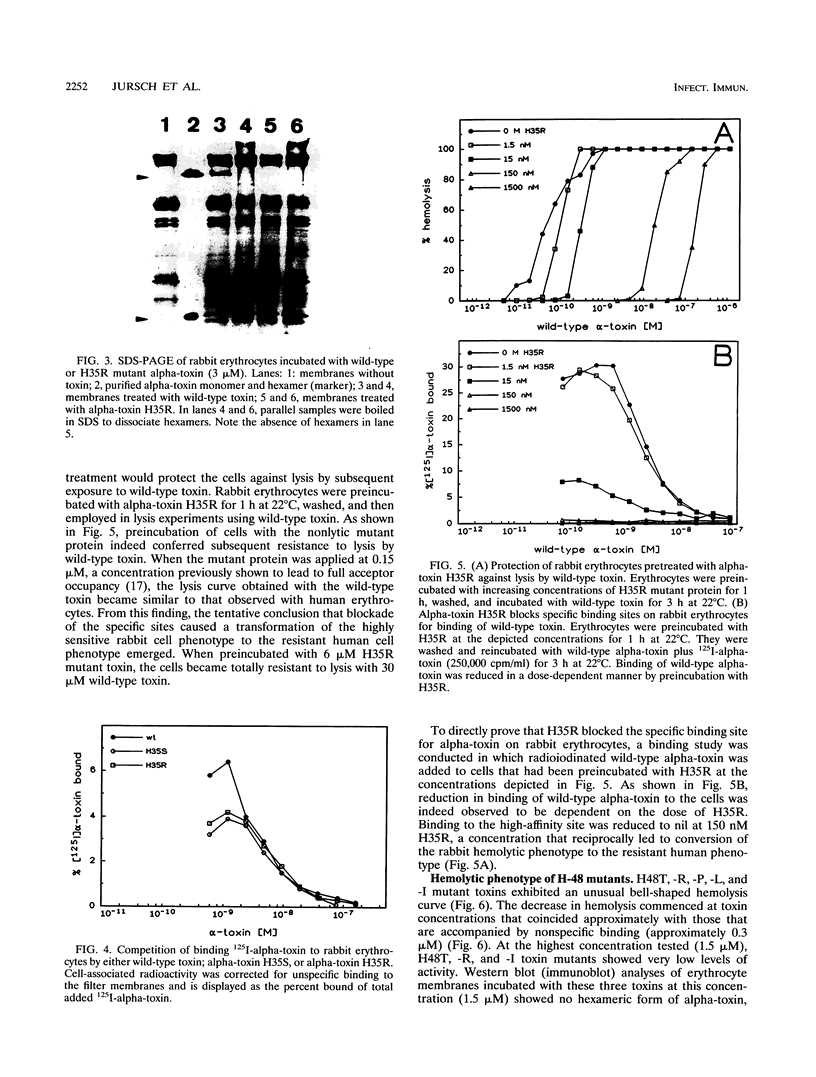
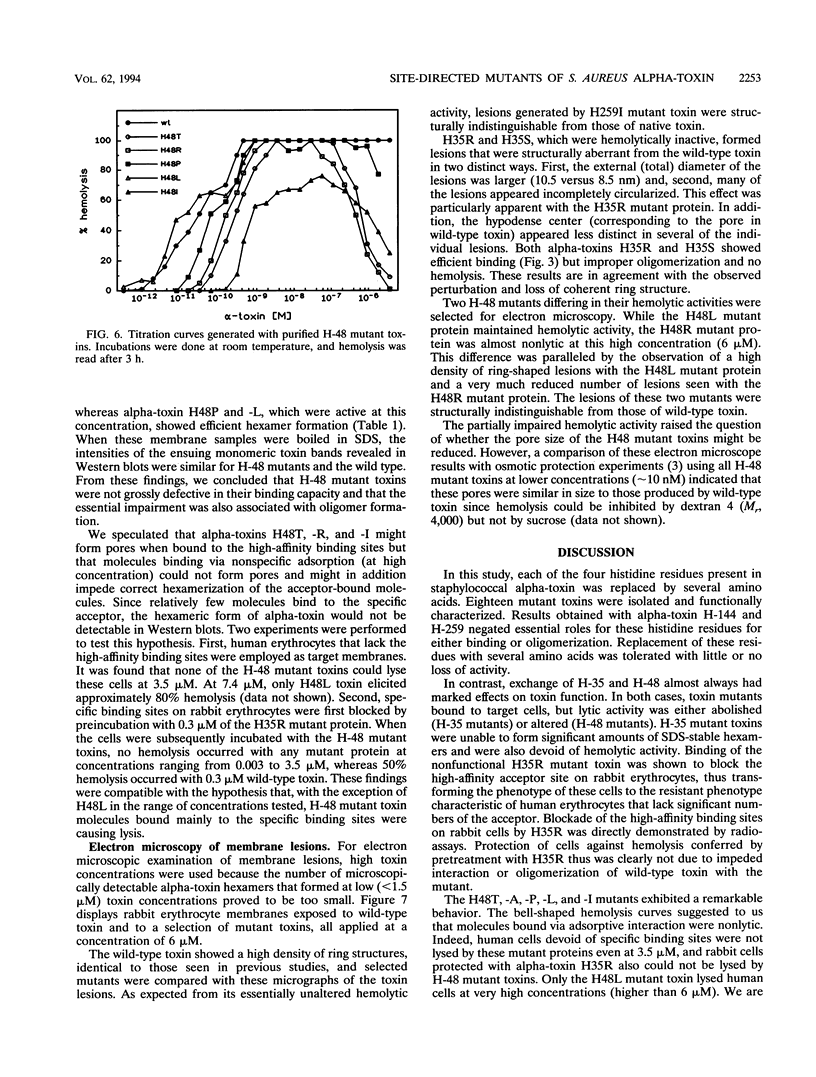
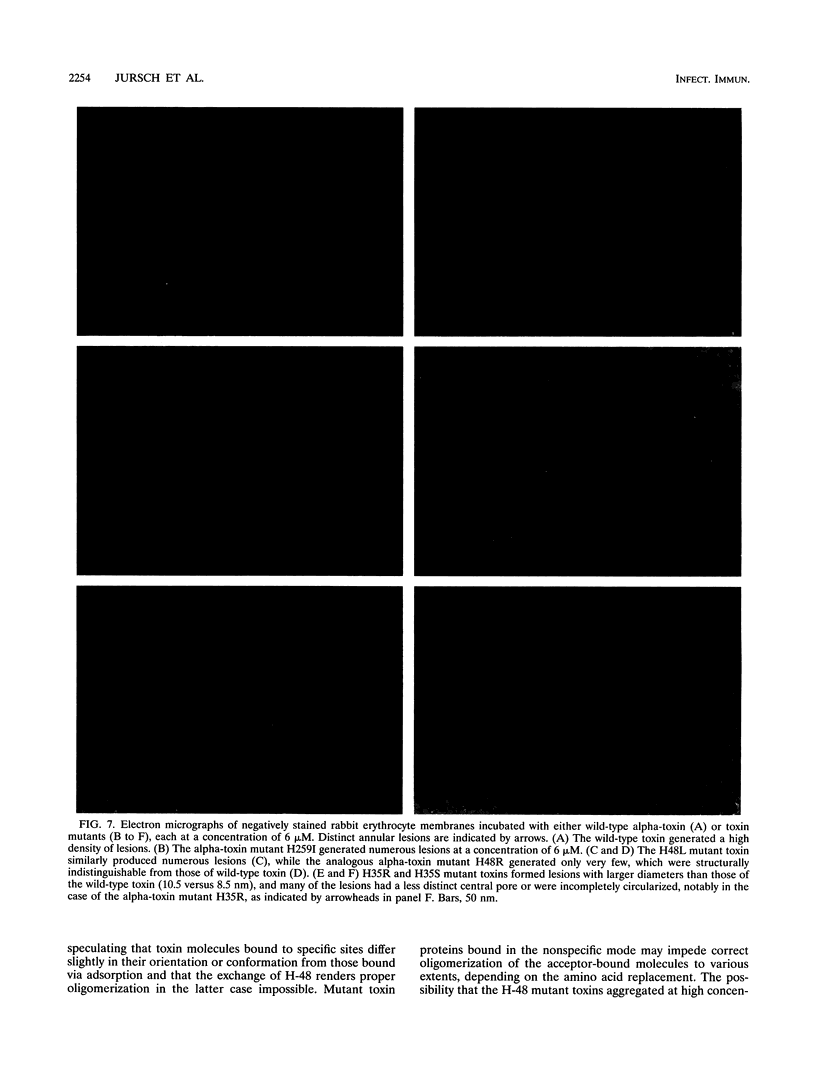
Chemical modification of histidine residues in staphylococcal alpha-toxin leads to loss of functional activity. Site-directed mutants of the toxin in which each of the four histidine residues was replaced by several amino acids were therefore produced. The mutant proteins were purified and characterized. Exchange of H-259 or H-144 was sometimes tolerated without reduction in hemolytic activity. These histidine residues are thus not essential for toxin function. Exchange of H-35 and H-48, however, had marked effects. H-35 mutant toxins bound with high affinity to rabbit erythrocytes but displayed faulty oligomerization and were unable to form pores. H-48 mutant toxins also had severely impaired hemolytic activity due probably to faulty hexamerization. We interpret these results to indicate that the N-terminal domain of alpha-toxin in the region of H-35 and H-48 is involved in protomer-protomer interactions that underlie the hexamerization and pore-forming process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Muhly M., Mannhardt U., Hugo F., Klapettek K., Mueller-Eckhardt C., Roka L. Staphylococcal alpha toxin promotes blood coagulation via attack on human platelets. J Exp Med. 1988 Aug 1;168(2):527–542. doi: 10.1084/jem.168.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991 Dec;55(4):733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S., Tranum-Jensen J. Damage to mammalian cells by proteins that form transmembrane pores. Rev Physiol Biochem Pharmacol. 1987;107:147–223. doi: 10.1007/BFb0027646. [DOI] [PubMed] [Google Scholar]
- Bhakdi S., Weller U., Walev I., Martin E., Jonas D., Palmer M. A guide to the use of pore-forming toxins for controlled permeabilization of cell membranes. Med Microbiol Immunol. 1993 Sep;182(4):167–175. doi: 10.1007/BF00219946. [DOI] [PubMed] [Google Scholar]
- Blomqvist L., Thelestam M. Oligomerization of 3H-labelled staphylococcal alpha-toxin and fragments on adrenocortical Y1 tumour cells. Microb Pathog. 1988 Mar;4(3):223–229. doi: 10.1016/0882-4010(88)90072-1. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Cassidy P., Harshman S. Studies on the binding of staphylococcal 125I-labeled alpha-toxin to rabbit erythrocytes. Biochemistry. 1976 Jun 1;15(11):2348–2355. doi: 10.1021/bi00656a016. [DOI] [PubMed] [Google Scholar]
- Chang S., Cohen S. N. High frequency transformation of Bacillus subtilis protoplasts by plasmid DNA. Mol Gen Genet. 1979 Jan 5;168(1):111–115. doi: 10.1007/BF00267940. [DOI] [PubMed] [Google Scholar]
- Fairweather N., Kennedy S., Foster T. J., Kehoe M., Dougan G. Expression of a cloned Staphylococcus aureus alpha-hemolysin determinant in Bacillus subtilis and Staphylococcus aureus. Infect Immun. 1983 Sep;41(3):1112–1117. doi: 10.1128/iai.41.3.1112-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freer J. H., Arbuthnott J. P. Toxins of Staphylococcus aureus. Pharmacol Ther. 1982;19(1):55–106. doi: 10.1016/0163-7258(82)90042-0. [DOI] [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. S., Kehoe M. Primary sequence of the alpha-toxin gene from Staphylococcus aureus wood 46. Infect Immun. 1984 Nov;46(2):615–618. doi: 10.1128/iai.46.2.615-618.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Harshman S., Boquet P., Duflot E., Alouf J. E., Montecucco C., Papini E. Staphylococcal alpha-toxin: a study of membrane penetration and pore formation. J Biol Chem. 1989 Sep 5;264(25):14978–14984. [PubMed] [Google Scholar]
- Harshman S., Sugg N., Gametchu B., Harrison R. W. Staphylococcal alpha-toxin: a structure-function study using a monoclonal antibody. Toxicon. 1986;24(4):403–411. doi: 10.1016/0041-0101(86)90200-x. [DOI] [PubMed] [Google Scholar]
- Hildebrand A., Pohl M., Bhakdi S. Staphylococcus aureus alpha-toxin. Dual mechanism of binding to target cells. J Biol Chem. 1991 Sep 15;266(26):17195–17200. [PubMed] [Google Scholar]
- Hugo F., Sinner A., Reichwein J., Bhakdi S. Quantitation of monomeric and oligomeric forms of membrane-bound staphylococcal alpha-toxin by enzyme-linked immunosorbent assay with a neutralizing monoclonal antibody. Infect Immun. 1987 Dec;55(12):2933–2939. doi: 10.1128/iai.55.12.2933-2939.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikigai H., Nakae T. Conformational alteration in alpha-toxin from Staphylococcus aureus concomitant with the transformation of the water-soluble monomer to the membrane oligomer. Biochem Biophys Res Commun. 1985 Jul 16;130(1):175–181. doi: 10.1016/0006-291x(85)90398-5. [DOI] [PubMed] [Google Scholar]
- Ikigai H., Nakae T. Interaction of the alpha-toxin of Staphylococcus aureus with the liposome membrane. J Biol Chem. 1987 Feb 15;262(5):2150–2155. [PubMed] [Google Scholar]
- Kreiswirth B. N., Löfdahl S., Betley M. J., O'Reilly M., Schlievert P. M., Bergdoll M. S., Novick R. P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983 Oct 20;305(5936):709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- Menestrina G., Belmonte G., Parisi V., Morante S. Structural features of the pore formed by Staphylococcus aureus alpha-toxin inferred from chemical modification and primary structure analysis. FEMS Microbiol Immunol. 1992 Sep;5(1-3):19–28. doi: 10.1111/j.1574-6968.1992.tb05882.x. [DOI] [PubMed] [Google Scholar]
- O'Reilly M., de Azavedo J. C., Kennedy S., Foster T. J. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb Pathog. 1986 Apr;1(2):125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- Palmer M., Weller U., Messner M., Bhakdi S. Altered pore-forming properties of proteolytically nicked staphylococcal alpha-toxin. J Biol Chem. 1993 Jun 5;268(16):11963–11967. [PubMed] [Google Scholar]
- Pederzolli C., Cescatti L., Menestrina G. Chemical modification of Staphylococcus aureus alpha-toxin by diethylpyrocarbonate: role of histidines in its membrane-damaging properties. J Membr Biol. 1991 Jan;119(1):41–52. doi: 10.1007/BF01868539. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Olofsson A., Blomqvist L., Hebert H. Oligomerisation of cell-bound staphylococcal alpha-toxin in relation to membrane permeabilisation. Biochim Biophys Acta. 1991 Feb 25;1062(2):245–254. doi: 10.1016/0005-2736(91)90399-s. [DOI] [PubMed] [Google Scholar]
- Tobkes N., Wallace B. A., Bayley H. Secondary structure and assembly mechanism of an oligomeric channel protein. Biochemistry. 1985 Apr 9;24(8):1915–1920. doi: 10.1021/bi00329a017. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranum-Jensen J. Electron microscopy: assays involving negative staining. Methods Enzymol. 1988;165:357–374. doi: 10.1016/s0076-6879(88)65053-1. [DOI] [PubMed] [Google Scholar]
- Walker B., Krishnasastry M., Bayley H. Functional complementation of staphylococcal alpha-hemolysin fragments. Overlaps, nicks, and gaps in the glycine-rich loop. J Biol Chem. 1993 Mar 5;268(7):5285–5292. [PubMed] [Google Scholar]
- Walker B., Krishnasastry M., Zorn L., Bayley H. Assembly of the oligomeric membrane pore formed by Staphylococcal alpha-hemolysin examined by truncation mutagenesis. J Biol Chem. 1992 Oct 25;267(30):21782–21786. [PubMed] [Google Scholar]
- Walker B., Krishnasastry M., Zorn L., Kasianowicz J., Bayley H. Functional expression of the alpha-hemolysin of Staphylococcus aureus in intact Escherichia coli and in cell lysates. Deletion of five C-terminal amino acids selectively impairs hemolytic activity. J Biol Chem. 1992 May 25;267(15):10902–10909. [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]