Abstract
RNA cap guanine-N2 methyltransferases such as Schizosaccharomyces pombe Tgs1 and Giardia lamblia Tgs2 catalyze methylation of the exocyclic N2 amine of 7-methylguanosine. Here we performed a mutational analysis of Giardia Tgs2, entailing an alanine scan of 17 residues within the minimal active domain. Alanine substitutions at Phe18, Thr40, Asp76, Asn103 and Asp140 reduced methyltransferase specific activity to <3% of wild-type Tgs2, thereby defining these residues as essential. Alanines at Pro142, Tyr148 and Pro185 reduced activity to 7–12% of wild-type. Structure–activity relationships at Phe18, Thr40, Asp76, Asn103, Asp140 and Tyr148, and at three other essential residues defined previously (Asp68, Glu91 and Trp143) were gleaned by testing the effects of 18 conservative substitutions. Our results engender a provisional map of the Tgs2 active site, which we discuss in light of crystal structures of related methyltransferases. A genetic analysis of S. pombe Tgs1 showed that it is nonessential. An S. pombe tgs1Δ strain grows normally, notwithstanding the absence of 2,2,7-trimethylguanosine caps on its U1, U2, U4 and U5 snRNAs. However, we find that S. pombe requires cap guanine-N7 methylation catalyzed by the enzyme Pcm1. Deletion of the pcm1+ gene was lethal, as were missense mutations in the Pcm1 active site. Thus, whereas m7G caps are essential in both S. pombe and S. cerevisiae, m2,2,7G caps are not.
INTRODUCTION
The 5′ m7GpppN cap is a distinctive feature of eukaryotic viral and cellular mRNA. Cap synthesis entails three enzymatic reactions: (i) the 5′ triphosphate end of the pre-mRNA is hydrolyzed to a diphosphate by RNA triphosphatase; (ii) the diphosphate RNA end is capped with GMP by RNA guanylyltransferase; and (iii) the GpppN cap is methylated by RNA (guanine-N7) methyltransferase (1). RNA guanylyltransferase is essential for cell growth in all organisms where its function has been tested, including: the fungi Saccharomyces cerevisiae (2–4), Schizosaccharomyces pombe (5) and Candida albicans (6); the nematode Caenorhabditis elegans (7,8); and cultured human cells (9). Cap guanine-N7 methyltransferase is essential for the viability of S. cerevisiae (10–13), but is reported to be nonessential in C. albicans (6).
A subset of capped RNAs contain one or two additional methyl groups attached to the exocyclic N2 of the cap guanosine. A 2,2,7-trimethylguanosine (TMG) cap is found on many small noncoding eukaryal RNAs such as small nuclear (sn) and small nucleolar (sno) RNAs and telomerase RNA (14,15) and on nematode mRNAs that undergo trans-splicing of a 5′-capped leader sequence (16). A 2,7-dimethylguanosine (DMG) cap is detected in the mRNAs of two RNA viruses: Sindbis virus and Semliki Forest virus (17,18). TMG synthesis has been of considerable interest to RNA biologists because of the involvement of snRNAs in pre-mRNA splicing (19–22). A breakthrough in defining the genetic pathway of TMG cap formation was made in 2002 when Remy Bordonné and colleagues identified the S. cerevisiae Tgs1 protein in an interaction screen using a yeast Sm protein as bait (23). The presence of a putative AdoMet binding motif in the Tgs1 polypeptide, mutation of which affected TMG formation in vivo (23,24), suggested that Tgs1 might be directly involved in TMG formation. Our biochemical studies of Schizosaccharomyces pombe Tgs1 showed that it is indeed the agent of TMG synthesis (25). Tgs1 catalyzes methyl transfer from AdoMet to m7GTP, m7GDP or m7GpppA, but is unreactive with GTP, GDP, GpppA, ATP, CTP, UTP or ITP. Thus, Tgs1 is a guanine-specific methyltransferase that requires prior methylation at N7 of the purine ring, indicating that TMG caps are formed by post-transcriptional methylation of standard m7G caps. We observed that the product of methyl transfer by Tgs1 to m7GDP under conditions of excess methyl acceptor is 2,7-dimethyl GDP. The initial m2,7GDP product is converted to m2,2,7GDP in the presence of excess AdoMet. We concluded that S. pombe Tgs1 acts via a distributive mechanism, and that the chemical steps of TMG synthesis do not require input from RNA or protein cofactors (25).
These findings were extended by studies of a cap guanine-N2 methyltransferase (Tgs2) from the primitive eukaryote Giardia lamblia (26). Tgs2 resembles S. pombe Tgs1 in its ability to catalyze AdoMet-dependent methylation of m7GTP, m7GDP or m7GpppA (but not GTP, GDP or GpppA). The m2,7GDP product formed by Tgs2 could be converted to m2,2,7GDP by S. pombe Tgs1. However, Giardia Tgs2 itself was unable to add a second methyl group at guanine-N2 (26).
The initial characterization of S. pombe Tgs1 and G. lamblia Tgs2 laid the foundation for a structure–function analysis aimed at mapping the active site of this interesting class of RNA processing enzymes. We are pursuing this problem using Giardia Tgs2 as a model for biochemical studies. We expect that Tgs proteins, which comprise a distinct clade within the AdoMet-dependent methyltransferase superfamily, will rely on structural motifs common to all superfamily members (e.g. for AdoMet binding), while exploiting novel structural determinants of m7G methyl acceptor specificity. Tgs-like proteins from diverse sources have similar primary structures, as illustrated in Figure 1, in which the sequence of Giardia Tgs2 is aligned to the sequence of a second Giardia paralog (Tgs1) and to the sequences of Tgs1 of S. pombe, S. cerevisiae and Homo sapiens. An AdoMet-binding site was predicted by Mouaikel et al. (24) to be composed of two conserved peptides, corresponding to 66VIDGTACVGG75 and 88VAIE91 in Tgs2. A binding site for the methyl acceptor was predicted to reside within a proline/glycine-containing peptide, corresponding to 140DPPWGGV146 in Tgs2. Bordonné and colleagues showed that Ala mutations of S. cerevisiae Tgs1 at three positions in these motifs (Asp103, Asp126 and Trp178, corresponding to Asp68, Glu91 and Trp143 in Giardia Tgs2) caused defects in TMG cap formation in vivo (23,24). To gauge the biochemical effects of such changes, we previously produced and purified Giardia Tgs2 mutants D68A, E91A and W143A. These proteins were inert in catalysis of methyl transfer from AdoMet to m7GDP (26). Based on the crystal structure of cap guanine-N7 methyltransferase bound to AdoMet and mutational analysis of that enzyme (13,27), we proposed that Asp68 and Glu91 coordinate the methionine amine and adenosine ribose hydroxyls, respectively (26).
Figure 1.
Conserved Tgs2 amino acids targeted for mutagenesis. The amino acid sequence of Giardia lamblia (Gla) Tgs2 from residues 17–190 is aligned to the sequence of its paralog GlaTgs1 and to the sequences of homologous Tgs1 polypeptides encoded by Schizosaccharomyces pombe (Spo), Saccharomyces cerevisiae (Sce) and Homo sapiens (Hsa). Gaps in the alignment are indicated by dashes. Positions targeted for alanine scanning in the present study are indicated by filled circles. Residues that were subjected previously to alanine substitution (26) are indicated by verticle lines. Essential side chains are highlighted in shaded boxes.
Here we conducted a more extensive biochemical structure–function analysis of Giardia Tgs2, including: (i) characterization of N- and C-terminal truncation mutants to delineate a minimal domain capable of methyltransferase activity in vitro and (ii) an alanine scan of conserved residues to identify individual essential side chains. The results of the alanine-scan engendered a round of conservative mutagenesis to determine structure–activity relationships at nine positions.
A major surprise accompanying the initial identification of S. cerevisiae Tgs1 was that a tgs1 deletion mutant was viable, even though the snRNAs and snoRNAs in the tgs1Δ strain lacked TMG caps (23). A pertinent question is whether other fungi can also survive without TMG caps. We addressed this question by deleting the S. pombe tgs1+ gene. It is not a foregone conclusion that dispensability of an RNA processing factor in S. cerevisiae can be extrapolated to S. pombe. For example, the pre-mRNA splicing factor Prp18 is not essential in S. cerevisiae, but is essential in S. pombe (B. Schwer, unpublished data). Also, deleting the intron lariat debranching enzyme Dbr1 is benign in S. cerevisiae, but elicits a severe growth defect in S. pombe (28). We report here that an S. pombe tgs1Δ strain grows normally, despite the absence of TMG caps on its snRNAs. However, we find that S. pombe requires cap guanine-N7 methylation, catalyzed by the enzyme Pcm1 (29), insofar as deletion of the pcm1+ gene is lethal, as are missense mutations in the active site of Pcm1.
METHODS
Tgs2 deletion mutants
N-terminal deletion mutants Tgs2-(14-258) and Tgs2-(31-258) were constructed by PCR amplification with sense-strand primers that introduced a BglII restriction site at the Met14 and Met31 codons, respectively, and an antisense-strand primer that introduced a XhoI site 3′ of the stop codon. C-terminal deletions Tgs2-(1-235) and Tgs2-(1-216) were constructed by PCR amplification with mutagenic antisense primers that introduced stop codons in place of the codons for Ala236 or Arg217 and a XhoI site 3′ of the new stop codon. The PCR products were digested with BglII and XhoI and then inserted between BamHI and XhoI sites in the plasmid pET28-His10Smt3, so as to fuse the truncated Tgs2 proteins in-frame with an amino-terminal His10Smt3 domain. The plasmid inserts were sequenced completely to exclude the acquisition of unwanted mutations during amplification and cloning.
Tgs2 missense mutants
Alanine and conservative mutations were introduced into the TGS2 gene by PCR amplification with mutagenic primers. The mutated open reading frames were inserted between the SacI and XhoI sites of a customized bacterial expression vector pET28-His10Smt3m (a derivative of pET28-His10Smt3 which has a unique SacI site downstream of the Smt3 cassette). The inserts were sequence completely to verify that no unwanted coding changes had been introduced.
Recombinant Tgs2
The pET28-His10Smt3-Tgs2 plasmids were transformed into E. coli BL21-CodonPlus(DE3). Cultures (500 ml) derived from single transformants were grown at 37°C in LB medium containing 50 µg/ml kanamycin and 50 µg/ml chloramphenicol until the A600 reached 0.6. The cultures were adjusted to 0.2 mM IPTG and 2% ethanol and incubation was continued for 20 h at 17°C. Cells were harvested by centrifugation and stored at −80°C. All subsequent procedures were performed at 4°C. Thawed bacteria were resuspended in 25 ml of buffer A (50 mM Tris-HCl, pH 8.0, 200 mM NaCl, 10% glycerol). PMSF and lysozyme were added to final concentrations of 500 µM and 100 µg/ml, respectively. After incubation on ice for 30 min, imidazole was added to a final concentration of 5 mM and the lysate was sonicated to reduce viscosity. Insoluble material was removed by centrifugation. The soluble extracts were mixed for 30 min with 1.6 ml of Ni2+-NTA-agarose (Qiagen) that had been equilibrated with buffer A containing 5 mM imidazole. The resins were recovered by centrifugation, resuspended in buffer A containing 5 mM imidazole, and poured into columns. The columns were washed with 10 ml of 20 mM imidazole in buffer A and then eluted step-wise with 2.5 ml aliquots of buffer A containing 50, 100, 250 and 500 mM imidazole. The elution profiles were monitored by SDS-PAGE. The 250 mM imidazole eluates containing the recombinant Tgs2 polypeptides were dialyzed against buffer containing 50 mM Tris-HCl, pH 8.0, 200 mM NaCl, 2 mM DTT, 1 mM EDTA, 10% glycerol and then stored at −80°C. The protein concentration was determined using the Bio-Rad dye binding method with BSA as the standard. Alternatively, the concentrations of some mutants were determined by SDS-PAGE analysis of the Tgs2 preparations in parallel with serial dilutions of a BSA standard. The gels were stained with Coomassie Blue, and the staining intensities of the Tgs2 and BSA polypeptides were quantified using a Fujifilm FLA-5000 digital imaging and analysis system. Tgs2 concentrations were calculated by interpolation to the BSA standard curve.
Methyltransferase assay
Reaction mixtures containing 50 mM Tris-HCl, pH 8.0, 5 mM DTT, 2.5 mM m7GDP, 50 µM [3H-CH3]-AdoMet, and enzyme were incubated for 15 min at 37°C. Aliquots (4 µl) were spotted on PEI-cellulose TLC plates, which were developed with 50 mM ammonium sulfate. The AdoMet- and m2,7GDP-containing portions of the lanes were cut out and the radioactivity in each was quantified by liquid scintillation counting.
Gene disruptions in S. pombe
We produced tgs1Δ and pcm1Δ gene disruption cassettes using a modified version of the long flanking homology PCR technique (30). The 5′-flanking DNA segments (360 and 380-bp upstream of the translation start codons of tgs1+ and pcm1+, respectively) were PCR-amplified from genomic DNA and inserted upstream of the kanMX gene in plasmid pFA6a-kanMX4. Then, 3′-flanking DNA segments (520 and 480-bp downstream of the stop codons for tgs1+ and pcm1+, respectively) were PCR-amplified from genomic DNA and inserted downstream of the kanMX gene. The tgs1::kanMX and pcm1::kanMX cassettes were excised by digestion with PvuII and SacII and used to transform a diploid strain of S. pombe.
The S. pombe diploid strain was generated by crossing two heterothallic strains FY527(ura4-D18 leu1-32 ade6-M216 his3-D1 h−) and FY528(ura4-D18 leu1-32 ade6-M210 his3-D1 h+) on ME plates at room temperature. After 24 h, the cells were streaked onto medium lacking adenine to select for diploids. The Ade+ diploids were verified by staining with phloxin B and a single diploid colony was picked and incubated in 100 ml of YES medium to prepare competent S. pombe cells. The transformations were performed using the lithium acetate method (31). The integrants were selected at 30°C on YES plates containing 200 µg/ml G418. Single colonies were restreaked on YES agar containing G418. Genomic DNA was prepared from individual isolates and the integration of the tgs1::kanMX or pcm1::kanMX cassettes into the correct locus was tested by PCR using diagnostic primers and confirmed by Southern blotting. The heterozygous diploids were sporulated on ME plates at room temperature. Tetrads were dissected from single asci and the spores were incubated at 30°C. All viable haploids were tested for growth on YES agar and YES agar containing 200 µg/ml G418. We found that 23 out of 23 tetrads derived from the tgs1+ tgs1::kanMX diploid yielded four viable spores, of which two were G418-sensitive and two were G418-resistant. In contrast, 14 out of 14 tetrads derived from the pcm1+ pcm1::kanMX diploid yielded two viable spores, all of which were G418-sensitive, i.e. none contained the pmc1::kanMX allele. We concluded that Tgs1 is nonessential for cell growth in S. pombe, whereas Pcm1 is essential.
RNA isolation and TMG analysis
Schizosaccharomyces pombe tgs1+ and tgs1Δ strains were grown in YES medium until A600 reached 1.0. Cells were harvested by centrifugation and washed with cold water. The washed cell pellets were stored frozen at −80°C prior to RNA isolation. Total cellular RNA was prepared using the hot-phenol method (32). TMG-containing RNA was immunoprecipitated with anti-TMG cap antibody R1131 (33; a generous gift of Dr R. Lührmann) as follows. Aliquots of the R1131 antibody (10 µg) were mixed with protein A sepharose beads [100 µl of a 50% slurry (w/v) in IPP-150 buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.1% Nonidet-P40)] and gently mixed at 4°C for 2 h. The beads were washed by 3 cycles of centrifugation and resuspened in 500 µl of cold IPP-150. Total S. pombe RNA (5 µg) was added to the bead pellet in 200 µl of IPP-150. The samples were mixed gently for 2 h at 4°C, then the supernatant was removed and the unbound RNA in the supernatant was recovered by phenol–chloroform extraction and ethanol precipitation. The bead pellet was washed three times with 500 µl cold IPP-150. The beads were then suspended in 200 µl IPP-150. The bead-bound RNA was extracted twice with phenol:chloroform and concentrated by ethanol precipitation. Aliquots of the input, bead-bound and supernatant RNAs (comprising 50% of the input RNA, 100% of the bead-bound RNA and 60% of the supernatant RNA) were analyzed by electrophoresis through a 6% polyacrylamide-urea gel. The gel contents were transferred to a Hybond membrane. Specific RNAs were detected by northern blotting, entailing sequential probing with 5′ 32P-labeled DNA oligonucleotides complementary to S. pombe U1 snRNA (5′-GCTGCAGAAACTCATGCCAGGTAAGT), U2 snRNA (5′-GAACAGATACTACACTTGATC), U4 snRNA (5′-GTTGGAGCGGTCAGGGTAATAG), U5 snRNA (5′-GATTACAAAAACTATACAGTCAAATTAGCAC) and 5.8 S RNA (5′-CTTCATCGATGCGAGAGCCAAGAGATCCGT). The hybridized probes were detected by autoradiography and quantified with a phosphorimager.
Pcm1 expression vectors
The S. cerevisiae expression plasmid pYN-Pcm1(6-389), in which S. pombe pcm1+ is under the transcriptional control of the TPI1 promoter (29) was digested with NdeI; the overhang was filled in with T4 DNA polymerase and the DNA was then digested with BamHI. The pcm1+ fragment was gel-purified and ligated into the S. pombe expression vector pREP81x (containing the selectable LEU2 gene), which had been digested with XhoI, filled in with T4 DNA polymerase, and then digested with BamHI. In the resulting pREP81x-Pcm1 plasmid, pcm1+ is under the transcriptional control of the nmt** promoter (34). The pcm1+ DNA fragment was also inserted into pDS472 (containing S. pombe ura4+ gene) to create pDS472-Pcm1, in which pcm1+ is driven by the nmt promoter (35). Missense mutations in pcm1+ were introduced via the two-stage overlap PCR method using pYN-Pcm1(6-389) as the template. The second-stage PCR products were digested with NdeI and BamHI and inserted into pYN132 to generate the S. cerevisiae expression plasmids pYN-Pcm1-D145A, pYN-Pcm1-R181A and pYN-Pcm1-Y225A. The pcm1+ inserts were sequenced completely to confirm the intended mutations and the absence of unintended coding changes. The mutated pcm1+ genes were subcloned into the S. pombe pREP81x vector as described above for the wild-type gene. The S. cerevisiae ABD1 gene was excised from p358-ABD1 and inserted into pREP81x using the same strategy.
Test of pcm1+ function in S. pombe by plasmid shuffle
The S. pombe pcm1+ pcm1::kanMX diploid strain was transformed with plasmid pDS472-Pcm1 (pcm1+ ura4+). Ura+ KanR haploids containing the pcm1::kanMX chromosomal locus and the plasmid-borne pcm1+ allele were recovered after sporulation. The pcm1Δ pDS472-Pcm1 isolates were unable to grow on agar medium containing 1 mg/ml 5-fluoroorotic acid (FOA), a drug that selects against the ura4+ plasmid. The pcm1Δ pDS472-Pcm1 strain was transformed with pREP81x (LEU2) plasmids bearing wild-type pcm1+, mutant alleles D145A, R181A and Y225A, or wild-type S. cerevisiae ABD1. A control transformation was performed with the empty pREP81x vector. Transformants were selected on minimal medium lacking leucine (36). Individual Leu+ isolates were then streaked to agar plates containing 1 mg/ml FOA. Only strains containing a plasmid encoding a biologically active cap guanine-N7 methyltransferase will give rise to FOA-resistant colonies.
RESULTS AND DISCUSSION
Mapping the Tgs2 active site by mutagenesis
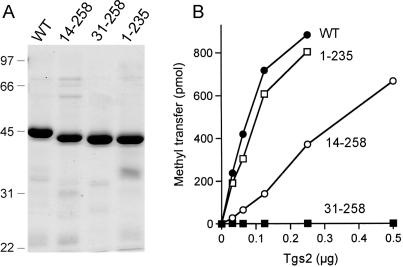
We initially sought to delineate a minimal functional domain of the 258-aa Tgs2 polypeptide by testing the effects of incremental deletions from the N- and C-termini. The full-length Tgs2 and truncated proteins Tgs2-(1-235), Tgs2-(14-258) and Tgs2(31-258) were produced in E. coli as N-terminal His10-Smt3 fusions and purified from soluble extracts by Ni-agarose chromatography (Figure 2A). Enzyme titrations in the presence of saturating substrate concentrations (2.5 mM m7GDP; 50 µM AdoMet) showed that Tgs2-(1-235) was as active as full-length Tgs2, signifying that the C-terminal 23-aa were dispensable for catalysis in vitro (Figure 2B). This result led us to construct a further C-terminal truncation, Tgs2-(1-216), but this derivative was insoluble when produced in bacteria (not shown). Whereas the first N-terminal truncation Tgs2-(14-258) retained about one-fifth of the wild-type activity, the next deletion, Tgs2(31-258) was catalytically inert (Figure 2B). We surmise that the segment between positions 15 and 30 either includes one or more critical functional groups or is needed for proper folding of the Tgs2 protein.
Figure 2.
Effects of N- and C-terminal deletions on Tgs2 activity. (A) Aliquots (4 µg) of the nickel-agarose preparations of wild-type (WT) Tgs2 and deletion mutants Tgs2-(14-258), Tgs2-(31-258), and Tgs2-(1-235) were analyzed by SDS-PAGE. Polypeptides were visualized by staining the gel with Coomassie Brilliant Blue dye. A scan of the stained gel is shown. The positions and sizes (in kDa) of marker proteins are indicated on the left. (B) Methyltransferase reaction mixtures (20 µl) containing 50 µM [3H-CH3]-AdoMet, 2.5 mM m7GDP and wild-type or mutant proteins as specified were incubated for 15 min at 37°C. The extent of methyl transfer per 20 µl reaction (containing 1000 pmol of input AdoMet) is plotted as a function of input enzyme.
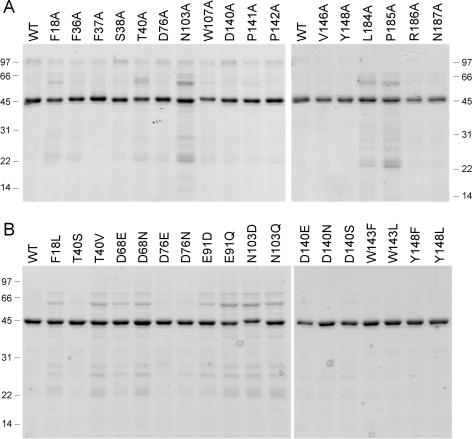
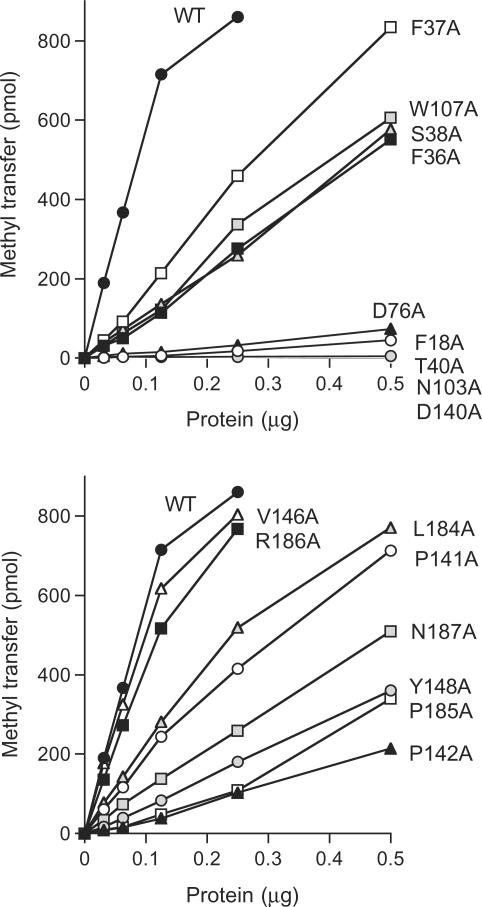
Guided by these results, we introduced single Ala mutations into the full-length Tgs2 protein at the 17 amino acids indicated by filled circles above the Tgs2 sequence in Figure 1. The targeted residues are located within the minimal domain and most are conserved among the Tgs homologs. The His10-Smt3-Tgs2-Ala proteins were produced in bacteria and purified by Ni-agarose chromatography (Figure 3A). Enzyme titrations revealed that 14 of the alanine mutants displayed detectable cap guanine-N2 methyltransferase activity and that the extent of m2,7GDP formation was proportional to input enzyme (Figure 4). Three of the mutants—T40A, N103A and D140A—had no activity at up to 0.5 µg of input protein (Figure 4). The specific activities of the Tgs2-Ala mutants were calculated from the slopes of the titration curves and normalized to the wild-type specific activity. The results are compiled in Table 1.
Figure 3.
Tgs2 missense mutants. Aliquots (2 µg) of the nickel-agarose preparations of wild-type (WT) Tgs2 and the indicated Tgs2-Ala mutants (panel A) or conservative mutants (panel B) were analyzed by SDS-PAGE. A scan of the Coomassie Blue-stained gel is shown. The positions and sizes (in kDa) of marker proteins are indicated on the left or right.
Figure 4.
Effects of alanine mutations on methyltransferase activity. Methyltransferase reaction mixtures (20 µl) containing 50 µM [3H-CH3]-AdoMet, 2.5 mM m7GDP and wild-type or mutant proteins as specified were incubated for 15 min at 37°C. The extent of methyl transfer per 20 µl reaction (containing 1000 pmol of AdoMet) is plotted as a function of input enzyme.
Table 1.
The methyltransferase-specific activities of wild-type (WT) Tgs2 and the various Tgs2 mutants were calculated from the slopes of the titration curves in Figures 4 and 5. The activities of the mutants were then normalized to the wild-type value (defined as 100%)
| Tgs2 | Methyltransferase (% of WT) |
|---|---|
| WT | 100 |
| F18A | 1.5 |
| F18L | 1.1 |
| F36A | 19 |
| F37A | 29 |
| S38A | 20 |
| T40A | <1 |
| T40S | 41 |
| T40V | <1 |
| D68A | <1 |
| D68E | 12 |
| D68N | 4.5 |
| D76A | 2.5 |
| D76E | 33 |
| D76N | 10 |
| E91A | <1 |
| E91D | 8.3 |
| E91Q | <1 |
| N103A | <1 |
| N103D | <1 |
| N103Q | <1 |
| W107A | 21 |
| D140A | <1 |
| D140E | <1 |
| D140N | <1 |
| D140S | <1 |
| P141A | 30 |
| P142A | 7.2 |
| W143A | <1 |
| W143F | 1.4 |
| W143L | <1 |
| V146A | 87 |
| Y148A | 12 |
| Y148F | 39 |
| Y148L | 4.7 |
| L184A | 37 |
| P185A | 11 |
| R186A | 73 |
| N187A | 18 |
We operationally defined an important side chain as one at which Ala substitution reduced specific activity to <15% of the wild-type value. By this criterion, we surmised that eight of the targeted amino acids are important: Phe18 (1.5% as active as wild-type when replaced by alanine); Thr40 (<1%); Asp76 (2.5%); Asn103 (<1%); Asp140 (<1%); Pro142 (7.2%); Tyr148 (12%); and Pro185 (11%). The eight important residues identified presently, and the three defined previously (Asp68, Glu91, Trp143) are highlighted in shaded boxes in Figure 1. The essentiality of Phe18 might explain the observed loss of methyltransferase activity upon deletion of the segment from aa 15–30 (Figure 2B). Nine residues did not meet the cutoff criterion for functional relevance in the alanine scan: Phe36 (19%); Phe37 (29%); Ser38 (20%); Trp107 (21%); Pro141 (30%); Val146 (87%); Leu184 (37%); Arg186 (73%); and Asn187 (18%). These nine residues were not subjected to further mutational analysis.
Structure–function relationships and mechanistic insights
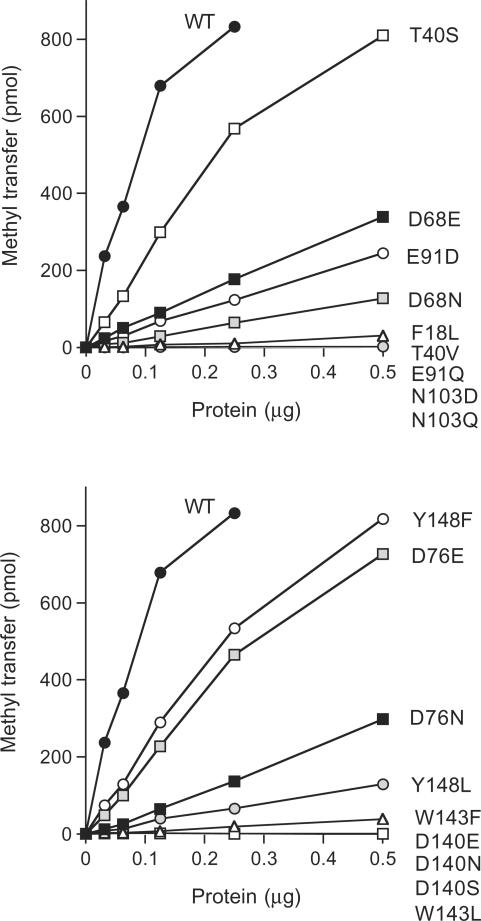
We introduced conservative substitutions at nine of the important/essential side chains (all except the two prolines). Eighteen conservative mutants were produced in bacteria and purified by Ni-agarose chromatography (Figure 3B). Their methyltransferase titration profiles are shown in Figure 5. Their specific activities were normalized to the wild-type value (Table 1). Interpretable structure–activity relationships were gleaned at every residue, as discussed below.
Figure 5.
Effects of conservative amino acid substitutions. Methyltransferase reaction mixtures (20 µl) containing 50 µM [3H-CH3]-AdoMet, 2.5 mM m7GDP and wild-type or mutant proteins as specified were incubated for 15 min at 37°C. The extent of methyl transfer per 20 µl reaction (containing 1000 pmol of AdoMet) is plotted as a function of input enzyme.
Asp68 and Asp76 are located within the Tgs2 counterpart (66VIDGTACVGGD76) of a canonical AdoMet-binding motif. Asp68 is strictly conserved in other Tgs homologs (Figure 1). We found that whereas the Tgs2 D68A mutant was inactive (<1% of wild-type), introducing a glutamate partially restored function (to 12% of wild-type), whereas asparagine was less beneficial (4.5%). Replacing Asp76 with glutamate revived activity to 33% of wild-type (compared to 2.5% for D76A), whereas asparagine was again less effective (10%). The requirement for a carboxylate at Tgs2 position 76 is notable in light of the fact that an amide functional group (Asn or Gln) is present naturally at the equivalent position in several Tgs2 homologs (Figure 1). The Tgs2 AdoMet-binding motif is similar to that of the microsporidian cap guanine-N7 methyltransferase Ecm1 (68VLDLGVGKGGD78), for which a crystal structure is available in the AdoMet-bound state (27). The structure–activity relationships for Tgs2 Asp68 and Asp76 are concordant with those seen at Ecm1 Asp70 and Asp78, i.e. Ala and Asn are defective in vitro and in vivo, while Glu restores Ecm1 activity (13). Reference to the Ecm1 structure suggests that Tgs2 Asp68 and Asp76 are likely to engage in a network of water-mediated contacts to the methionine amino group of AdoMet. The equivalent of Tgs2 Asp68 in Ecm1 coordinates two AdoMet-bridging waters (one from each carboxylate oxygen), whereas the equivalent of Tgs2 Asp76 coordinates one water.
Tgs2 Glu91 is located within a putative counterpart (VAIE91) of another AdoMet binding motif. In many other methyltransferases, the equivalent of Glu91 is an aspartate (e.g. YGVD94 in Ecm1) and, indeed, the budding yeast and human Tgs1 proteins have an aspartate at this position (Figure 1). We find that the carboxylate of Tgs2 Glu91 is essential for activity, insofar as the E91Q change (<1% activity) phenocopied E91A. Yet, the aspartate substitution resulted in only a small gain of function (to 8.3% of wild-type). Based on the Ecm1 structure (27), we predict that Tgs2 Glu91 coordinates the two adenosine ribose hydroxyls of AdoMet. In the case of Ecm1, replacing the equivalent Asp94 with Ala or Asn, abolishes activity in vitro and in vivo, while a glutamate restores activity (13). We surmise that Tgs2 requires a longer main-chain to carboxylate linker to attain this essential AdoMet contact than does Ecm1.
We find that four of the important residues of Tgs2 map to a conserved peptide (140DPPWGGVDY148) of the Tgs clade (Figure 1) that was proposed to comprise a binding site for the m7G methyl acceptor. A similar peptide (291DPPYGIRESI) is found in the S. cerevisiae Trm11 protein, which is required for the formation of a modified N2-monomethylguanosine nucleoside in yeast tRNA (37). An alanine mutation at Asp291 of yeast Trm11 virtually abolished tRNA guanosine N2-methylation activity in yeast extracts (37). We were intrigued by the prospect that the mutational analysis of this motif in Tgs2 might illuminate the distinctions between Giardia Tgs2 and S. pombe Tgs1 with respect to the ability to add one versus two methyl groups at guanine N2. We focused especially on Asp140, which is a serine in S. pombe Tgs1 and other Tgs1 homologs (Figure 1). Thus, we included a serine change (along with conservative mutations of aspartate to asparagine and glutamate), thinking that D140S might confer on Tgs2 the ability to synthesize a TMG cap in vitro. We found that every substitution for Asp140 abolished Tgs2 methyltransferase activity (Table 1), signifying that Asp140 is stringently required for catalysis by the Giardia enzyme. This finding contrasts with the previous report that TMG cap formation in vivo was unaffected when the corresponding serine of S. cerevisiae Tgs1 (Ser175) was replaced by alanine (24).
Trp143 in the 140DPPWGGVDY motif was also strictly essential for Tgs2 activity, insofar as its replacement with phenylalanine (an alternative aromatic side chain) or leucine (a bulky hydrophobic side chain) phenocopied the severe effects of the alanine change. Trp143 is a good candidate to engage in a cation-π stacking interaction with the m7G substrate. An intriguing prospect is that the tryptophan ring nitrogen might also engage in a critical hydrogen bond, which cannot be achieved by Phe or Leu. Different structure–activity relationships were apparent at the other important aromatic side chain of the 140DPPWGGVDY motif, Tyr148, whereby phenylalanine resulted in a gain of function (to 39%) over Y148A (12%) or Y148L (4.7%). Thus, the aromatic quality of this residue is the key property.
Asn103 is strictly essential for Tgs2 methyltransferase activity; the N103D and N103Q mutants were catalytically defective (<1% activity), just like N103A (Table 1). Asn103 is conserved in all the Tgs homologs (Figure 1). Although there is no atomic structure available for any member of the Tgs family, it has been proposed (24) that they might be structurally similar to a putative Methanococcus methyltransferase (Mj082) for which a crystal structure of the apoprotein has been solved (38). Reference to the Mj082 structure shows that its putative equivalent of Tgs2 Asn103 (Asn96 in Mj082) is not situated anywhere near the probable active site; rather it donates two hydrogen bonds from Asn-Nδ to the backbone carbonyl atoms of two secondary structure elements that form the predicted AdoMet binding pocket. We suspect that Asn103 plays a similar critical structural role in insuring the fold of Giardia Tgs2, thus accounting for the drastic effects of all Asn103 mutations.
A clear requirement for the hydroxyl group at essential Tgs2 residue Thr40 emerged from the conservative mutational effects, whereby valine (<1% activity) phenocopied the catastrophic effects of the alanine change, while serine restored activity to 41% of the wild-type level (Table 1). We surmise that the Thr40 hydroxyl engages in a critical hydrogen bond, but we have no basis at present to infer whether this is an interaction with substrates or other constituents of the enzyme. The most proximally located of the essential residues, Phe18, appears to function via its aromatic character, because the conservative leucine change mimicked the drastic effects of F18A (Table 1).
The TMG cap is nonessential in S. pombe
Given the ubiquity of TMG caps in eukaryal species, and the essential roles of TMG-capped RNAs in pre-mRNA splicing and other RNA transactions, it was surprising that S. cerevisiae was viable when TGS1 was deleted and the snRNAs and snoRNAs were consequently deprived of TMG caps (23). Here we conducted a genetic analysis, which showed that TMG caps are also nonessential for growth of S. pombe. We inactivated one tgs1+ locus in a diploid fission yeast strain by deleting the coding sequence and replacing it with a kanMX gene, which confers resistance to G418. Sporulation and tetrad dissection resulted in recovery of four viable haploid progeny, two of which were G418-resistant and contained the tgs1::kanMX disruption, but lacked the wild-type gene (as determined by Southern blotting; not shown). The tgs1Δ cells grew as well as the wild-type tgs1+ sisters at 30°C (not shown). Thus, the Tgs1 protein is nonessential for growth.
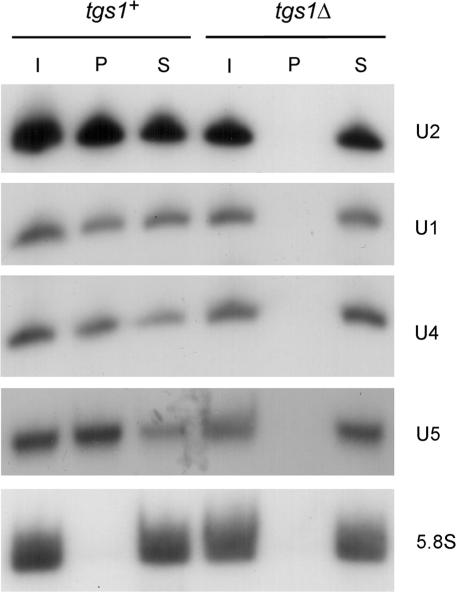
We considered the possibility that S. pombe might have an alternative means to generate TMG caps. We used the polyclonal TMG-specific antibody R1131 (33) to assay whether S. pombe snRNAs have TMG caps in the tgs1Δ strain (Figure 6). Total input cellular RNA (lane I), anti-TMG precipitated RNA (lane P) and the supernatant RNA from the immunoprecipitation (lane S) were resolved by urea-PAGE, transferred to a membrane, and analyzed by northern blotting with snRNA-specific probes. Our experiments showed that whereas U1, U2, U4 and U5 snRNAs all have TMG caps in wild-type tgs1+ S. pombe cells (i.e. at least half of the RNA was recovered in the TMG-containing fraction in lane P), these RNAs are devoid of TMG caps in the tgs1Δ strain (no RNA detected in lane P) (Figure 6). Controls showed that uncapped 5.8S RNA was recovered exclusively in the supernatant in both strains (Figure 6). We surmise that Tgs1 is the sole enzyme capable of TMG capping in S. pombe (at least as far as snRNAs are concerned) and that the TMG cap structure is not essential in fission yeast. In contrast, TMG synthesis is essential in Drosophila, i.e. mutations in the putative Tgs1 active site caused lethality at the early pupal stage correlated with depletion of TMG-containing RNAs (39).
Figure 6.
Cap guanine-N2 methylation is not essential in S. pombe. Total RNA from tgs1+ and tgs1Δ strains of S. pombe was subjected to immunoprecipitation with anti-TMG antibody bound to protein A sepharose beads. Aliquots of input RNA (I), immunoprecipitated RNA (P) and unbound RNA from the supernatant (S) were analyzed by northern blotting using probes to detect U1, U2, U4 and U5 snRNA and the uncapped 5.8S RNA as described in methods section. Autoradiograms of the blots are shown.
The m7G monomethyl cap is essential in S. pombe
The report that m7G synthesis can be dispensed with C. albicans (6) is counter to expectations regarding the dependence of translation on the cap N7 methyl group. To discern if Candida is exceptional among fungi in this regard, we queried the effects of ablating the cap guanine-N7 methyltransferase (Pcm1) of S. pombe. We inactivated one pcm1+ allele in a diploid strain (by replacing the coding sequence with a kanMX cassette) and confirmed correct gene targeting by southern blotting (not shown). Sporulation and tetrad dissection failed to yield any viable haploid progeny containing the pcm1Δ locus. Therefore, we conclude that the cap N7 methyltransferase enzyme is essential in fission yeast.
To explore whether cap methylation activity is essential (as opposed to some other property of the Pcm1 protein), we established a plasmid shuffle assay for complementation of the S. pombe pcm1Δ strain. We introduced into the pcm1+ pcm1Δ diploid a wild-type pcm1+ gene on a plasmid marked with the S. pombe ura4+ gene. This maneuver allowed us to recover uracil-prototrophic G418-resistant pcm1Δ haploids after sporulation, because the pcm1+ plasmid complements the chromosomal null mutation. Into this strain, we introduced plasmids marked with LEU2 and bearing or lacking a wild-type pcm1+ gene. Leu+ transformants were screened for growth on medium containing FOA (5-fluoroorotic acid), a drug that selects against maintenance of the ura4+ plasmid. We found that the strain containing the LEU2 pcm1+ plasmid gave rise to FOA-resistant colonies, whereas the strain containing the LEU2 vector failed to do so (Figure 7). With the plasmid complementation assay validated, we proceeded to test whether a heterologous cap guanine-N7 methyltransferase could support S. pombe growth. We found that a LEU2 plasmid bearing the ABD1 gene encoding S. cerevisiae cap methyltransferase was able to support growth under FOA selection (Figure 7). Thus, budding yeast Abd1 can function in S. pombe in lieu of Pcm1. We had shown previously that S. pombe Pcm1 could replace Abd1 in S. cerevisiae (29).
Figure 7.
Cap guanine-N7 methyltransferase is essential in S. pombe. A pcm1Δ p(ura4+ pcm1+) haploid strain of S. pombe was transformed with LEU2 plasmids bearing wild-type pcm1+, pcm1 mutants D145A, R181A and Y225A or wild-type S. cerevisiae ABD1. A control transformation was performed with the empty pREP81x vector. Leu+ transformants were tested for growth on medium containing FOA. The plates were photographed after 3 days of incubation at 30°C.
Finally, we tested three point mutations in Pcm1 for complementation of fungal growth. Alanine substitutions were introduced at Pcm1 residues Asp145, Arg181 and Tyr225, which correspond to active site constituents that are essential for cap guanine-N7 methylation by Abd1, Ecm1 and Hcm1 (13,29,40). The D145A, R181A and Y225A alleles were unable to complement the S. pombe pcm1Δ strain in the plasmid shuffle assay (Figure 7). These three Pcm1 mutants were also unable to complement growth of an S. cerevisiae abd1Δ strain in a plasmid shuffle assay (not shown). We conclude that Pcm1 and its cap guanine-N7 methyltransferase activity are essential for growth of S. pombe. Given that Tgs1 is not essential in S. pombe, we infer that a monomethyl cap is sufficient for the function of the small RNAs that normally have TMG caps in fission yeast.
ACKNOWLEDGEMENTS
This research was supported by NIH grant GM52470 (to S.S. and B.S.). A.R. is supported by NIH predoctoral training grant T32-GM008539. S.S. is an American Cancer Society Research Professor. We thank Anna Aronova for expert technical assistance and Reinhard Lührmann for the gift of anti-TMG antibody. Funding to pay the Open Access publication charge was provided by NIH grant GM52470.
Conflict of interest statement. None declared.
REFERENCES
- 1.Shuman S. The mRNA capping apparatus as drug target and guide to eukaryotic phylogeny. Cold Spring Harbor Symp. Quant. Biol. 2001;66:301–312. doi: 10.1101/sqb.2001.66.301. [DOI] [PubMed] [Google Scholar]
- 2.Shibagaki Y, Itoh N, Yamada H, Nagata S, Mizumoto K. mRNA capping enzyme: isolation and characterization of the gene encoding mRNA guanylyltransferase subunit from Saccharomyces cerevisiae. J. Biol. Chem. 1992;267:9521–9528. [PubMed] [Google Scholar]
- 3.Schwer B, Shuman S. Mutational analysis of yeast mRNA capping enzyme. Proc. Natl. Acad. Sci. U.S.A. 1994;91:4328–4332. doi: 10.1073/pnas.91.10.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fresco LD, Buratowski S. Active site of the mRNA capping enzyme guanylyltransferase from Saccharomyces cerevisiae: similarity to the nucleotidyl attachment motif of DNA and RNA ligases. Proc. Natl. Acad. Sci. U.S.A. 1994;91:6624–6628. doi: 10.1073/pnas.91.14.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pei Y, Schwer B, Saiz J, Fisher RP, Shuman S. RNA triphosphatase is essential in Schizosaccharomyces pombe and Candida albicans. BMC Microbiol. 2001;1:29. doi: 10.1186/1471-2180-1-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunyak DS, Everdeen DS, Albanese JG, Quinn CL. Deletion of individual mRNA capping genes is unexpectedly not lethal to Candida albicans and results in modified mRNA cap structures. Eukaryot. Cell. 2002;1:1010–1020. doi: 10.1128/EC.1.6.1010-1020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasan P, Piano F, Shatkin AJ. mRNA capping enzyme requirement for Caenorhabditis elegans viability. J. Biol. Chem. 2003;278:14168–14173. doi: 10.1074/jbc.M212102200. [DOI] [PubMed] [Google Scholar]
- 8.Takagi T, Walker AK, Sawa C, Diehn F, Takase Y, Blackwell TK, Buratowski S. The Caenorhabditis elegans mRNA 5′-capping enzyme: in vitro and in vivo characterization. J. Biol. Chem. 2003;278:14174–14184. doi: 10.1074/jbc.M212101200. [DOI] [PubMed] [Google Scholar]
- 9.Ping YH, Chu C, Cao H, Jacque JM, Stevenson M, Rana TM. Modulating HIV-1 replication by RNA interference directed against human transcription elongation factor SPT5. Retrovirology. 2004;1:46. doi: 10.1186/1742-4690-1-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mao X, Schwer B, Shuman S. Yeast mRNA cap methyltransferase is a 50-kilodalton protein encoded by an essential gene. Mol. Cell Biol. 1995;15:4167–4174. doi: 10.1128/mcb.15.8.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao X, Schwer B, Shuman S. Mutational analysis of the Saccharomyces cerevisiae ABD1 gene: cap methyltransferase activity is essential for cell growth. Mol. Cell Biol. 1996;16:475–480. doi: 10.1128/mcb.16.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwer B, Saha N, Mao X, Chen HW, Shuman S. Structure-function analysis of yeast mRNA cap methyltransferase and high-copy suppression of conditional mutants by AdoMet synthase and the ubiquitin conjugating enzyme Cdc34p. Genetics. 2000;155:1561–1576. doi: 10.1093/genetics/155.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng S, Hausmann S, Liu Q, Ghosh A, Schwer B, Lima CD, Shuman S. Mutational analysis of Encephalitozoon cuniculi mRNA cap (guanine-N7) methyltransferase, structure of the enzyme bound to sinefungin, and evidence that cap methyltransferase is the target of sinefungin's antifungal activity. J. Biol. Chem. 2006;281:35904–35913. doi: 10.1074/jbc.M607292200. [DOI] [PubMed] [Google Scholar]
- 14.Busch H, Reddy R, Rothblum L, Choi YC. SnRNAs, SnRNPs, and RNA processing. Annu. Rev. Biochem. 1982;51:617–654. doi: 10.1146/annurev.bi.51.070182.003153. [DOI] [PubMed] [Google Scholar]
- 15.Seto AG, Zaug AJ, Sobel SG, Wolin SL, Cech TR. Saccharomyces cerevisiae telomerase is a small nuclear ribonucleoprotein particle. Nature. 1999;401:177–180. doi: 10.1038/43694. [DOI] [PubMed] [Google Scholar]
- 16.Liou RF, Blumenthal T. trans-Spliced Caenorhabditis elegans mRNA retain trimethylguanosine caps. Mol. Cell Biol. 1990;10:1764–168. doi: 10.1128/mcb.10.4.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HsuChen CC, Dubin DT. Di- and trimethylated congeners of 7-methylguanine in Sindbis virus mRNA. Nature. 1976;264:190–191. doi: 10.1038/264190a0. [DOI] [PubMed] [Google Scholar]
- 18.Van Duijn LP, Kasperaitis M, Ameling C, Voorma HO. Additional methylation at the N(2)-position of the cap of 26S Semliki Forest virus late mRNA and initiation of translation. Virus Res. 1986;5:61–66. doi: 10.1016/0168-1702(86)90065-1. [DOI] [PubMed] [Google Scholar]
- 19.Mattaj IW. Cap trimethylation of U snRNA is cytoplasmic and dependent of U snRNP protein binding. Cell. 1986;46:905–911. doi: 10.1016/0092-8674(86)90072-3. [DOI] [PubMed] [Google Scholar]
- 20.Terns MP, Grimm C, Lund E, Dahlberg JE. A common maturation pathway for small nucleolar RNAs. EMBO J. 1995;14:4860–4871. doi: 10.1002/j.1460-2075.1995.tb00167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speckmann WA, Terns RM, Terns MP. The box C/D motif directs snoRNA 5′-cap hypermethylation. Nucleic Acids Res. 2000;28:4467–4473. doi: 10.1093/nar/28.22.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plessel G, Fischer U, Lührmann R. m3G cap hypermethylation of U1 small nuclear ribonucleoprotein (snRNP) in vitro: evidence that the U1 small nuclear RNA-(guanosine-N2)-methyltransferase is a non-snRNP protein that requires a binding site on the Sm core domain. Mol. Cell Biol. 1994;14:4160–4172. doi: 10.1128/mcb.14.6.4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mouaikel J, Verheggen C, Bertrand E, Tazi J, Bordonné R. Hypermethylation of the cap structure of both yeast snRNAs and snoRNAs requires a conserved methyltransferase that is localized to the nucleolus. Mol. Cell. 2002;9:891–901. doi: 10.1016/s1097-2765(02)00484-7. [DOI] [PubMed] [Google Scholar]
- 24.Mouaikel J, Bujnicki JM, Tazi J, Bordonné R. Sequence-structure-function relationships of Tgs1, the yeast snRNA/snoRNA cap hypermethylase. Nucleic Acids Res. 2003;31:4899–4909. doi: 10.1093/nar/gkg656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hausmann S, Shuman S. Specificity and mechanism of RNA cap guanine-N2 methyltransferase (Tgs1) J. Biol. Chem. 2005;280:4021–4024. doi: 10.1074/jbc.C400554200. [DOI] [PubMed] [Google Scholar]
- 26.Hausmann S, Shuman S. Giardia lamblia RNA cap guanine-N2 methyltransferase (Tgs2) J. Biol. Chem. 2005;280:32101–32106. doi: 10.1074/jbc.M506438200. [DOI] [PubMed] [Google Scholar]
- 27.Fabrega C, Hausmann S, Shen V, Shuman S, Lima CD. Structure and mechanism of cap (guanine-N7) methyltransferase. Mol. Cell. 2004;13:77–89. doi: 10.1016/s1097-2765(03)00522-7. [DOI] [PubMed] [Google Scholar]
- 28.Nam K, Lee G, Trambley J, Devine SE, Boeke JD. Severe growth defect in a Schizosaccharomyces pombe mutant defective in intron lariat degradation. Mol. Cell Biol. 1997;17:809–818. doi: 10.1128/mcb.17.2.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saha N, Schwer B, Shuman S. Characterization of human, Schizosaccharomyces pombe and Candida albicans mRNA cap methyltransferases and complete replacement of the yeast capping apparatus by mammalian enzymes. J. Biol. Chem. 1999;274:16553–16562. doi: 10.1074/jbc.274.23.16553. [DOI] [PubMed] [Google Scholar]
- 30.Wach A. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast. 1996;12:259–265. doi: 10.1002/(SICI)1097-0061(19960315)12:3%3C259::AID-YEA901%3E3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 31.Gietz D, St Jean A, Woods RA, Schiestl RH. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrick D, Parker R, Jacobsen A. Identification and comparison of stable and unstable mRNAs in Saccharomyces cerevisiae. Mol. Cell Biol. 1990;10:2269–2284. doi: 10.1128/mcb.10.5.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemm I, Girard C, Kuhn AN, Watkins NJ, Schneider M, Bordonné R, Lührmann R. Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies. Mol. Biol. Cell. 2006;17:3221–3231. doi: 10.1091/mbc.E06-03-0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forsburg SL. Comparison of different Schizosaccharomyces pombe expression systems. Nucleic Acids Res. 1993;21:2955–2956. doi: 10.1093/nar/21.12.2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forsburg SL, Sherman DA. General purpose tagging vectors for fission yeast. Gene. 1997;191:191–195. doi: 10.1016/s0378-1119(97)00058-9. [DOI] [PubMed] [Google Scholar]
- 36.Moreno S, Klar A, Nurse P. Molecular genetic analysis of the fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 37.Purushothaaman SK, Bujnicki JM, Grosjean H, Lapeyre B. Trm11p and Trm112p are both required for the formation of 2-methylguanosine at position 10 in yeast tRNA. Mol. Cell Biol. 2005;25:4359–4370. doi: 10.1128/MCB.25.11.4359-4370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang L, Hung L, Odell M, Yokota H, Kim R, Kim SH. Structure-based experimental confirmation of biochemical function to a methyltransferase, MJ0882, from hyperthermophile Methanococcus jannaschii. J. Struct. Funct. Genomics. 2002;2:121–127. doi: 10.1023/a:1021279113558. [DOI] [PubMed] [Google Scholar]
- 39.Komonyi O, Papai G, Enunlu I, Muratoglu S, Pankotai T, Kopitova D, Maróy P, Udvarty A, Boros I. DTL, the Drosophila homolog of PIMT/Tgs1 nuclear receptor coactivator-interacting protein/RNA methyltransferase, has an essential role in development. J. Biol. Chem. 2005;280:12397–12404. doi: 10.1074/jbc.M409251200. [DOI] [PubMed] [Google Scholar]
- 40.Wang SP, Shuman S. Structure-function analysis of the mRNA cap methyltransferase of Saccharomyces cerevisiae. J. Biol. Chem. 1997;272:14683–14689. doi: 10.1074/jbc.272.23.14683. [DOI] [PubMed] [Google Scholar]