Abstract
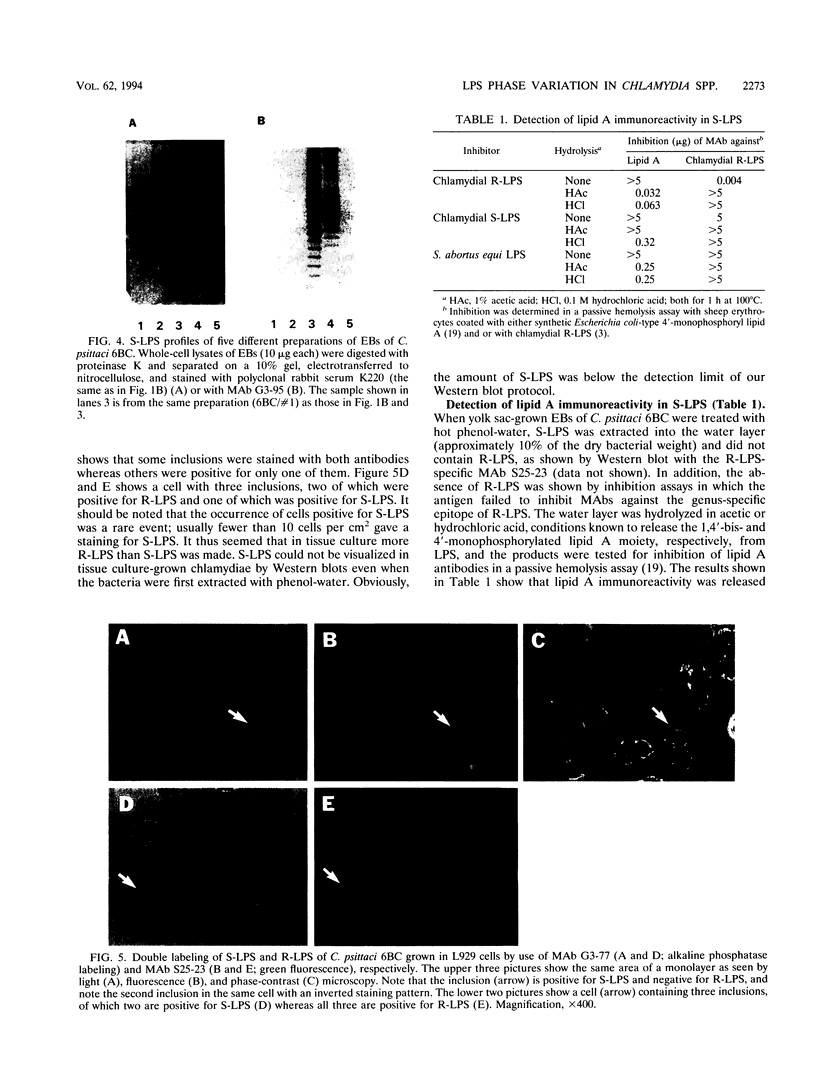
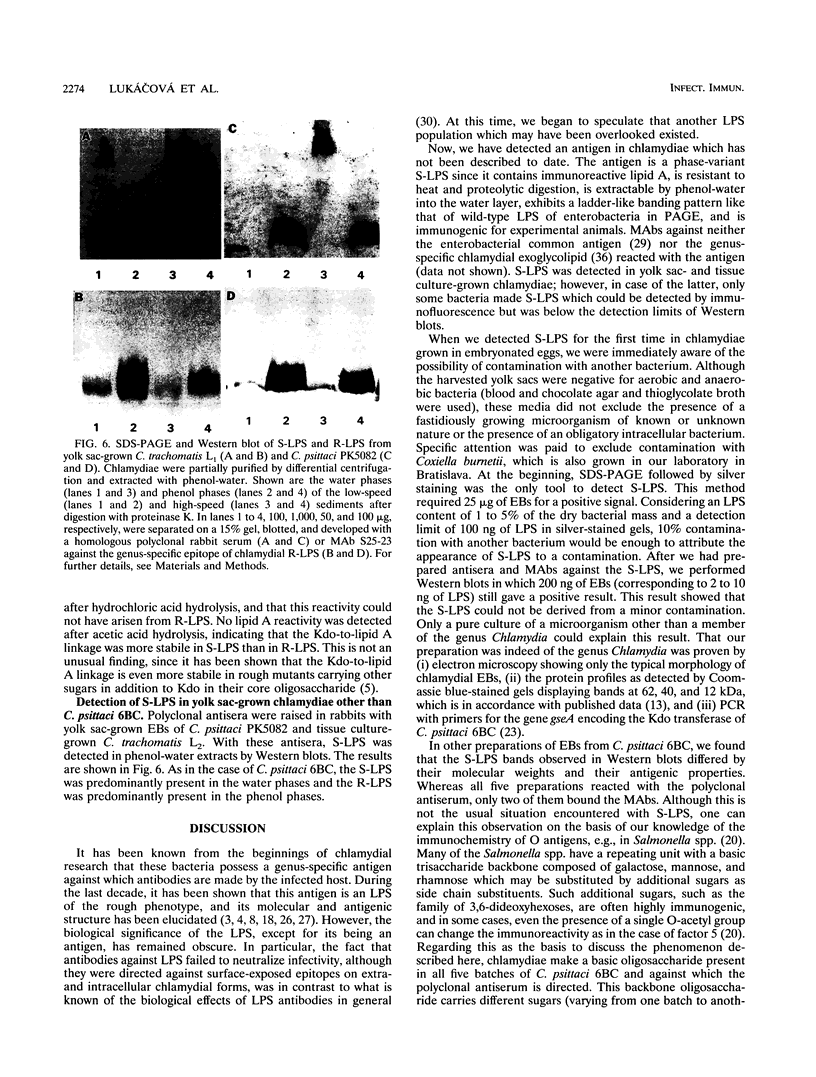
In two strains of Chlamydia psittaci and in Chlamydia trachomatis serotype L1, we have detected a so-far-unknown antigen which (i) is resistant to heat and proteolytic digestion, (ii) can be extracted with phenol-water into the water phase, (iii) gives a ladder-like banding pattern in sodium dodecyl sulfate-polyacrylamide gel electrophoresis, (iv) is immunogenic in rabbits and mice, and (v) contains immunoreactivity of lipid A, a common and characteristic component of gram-negative lipopolysaccharides (LPS). Thus, chlamydiae contain, in addition to the known rough-type LPS, another LPS type which is phenotypically smooth (S-LPS). S-LPS was observed preferentially in chlamydiae grown in the yolk sac of embryonated eggs; it was, however, also detected by immunofluorescence in tissue culture-grown chlamydiae with a monoclonal antibody against S-LPS.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bastin D. A., Romana L. K., Reeves P. R. Molecular cloning and expression in Escherichia coli K-12 of the rfb gene cluster determining the O antigen of an E. coli O111 strain. Mol Microbiol. 1991 Sep;5(9):2223–2231. doi: 10.1111/j.1365-2958.1991.tb02152.x. [DOI] [PubMed] [Google Scholar]
- Baumann M., Brade L., Fasske E., Brade H. Staining of surface antigens of Chlamydia trachomatis L2 in tissue culture. Infect Immun. 1992 Oct;60(10):4433–4438. doi: 10.1128/iai.60.10.4433-4438.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belunis C. J., Mdluli K. E., Raetz C. R., Nano F. E. A novel 3-deoxy-D-manno-octulosonic acid transferase from Chlamydia trachomatis required for expression of the genus-specific epitope. J Biol Chem. 1992 Sep 15;267(26):18702–18707. [PubMed] [Google Scholar]
- Brade H., Brade L., Nano F. E. Chemical and serological investigations on the genus-specific lipopolysaccharide epitope of Chlamydia. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2508–2512. doi: 10.1073/pnas.84.8.2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brade H., Galanos C., Lüderitz O. Differential determination of the 3-Deoxy-D-mannooctulosonic acid residues in lipopolysaccharides of Salmonella minnesota rough mutants. Eur J Biochem. 1983 Mar 1;131(1):195–200. doi: 10.1111/j.1432-1033.1983.tb07249.x. [DOI] [PubMed] [Google Scholar]
- Brade L., Schramek S., Schade U., Brade H. Chemical, biological, and immunochemical properties of the Chlamydia psittaci lipopolysaccharide. Infect Immun. 1986 Nov;54(2):568–574. doi: 10.1128/iai.54.2.568-574.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. R., Whitfield C. Molecular cloning of the rfb region of Klebsiella pneumoniae serotype O1:K20: the rfb gene cluster is responsible for synthesis of the D-galactan I O polysaccharide. J Bacteriol. 1992 Jul;174(14):4614–4621. doi: 10.1128/jb.174.14.4614-4621.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Baumann M., Kosma P., Brade L., Brade H. A synthetic glycoconjugate representing the genus-specific epitope of chlamydial lipopolysaccharide exhibits the same specificity as its natural counterpart. Infect Immun. 1992 Apr;60(4):1314–1321. doi: 10.1128/iai.60.4.1314-1321.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Hatano K., Meluleni G. S., Pier G. B. Cloning and surface expression of Pseudomonas aeruginosa O antigen in Escherichia coli. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10716–10720. doi: 10.1073/pnas.89.22.10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiss J. M., Schneider H., Mandrell R. E., Yamasaki R., Jarvis G. A., Kim J. J., Gibson B. W., Hamadeh R., Apicella M. A. Lipooligosaccharides: the principal glycolipids of the neisserial outer membrane. Rev Infect Dis. 1988 Jul-Aug;10 (Suppl 2):S287–S295. doi: 10.1093/cid/10.supplement_2.s287. [DOI] [PubMed] [Google Scholar]
- Gupta D. S., Shashkov A. S., Jann B., Jann K. Structures of the O1B and O1C lipopolysaccharide antigens of Escherichia coli. J Bacteriol. 1992 Dec;174(24):7963–7970. doi: 10.1128/jb.174.24.7963-7970.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi G. E., Zähringer U., Jann B., Jann K., Hull R. A., Hull S. I. Genetic characterization of the O4 polysaccharide gene cluster from Escherichia coli. Microb Pathog. 1991 May;10(5):351–361. doi: 10.1016/0882-4010(91)90080-t. [DOI] [PubMed] [Google Scholar]
- Hatch T. P., Allan I., Pearce J. H. Structural and polypeptide differences between envelopes of infective and reproductive life cycle forms of Chlamydia spp. J Bacteriol. 1984 Jan;157(1):13–20. doi: 10.1128/jb.157.1.13-20.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J. Analyses of gonococcal lipopolysaccharide in whole-cell lysates by sodium dodecyl sulfate-polyacrylamide gel electrophoresis: stable association of lipopolysaccharide with the major outer membrane protein (protein I) of Neisseria gonorrhoeae. Infect Immun. 1984 Oct;46(1):202–212. doi: 10.1128/iai.46.1.202-212.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst O., Broer W., Thomas-Oates J. E., Mamat U., Brade H. Structural analysis of two oligosaccharide bisphosphates isolated from the lipopolysaccharide of a recombinant strain of Escherichia coli F515 (Re chemotype) expressing the genus-specific epitope of Chlamydia lipopolysaccharide. Eur J Biochem. 1993 Jun 15;214(3):703–710. doi: 10.1111/j.1432-1033.1993.tb17971.x. [DOI] [PubMed] [Google Scholar]
- Kosma P., Bahnmüller R., Schulz G., Brade H. Synthesis of a tetrasaccharide of the genus-specific lipopolysaccharide epitope of Chlamydia. Carbohydr Res. 1990 Dec 15;208:37–50. doi: 10.1016/0008-6215(90)80083-f. [DOI] [PubMed] [Google Scholar]
- Kuhn H. M., Brade L., Appelmelk B. J., Kusumoto S., Rietschel E. T., Brade H. Characterization of the epitope specificity of murine monoclonal antibodies directed against lipid A. Infect Immun. 1992 Jun;60(6):2201–2210. doi: 10.1128/iai.60.6.2201-2210.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüderitz O., Staub A. M., Westphal O. Immunochemistry of O and R antigens of Salmonella and related Enterobacteriaceae. Bacteriol Rev. 1966 Mar;30(1):192–255. doi: 10.1128/br.30.1.192-255.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson D. F., Morona R., Beger D. W., Cheah K. C., Manning P. A. Genetic analysis of the rfb region of Shigella flexneri encoding the Y serotype O-antigen specificity. Mol Microbiol. 1991 Jun;5(6):1491–1499. doi: 10.1111/j.1365-2958.1991.tb00795.x. [DOI] [PubMed] [Google Scholar]
- Mamat U., Baumann M., Schmidt G., Brade H. The genus-specific lipopolysaccharide epitope of Chlamydia is assembled in C. psittaci and C. trachomatis by glycosyltransferases of low homology. Mol Microbiol. 1993 Dec;10(5):935–941. doi: 10.1111/j.1365-2958.1993.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Mandrell R. E., Lesse A. J., Sugai J. V., Shero M., Griffiss J. M., Cole J. A., Parsons N. J., Smith H., Morse S. A., Apicella M. A. In vitro and in vivo modification of Neisseria gonorrhoeae lipooligosaccharide epitope structure by sialylation. J Exp Med. 1990 May 1;171(5):1649–1664. doi: 10.1084/jem.171.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino P. A., McGrath B. C., Osborn M. J. Energy dependence of O-antigen synthesis in Salmonella typhimurium. J Bacteriol. 1991 May;173(10):3128–3133. doi: 10.1128/jb.173.10.3128-3133.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nano F. E., Caldwell H. D. Expression of the chlamydial genus-specific lipopolysaccharide epitope in Escherichia coli. Science. 1985 May 10;228(4700):742–744. doi: 10.1126/science.2581315. [DOI] [PubMed] [Google Scholar]
- Nurminen M., Leinonen M., Saikku P., Mäkelä P. H. The genus-specific antigen of Chlamydia: resemblance to the lipopolysaccharide of enteric bacteria. Science. 1983 Jun 17;220(4603):1279–1281. doi: 10.1126/science.6344216. [DOI] [PubMed] [Google Scholar]
- Parsons N. J., Andrade J. R., Patel P. V., Cole J. A., Smith H. Sialylation of lipopolysaccharide and loss of absorption of bactericidal antibody during conversion of gonococci to serum resistance by cytidine 5'-monophospho-N-acetyl neuraminic acid. Microb Pathog. 1989 Jul;7(1):63–72. doi: 10.1016/0882-4010(89)90112-5. [DOI] [PubMed] [Google Scholar]
- Peters H., Jürs M., Jann B., Jann K., Timmis K. N., Bitter-Suermann D. Monoclonal antibodies to enterobacterial common antigen and to Escherichia coli lipopolysaccharide outer core: demonstration of an antigenic determinant shared by enterobacterial common antigen and E. coli K5 capsular polysaccharide. Infect Immun. 1985 Nov;50(2):459–466. doi: 10.1128/iai.50.2.459-466.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rietschel E. T., Brade H. Bacterial endotoxins. Sci Am. 1992 Aug;267(2):54–61. doi: 10.1038/scientificamerican0892-54. [DOI] [PubMed] [Google Scholar]
- Schachter J. The intracellular life of Chlamydia. Curr Top Microbiol Immunol. 1988;138:109–139. [PubMed] [Google Scholar]
- Stroeher U. H., Karageorgos L. E., Morona R., Manning P. A. Serotype conversion in Vibrio cholerae O1. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2566–2570. doi: 10.1073/pnas.89.7.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart E. S., Tirrell S. M., MacDonald A. B. Characterization of an antigen secreted by Chlamydia-infected cell culture. Immunology. 1987 Aug;61(4):527–533. [PMC free article] [PubMed] [Google Scholar]
- Stähli C., Staehelin T., Miggiano V. Spleen cell analysis and optimal immunization for high-frequency production of specific hybridomas. Methods Enzymol. 1983;92:26–36. doi: 10.1016/0076-6879(83)92006-2. [DOI] [PubMed] [Google Scholar]
- Sádecký E., Trávnicek M., Balascák J., Brezina R., Kazár J., Urvölgyi J., Flesár I., Cizmár J. Enzootický potrat oviec v okrese Roznava. Vet Med (Praha) 1978 Jan;23(1):25–28. [PubMed] [Google Scholar]
- Whitfield C., Perry M. B., MacLean L. L., Yu S. H. Structural analysis of the O-antigen side chain polysaccharides in the lipopolysaccharides of Klebsiella serotypes O2(2a), O2(2a,2b), and O2(2a,2c). J Bacteriol. 1992 Aug;174(15):4913–4919. doi: 10.1128/jb.174.15.4913-4919.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]