Abstract
BACKGROUND
The regulation of the prostate size by androgens may be partly the result of androgen effects on the prostatic vasculature. We examined the effect of changes in androgen levels on the expression of a variety of angiogenic factors in the mouse prostate and determined if vascular endothelial growth factor (VEGF)-A and the angiopoietins are involved in the vascular response to androgens.
METHODS
Expression of angiogenic factors in prostate was quantitated using real-time PCR at different times after castration and after administration of testosterone to castrated mice. Angiopoietins were localized in prostate by immunohistochemistry and in situ hybridization. The roles of VEGF and the angiopoietins in regeneration of the prostate were examined in mice inoculated with cells expressing soluble VEGF receptor-2 or soluble Tie-2.
RESULTS
Castration resulted in a decrease in VEGF-A, VEGF-B, VEGF-C, placenta growth factor, FGF-2, and FGF-8 expression after one day. In contrast, VEGF-D mRNA levels increased. No changes in angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), hepatocyte growth factor, VEGF receptor-1, VEGF receptor-2 or tie-2 mRNA levels were observed. Administration of testosterone to castrated mice had the opposite effect on expression of these angiogenic factors. Ang-2 was expressed predominately in prostate epithelial cells whereas Ang-1 was expressed in epithelium and smooth muscle. Inoculation of mice with cells expressing soluble VEGF receptor-2 or Tie-2 blocked the increase in vascular density normally observed after administration of testosterone to castrated mice. The soluble receptors also blocked the increase in prostate weight and proliferation of prostatic epithelial cells.
CONCLUSION
VEGF-A and angiopoietins are required for the vascular response to androgens and for the ability of the prostate to regenerate in response to androgens.
Keywords: testosterone, vascular endothelial growth factor, angiopoietin, prostate
INTRODUCTION
The rodent prostate involutes rapidly after castration, with apoptosis of prostate epithelial cells observable within 48 h after castration (1,2). Similarly, the prostate regenerates rapidly after administration of testosterone to castrated animals with major increases in growth of the epithelial compartment occurring during the first three days (3). In addition to the prostate epithelial cells, the prostate vasculature seems particularly sensitive to androgen status. After castration, apoptosis of the blood vessel endothelial cells precedes the apoptosis of the prostate epithelium (4,5). On administration of testosterone, the prostatic vasculature is one of the first compartments to respond, and proliferation of endothelial cells can be observed within 24 h (6,7). Thus, the vasculature appears to be a primary target of androgen action in the prostate.
Although the vasculature is a primary target of androgens, the vascular endothelium appears not to be a direct target as androgen receptors have not been detected in prostatic endothelial cells (8). The vascular response to androgens may be mediated by factors that stimulate vascular growth (angiogenic factors) that are produced by the epithelial cells or other stromal cells in an androgen-dependent manner. The endothelial cell-specific growth factor, vascular endothelial growth factor (VEGF-A), has been shown to be regulated by androgens in prostate cells both in vitro and in vivo (9-15). VEGF-A is a particularly attractive candidate as the mediator of androgen action as the vasculature is exquisitely sensitive to changes in VEGF-A levels. Even a 50% reduction in VEGF-A expression in embryos disrupts vascular development (16,17). Furthermore, administration of exogenous VEGF-A can induce the growth of some mature vascular beds (18).
Although VEGF-A is a leading candidate for the mediator of androgen action, other angiogenic factors may modify its effects. Placenta growth factor (PlGF), VEGF-B, VEGF-C, and VEGF-D are additional members of the VEGF family (19-22) that also bind to VEGF receptor-1 (VEGFR-1)/flt-1 and VEGF receptor-2 (VEGFR-2)/flk-1, the same receptors that bind VEGF-A (23). These molecules may induce angiogenesis on their own or may modify responses to VEGF-A (23). The effect of changes in androgen status on the expression of the VEGF family members in prostate has not been reported. Fibroblast growth factor (FGF) -2 was originally purified as an angiogenic factor and is strongly angiogenic when inoculated in vivo (24). Hepatocyte growth factor (HGF) also induces angiogenesis in model systems (25). FGF-2 and HGF both are expressed in prostate and are reported to be affected by castration (26). The potential roles of these molecules in modifying the vascular response to androgens have also received little attention.
Finally, the angiopoietins constitute a family of vascular regulatory molecules that interact with a receptor, Tie-2, that is generally restricted to endothelial cells (27,28). The receptor agonist, angiopoietin-1 (Ang-1), can induce angiogenesis under some conditions (29,30). However, Ang-1 is not required for angiogenesis as a primitive vasculature develops in Ang-1- or Tie-2-deficient embryos. However, in the absence of Ang-1, the embryonic vasculature fails to remodel and form mature, stable vessels (31,32). Angiopoietin-2 (Ang-2) acts as a functional antagonist of the Ang-1/Tie2 interaction and is expressed primarily in areas undergoing vascular remodeling (28). Ang-2 can cause either vessel regression (in the absence of VEGF-A) or enhanced vessel sprouting (in the presence of VEGF-A) (33,34). Thus, angiopoietins may also modify the response to VEGF-A. Angiopoietins are expressed in mouse prostate, but there are conflicting claims about the response of Ang-1 to androgens (35,36).
Here we have examined the dynamic changes in expression of the major angiogenic molecules in vivo when mice were castrated, as well as changes in expression upon testosterone replacement to clarify the potential roles of these angiogenic factors in the response of the prostatic vasculature to androgens. As expression of Ang-2 was found to be high in normal, resting prostate in contrast to what has been reported for other tissues, we also examined the source of Ang-1 and Ang-2 in the mouse prostate. Finally, we have also determined whether the endothelial-specific ligands of the VEGF and angiopoietin families are required for the regeneration of the prostate in response to testosterone. An understanding of the changes in these angiogenic regulators brought about by alterations in testosterone levels will expand our knowledge of the regulation of vascular growth in normal prostate and in prostate tumors.
MATERIALS AND METHODS
Animals
Two-month-old Balb/C male mice were purchased from Taconic Laboratories, and male nude mice were obtained from the National Institutes of Health. Mice were housed at constant temperature in an automatically controlled 12-h light cycle environment. All animal experimental procedures described here were approved by the Institutional Animal Care and Use Committee of New York University School of Medicine.
Analysis of Angiogenic Factor mRNA in Castrated and Testosterone-treated Mice
For analysis of angiogenic factor mRNA levels as a function of time after castration and testosterone replenishment, 36 Balb/C mice were castrated, and 6 intact mice served as normal controls (CTL). Six animals were sacrificed each day at 1, 3, and 10 days after castration. On day 10 after castration, the remaining 18 mice were injected daily subcutaneously (s.c.) with 40 mg/kg/day testosterone (Sigma). Six animals were sacrificed each day at 1, 3, and 10 days after testosterone replacement. After sacrifice of the mice, ventral, dorsal, and lateral prostates were rapidly dissected as a unit, and total prostate RNA was extracted immediately using the High Pure RNA Tissue kit (Roche Diagnostics Corporation, Indianapolis, IN). The RNA was quantitated from its optical density, and its integrity assessed by 1.2% formaldehyde gel electrophoresis.
To quantify the content of specific mRNA species in the prostates, a real-time PCR approach was used. Total RNA (2 μg) from each sample was reverse transcribed into first-strand cDNAs using an oligo (dT)15 primer and Transcriptor reverse transcriptase (Roche Diagnostics Corporation, Indianapolis, IN) according to the manufacturer’s directions. The primers for real-time PCR assay listed in Table 1 were designed using ABI PRISM Primer Express version 2.0 software to meet several requirements concerning GC-content, annealing temperature, and amplicon length and were synthesized by Qiagen Science (Alameda CA). After assessing correct product formation by regular RT-PCR, quantitative real-time PCR was conducted on an ABI PRISM 7900 Sequence Detection System apparatus (Applied Biosystems) with the SYBR Green PCR master mix (Applied Biosystems) in a 10-μl volume using a 384-well plate format. SYBR Green PCR master mix was purchased from AB Applied Biosystems (Foster City, CA). cDNAs derived from 200 ng total RNAs from each sample were used for PCR amplification. The PCR profile was as follows: 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. The parameter Ct (threshold cycle) was defined as the cycle number at which the fluorescent signal passed a fixed value (threshold) above baseline. Relative abundance of mRNA levels were determined by using the formula 2-δCt (37). For each sample a replicate was run, and a template negative control was run for each primer without addition of sample cDNA. The mRNA levels were expressed relative to an internal control, hypoxanthine phosphoribosyltransferase (hprt). Melting curve analysis was conducted to determine the specificity of the amplified products and the absence of primer-dimer formation.
TABLE 1.
Sequences of Oligonucleotide Primers Used for Real-time PCR Analyses
| Transcript | Sense 5′ to 3′ | Antisense 5′ to 3′ | Size (bp) | LOCUS NUMBER |
|---|---|---|---|---|
| Hprt | 577CTGGTGAAAAGGACCTCTCG596 | 667CAAGGGCATATCCAACAACA648 | 90 | J00423 |
| VEGF-A | 768GCAGATGTGAATGCAGACCAAA789 | 868CTGCGGATCTTGGACAAACA849 | 100 | NM009505 |
| VEGF-B | 214CTGCTGCTTGTTGCACTGCT233 | 314ACGTCTATCCATGGCACCACT294 | 100 | NM011697 |
| VEGF-C | 809CAATTATTAGACGTTCTCTGCCAGC833 | 909GCATCGGCACATGTAGTTATTCC887 | 100 | NM009506 |
| VEGF-D | 441TGCAGCCCTAGAGAGACATGC461 | 541CCTCCACACCGGAAGACATT522 | 100 | D89628 |
| PlGF | 642CCAATCGGGATCCACATTTC661 | 742TTTCCTCCTTTCTGCCTTTGTC721 | 100 | NM008827.2 |
| HGF | 938GCTACACTCTTGACCCTGACACC960 | 1038TTCAGTTGTTTCCATAGGGACATC1035 | 100 | NM010427.2 |
| VEGFR-1 | 3001ACCTGTCCAACTACCTCAAGAGC3023 | 3096CTGGTTCCAGGCTCTCTTTCTT3075 | 95 | NM010228 |
| VEGFR-2 | 590CGACATAGCCTCCACTGTTTATG612 | 698TTTGTTCTTGTTCTCGGTGATGT676 | 108 | NM010612 |
| FGF-2 | 111CTTCTTCCTGCGCATCCATC130 | 211CAACTCCTCTCTCTTCTGCTTGG189 | 100 | NM008006.1 |
| FGF-8 | 558GAAGCAGAGTCCGAGTTCGC577 | 660ACGCAGTCCTTGCCTTTGC642 | 102 | BC048734.1 |
| Ang-1 | 711CCATGCTTGAGATAGGAACCAG732 | 814TTCAAGTCGGGATGTTTGATTT793 | 103 | NM009640 |
| Ang-2 | 788AGCAGATTTTGGATCAGACCAG809 | 907GCTCCTTCATGGACTGTAGCTG886 | 119 | NM007426 |
| Tie-2 | 366CGGCTTAGTTCTCTGTGGAGTC387 | 465GGCATCAGACACAAGAGGTAGG444 | 99 | NM013690 |
Molecular sequences of oligonucleotides and expected lengths (in bp) of RT-PCR products in this study. Also listed are the positions within coding sequences and the GenBank accession numbers, which were used to design PCR oligonucleotides.
In Situ Hybridization (ISH)
ISH was performed with digoxigenin (DIG)-labeled RNA probes. The probes were prepared by using a set of primers, designed to avoid regions of homology between the angiopoietins. The primers for Ang-2 were: 5′-AACATTCTAGAGAACAACACACA-3′(forward) and 5′-CTGGTTCTGCACCACATTCTG-3′ (reverse). The expected cDNA product of 108 bp between base pairs 525 and 633 (NM007426) was PCR amplified and sub-cloned into pGEM-T Easy Vector System (Promega, Madison, WI), containing T7 and SP6 promoters. After sequencing to confirm the identity and orientation of the insert, the recombinant plasmids were linearized with appropriate restriction enzymes. Using T7 and SP6 RNA polymerase respectively, the DIG-labeled single-stranded anti-sense and sense RNA riboprobes were synthesized according to the labeling protocol in a DIG Labeling Kit (Roche Diagnostics Corporation, Indianapolis, IN). The probes were incubated with DNAse at 37°C for 10 min to remove the plasmid template before phenol extraction and ethanol precipitation. RNA probes were visualized by gel electrophoresis and the concentrations were estimated by dot-blot analysis, using DIG-labeled control RNA.
Dissected prostates were fixed in 4% paraformaldehyde/PBS for 3 hours, equilibrated in 30% sucrose overnight, and snap-frozen in Tissue Tek O.C.T (Triangle Biomedical Science, Durham, NC). Transverse 10 μm sections were cut at -20° C, transferred to slides, and allowed to dry 2h at room temperature before use. After postfixation in 4% paraformaldehyde-PBS for 20 minutes, sections were treated with proteinase K (1 μg/ml) in Tris-EDTA (pH 8.0) for 30 min at 37°C. Slides were then immersed in 0.25% acetic anhydride for 10 minutes, washed in Trisbuffered saline, dehydrated in graded concentrations of ethanol, dried, and processed for ISH. Prehybridization was performed at 55°C for 2 hours with 100 μl of hybridization buffer containing 50% (vol/vol) deionized formamide, 5x SSC, 10% (wt/vol) dextran sulfate, 1x Denhardt’s solution (Sigma), 0.2 mg/ml yeast RNA and 0.5 mg/ml sheared salmon sperm DNA. After denaturing the probes for 10 minutes at 70°C, the probe mixture was added to the hybridization buffer to obtain a probe concentration of 500 ng/ml. Hybridization was performed at 55°C for 16 hours in a Parafilm-(American Can Company, Greenwich, CT) covered chamber humidified with 50% (vol/vol) formamide-2x SSC. Slides were then washed in 2x SSC-50% (vol/vol) formamide at 55°C for 30 min and in 1x SSC at 55°C for 30 min. Slides were treated with 2 μg/ml RNAse in 0.5 M NaCl in Tris-EDTA for 30 min at 37°C. After washing in 0.1 M maleic acid, 0.15 M NaCl (pH 7.5), slides were blocked in 1% blocking reagent for 1 h at RT. The sections were incubated for 2 hours at 4°C with alkaline phosphatase-coupled anti-DIG antibody (Roche) diluted 1:500 in 1 % DIG-blocking reagent (Roche). The sections were equilibrated in 100 mM Tris, 100 mM NaCl, and 50 mM MgCl2 (pH 9.5), for 5 minutes. Color was developed at room temperature with 0.0175% 5-bromo-4-chloro-3-indolyl-phosphate (Roche), 0.045% nitroblue tetrazolium chloride (Roche), 100 mM NaCl, and 50 mM MgCl2 (pH 9.5). Staining was stopped by incubation in Tris-EDTA buffer (pH 8.0) for 15 minutes.
Immunocytochemistry
Three snap-frozen prostates from each group were examined by immunohistochemistry for expression of Ang-1, Ang-2, or CD 31 (PECAM-1). 8-μm-thick-frozen sections were post fixed in acetone for 10 minutes, treated with 0.3% hydrogen peroxide/PBS for 15 minutes, blocked in 5% normal rabbit serum or goat serum for 30 minutes, and biotin/avidin blocking reagents (Vector Laboratories, Inc.) for 15 minutes each. The sections were then incubated with rabbit antiserum raised against Ang-2 (diluted 1:200) (Zymed Laboratories Inc., S. San Francisco, CA), goat anti-Ang-1 (diluted 1:100) (sc-6320 from Santa Cruz Biotechnology Inc, Santa Cruz, CA) or rat anti-mouse CD31 antibodies (diluted 1:200; Pharmingen) for 1 h at room temperature. The antibody-antigen complexes were visualized by the avidin/biotin method (Vectastain, Elite, ABC Kit, Vector Laboratories, Inc., Burlingame, CA) according to the manufacturer’s instructions using diaminobenzidine as a substrate (Peroxidase Substrate Kit, Vector Laboratories, Inc.). The sections were counterstained with Mayer’s hematoxylin (Sigma), dehydrated in graded concentrations of alcohol, and coverslipped with resin (Permount, Fisher Scientific Co.). Control sections were incubated with non-immune rabbit immunoglobulins or blocking peptide using the same working dilution as the primary antibody.
Five randomly selected fields for each of three non-adjacent sections per prostate were captured using a Leica DMLB microscope equipped with 20× objective lens and a SPOT INSIGHT digital camera (Model 3.2.0, Diagnostic Instruments Inc. Michigan City, IN) interfaced to a computer. Blood vessels density per field was expressed as the percent of the area of the field covered by the immunostained vessels as calculated by a computer image analysis program. In some experiments, individual blood vessels in each field were counted. The number of blood vessels per unit area correlated with the percent of the area covered by immunostained vessels (data not shown).
Angiopoietin Immunoprecipitation and Western Blotting
For immunoprecipitation experiments, extracts were made from pools of 3 prostates for each time point of castration (CTL, day1, day3, and day10), and the whole experiment was performed twice. Prostates were homogenized in lysis buffer (30 μg/ml of aprotinin; 150 mM NaCl; 10 mM Tris-HCl; 10 mM EDTA; 1% nonidet P-40; 0.5% sodium deoxycholate; and 0.1% SDS) at 4°C, and supernatants were collected after centrifugation at 13,000 rpm for 30 min. Supernatants were used for protein determination (BCA protein assay; Pierce, Rockford, IL). Extracts containing 1-3 mg protein (in 0.5 ml) were incubated at 4°C with 10 ml conditioned medium from insect cells expressing a chimeric protein consisting of the extracellular domain of tie-2 receptor fused to the Fc region of human immunoglobulin (gift from Dr. Yao-Qi. Huang, NYU School of Medicine, NY). After 2h, 15 μl of Fc-specific anti-human IgG conjugated to agarose (Sigma) was added, and the mixture was incubated overnight at 4°C with agitation. The beads were washed with phosphate-buffered saline four times and collected by centrifugation at 2500 rpm for 5 min at 4°C. After the final wash, the supernatant was aspirated and discarded, and the pellet was resuspended in 30 μl of electrophoresis buffer. The samples were separated on a 10% SDS-polyacrylamide electrophoresis gel (Invitrogen). Proteins were transferred to a nitrocellulose membrane (Schleicher & Schuell Protran, BioSciences) by electroblotting (Bio Rad Laboratories, Hercules, CA). The membrane was blocked with 5% (wt/vol) fat-free milk powder, 0.5% (vol/vol) Tween 20 in phosphate-buffered saline overnight at 4°C and incubated with goat anti-human Ang-1 antibody at a dilution of 1:200 (Santa Cruz Biotechnology, Santa Cruz, CA) for 2 h at room temperature. After washing in TBS, blots were incubated at room temperature for 1 h with a 1:2000 dilution of horseradish peroxidase-conjugated anti-goat IgG secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA). Blots were washed three times with TBS. Immunocomplexes were developed using Western Lightning Chemiluminescence Reagent Plus (PerkinElmer Life Science, Inc, Boston, MA). The membrane was exposed to an X-ray film for imaging. Proteins were sized with Rainbow markers (Amersham Pharmacia Biotech). After visualizing the immobilized proteins, the antibody complex was stripped by rocking the blot in a hybridization tube with 2% SDS and 100 mM 2 mercaptoethanol in Tris buffered saline (Sigma) for 30 min at 50°C. The blot was then reprobed with anti-Ang-2 antibody (Santa Cruz) using the same procedure described above.
Treatment of Castrated Mice with Soluble Angiogenic Factor Receptors
Adult male nude mice were castrated by the scrotal route. Seven days later, collagen gels containing 2.5 x 107 293 cells expressing the extracellular domain of VEGFR-2 fused to the Fc domain of human immunoglobulin or the extracellular domain of Tie-2 fused to Fc were implanted into the peritoneal cavity of these mice. The collagen gels were formed by suspending pelleted 293 cells in 30 μl type I collagen (BD Biosciences) and incubating at 37° C. The 293 cells expressing the VEGFR-2 - Fc and Tie-2 - Fc fusion proteins were a gift from Dr. Yao-Qi Huang, New York University School of Medicine (38). As a control, collagen gels containing 2.5 x 107 293 cells transfected with the vector alone were implanted into a third group of mice. To implant the gels, a 1 cm incision was made medially along the lower abdomen, two gels containing 293 cells were placed into the peritoneal cavity, one on each side of the prostate, and the incision was closed with sutures. The mice were allowed to recover from surgery for three days so that levels of soluble receptor could build up in the animals. No significant change in prostate weight occurred during this period (data not shown). Then, two-thirds of the mice in each group were given daily injections of 40 mg/kg testosterone, while the remaining mice were inoculated with vehicle alone. After a 3 day treatment with testosterone, the mice were sacrificed. One and two hours prior to sacrifice, the mice were inoculated with bromodeoxyuridine (BrdU, 40 μg/g body weight, Roche) to label dividing cells. At sacrifice, ventral, dorsal and lateral prostates were removed as a unit, weighed, and analyzed for changes in vascular density and BrdU labeling index. In addition, the implanted collagen pellets were removed, frozen, and processed for histology. Histological analysis showed that the implanted cells survived and were healthy. At the time of sacrifice, the peritoneal cavity was also lavaged with phosphate-buffered saline. Western blot analysis of the recovered fluid with antibodies to human Fc showed that cells expressing VEGFR-2 - Fc and cells expressing Tie-2 - Fc secreted similar amounts of fusion protein in vivo (data not shown).
Statistical Analysis
Data are expressed as the mean ± SEM. Differences between the treatment group and the appropriate control group were assessed by Student’s t-test using GraphPad InStat software (GraphPad Software Inc. San Diego, CA). Differences were regarded as significant at P<0.05.
RESULTS
Response of Prostatic Blood Vessels to Castration and Testosterone Replacement
We examined the changes in the mouse prostate microvasculature induced by castration and testosterone replacement. As shown in Fig. 1 A, castration caused a decrease in prostate weight (20.4 ± 9.4 %) that could be detected as early as one day after the event. Prostate weight was significantly decreased (52.2 ± 10.2 %) by three days after castration and reached its lowest point by day 10 (57.9 ± 9.4 %, P < 0.01). Anti-CD31 immunostaining (Fig. 1 B) revealed that changes in vascular density were even more dramatic than the changes in prostate weight at early times. Vascular density in prostatic tissue decreased by 32.4 ± 9.3 % one day after castration and reached its lowest level (45.6 ± 8.5 % of control) by three days. By 10 days after castration, vascular density had stabilized at a level 33.9 ± 10.6 % lower than that of intact prostates. When testosterone was administered to mice that had been castrated 10 days earlier, the prostate regenerated and a significant increase in prostate weight could be detected three days later (Fig. 1 A). The prostate grew to a size even greater than that of an intact animal by 10 days of treatment (Fig. 1 A). Similar to the observation in castrated animals, an increase in vascular density accompanied the early changes in prostatic weight. A 17.7 ± 5.0 % increase in vascular density was observed one day after administration of testosterone to castrated animals. Blood vessel density reached a maximum by three days after replacement of testosterone (63.2 ± 17.8 % greater than 10-day castrated prostate, P<0.05) and returned to normal at 10 days (Fig. 1). These results show that the prostate vasculature responds rapidly to changes in androgen status. Therefore, changes in angiogenic factors that are relevant to androgen effects on vascularization are likely to occur early after changes in androgen status. We next examined whether alterations in expression of angiogenic factors correlated with these changes in the blood vessels.
Figure 1.

Changes in prostate weight and vascular density in response to castration and androgen repletion. Mice were castrated, and prostates were removed after 1, 3, and 10 days, weighed, and analyzed by immunohistochemistry for blood vessel density. The remaining animals that had been castrated for 10 days were inoculated daily with testosterone. Prostates were removed after 1, 3 and 10 days, and analyzed as above. A. Castration caused a decrease of prostate weight (broken lines) to 60% and 40% of CTL (intact mice) 3 and 10 days after castration (P< 0.01), respectively. Administration of testosterone to the castrated group restored the prostate weight in a time-dependent manner (P < 0.01). Blood vessels were immunostained with anti-CD-31 antibody, and vessel density was determined by counting vessels in 5 random fields from each of three sections from each prostate (solid lines). In order to compare effects on prostate weight and blood vessel density, all data are shown as a ratio to control values. B. Representative fields showing vessel (arrow) density in control prostate (a), prostate from a mouse castrated 3 days earlier (b), or prostate from a castrated mouse administered testosterone for 3 days (c). Microvascular density was significantly decreased after castration compared to CTL group (P< 0.05 on d 1 and P< 0.01 on d 3). In contrast, testosterone replacement restored the vascular density in mouse prostate on T day1 (P< 0.05) and on T day3 (P< 0.01, ANOVA assay). Error bars depict the standard error of the mean. Scale bar (bottom right panel), 40 μm.
Relative Abundance of mRNA for Angiogenic Factors in Mouse Prostate
To determine which angiogenic factors might be responsible for these changes in the vasculature, we quantified expression levels of the VEGF family, FGFs, HGF, and the angiopoietins in normal mouse prostate (Fig. 2). Using real-time RT-PCR, we found that VEGFA was the most abundant angiogenic factor expressed in mouse prostate, followed by VEGF-B. VEGF-C and VEGF-D were expressed at moderate levels and PlGF was expressed at low levels. FGF-2, FGF-8, HGF, and Ang-1 were also expressed at low levels. Interestingly, Ang-2, which has been reported to be expressed mainly in tissues undergoing vascular turnover, was expressed at 3.5-fold higher levels than Ang-1 in normal, resting mouse prostate (Fig. 2). Thus, the major angiogenic factors expressed by the prostate are members of the VEGF family.
Figure 2.
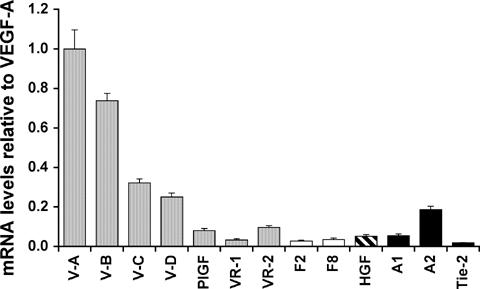
Relative expression of mRNA for angiogenic factors and their receptors in normal mouse prostate. Levels of specific mRNA species in mouse prostate were assessed by real timequantitative RT-PCR and normalized to an internal (Hprt) control. For each data point, the RNA from 3 prostates was pooled and assayed by real-time PCR in triplicate. Results show the mean ± SEM. The mean derived value for VEGF-A was arbitrarily designated as “1”. Note VEGF-A expression in mouse prostate was higher than each of the other family members. Ang-2 expression by mouse prostate was greater than Ang-1.
Effect of Castration and Testosterone Replacement on the Expression of the VEGF Family
We examined whether expression of mRNA for these angiogenic factors was altered by changes in androgen availability in a manner consistent with effects on vascular density. All VEGF family members responded rapidly to testosterone deficiency or administration. Real time RT-PCR analyses showed a significant decline in VEGF-A (40 % decrease), VEGF-B (28 %), VEGF-C (39 %), and PlGF (85 %) one day after castration (Fig. 3 A). In contrast, VEGF-D increased in expression (39 %) one day after castration. Expression of all family members increased by the third day after castration and resolved to a level equal to (VEGF-B and VEGF-D) or below (VEGF-A, 61%, VEGF-C, 70% and PlGF, 54%) that of prostates from intact animals at ten days after castration (Table 2).
Figure 3.

Effect of castration or testosterone administration on the expression of angiogenic factor mRNA. Mice were castrated and prostates were harvested after 1 day (A). Other animals that had been castrated for 10 days were inoculated with testosterone and their prostates were harvested 24 h later (B). Total RNA from whole prostates of control, castrated, and testosterone-treated mice was reverse transcribed and amplified by real time-quantitative RT-PCR using primers specific for the VEGF family (shaded bars), FGF family (open bars), HGF (hatched bar), and the angiopoietins (filled bars). Results were normalized to an internal (Hprt) control and show the mean ± SEM of three repeat experiments. Each of the three experiments displayed a consistent pattern and level of expression for each of the mRNAs. The mean derived value for each gene at the CTL group was arbitrarily designated as “1”. VEGF family (except VEGF-D) and FGF family rapidly decreased one day after castration (A), and the effect was reversed by testosterone replacement (B). Angiopoietins and HGF did not respond rapidly to androgen deficiency or readministration.
TABLE 2.
Changes in mRNA Expression of Angiogenic Growth Factors in Mouse Prostate at Different Times after Castration
| CTL* | Day1* | Day3* | Day10* | |
|---|---|---|---|---|
| VEGF-A | 1.00 ± 0.29 | 0.61 ± 0.10 | 1.29 ± 0.12 | 0.39 ± 0.16 |
| VEGF-B | 1.00 ± 0.20 | 0.73 ± 0.12 | 3.39 ± 0.03 | 1.14 ± 0.09 |
| VEGF-C | 1.00 ± 0.15 | 0.59 ± 0.17 | 1.61 ± 0.10 | 0.30 ± 0.04 |
| VEGF-D | 1.00 ± 0.16 | 1.39 ± 0.15 | 4.32 ± 0.07 | 1.27 ± 0.02 |
| PlGF | 1.00 ± 0.11 | 0.15 ± 0.11 | 0.96 ± 0.13 | 0.46 ± 0.11 |
| VEGFR-1 | 1.00 ± 0.23 | 0.93 ± 0.26 | 6.25 ± 0.41 | 1.53 ± 0.05 |
| VEGFR-2 | 1.00 ± 0.31 | 1.08 ± 0.26 | 11.04 ± 0.17 | 1.04 ± 0.11 |
| FGF-2 | 1.00 ± 0.23 | 0.64 ± 0.03 | 5.78 ± 0.34 | 0.71 ± 0.04 |
| FGF-8 | 1.00 ± 0.31 | 0.47 ± 0.20 | 6.56 ± 0.07 | 0.10 ± 0.08 |
| HGF | 1.00 ± 0.36 | 1.15 ± 0.18 | 7.52 ± 0.49 | 1.03 ± 0.09 |
| Ang-1 | 1.00 ± 0.17 | 1.09 ± 0.18 | 35.52 ± 0.10 | 1.75 ± 0.15 |
| Ang-2 | 1.00 ± 0.18 | 0.94 ± 0.19 | 4.78 ± 0.29 | 0.91 ± 0.19 |
| Tie-2 | 1.00 ± 0.36 | 0.96 ± 0.63 | 3.15 ± 0.38 | 1.22 ± 0.22 |
Mean ± SEM, n=3. The mean derived value for each gene in the CTL group was arbitrarily designated as “1”.
CTL, prostates from intact mice; Day 1, Day 3, Day 10, prostates from mice 1, 3, or 10 days after castration.
Mice that had been castrated ten days earlier were given daily inoculations of testosterone, and the effects on expression of the VEGF family members in the prostate were examined. VEGF-A, VEGF-B, VEGF-C, and PlGF expression increased on the first day after testosterone administration (180%, 18%, 79% and 300%, respectively compared to 10 day castrated prostate), and VEGF-D expression decreased (40%, Fig. 3 B). After 10 days of testosterone treatment, expression of VEGF-A, VEGF-B, VEGF-C, and PlGF remained at these elevated levels (Table 3). Castration had no significant effects on expression of the major signaling receptors for the VEGF family, VEGFR-1 and VEGFR-2 at one day (Table 2). However, testosterone administration increased expression of both receptors (Table 3). Taken together, our data suggest that the VEGF family responds acutely to changes in androgen availability in the mouse prostate in vivo, and the changes are consistent with prostatic vascular regression and regeneration after castration and testosterone replacement.
TABLE 3.
Changes in mRNA Expression of Angiogenic Growth Factors in Mouse Prostate at Different Times after Testosterone Replacement
| CTL* | T day 1* | T day 3* | T day 10* | |
|---|---|---|---|---|
| VEGF-A | 1.00 ± 0.16 | 2.79 ± 0.11 | 2.49 ± 0.18 | 3.69 ± 0.07 |
| VEGF-B | 1.00 ± 0.09 | 1.18 ± 0.05 | 1.52 ± 0.21 | 1.58 ± 0.11 |
| VEGF-C | 1.00 ± 0.04 | 1.80 ± 0.17 | 1.83 ± 0.08 | 1.93 ± 0.11 |
| VEGF-D | 1.00 ± 0.02 | 0.60 ± 0.01 | 0.95 ± 0.13 | 0.61 ± 0.12 |
| PlGF | 1.00 ± 0.11 | 4.09 ± 0.06 | 3.15 ± 0.22 | 3.33 ± 0.15 |
| VEGFR-1 | 1.00 ± 0.05 | 1.25 ± 0.19 | 1.60 ± 0.13 | 1.46 ± 0.27 |
| VEGFR-2 | 1.00 ± 0.11 | 1.65 ± 0.42 | 3.89 ± 0.22 | 2.35 ± 0.21 |
| FGF-2 | 1.00 ± 0.04 | 3.56 ± 0.14 | 6.08 ± 0.48 | 2.54 ± 0.28 |
| FGF-8 | 1.00 ± 0.08 | 6.10 ± 0.30 | 13.9 ± 0.27 | 12.0 ± 0.36 |
| HGF | 1.00 ± 0.09 | 1.17 ± 0.20 | 1.23 ± 0.50 | 0.97 ± 0.72 |
| Ang-1 | 1.00 ± 0.15 | 1.23 ± 0.21 | 0.82 ± 0.09 | 0.51 ± 0.17 |
| Ang-2 | 1.00 ± 0.19 | 0.93 ± 0.28 | 1.35 ± 0.04 | 1.21 ± 0.24 |
| Tie-2 | 1.00 ± 0.22 | 1.71 ± 0.11 | 3.56 ± 0.23 | 2.20 ± 0.34 |
Mean ± SEM, n=3. The mean derived value for each gene in the CTL group was arbitrarily designated as “1”.
CTL, prostates from 10 day-castrated mice; T day 1, T day 3, T day 10, prostates from mice 1, 3, or 10 days after testosterone treatment.
Effect of Castration and Testosterone Replacement on FGF and HGF Expression
The effect of castration on expression of FGF-2, FGF-8, and HGF mRNA was examined. Expression of FGF-2 and FGF-8 decreased 36 and 53 %, respectively, one day after castration (Fig. 3 A). There was no change in HGF expression immediately after castration. Expression of FGF-2, FGF-8, and HGF all increased 5-7.5-fold by the third day after castration (Table 2). Expression of HGF returned to control values by 10 days after castration, but FGF-2 remained below levels observed in intact animals and FGF-8 levels were almost undetectable (Table 2). When mice that had been castrated for 10 days were administered testosterone, FGF-2 and FGF-8 levels increased 4- to 6-fold one day later (Fig. 3 B). FGF-8 levels remained elevated 10 days after testosterone administration, whereas FGF-2 levels decreased but remained above levels found in control animals (Table 3). HGF levels did not change after testosterone administration. These data suggest that FGFs also respond acutely to changes in androgen status in the mouse prostate, but HGF expression in prostate is androgen independent.
Effects of Castration and Testosterone Replacement on the Expression of the Angiopoietins
Real time RT-PCR analyses showed that Ang-1 and Ang-2 mRNA expression were not changed one day after castration (Fig. 3 A). Ang-1 expression increased 35-fold and Ang-2 expression increased 5-fold by the third day after castration, and then returned to near control values by ten days after castration (Table 2). Similarly, testosterone replacement had no significant effect on Ang-1 or Ang-2 expression (Fig. 3 B). Expression of the receptor for the angiopoietins, Tie-2, increased three days after castration but returned to control values by 10 days (Table 2). Expression of tie-2 in castrated animals treated with testosterone increased above the levels observed in intact animals (Table 3). These results indicate that the expression of Ang-1 and Ang-2 mRNAs is not directly regulated by testosterone.
As increased Ang-2 expression accompanies blood vessel regression in other systems, we examined whether there might be changes in angiopoietin protein expression after castration. Ang-1 and Ang-2 protein expression were analyzed by immunoblot analysis (Fig. 4 A), and NIH image J software was used to quantitate the band density (Fig. 4 B). In contrast to the mRNA data, the relative ratio of Ang-1/Ang-2 protein declined by 60 % one day after castration but returned to normal on day three and increased nearly two-fold over control by day 10 (Fig. 4 A and B). The change in Ang 1/Ang 2 ratio was mainly the result of changes in expression of Ang 1 protein as there was no change in Ang-2 protein after castration (data not shown). Thus, although angiopoietin mRNA expression did not respond to androgens, changes in testosterone availability significantly modulated the Ang1/Ang2 protein ratio at times consistent with effects on blood vessels.
Figure 4.

Hormonal regulation of Ang-1 and Ang-2 protein expression. Angiopoietin protein expression was examined in prostates isolated from normal mice and mice castrated 1, 3, or 10 days earlier. Prostate protein was extracted, and 1-3 mg from each sample was incubated with soluble Tie-2-Fc fusion protein. After immunoprecipitation of the complexes, immunoblots were performed using anti-Ang-1 antibody. The blots were then stripped, and reprobed with anti-Ang-2 antibody. A. The top panel shows the immunoblot with antibodies to Ang-1 (∼75 kDa), and the lower panel shows the same immunoblot reprobed with antibodies to Ang-2 (∼70 kDa). Conditioned medium from cells transfected with Ang-1 or Ang-2 was used as a positive control (+). B. Changes in Ang1/Ang2 ratio after castration. Densitometric analysis of protein expression for Ang-1 and Ang-2 was performed on 2 blots including the one presented in A. The ratio of Ang-1 to Ang-2 expression was calculated and normalized to the ratio in intact animals (CTL), which was arbitrarily designated as “1”. Note the approximately 60 % decline in the ratio of Ang-1/Ang-2 protein compared with CTL one day after castration.
Angiopoietin Localization in Mouse Prostate
As the distribution of the angiopoietins in the prostate has received little attention, we examined the localization of angiopoietin expression in vivo. In situ hybridization (Fig 5 a-c) and immunohistochemitry (Fig 5 d-f) revealed that Ang-2 mRNA and protein was predominantly expressed in prostate epithelial cells. Immunohistochemistry showed a weak signal for Ang-1 in epithelial cells and stromal cells, especially smooth muscle cells (Fig. 5 g-i). The changes in angiopoietin expression observed by real-time PCR were reflected in in situ hybridization and immunohistochemistry (Fig. 5).
Figure 5.

Angiopoietin localization in mouse prostate. Ang-2 expression was examined by in situ hybridization (a-c) and immunohistochemistry (d-f). Ang-2 mRNA and protein were predominantly expressed by epithelial cells (black arrow), and expressed faintly in stromal cells and blood vessels, whereas smooth muscle cells were negative. The changes in expression observed by real-time PCR were reflected in the in situ hybridization and immunohistochemistry (a, d, intact; b, e, day3 after castration; and c, f, day3 after testosterone administration). Hybridization with the Ang-1 sense probe did not produce a signal that significantly differed from the Ang-1 antisense signal (data not shown). Immunohistochemistry showed a weak staining for Ang-1 in epithelial cells and stromal cells, especially smooth muscle cells (white arrow), of the prostate, (g, intact; and h, day3 after castration) whereas there was strong staining for Ang-1 in stromal cells of the ovary (positive control, i). Scale bar (bottom left panel) 40 μm (the same scale for all panels).
Soluble VEGFR-2 or Tie-2 receptors inhibit prostate regeneration
To determine if the changes in VEGF availability were relevant to prostate regeneration, the ability of mice to regenerate prostates in the presence of soluble VEGFR-2 - Fc or Tie-2 - Fc fusion proteins was examined. These soluble receptor constructs are expected to bind their cognate ligands and sequester them from cell-surface receptors. Castrated nude mice were inoculated intraperitoneally with 293 cells expressing VEGFR-2 - Fc fusion protein or Tie-2 -Fc fusion protein. Three days later, testosterone was administered to these animals daily for 3 days. The mice were sacrificed, and effects on prostate regeneration were determined. As observed in Fig. 6 A, prostates of animals that were inoculated with control 293 cells not expressing the fusion proteins increased 2.9-fold in weight three days after testosterone administration. However, prostate weight increased only 60% in mice inoculated with 293 cells expressing VEGFR-2 - Fc (Fig 6 A). In mice inoculated with 293 cells expressing Tie-2 - Fc fusion proteins, regeneration of the prostate was also inhibited, but to a lesser extent (Fig. 6 A). As expected, VEGFR-2 - Fc fusion protein blocked the increased vascular density observed in regenerating prostates (Fig. 6 B and C). The increase in vascular density was also blocked in animals exposed to the Tie-2 - Fc fusion protein (Fig. 6 B and C). Thus, the angiogenesis induced in regenerating prostates is dependent on the VEGF and angiopoietin signals that arise in these tissues.
Figure 6.

Inhibition of prostate regeneration by soluble VEGF and angiopoietin receptors. Nude mice that had been castrated seven days earlier were inoculated with collagen pellets containing 2 x 107 293 cells expressing VEGFR-2 - Fc fusion protein (VEGFR) or Tie-2 - Fc fusion protein (Tie2). Control animals were inoculated with pellets containing 293 cells transfected with the vector alone (Vec). Three days later, mice from each group were inoculated daily with vehicle alone (CTL) or with testosterone. After 3 days, the mice were sacrificed, prostates were removed, weighed, and processed for histology. As the prostates from mice inoculated with vehicle alone were indistinguishable, the data from these prostates were pooled (CTL). Values are given as means ± SEM for 5 animals per group. * significant difference (P<0.01) from Vec; ** significant difference (P<0.05) from CTL or Vec. A. Wet weight of recovered prostates. B. Sections were immunostained with antibodies to CD31, and blood vessel density was determined. Blood vessel density is expressed as the percent of the field covered by the immunostained vessels. C. Representative fields showing blood vessel densities. Scale bar (bottom left panel), 50 μm.
The effect of blocking angiogenesis on the response of prostatic epithelial cells to testosterone was also examined. The proportion of proliferating epithelial cells was determined by counting cells labeled with BrdU in random sections from the prostates of the animals in this experiment. Only 0.3 % of epithelial cells were labeled with BrdU in castrated animals (Fig. 7). In animals inoculated with control 293 cells, testosterone increased BrdU labeling 21-fold in the epithelial cell compartment (Fig. 7). In contrast, the proliferative response to testosterone was decreased by 70% in mice inoculated with 293 cells expressing VEGFR-2 - Fc fusion protein or Tie-2 - Fc fusion protein (Fig. 7). Thus, inhibition of angiogenesis prevents the normal prostate epithelial response to testosterone.
Figure 7.
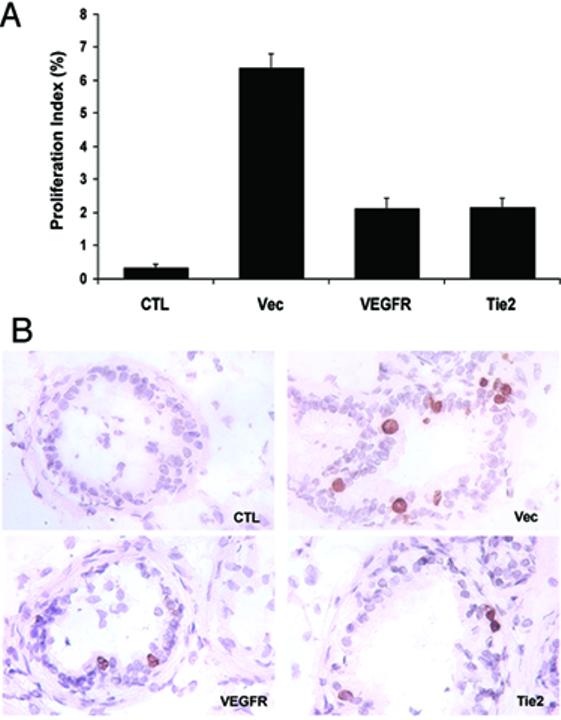
Inhibition of angiogenesis inhibits prostate epithelial cell proliferation. One and two hours prior to sacrifice, the mice from the experiment of Fig. 6 were inoculated with BrdU. Sections of the prostates were immunostained with antibodies to BrdU and labeled epitehelial cell nuclei were counted. Total epithelial cell nuclei were determined after counting epithelial nuclei labeled by hematoxylin. A. A proliferation index was calculated by dividing BrdU labeled nuclei by total nuclei. Values are given as means ± SEM for 5 animals per group. * significant difference (P<0.01) from Vec; ** significant difference (P<0.05) from CTL or Vec. B. Representative fields showing BrdU-labeled nuclei. Scale bar (bottom left panel), 100 μm.
DISCUSSION
Our results show that the rapid involution of the prostate after castration is accompanied by an attendant reduction in blood vessels in the prostate, consistent with previous reports (6). Increasing evidence has suggested that testosterone, which is not an angiogenic factor per se, regulates the prostate vasculature through its action on synthesis of angiogenic growth factors (39). Most previous investigations have examined the role of VEGF-A in the response to androgen. Here we have examined the effects of androgen availability on a constellation of angiogenic molecules that may modulate the responses to androgens. Indeed, we find that the expression of the angiogenic factors VEGF-A, VEGF-B, VEGF-C, PlGF, FGF-2, and FGF-8 all decrease prior to the time that major changes are observed in blood vessel density as a result of castration, whereas HGF and angiopoietin mRNAs remain constant during these early times. Similarly, the rapid growth of blood vessels that accompanies regeneration of the prostate upon administration of testosterone to castrated animals occurs at the same time as an increase in expression of the VEGF and FGF family members. These results suggest that the response of prostatic blood vessels to changes in androgens may be the concerted effect of changes in a number of angiogenic regulators.
VEGF-A was the major angiogenic factor detected in prostate, and significant changes in VEGF-A expression were observed within 1 day after castration and testosterone replacement. As even a 50% reduction in VEGF-A expression in embryos results in disruption of blood vessel development (16,17), these changes in VEGF-A may be sufficient for the observed effects on blood vessel density in the prostate. However, as the majority of VEGF-A produced by prostate epithelial cells is secreted apically and large amounts of VEGF-A are found in prostatic secretions (35,40), it was not clear how much of the VEGF-A produced in the prostate is available to the blood vessels. The ability of VEGFR-2 - Fc fusion proteins to block angiogenesis in the regenerating prostate demonstrates that sufficient VEGF-A is available to the blood vessels to regulate their response to testosterone and that VEGF-A is essential to the angiogenic response.
We found that VEGF-B was also expressed at high levels in prostate and PlGF was expressed at low levels. The precise role of VEGF-B and PlGF in angiogenesis is not clear as they do not interact with VEGFR-2, the major mediator of VEGF angiogenic signals (41). VEGF-B and PlGF do bind to VEGFR-1 (41), and perhaps influence angiogenesis by displacing VEGF-A from this receptor, making more VEGF-A available to VEGFR-2. VEGF-B and PlGF expression both change dramatically in response to castration and administration of testosterone, and these alterations may influence the vascular response. Interestingly, VEGF-C and VEGF-D both respond to changes in androgen availability, but in opposite directions. VEGF-C and VEGF-D both also interact with VEGFR-2 (21,22) and may affect angiogenic responses, but the major roles of these molecules is in regulating lymphangiogenesis (23). Both VEGFR-1 and VEGFR-2 are expressed in prostate and their expression is not altered by castration. However, expression of both receptors is upregulated in the regenerating prostate, as has been noted in other systems undergoing active angiogenesis (23).
In contrast to the early decreases in expression of the VEGF family and FGFs one day after castration, expression of these same molecules was increased three days after castration. This biphasic response to castration was previously noted for VEGF-A (14). In addition, expression of HGF and the angiopoietins was increased three days after castration. These changes in expression may be initiated by other signals that occur as the prostate approaches its maximum involution. The transitory increased expression of these angiogenic factors may be necessary for the stabilization of blood vessels at a new lower density in the regressed prostate. The late increases in FGF-2 and HGF may account for the previous report that FGF-2 and HGF expression increase after castration (26). These results emphasize the importance of timing in assaying changes after castration. As the changes in prostate epithelium and associated changes in blood vessels are initiated in the first 24 h after testosterone withdrawal, changes in angiogenic factor expression in androgen sensitive epithelium and stroma that occur early are likely to be involved in changes in prostate vascular response to androgen.
Previous studies have shown that Ang-1 is widely expressed in adult mouse, while Ang-2 appears to be primarily expressed in organs that undergo blood vessel turnover, such as the ovary, uterus, and placenta (28). Interestingly, we demonstrate that Ang-2 was expressed at higher levels than Ang-1 in normal, resting adult mouse prostate. In other systems, Ang-2 expression increases immediately before vessel regression or sprouting (28). Ang-2 is thought to destabilize endothelial cell interactions with smooth muscle cells or pericytes in the vessel wall in preparation for vessel turnover or angiogenesis. In contrast to this model, we found no increase in Ang-2 expression prior to prostate involution in response to castration and no increase prior to the angiogenic events that accompany regeneration of the prostate. Nevertheless, angiopoietin signaling appears to have a critical role in the vascular response to testosterone, as the Tie-2 - Fc fusion protein was able to block vascular growth in regenerating prostate. A potential resolution of this apparent discrepancy is suggested by our Ang-2 localization studies. In systems in which Ang-2 expression increased before vascular regression, Ang-2 expression was localized to the regressing blood vessels (28). In the prostate, Ang-2 expression was localized by both immunohistochemistry and in situ hybridization primarily to prostate epithelium and, to a much lesser extent, to blood vessels. Because of the abundant expression of Ang-2 in the epithelium, relevant changes in Ang-2 expression in the blood vessels may have been masked. Changes in Ang-2 expression in the blood vessels may be more important in determining vascular turnover than the Ang-2 expressed by the epithelium. Furthermore, the majority of the Ang-2 expressed by the epithelium, like VEGF-A, may be secreted into the prostatic lumen and never interact with blood vessels. However, a portion of the epithelial-derived Ang-2 may reach prostate duct-associated vessels, counteracting the effect of Ang-1. It is possible that this epithelium-derived Ang-2 has non-angiogenic role in the prostate. As Ang-1 has been shown to decrease vessel permeability (30), it is possible that the high level of expression of Ang-2 counteracts Ang-1 effects on permeability, leading to increased permeability of prostatic vessels.
Administration of VEGFR-2 - Fc or Tie-2 - Fc fusion proteins to castrated mice not only ablated the vascular response to testosterone but also inhibited prostate regeneration and prostate epithelial cell proliferation. In similar experiments by Lissbrant et al. (42), administration of soluble VEGFR-1 also inhibited both vascular growth and prostate regeneration. However, in contrast to our data, Lissbrant et al. found that VEGF inhibition did not affect prostate epithelial proliferation but increased epithelial cell apoptosis (42). The lack of effect on epithelial proliferation using VEGFR-1 is likely not due to a difference between the receptors, as soluble VEGFR-1 was able to inhibit endometrial epithelial proliferation in response to 17β-estradiol administration to ovariectomized mice (43). Our results also contrast with a report that Tie-2— Fc fusion protein decreased prostate vascular maturation but did not affect prostate growth in castrated mice administered testosterone (36). Perhaps these differences are due to the mode of delivery of the fusion proteins. Implantation of cells expressing the fusion proteins may result in higher local levels of the protein than simple inoculation of purified fusion protein. The finding that the Tie-2—Fc protein blocks the increase in blood vessel density after testosterone administration is in agreement with reports that soluble Tie-2 receptors can block angiogenesis and growth of tumors (44,45). This suggests that the angiopoietins are necessary for both normal vascular growth and tumor angiogenesis in adult organisms. Our results together with those of Lissbrant et al. demonstrate that growth of the prostate in response to androgens is dependent on expansion of the vasculature.
Our results demonstrate that in vivo alterations of testosterone levels regulate the expression of a variety of endothelial specific effectors. Thus, testosterone appears to maintain the prostate vasculature through its effects on expression of VEGF, FGF, and angiopoietin family members. As vascular stability depends on the balance between angiogenic promoters and inhibitors, lack of testosterone may induce endothelial cell apotosis by altering the balance between the pro-angiogenic activities of the VEGF and FGF families and the inhibitory activity of Ang-2. The decreased expression of VEGF and FGF family members in conjunction with constant Ang-2 expression in castrated animals would be expected to reduce angiogenic signals, and thereby destabilize prostatic blood vessels. Similarly, because neovascularization is dependent on the dominance of angiogenic promoters over inhibitors, constant Ang-2 expression in the context of increased signaling from the VEGF and FGF families in testosterone-treated castrated animals may lead to pro-angiogenic signals and restoration of the prostatic vasculature. The changes in the vasculature brought about by these shifting balances of angiogenic factors are critical to the growth of the prostate.
ACKNOWLEDGEMENTS
We are indebted to Dr. Jiri Zavadil (NYU Cancer Institute, New York, NY) and Dr. Yukio Hosomi (NYU School of Medicine) for their technical assistance in real-time PCR assay. We are also grateful to the NYU Cancer Institute Genomics Facility for providing access to relevant instrumentation. We thank Drs. E. Lynette Wilson and Yao-Qi Huang for comments on manuscript.
Abbreviations
- Ang-1
angiopoietin-1
- Ang-2
angiopoietin-2
- BrdU
bromodeoxyuridine
- HGF
hepatocyte growth factor
- PlGF
placental growth factor
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
- FGF
fibroblast growth factor
Footnotes
This work was supported by grants DK52644 and CA90593 from the National Institutes of Health.
REFERENCES
- 1.Buttyan R, Shabsigh A, Perlman H, Colombel M. Regulation of Apoptosis in the Prostate Gland by Androgenic Steroids. Trends Endocrinol Metab. 1999;10(2):47–54. doi: 10.1016/s1043-2760(98)00104-0. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee PP, Banerjee S, Tilly KI, Tilly JL, Brown TR, Zirkin BR. Lobe-specific apoptotic cell death in rat prostate after androgen ablation by castration. Endocrinology. 1995;136(10):4368–4376. doi: 10.1210/endo.136.10.7664656. [DOI] [PubMed] [Google Scholar]
- 3.Coffey DS, Shimazaki J, Williams-Ashman HG. Polymerization of deoxyribonucleotides in relation to androgen-induced prostatic growth. Arch Biochem Biophys. 1968;124(1):184–198. doi: 10.1016/0003-9861(68)90319-6. [DOI] [PubMed] [Google Scholar]
- 4.Shabsigh A, Chang DT, Heitjan DF, Kiss A, Olsson CA, Puchner PJ, Buttyan R. Rapid reduction in blood flow to the rat ventral prostate gland after castration: preliminary evidence that androgens influence prostate size by regulating blood flow to the prostate gland and prostatic endothelial cell survival. Prostate. 1998;36(3):201–206. doi: 10.1002/(sici)1097-0045(19980801)36:3<201::aid-pros9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Shabisgh A, Tanji N, D’Agati V, Burchardt M, Rubin M, Goluboff ET, Heitjan D, Kiss A, Buttyan R. Early effects of castration on the vascular system of the rat ventral prostate gland. Endocrinology. 1999;140(4):1920–1926. doi: 10.1210/endo.140.4.6644. [DOI] [PubMed] [Google Scholar]
- 6.English HF, Drago JR, Santen RJ. Cellular response to androgen depletion and repletion in the rat ventral prostate: autoradiography and morphometric analysis. Prostate. 1985;7:41–51. doi: 10.1002/pros.2990070106. [DOI] [PubMed] [Google Scholar]
- 7.Franck-Lissbrant I, Haggstrom S, Damber JE, Bergh A. Testosterone stimulates angiogenesis and vascular regrowth in the ventral prostate in castrated adult rats. Endocrinology. 1998;139(2):451–456. doi: 10.1210/endo.139.2.5683. [DOI] [PubMed] [Google Scholar]
- 8.Prins G, Birch L, Green G. Androgen receptor localization in different cell types of the adult rat prostate. Endocrinology. 1991;129:3187–3199. doi: 10.1210/endo-129-6-3187. [DOI] [PubMed] [Google Scholar]
- 9.Joseph IB, Isaacs JT. Potentiation of antiangiogenic ability of linomide by androgen ablation involves down-regulation of vascular endothelial growth factor in human androgen-responsive prostate cancers. Cancer Res. 1997;57:1054–1057. [PubMed] [Google Scholar]
- 10.Sordello S, Bertrand N, Plouet J. Vascular endothelial growth factor is up-regulated in vitro and in vivo by androgens. Biochem Biophys Res Commun. 1998;251(1):287–290. doi: 10.1006/bbrc.1998.9328. [DOI] [PubMed] [Google Scholar]
- 11.Levine AC, Liu XH, Greenberg PD, Eliashvili M, Schiff JD, Aaronson SA, Holland JF, Kirschenbaum A. Androgens induce the expression of vascular endothelial growth factor in human fetal prostatic fibroblasts. Endocrinology. 1998;139(11):4672–4678. doi: 10.1210/endo.139.11.6303. [DOI] [PubMed] [Google Scholar]
- 12.Haggstrom S, Lissbrant IF, Bergh A, Damber JE. Testosterone induces vascular endothelial growth factor synthesis in the ventral prostate in castrated rats. J Urol. 1999;161(5):1620–1625. [PubMed] [Google Scholar]
- 13.Benjamin LE, Golijanin D, Itin A, Pode D, Keshet E. Selective ablation of immature blood vessels in established human tumors follows vascular endothelial growth factor withdrawal. J Clin Invest. 1999;103(2):159–165. doi: 10.1172/JCI5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burchardt M, Burchardt T, Chen MW, Hayek OR, Knight C, Shabsigh A, de La Taille A, Buttyan R. Vascular endothelial growth factor-A expression in the rat ventral prostate gland and the early effects of castration. Prostate. 2000;43(3):184–194. doi: 10.1002/(sici)1097-0045(20000515)43:3<184::aid-pros4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Stewart RJ, Panigrahy D, Flynn E, Folkman J. Vascular endothelial growth factor expression and tumor angiogenesis are regulated by androgens in hormone responsive human prostate carcinoma: evidence for androgen dependent destabilization of vascular endothelial growth factor transcripts. J Urol. 2001;165(2):688–693. doi: 10.1097/00005392-200102000-00095. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kleckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 18.LeCouter J, Moritz DR, Li B, Phillips GL, Liang XH, Gerber HP, Hillan KJ, Ferrara N. Angiogenesis-independent endothelial protection of liver: role of VEGFR-1. Science. 2003;299(5608):890–893. doi: 10.1126/science.1079562. [DOI] [PubMed] [Google Scholar]
- 19.Maglione D, Guerriero V, Viglietto G, Delli-Bovi P, Persico MG. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc Natl Acad Sci U S A. 1991;88(20):9267–9271. doi: 10.1073/pnas.88.20.9267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, Orpana A, Pettersson RF, Alitalo K, Eriksson U. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci U S A. 1996;93(6):2576–2581. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996;15:290–298. [PMC free article] [PubMed] [Google Scholar]
- 22.Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95(2):548–553. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tammela T, Enholm B, Alitalo K, Paavonen K. The biology of vascular endothelial growth factors. Cardiovasc Res. 2005;65(3):550–563. doi: 10.1016/j.cardiores.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- 25.Grant D, Kleinman H, Goldberg I, Bhargava M, Nickoloff B, Kinsella J, Polverini P, Rosen E. Scatter factor induces blood vessel formation in vivo. Proc Natl Acad Sci USA. 1993;90:1937–1941. doi: 10.1073/pnas.90.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishi N, Oya H, Matsumoto K, Nakamura T, Miyanaka H, Wada F. Changes in gene expression of growth factors and their receptors during castration-induced involution and androgen-induced regrowth of rat prostates. Prostate. 1996;28:139–152. doi: 10.1002/(SICI)1097-0045(199603)28:3<139::AID-PROS1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 27.Davis S, Aldrich TH, Jones PF, Acheson A, Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre PC, Yancopoulos GD. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87(7):1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 28.Maisonpierre P, Suri C, Jones P, Bartunkova S, Wiegand S, Radziejewski C, Compton D, McClain J, Aldrich T, Papadopoulos N, Daly T, Davis S, Sato T, Yancopoulos G. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 29.Suri C, McClain J, Thurston G, McDonald DM, Zhou H, Oldmixon EH, Sato TN, Yancopoulos GD. Increased vascularization in mice overexpressing angiopoietin-1. Science. 1998;282(5388):468–471. doi: 10.1126/science.282.5388.468. [DOI] [PubMed] [Google Scholar]
- 30.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286(5449):2511–2514. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 31.Suri C, Jones P, Patan S, Bartunkova S, Maisonpierre P, Davis S, Sato T, Yancopoulos G. Requisite role of angiopoietin-1, a ligand for the TIE2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- 32.Dumont DJ, Gradwohl G, Fong GH, Puri MC, Gertsenstein M, Auerbach A, Breitman ML. Dominant-negative and targeted null mutations in the endothelial receptor tyrosine kinase, tek, reveal a critical role in vasculogenesis of the embryo. Genes Dev. 1994;8(16):1897–1909. doi: 10.1101/gad.8.16.1897. [DOI] [PubMed] [Google Scholar]
- 33.Holash J, Wiegand SJ, Yancopoulos GD. New model of tumor angiogenesis: dynamic balance between vessel regression and growth mediated by angiopoietins and VEGF. Oncogene. 1999;18(38):5356–5362. doi: 10.1038/sj.onc.1203035. [DOI] [PubMed] [Google Scholar]
- 34.Lobov IB, Brooks PC, Lang RA. Angiopoietin-2 displays VEGF-dependent modulation of capillary structure and endothelial cell survival in vivo. Proc Natl Acad Sci U S A. 2002;99(17):11205–11210. doi: 10.1073/pnas.172161899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richard C, Kim G, Koikawa Y, Salm SN, Tsujimura A, Wilson EL, Moscatelli D. Androgens modulate the balance between VEGF and angiopoietin expression in prostate epithelial and smooth muscle cells. Prostate. 2002;50(2):83–91. doi: 10.1002/pros.10035. [DOI] [PubMed] [Google Scholar]
- 36.Johansson A, Rudolfsson SH, Wikstrom P, Bergh A. Altered levels of angiopoietin 1 and tie 2 are associated with androgen-regulated vascular regression and growth in the ventral prostate in adult mice and rats. Endocrinology. 2005;146(8):3463–3470. doi: 10.1210/en.2004-1480. [DOI] [PubMed] [Google Scholar]
- 37.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Caunt M, Huang Y-Q, Brooks PC, Karpatkin S. Thrombin induces neoangiogenesis in the chick chorioallantoic membrane. Journal of Thrombosis and Haemostasis. 2003;1:2097–2102. doi: 10.1046/j.1538-7836.2003.00426.x. doi: 2010.1046/j.1538-7836.2003.00426.x. [DOI] [PubMed] [Google Scholar]
- 39.Scolnik M, Tykochinsky G, Servadio C, Abramovici A. The development of vascular supply of normal rat prostate during the sexual maturation: an angiographic study. Prostate. 1992;21:1–14. doi: 10.1002/pros.2990210102. [DOI] [PubMed] [Google Scholar]
- 40.Brown LF, Yeo KT, Berse B, Morgentaler A, Dvorak HF, Rosen S. Vascular permeability factor (vascular endothelial growth factor) is strongly expressed in the normal male genital tract and is present in substantial quantities in semen. J Urol. 1995;154:576–579. doi: 10.1097/00005392-199508000-00073. [DOI] [PubMed] [Google Scholar]
- 41.Olofsson B, Pajusola K, Kaipainen A, von Euler G, Joukov V, Saksela O, Orpana A, Pettersson R, Alitalo K, Eriksson U. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci USA. 1996;93:2576–2581. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lissbrant IF, Hammarsten P, Lissbrant E, Ferrara N, Rudolfsson SH, Bergh A. Neutralizing VEGF bioactivity with a soluble chimeric VEGF-receptor protein flt(1-3)IgG inhibits testosterone-stimulated prostate growth in castrated mice. Prostate. 2004;58(1):57–65. doi: 10.1002/pros.10312. [DOI] [PubMed] [Google Scholar]
- 43.Hastings JM, Licence DR, Burton GJ, Charnock-Jones DS, Smith SK. Soluble vascular endothelial growth factor receptor 1 inhibits edema and epithelial proliferation induced by 17beta-estradiol in the mouse uterus. Endocrinology. 2003;144(1):326–334. doi: 10.1210/en.2002-220641. [DOI] [PubMed] [Google Scholar]
- 44.Lin P, Buxton JA, Acheson A, Radziejewski C, Maisonpierre PC, Yancopoulos GD, Channon KM, Hale LP, Dewhirst MW, George SE, Peters KG. Antiangiogenic gene therapy targeting the endothelium-specific receptor tyrosine kinase Tie2. Proc Natl Acad Sci U S A. 1998;95(15):8829–8834. doi: 10.1073/pnas.95.15.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest. 1997;100(8):2072–2078. doi: 10.1172/JCI119740. [DOI] [PMC free article] [PubMed] [Google Scholar]


