Abstract
Bilobalide, a constituent of Ginkgo biloba, has neuroprotective properties. Its mechanism of action is unknown but it was recently found to block GABAA receptors. The goal of this study was to test the potential role of a GABAergic mechanism for the neuroprotective activity of bilobalide. In rat hippocampal slices exposed to NMDA, release of choline indicates breakdown of membrane phospholipids. NMDA-induced choline release was almost completely blocked in the presence of bilobalide (10 μM) and under low-chloride conditions. Bicuculline (100 μM), a competitive antagonist at GABAA receptors, reduced NMDA-induced choline release to a small extent (−23%). GABA (100 μM) partially antagonized the inhibitory action of bilobalide. Exposure of hippocampal slices to NMDA also caused edema formation as measured by increases of tissue water content. NMDA-induced edema formation was suppressed by bilobalide and by low-chloride conditions. Bicuculline exerted partial protection (by 30%) while GABA reduced bilobalide's effect by about one third.
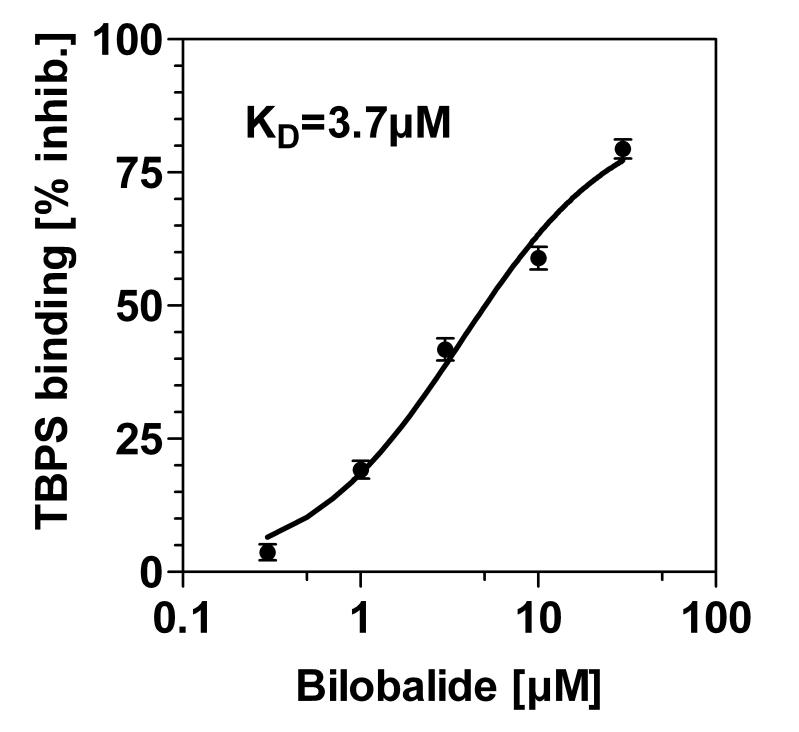
To investigate bilobalide's interaction with GABAA receptors directly, we measured binding of [35S-TBPS] to rat cortical membranes. TBPS binding was competitively inhibited by bilobalide in the low micromolar range (IC50=3.7 μM). As a functional test, we determined 36chloride flux in rat corticohippocampal synaptoneurosomes. GABA (100μM) significantly increased 36chloride flux (+65 %), and this increase was blocked by bilobalide, but with low potency (IC50: 39 μM). We conclude that, while antagonism of GABAA receptors may contribute to bilobalide's neuroprotective effects, additional mechanisms must be postulated to fully explain bilobalide's actions.
Keywords: bicuculline, edema formation, GABAA receptors, Ginkgo biloba, low-chloride condition, water content
1. Introduction
Ginkgo biloba extracts such as EGb 761 are widely used for the treatment of chronic neurodegenerative disorders such as Alzheimer's disease (Oken et al., 1998; DeFeudis and Drieu, 2000). Experimental work during the last ten years has shown that Ginkgo extracts and their constituents, such as ginkgolides and bilobalide, also exert beneficial effects in animal models of acute neurodegeneration, e.g. in cerebral hypoxia and ischemia (Krieglstein et al., 1995; Chandrasekaran et al., 2001). In an experimental model of hypoxia-induced phospholipid breakdown, we found that bilobalide, a sesquiterpene lactone which constitutes ca. 3 % of EGb761, was the active constituent of the extract (Klein et al., 1997) acting in the submicromolar range (IC50=0.38 μM). In further work, we described the antagonistic effect of bilobalide on NMDA receptor-induced choline release in the low micromolar range (IC50=2.3 μM) (Weichel et al., 1999). Other groups reported neuroprotection by bilobalide in neuronal cell cultures where it counteracted apoptotic cell death induced by amyloid (Luo et al., 2002) and serum deprivation (Ahlemeyer et al., 1999). The neuroprotective properties of bilobalide have recently been reviewed (DeFeudis, 2002; Ahlemeyer and Krieglstein, 2003).
While evidence for neuroprotective properties of bilobalide is available from a variety of models, its mechanism of action is elusive. Bilobalide has been found to interfere with glutamatergic transmission, with mitochondrial function and apoptosis and it has genomic as well as proteomic effects (DeFeudis, 2002; Ahlemeyer and Krieglstein, 2003). The best available evidence, however, points to an interference with GABAergic neurotransmission. Electrophysiological data in hippocampal slices (Sasaki et al., 1995) as well as antagonistic effects of bilobalide on barbital-induced sleeping time (Brochet et al., 1999) gave the first indirect evidence for an interference of bilobalide with GABAergic actions. Further data on GABAergic actions of bilobalide were reported by us in neurochemical models (Klein et al., 2003) and by others in electrophysiological studies (Chatterjee et al., 2003). Subsequently, it was demonstrated in careful patch-clamp studies that bilobalide is a noncompetitive antagonist (open channel blocker) at GABAA receptors in embryonic cortical slices (Ivic et al., 2003) and at recombinant GABAA receptors and GABAC receptors (Huang et al., 2003, 2006). Moreover, it was reported that bilobalide structurally resembles picrotoxin as a ligand of GABA and glycine channels (Hawthorne and Lynch, 2005).
Several groups have reported that blockade of GABAA receptors can be neuroprotective in certain situations (Erdö et al., 1991; Muir et al., 1996; Chen et al., 1999). It remains unclear, however, if the recently described GABAergic antagonism contributes to the neuroprotective properties of bilobalide. In the present communication, we tested the potential of GABAergic antagonism in two models of excitotoxicity, and we determined if bilobalide's neuroprotective effects may be due to interactions with the GABAA receptor.
2. Results
2.1 Choline release from hippocampal slices
The model of NMDA-induced choline release was established in our laboratory and can be used to follow NMDA receptor-induced excitotoxicity and membrane brakdown (Weichel et al., 1999; Klein, 2000). In the present study, basal efflux of choline from rat hippocampal slices was 1.5±0.1 pmol/10μl (N=24); it increased slightly (by 15-20%) during the experimental period (30 min; Fig. 1). Basal choline efflux was unchanged in the absence or presence of magnesium (data not shown). Upon addition of NMDA (100 μM) in magnesium-free solution, we observed an immediate, approximately two-fold increase of choline release that lasted beyond the experimental period of observation (30 min; Figs. 1 and 2).
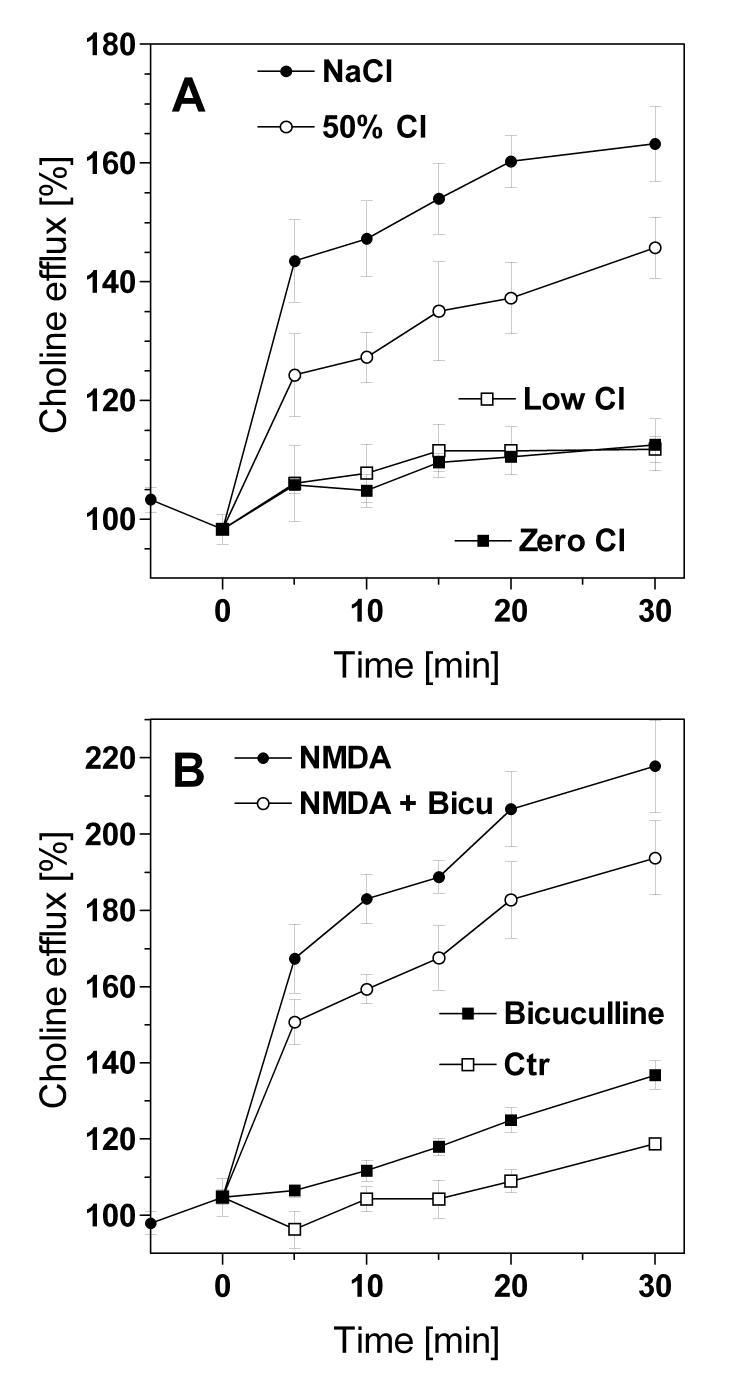
Fig. 1.
Choline release from hippocampal slices induced by NMDA receptor activation: effects of low chloride and bicuculline - Hippocampal slices were superfused with a magnesium-free solution, and the superfusion fluid was switched to one containing N-methyl-D-aspartate (NMDA, 100 μM) at time zero. Choline efflux was measured using a chemoluminescence assay. (A) Effect of NMDA in solutions with varying concentrations of chloride. In these experiments, NaCl (142 mM in controls, “NaCl”) was partially (“50% Cl”) or completely (“Low Cl”) replaced by Na2SO4 in an isoosmotic manner. For the zero-chloride (“Zero Cl”) condition, KCl and CaCl2 were additionally replaced by their nitrate salts. (B) Effect of bicuculline (100 μM), a competitive antagonist at GABAA receptors. In these experiments, choline efflux was recorded under basal conditions (“Ctr”) or in the presence of bicuculline (“Bicuculline”). When NMDA was present, it was added at time zero in the absence (“NMDA”) or presence (“NMDA + Bicu”) of bicuculline. Data are given as relative changes of the basal choline efflux (determined from three consecutive samples before addition of NMDA) and are means ± S.E.M. of 4-6 experiments.
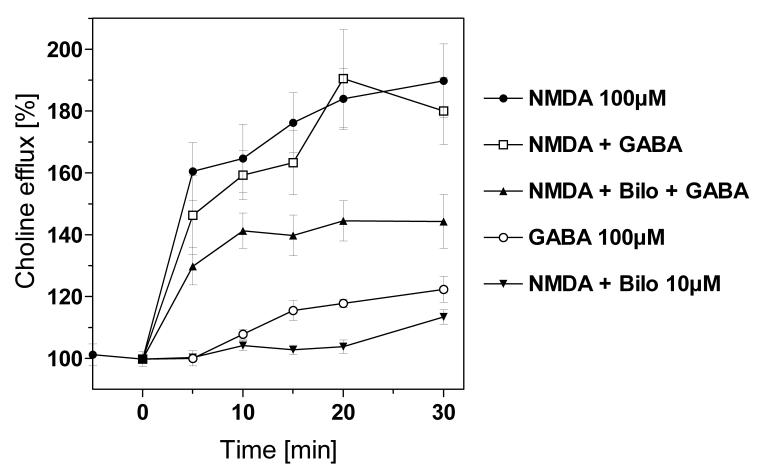
Fig. 2.
Choline release from hippocampal slices induced by NMDA receptor activation: effects of bilobalide and GABA - Hippocampal slices were superfused with a magnesium-free solution. When N-methyl-D-aspartate (NMDA) was present, it was added at time zero. Choline efflux was measured using a chemoluminescence assay. Data are as follows: “NMDA 100μM” indicates the effect of NMDA alone. “NMDA + Bilo”, “NMDA + GABA” and “NMDA + Bilo + GABA” show the responses of NMDA in the presence of bilobalide (Bilo, 10 μM) or GABA (100 μM), or both. Bilobalide and GABA, when present, were added together with NMDA. “GABA 100 μM” illustrates the effect of GABA alone, in the absence of NMDA. Bilobalide, when given alone, did not affect choline efflux (data not illustrated). Data are given as relative changes of the basal choline efflux (determined from three consecutive samples before addition of NMDA) and are means ± S.E.M. of 4-6 experiments.
In this model of NMDA receptor-mediated excitotoxicity, we tested three conditions: the effect of low chloride conditions, of bicuculline, and of bilobalide. Replacement of sodium chloride with sodium sulfate (Fig. 1A) resulted in a close to linear reduction of NMDA-induced choline release indicating a strict requirement of the chloride ion for NMDA's actions. For the quantification of effects, we calculated the “areas under the curve” (AUC) values as a measure of the total amount of free choline that was released by NMDA receptor activation during 30 min of superfusion (Table 1). Accordingly, NMDA-induced choline release was 43 % of NMDA in control buffer when half of the chloride was exchanged for sulfate; it was less than 20% with low (6.3 mM) or zero chloride solutions (Table 1).
Table 1.
Area under the curve (AUC) values from choline efflux experiments.
| Data | Condition | AUC value1 | N |
|---|---|---|---|
| Figs. 1-2 | NMDA | 2000 ± 415 | 16 |
| Fig. 1A | NMDA/50% chloride | 870 ± 282a | 4 |
| NMDA/Low chloride | 323 ± 59a | 4 | |
| NMDA/Zero chloride | 363 ± 158a | 4 | |
| Fig. 1B | NMDA + Bicuculline | 1532 ± 227b | 6 |
| Bicuculline alone | 335 ± 166c | 6 | |
| Controls | 119 ± 90b | 6 | |
| Fig. 2 | NMDA + GABA | 1844 ± 656 | 4 |
| NMDA + Bilobalide | 146 ± 149a | 5 | |
| NMDA + Bilo + GABA | 1063 ± 184a | 4 | |
| GABA alone | 370 ± 128c | 4 |
AUC values were calculated from the data shown in Figs. 1 and 2, for 30 minutes of choline release. They are given as arbitrary units (AU, formally representing [%] · min) normalized to 2000 AU for NMDA effects from different experiments (absolute values for NMDA-induced choline releases were between 1200 and 2500 AU in individual experiments). Statistical evaluation (ANOVA with Dunnett post-test):
p<0.01 vs. NMDA.
p<0.05 vs. NMDA.
p<0.05 vs. controls.
Bicuculline is a competitive antagonist at the GABAA receptor. The presence of bicuculline (100 μM) per se caused a small increase of basal choline release (Fig. 1B). When bicuculline was given together with NMDA, it reduced NMDA-induced choline release by 23% based on AUC values (p<0.05; Table 1). Bilobalide (10 μM), given alone, did not affect basal choline release (data not shown), but it suppressed NMDA-induced choline release by more than 90% (Fig. 2) (p<0.01; Table 1). GABA (100 μM) slightly increased choline release per se (p<0.05; Table 1) but did not significantly affect NMDA-induced release of choline (Fig. 2; Table 1). However, GABA partially counteracted bilobalide's effect (Fig. 2). Based on AUC values, the NMDA effect in the presence of GABA was 1474 AU (1844 minus 370); in the presence of GABA, bilobalide reduced the NMDA effect by 781 AU (1844 minus 1063) which is equivalent to a 47% reduction of this response.
In two experiments, this GABA effect was apparently blocked in the presence of picrotoxin, a well-characterized GABAA channel blocker (data not shown); however, picrotoxin interfered with the choline oxidase-based detection of choline in this assay, and further experiments with picrotoxin were not performed.
2.2 Brain edema induced by excitotoxicity
As a second model of tissue damage induced by excitotoxicity, we exposed rat hippocampal slices to NMDA and measured slice water contents as an indicator of cytotoxic edema formation. In our hands, water contents in untreated slices superfused for 30 minutes with low-magnesium Tyrode solution were 77.8 ± 0.7 % (mean ± S.D., N=18). While basal values of water contents varied somewhat (from 76-80 %), relative changes of water contents after NMDA exposure were highly reproducible (Figs. 3 and 4). We here report that the excitotoxic agent, NMDA, caused an increase of the slice water content by approximately 2 % (Figs. 3 and 4).
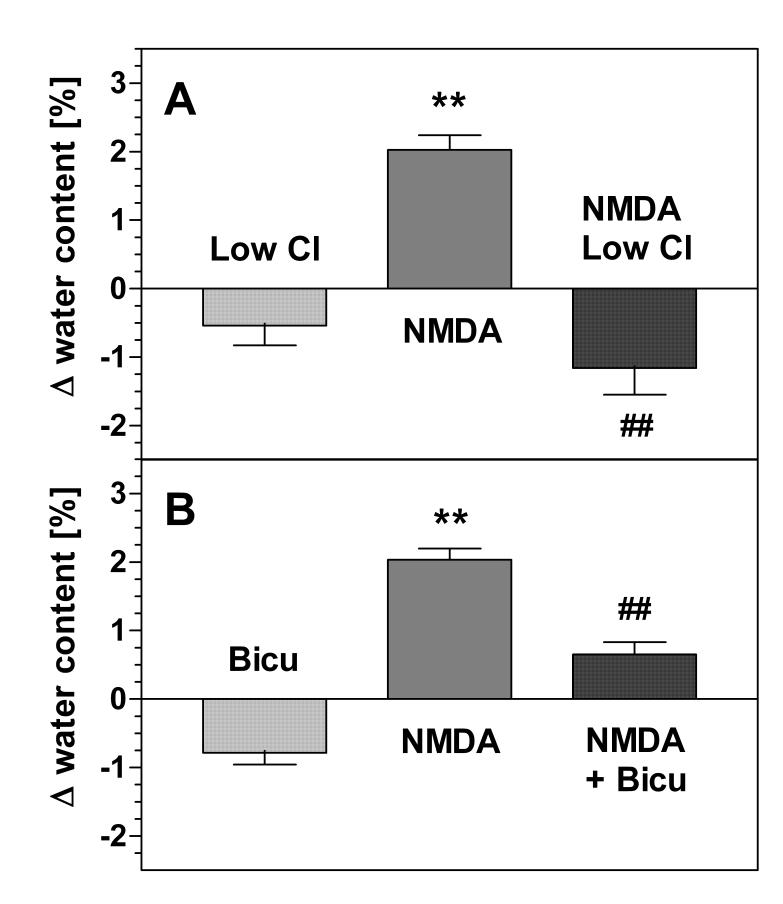
Fig. 3.
Edema formation in hippocampal slices: effects of low chloride and bicuculline. (A) Effect of low chloride. Slices were superfused for 30 min with control (low-magnesium) buffer (data not shown) or with low chloride buffer (“Low Cl”) in which NaCl was replaced by sodium sulfate keeping isoosmolarity. As indicated, slices were exposed to NMDA (300 μM) in control buffer (“NMDA”) or in low-chloride buffer (“NMDA Low Cl”). (B) Effect of bicuculline. Slices were superfused with control buffer (not shown), control buffer containing 100 μM bicuculline (“Bicu”), with NMDA (300 μM, “NMDA”) or with NMDA in the presence of bicuculline (“NMDA + Bicu”). Superfusion buffers contained 0.1% DMSO in these experiments. Water contents in the slices were determined at the end of the superfusion procedure by differential weighing before and after drying the slices. Results are expressed as differences of water contents when compared to control incubations (low magnesium buffer, no drugs). Statistical significance was evaluated by paired ANOVA. **, p<0.01 vs controls. ##, p<0.01 vs. NMDA (N=6 for each series of experiments).
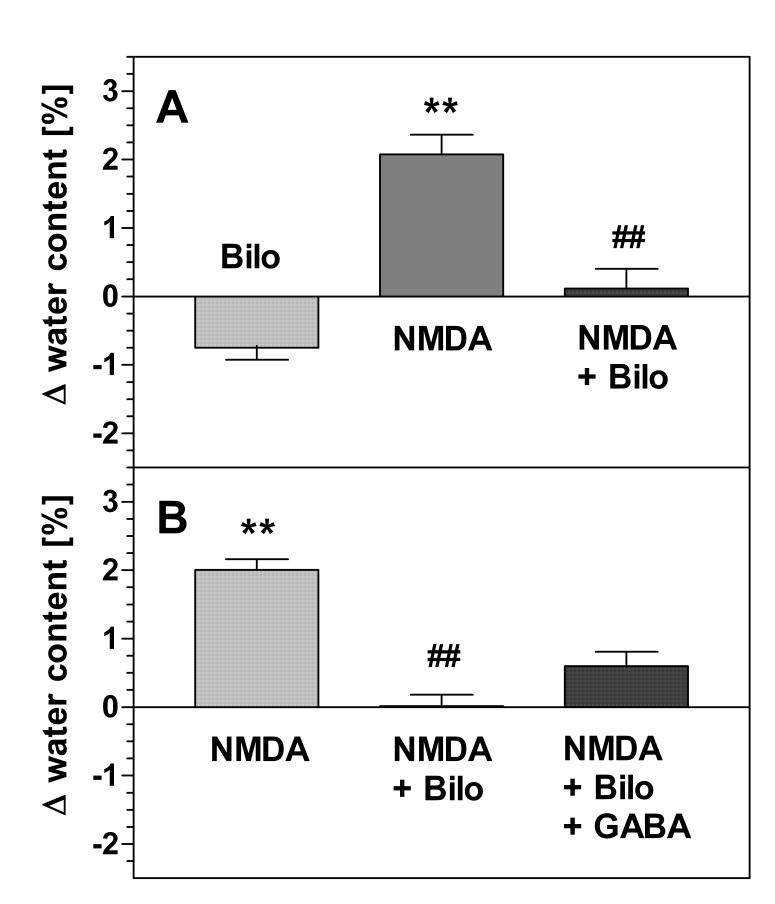
Fig. 4.
Edema formation in hippocampal slices: effects of bilobalide and GABA. (A) Effect of bilobalide. Slices were superfused for 30 min with control (low-magnesium) buffer (data not shown) or with 10 μM bilobalide (“Bilo”). As indicated, slices were exposed to NMDA (300 μM) in the absence (“NMDA”) or presence (“NMDA + Bilo”) of bilobalide. When present, bilobalide was added five minutes before NMDA. (B) Effect of bilobalide plus GABA. Slices were superfused for 30 min with control (low-magnesium) buffer (data not shown) or with buffer containing NMDA (“NMDA”, 300 μM), NMDA plus 10 μM bilobalide (“NMDA + Bilo”) or NMDA plus 10 μM bilobalide plus 100 μM GABA (“NMDA + Bilo + GABA). When present, bilobalide was added five minutes before NMDA. All superfusion buffers in (A) and (B) contained 0.1% DMSO. Water contents in the slices were determined at the end of the superfusion procedure by differential weighing before and after drying the slices. Results are expressed as differences of water contents when compared to control incubations (low magnesium buffer, no drugs). Statistical significance was evaluated by paired ANOVA. **, p<0.01 vs controls. ##, p<0.01 vs. NMDA (N=6 for each series of experiments).
In this model, we tested the same conditions as with the choline release experiments. First, we noted that superfusion with a low-chloride solution caused a slight reduction of water contents under basal conditions (p>0.05; Fig. 3A). Importantly, the low-chloride condition completely prevented NMDA-induced edema formation (Fig. 3A). Moreover, the increase of tissue water induced by NMDA (+2.02 ±0.53 %, N=6, column 2 in Fig. 3A) was reversed to a decrease when NMDA was applied under low-chloride conditions (−0.62 ± 0.86 %; N=6, calculated from columns 1 and 3 in Fig. 3A). The latter condition actually produced lower than control values (p<0.05) indicating a loss of water from the slices when the slices were exposed to NMDA under low-chloride conditions.
Bicuculline (100 μM) caused a reduction of hippocampal water contents when infused alone (p<0.05 vs. controls, Fig. 3B). More importantly, bicuculline also reduced NMDA-induced edema (Fig. 3B). In the absence of bicuculline, tissue water in NMDA-exposed slices increased by 2.04 ± 0.40 % (mean ± SEM, N=6); in the presence of bicuculline, this value changed to 1.43 ± 0.59 % (calculated from columns 1 and 3 in Fig. 3B). Thus, bicuculline reduced the NMDA effect by 30% (p=0.06, t-test).
The effects of bilobalide on excitotoxic in vitro brain edema formation are shown in Fig. 4. Bilobalide reduced basal water contents to a similar degree as bicuculline and the low-chloride condition (−0.75% vs. controls, p>0.05). It also largely prevented NMDA-induced edema formation; in fact, the NMDA-induced increase of tissue water was not significant vs. controls in the presence of bilobalide (Fig. 4A). The NMDA-induced increase of tissue water was 2.06 ± 0.70 % in control slices (Fig. 4A, column 2, N=6) and 0.87 ± 1.03 % in the presence of bilobalide (Fig. 4A, columns 1 and 3, N=6); this corresponds to a reduction of the NMDA effect by 58% (p<0.01). In a second series of experiments, we compared the effect of bilobalide in the absence and presence of GABA (100 μM). As shown in Fig. 4B, GABA partially prevented the effect of bilobalide; it restored the effect of NMDA to about 1/3 of its effect in the absence of bilobalide. In parallel experiments, GABA alone did not affect NMDA-induced edema formation (data not shown).
2.3 TBPS binding assay
The following experiments directly addressed the interaction of bilobalide with GABAA receptors. While previous work had described GABAA–antagonistic properties of bilobalide (see Introduction), binding studies had not been performed. In the present study, specific binding of the GABAA receptor ligand, [35S]-TBPS, to rat corticohippocampal membranes was completely prevented by picrotoxin (10 μM) (data not shown). [35S]-TBPS was also displaced in the presence of bilobalide which displayed a KD value of 3.7 μM (Fig. 5); the confidence interval (95%) was 2.7-4.6 μM, the correlation coefficient R2 was 0.975.
Fig. 5.
Displacement of 35S-TBPS binding by bilobalide - The binding assay was carried out in rat cortical membranes, as described in Methods. Data were from four different experiments, each averaged from incubations run in triplicates. The KD value was calculated as 3.7 ± 0.4 μM (R2=0.975).
2.4 36Chloride flux assay
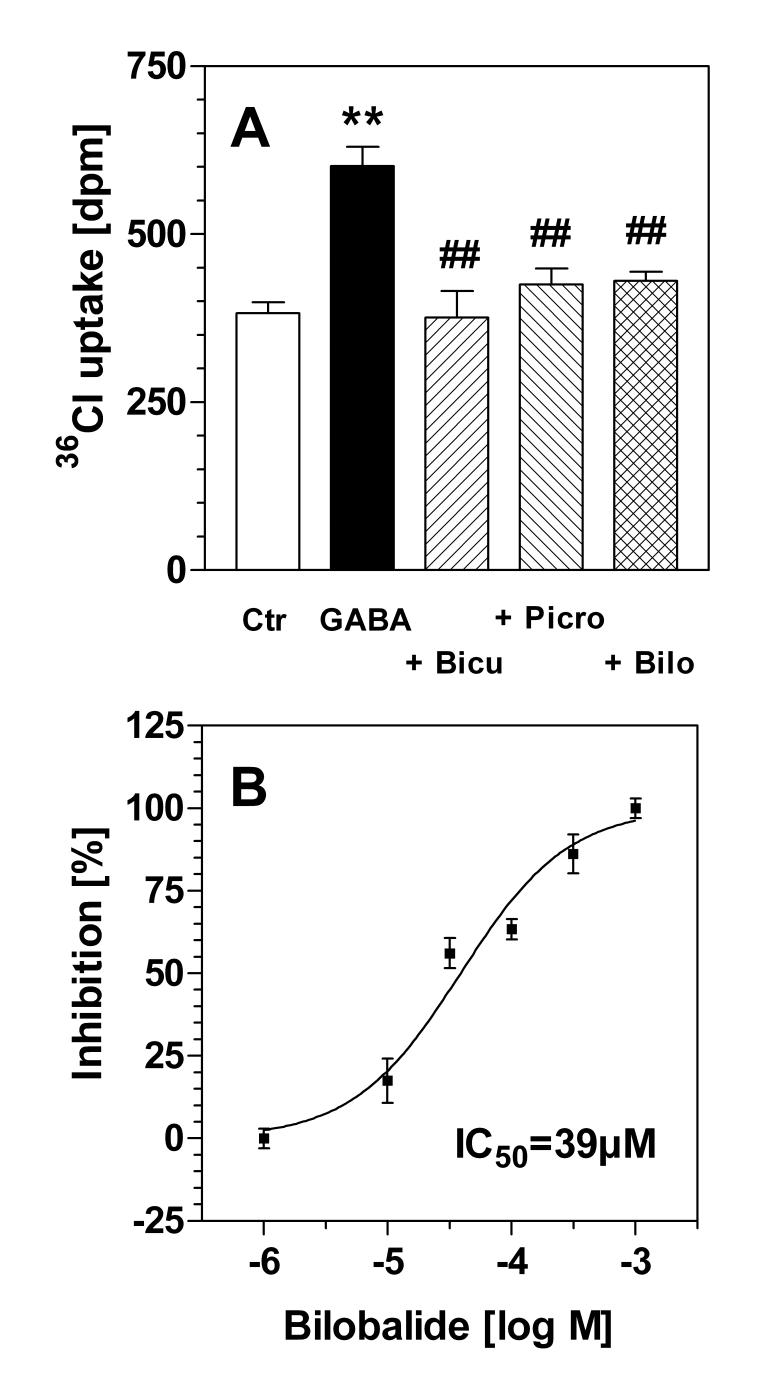
To compare the binding data with a functional assay, we measured the GABA-induced influx of chloride in corticohippocampal synaptoneurosomes. Control experiments verified that GABA (100 μM) significantly increased 36Cl uptake in synaptoneurosomes (+ 65%; p<0.01, N=12) (Fig. 6A). The GABA effect was completely inhibited in the presence of bicuculline (100 μM), a competitive antagonist of GABA at GABAA receptors (Fig. 6A). It was also inhibited by 81% in the presence of picrotoxin (100 μM). Bilobalide (1-1000 μM), in high concentrations, completely blocked GABA-induced chloride uptake in synaptoneurosomes. Its potency, however, was relatively low; the IC50 value was calculated as 39 μM (95% confidence interval: 24-63 μM; R2=0.991) (Fig. 6B).
Fig. 6.
36Chloride flux in synaptoneurosomes - Incubations were performed in rat corticohippocampal synaptoneurosomes as described in Methods. (A) 36Cl uptake under control conditions (“Ctr”), in the presence of 100 μM GABA (“GABA”), and in the presence of GABA plus bicuculline (“Bicu”, 100 μM), picrotoxin (“Picro”, 100 μM), or bilobalide (“Bilo”, 100 μM). Shown are data ± SEM from N=6-12 separate experiments run in triplicate. **, p<0.01 vs control (Ctr). ##, p<0.01 vs. GABA. (B) Inhibition of 36Cl flux induced by GABA (100 μM) in the presence of bilobalide. The IC50 for bilobalide was calculated from six individual experiments by non-linear regression (sigmoidal dose-response function) as 39 μM (confidence interval: 24-63 μM; R2=0.99).
3. Discussion
The goal of the present project was to investigate the interaction of bilobalide with GABAA receptors, and to test the contribution of GABAergic blockade by bilobalide to its neuroprotective actions. The project was inspired by recent reports showing that bilobalide blocks GABAergic responses in electrophysiological experiments (see Introduction). We here applied two assays of cellular toxicity to screen conditions of low chloride availability, GABAA receptor blockade, and GABA-bilobalide interactions.
3.1 Neuroprotective actions of bilobalide in assays of excitotoxicity
Choline release secondary to NMDA receptor activation was previously found to depend on calcium influx and activation of phospholipase A2, leading to hydrolysis of phosphatidylcholine and membrane breakdown, a typical consequence of excitotoxicity (Weichel et al., 1999; Klein, 2000). Edema formation in hippocampal slices was previously reported to occur upon exposure of slices to ischemia (oxygen-glucose deprivation), and the model has been used to investigate the roles of various receptors and ion channels which are linked to ischemia-induced edema formation (LoPachin et al., 2001; MacGregor et al., 2003). We here report that this model is also useful to measure excitotoxicity induced by exposure to NMDA (in low-magnesium buffer). In our hands, water contents of untreated slices varied between 76 and 80 %, but the NMDA-induced increases were highly reproducible (cf. Figs. 3 and 4).
We first used low-chloride conditions to test the idea that interference with chloride influx (e.g. through GABAA receptors) would indeed modulate excitotoxicity. NMDA receptor activation is known to cause influx of chloride ions in hippocampal slices (Inglefield and Schwartz-Bloom, 1998), and NMDA receptor-mediated neuronal cell death is known to depend on chloride influx (Rothman, 1985; Olney et al., 1986; Choi, 1987). The influx pathways of chloride following NMDA receptor activation have not been conclusively investigated, however, and the role of GABAA receptors and other chloride influx pathways are under scrutiny (Takahashi et al., 1995; Hasbani et al., 1998; Sun and Murali, 1998; Schwartz-Bloom and Sah, 2001). In the present study, NMDA-induced choline release was found to be strictly dependent on extracellular chloride; partial or complete substitution of chloride with sulfate (which does not pass chloride channels) was found to cause a corresponding decrease of choline release (Fig. 1A). In agreement with the choline release experiments, absence of chloride in the superfusion buffer also completely blocked NMDA-induced edema formation (Fig. 3A). In fact, NMDA even caused a reduction of slice water contents during low-chloride conditions (Fig. 3A), a finding that may be explained by reversal of the chloride gradient across the cell membrane under lowchloride conditions (Schwartz-Bloom and Sah, 2001). While more work is required to confirm this speculation, our data with low-chloride solutions confirm that blockade of chloride influx is a potential mechanism of action for neuroprotective drugs in assays of excitotoxicity.
Bicuculline, the competitive antagonists at GABAA receptors, was used to test the contribution of endogenous GABA on NMDA-induced excitotoxicity. NMDA receptor activation is known to induce GABA release from hippocampal neurons (Janaky et al., 1993; Fontana et al., 1997). In the choline release assay, bicuculline caused a significant decrease of NMDA-induced choline release by 23 % (p<0.05; Fig. 1B, Table 1). Bicuculline showed similar activity in the edema assay where it reduced NMDA-induced edema formation by 30 % (Fig. 3B). These data indicate that GABAA receptors likely contribute to NMDA-induced excitotoxicity and chloride influx under our experimental conditions; however, other chloride influx pathways that are not blocked by bicuculline must also be operative.
Bilobalide, the drug under study, completely blocked NMDA-induced choline release (Fig. 2), a finding that is in agreement with previous reports from our group (Weichel et al., 1999; Klein et al., 2003). We now report that bilobalide also reduces NMDA-induced edema formation. In fact, bilobalide completely prevented NMDA-induced edema formation when compared to control slices. When its effect on basal water content was taken into account (Fig. 2A), the inhibition of NMDA-induced edema (in the absence vs. in the presence of NMDA) by bilobalide was calculated as 58 % (p<0.01; see Results). Effects of bilobalide on basal water contents may be explained by the fact that freshly prepared hippocampal slices are known to undergo some swelling even under control conditions. This process is accompanied by uptake of sodium, chloride, and water (Siklos et al., 1997), and it was attenuated by low-chloride conditions, bicuculline, and bilobalide in the present study (cf. Figs. 3 and 4).
To test if bilobalide's effects were mediated by GABA receptor blockade, we carried out another set of experiments in which bilobalide's actions were tested in the presence of GABA. In these experiments, GABA was found to partially antagonize bilobalide's inhibitory actions. In both assays – choline release and edema formation - the presence of GABA reduced bilobalide's effect although bilobalide was still inhibitory even in the presence of a high concentration (100 μM) of GABA. In the choline release assay, bilobalide was more effective inhibiting the NMDA effect than either bicuculline or bilobalide plus GABA combined (Fig. 2 and Table 1). The same observation was made in the edema assay (Fig. 4B).
3.2 GABAA receptor binding and blockade by bilobalide
As the excitotoxicity assays indicated a potential relevance for the interaction of bilobalide with GABAA receptors, we decided to directly measure GABAergic activities of bilobalide by two approaches, the TBPS binding assay and the 36chloride flux assay. TBPS (t-butylbicyclo-phosphorothionate) is a synthetic substance that binds with high affinity (KD=25 nM) in the GABAA-associated chloride channel (Squires et al., 1983). Competition with TBPS binding is often used to characterize GABAA channel blockers (Holland et al., 1993; Vogel and Vogel, 2002). In this study, bilobalide was found to displace [35S]-TBPS in rat cortical membranes with a IC50 value of 3.7 μM (Fig. 5). The ability of bilobalide to displace TBPS corresponds to its structural similarity to picrotoxin, another compound binding the GABAA receptor channel (Ivic et al., 2003; Hawthorne and Lynch, 2005). The IC50 value determined for bilobalide in this assay (3.7 μM, Fig. 5) is reasonably close to the value reported for blockade of recombinant GABAA receptors by bilobalide (4.6 μM; Huang et al., 2003) and to our earlier data showing blockade of NMDA-induced choline release (2.3 μM; Weichel et al., 1999).
Blockers of the GABAA receptor, such as picrotoxin or bicuculline, are well-known convulsants. In contrast, bilobalide, in spite of its apparent GABAergic antagonism, is totally inactive as a convulsant even in high doses; it actually exhibits some anti-convulsant properties (Sasaki et al., 1995; Weichel et al., 1999; DeFeudis, 2002). To elucidate this apparent contradiction, we decided to carry out a functional assay of GABAA receptor activity, namely the 36Cl flux assay in synaptoneurosomes (Harris and Allan, 1985; Bloomquist and Soderlund, 1985). In our hands, GABA induced a significant increase of 36Cl uptake into corticohippocampal synaptoneurosomes which was blocked in the presence of bicuculline and picrotoxin (Fig. 6A). Bilobalide was found to block GABA-induced 36Cl uptake (Fig. 6B); however, the IC50 value was high at 39 μM. This value is at variance with the TBPS binding assay but it resembles the concentration of bilobalide which was required to block GABAergic currents in rat cortical neurons (46 μM; Ivic et al., 2003). We conclude that bilobalide binding to the GABAA receptor is not sufficient to block chloride flux through the receptor pore. Bilobalide is a naturally occurring γ-butyrolactone, and its behaviour at GABAA receptors resembles findings with synthetic γ-butyrolactones in earlier studies that displaced TBPS binding without blocking GABAergic function (Weissman et al., 1984; Holland et al., 1990). Later work with synthetic γ-butyrolactones demonstrated that they bind at sites that are allosterically coupled to the TBPS binding site (Holland et al., 1993). We speculate that bilobalide may also bind to an allosteric site on the GABAA receptor thereby avoiding direct interference with chloride flux.
Summarizing, our data indicate that bilobalide binds GABAA receptors with an affinity in the low micromolar range but that blockade of GABAA receptor function, as reflected in chloride flux, is only achieved at ten-fold higher concentrations. This type of behaviour explains the lack of convulsive activity of the drug because concentrations above 10 μM likely will not be reached in vivo. While there are no pharmacokinetic studies with pure bilobalide, rats dosed orally with 30-100 mg/kg Ginkgo extract EGb761 (which contains 3 % bilobalide) had plasma levels of bilobalide of 0.5-1.3 μM (Biber, 2003). Thus, activities of bilobalide observed in the low micromolar range may be therapeutically relevant while much higer concentrations are probably irrelevant for therapeutic use.
Our current findings indicate that GABAergic antagonism plays a minor role in the neuroprotective properties of bilobalide. First, bilobalide has low potency at inhibiting chloride fluxes through the GABAA receptor channel. Second, bilobalide was consistently more active than bicuculline in the excitotoxicity assays. Moreover, the concentration of bilobalide used in the excitotoxicity assays (10 μM) would not be expected to inhibit GABAergic chloride flux by more than 20% whereas bilobalide's blocking actions on the NMDA effects were consistently above 50%. Thus, while the well-known GABAA receptor antagonism may contribute to bilobalide's neuroprotective effects to a small extent, additional activities of bilobalide must be postulated to explain the bulk of its neuroprotective effects. One possibility would be blockade of other chloride channels because the low-chloride conditions were equally effective as bilobalide in both assays of excitotoxicity. Alternative mechanisms of action of bilobalide are currently being investigated in our laboratory.
4. Experimental procedures
4.1 Materials
Bilobalide was isolated in 99 % purity from Ginkgo biloba leaves as described (Weinges and Bähr, 1969) and was made available by Dr. Michael Nöldner (Dr. Willmar Schwabe Pharmaceuticals, Karlsruhe, Germany). [35S]-TBPS (NEG049; 2 mCi/ml) was from Perkin Elmer (Boston, MA). 36Cl as sodium chloride (ARX-104; 0.6 Ci/mol) was from American Radiolabeled Chemicals (St. Louis, MO). All other chemicals were from Sigma at the highest purity available.
4.2 Animals
Male Sprague-Dawley rats (250-350g; Charles River) were kept under standardised light/dark (12h), temperature (22°C) and humidity (70 %) conditions, with rat chow and water available ad libitum. Animal procedures were in accordance with NIH regulations and were registered with the Institutional Animal Care and Use Committee of TTUHSC (protocol #04003-02).
4.3 Choline release from rat hippocampal slices
Hippocampal slices (400 µm) were prepared from male rats as previously described (Klein et al., 1997; Weichel et al., 1999) and superfused (0.7ml/min) at 35°C with Tyrode solution of the following composition: 142 mM NaCl, 2.7 mM KCl, 1.8 mM CaCl2, 1.2 mM MgCl2, 11.9 mM NaHCO3, 5.6 mM glucose. All superfusion solutions were continuously gassed with carbogen (95% O2, 5% CO2). The slices were first incubated with 0.1 mM di-isopropyl-fluorophosphate (DFP) for 30 minutes in order to prevent choline release from acetylcholine by the action of acetylcholinesterase. Subsequently, the slices were washed for 40 minutes with magnesium-free Tyrode solution, and basal choline efflux was determined. Then, the superfusion solutions were switched to those containing NMDA (100 μM) and/or bilobalide (10 μM) or GABA (100 μM) in magnesium-free Tyrode solution. For low chloride conditions, sodium chloride was partially or completely replaced by sodium sulfate (osmolarity was kept constant). For zero chloride conditions, KCl and CaCl2 were additionally replaced by their respective nitrate salts.
The superfusates were collected at 5 or 10 min intervals and analyzed for choline content. Choline was determined by a chemoluminescence assay (Klein et al., 1997; Weichel et al., 1999). Briefly, 10 μl aliquots of the superfusates were given to a reaction mixture consisting of 20 mM Tris buffer pH 8.6, 1 μg luminol, 10 μg peroxidase, and 1.25 U choline oxidase, and the chemiluminescence resulting from oxidation of choline to betaine was measured at 425 nm in a LKB-Wallac luminometer. The assay was linear from 1-5 pmol choline. The data for choline efflux (Fig. 2) are expressed in % of basal choline efflux which was 71.6 ± 5.4 pmol/min/mg protein (n=24). Three values for choline efflux were taken at 30, 20 and 10 minutes before addition of NMDA, and the average of these three values was set as 100% and used to calculate the relative changes of choline efflux after addition of NMDA in Figs. 1 and 2. Areas under the curve (AUC) values were calculated for each individual experiment; they represent the amount of choline that was released by NMDA receptor activation during 30 min of superfusion.
4.4 Edema formation in hippocampal slices
Rat hippocampal slices were prepared and superfused with Tyrode solution as described above. During the equilibration period, all superfusion solutions were continuously gassed with carbogen (95% O2, 5% CO2). To induce cytotoxic edema formation, the slices were superfused with NMDA (300 μM), and the concentration of magnesium chloride in the Tyrode solution was lowered to 1/10 (0.12 mM). Bilobalide, when used, was pre-incubated for 5 min before NMDA was added. Bicuculline and GABA were added together with NMDA. Stock solutions of bicuculline and bilobalide were prepared in DMSO; during superfusion of slices with these compounds, all solutions contained 0.1% DMSO. Four lanes of slices were superfused in parallel for 30 minutes. At the end of the superfusion period, slices from each lane were collected, superficially dried, transferred to aluminum foil, and weighed (“wet weight”). They were then dried over night at 105°C in a desiccating oven and weighed again (“dry weight”). Total tissue brain water was calculated according to [(wet weight – dry weight)/ wet weight] × 100.
4.5 TBPS binding assay
Preparation of rat corticohippocampal membranes and measurement of [35S-]TBPS binding were carried out as previously described (Squires et al., 1983; Vogel and Vogel, 2002). Briefly, aliquots of membranes were incubated with [35S]-TBPS (2 nM) in the presence of bilobalide (0.3-30 μM, dissolved in DMSO). Incubations with DMSO were used as negative controls, those with non-labelled TBPS (10 μM) to determine specific binding; specific binding was 77-85% of total binding. After incubation at 25°C for 150 min, the assay was terminated by rapid filtration over Whatman GF/B filters, followed by four washes (5 ml each) with incubation buffer (5 mM Tris buffer, pH 7.4, with 0.1 M KCl). Radioactivity on filters was counted in a Beckman Coulter LS 6000 scintillation counter. All experiments were done in triplicate.
4.6 36Chloride flux assay in synaptoneurosomes
Synaptoneurosomes were prepared from rat corticohippocampal tissue essentially as described (Hollingsworth et al., 1985; Bloomquist and Soderlund, 1985). For 36Cl uptake assays, corticohippocampal vesicles were suspended in buffer A (20 mM HEPES buffer pH 7.4 with 54 mM glucose, 118 mM NaCl, 4.7 mM KCl, 1.2 mM MgSO4, 2.5 mM CaCl2), and chemicals (bilobalide; DMSO for negative control; picrotoxin and bicuculline for positive controls) were added and preincubated for 10 min at room temperature. Vials were then transferred to a heating block and incubated for 60 sec at 30°C in the presence of 100 μM DIDS and 10 μM furosemide (Bloomquist and Soderlund, 1985). Aliquots of the incubations were then rapidly mixed with buffer B (composition identical to buffer A, except that NaCl was 145 mM and glucose was 27 mM) containing 36chloride (1 μCi/ml). GABA (100 μM), when present, was added to buffer B. After 5 sec of incubation, the assay tubes (0.4 ml) were mixed with a surplus (4 ml) of ice-cold buffer B and rapidly filtered over GF/C Whatman filters in a vacuum filter unit. Filters were washed twice, dried and counted for radioactivity in a Beckman Coulter LS 6000 scintillation counter.
4.7 Statistics
Statistical calculations were performed by GraphPad InStat 3.0 program package, using analysis of variance (ANOVA) of paired or unpaired data as indicated in text and figure legends. Curve fitting and calculation of inhibition constants (Figs 5 and 6) was done by nonlinear regression using GraphPad Prism 3.0.
Acknowledgments
The authors are grateful to Drs. Shyam S. Chatterjee and Michael Nöldner, Dr. Willmar Schwabe Pharmaceuticals (Karlsruhe, Germany) for helpful discussions and for supplying pure bilobalide; to Markus Hillert for expert assistance with brain slices; and to the Alzheimer Association, the National Center for Complementary and Alternative Medicine, and to Texas Tech University Health Science Center (Cardiovascular Seed Grant) for financial support.
Abbreviations
- AU
arbitrary units
- AUC
area under the curve
- GABA
γ-aminobutyric acid
- NMDA
N-methyl-d-aspartate
- TBPS
t-butylbicyclo-phosphorothionate
Footnotes
Section: Neurophysiology, Neuropharmacology and other forms of Intercellular Communication
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahlemeyer B, Krieglstein J. Neuroprotective effects of Ginkgo biloba extract. Cell. Mol. Life Sci. 2003;60:1779–1792. doi: 10.1007/s00018-003-3080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlemeyer B, Möwes A, Krieglstein J. Inhibition of serum deprivation- and staurosporine-induced neuronal apoptosis by Ginkgo biloba extract and some of its constituents. Eur. J. Pharmacol. 1999;367:423–430. doi: 10.1016/s0014-2999(98)00903-0. [DOI] [PubMed] [Google Scholar]
- Biber A. Pharmacokinetics of Ginkgo biloba extracts. Pharmacopsychiatry. 2003;36(Suppl 1):S32–S37. doi: 10.1055/s-2003-40446. [DOI] [PubMed] [Google Scholar]
- Bloomquist JR, Soderlund DM. Neurotoxic insecticides inhibit GABA-dependent chloride uptake by mouse brain vesicles. Biochem. Biophys. Res. Commun. 1985;133:37–43. doi: 10.1016/0006-291x(85)91838-8. [DOI] [PubMed] [Google Scholar]
- Brochet D, Chermat R, DeFeudis FV, Drieu K. Effects of single intraperitoneal injections of an extract of Ginkgo biloba (EGb761) and its terpene trilactone constituents on barbital-induced narcosis in the mouse. Gen. Pharmacol. 1999;33:249–256. doi: 10.1016/s0306-3623(99)00018-x. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran K, Mehrabian Z, Spinnewyn B, Drieu K, Fiskum G. Neuroprotective effects of bilobalide, a component of the Ginkgo biloba extract (EGb761), in gerbil global brain ischemia. Brain Res. 2001;922:282–292. doi: 10.1016/s0006-8993(01)03188-2. [DOI] [PubMed] [Google Scholar]
- Chen Q, Moulder K, Tenkova T, Hardy K, Olney JW, Romano C. Excitotoxic cell death dependent on inhibitory receptor activation. Exp. Neurol. 1999;160:215–225. doi: 10.1006/exnr.1999.7179. [DOI] [PubMed] [Google Scholar]
- Chatterjee SS, Kondratskaya EL, Krishtal OA. Structure-activity studies with Ginkgo biloba extract constituents as receptor-gated chloride channel blockers and modulators. Pharmacopsychiatry. 2003;36(Suppl 1):S68–S77. doi: 10.1055/s-2003-40455. [DOI] [PubMed] [Google Scholar]
- Choi DW. Ionic dependence of glutamate neurotoxicity. J. Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeudis FV. Bilobalide and neuroprotection. Pharmacol. Res. 2002;46:565–568. doi: 10.1016/s1043-6618(02)00233-5. [DOI] [PubMed] [Google Scholar]
- DeFeudis FV, Drieu K. Ginkgo biloba extract (EGb761) and CNS functions: basic studies and clinical applications. Curr. Drug Targets. 2000;1:25–58. doi: 10.2174/1389450003349380. [DOI] [PubMed] [Google Scholar]
- Erdö SL, Michler A, Wolff JR. GABA accelerates excitotoxic cell death in cortical cultures: Protection by blockers of GABA-gated chloride channels. Brain Res. 1991;542:254–258. doi: 10.1016/0006-8993(91)91575-l. [DOI] [PubMed] [Google Scholar]
- Fontana G, Valenti L, Raiteri M. Gp120 can revert antagonism at the glycine site of NMDA receptors mediating GABA release from cultured hippocampal neurons. J. Neurosci. Res. 1997;49:732–738. doi: 10.1002/(SICI)1097-4547(19970915)49:6<732::AID-JNR7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Harris RA, Allan AM. Functional coupling of gamma-aminobutyric acid receptors to chloride channels in brain membranes. Science. 1985;228:1108–1110. doi: 10.1126/science.2581319. [DOI] [PubMed] [Google Scholar]
- Hasbani MJ, Hyrc KL, Faddis BT, Romano C, Goldberg MP. Distinct roles for sodium, chloride, and calcium in excitotoxic dendritic injury and recovery. Exp. Neurol. 1998;154:241–258. doi: 10.1006/exnr.1998.6929. [DOI] [PubMed] [Google Scholar]
- Hawthorne R, Lynch JW. A picrotoxin-specific conformational change in the glycine receptor M2-M3 loop. J. Biol. Chem. 2005;280:35836–35843. doi: 10.1074/jbc.M506645200. [DOI] [PubMed] [Google Scholar]
- Holland KD, Bouley MG, Covey DF, Ferrendelli JA. Alkyl-linked γ-butyrolactones act at a distinct site allosterically linked to the TBPS/picrotoxinin site on the GABAA receptor complex. Brain Res. 1993;615:170–174. doi: 10.1016/0006-8993(93)91128-f. [DOI] [PubMed] [Google Scholar]
- Holland KD, Ferrendelli JA, Covey DF, Rothman SM. Physiological regulation of the picrotoxin receptor by γ-butyrolactones and γ-thiobutyrolactones in cultured hippocampal neurons. J. Neurosci. 1990;10:1719–1727. doi: 10.1523/JNEUROSCI.10-06-01719.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth EB, McNeal ET, Burton JL, Williams RJ, Daly JW, Creveling CR. Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex. J. Neurosci. 1985;5:2240–2253. doi: 10.1523/JNEUROSCI.05-08-02240.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SH, Duke RK, Chebib M, Sasaki K, Wada K, Johnston GAR. Bilobalide,a sesquiterpene trilactone from Ginkgo biloba, is an antagonist at recombinant α1ß2γ2L GABAA receptors. Eur. J. Pharmacol. 2003;464:1–8. doi: 10.1016/s0014-2999(03)01344-x. [DOI] [PubMed] [Google Scholar]
- Huang SH, Duke RK, Chebib M, Sasaki K, Wada K, Johnston GAR. Mixed antagonistic effects of bilobalide at ρ1 GABAC receptor. Neuroscience. 2006;137:807–817. doi: 10.1016/j.neuroscience.2005.08.071. [DOI] [PubMed] [Google Scholar]
- Inglefield JR, Schwartz-Bloom RD. Activation of excitatory amino acid receptors in the rat hippocampal slice increases intracellular Cl− and cell volume. J. Neurochem. 1998;71:1396–1404. doi: 10.1046/j.1471-4159.1998.71041396.x. [DOI] [PubMed] [Google Scholar]
- Ivic L, Sands TT, Fishkin N, Nakanishi K, Kriegstein AR, Stromgaard K. Terpene trilactones from Ginkgo biloba are antagonists of cortical glycine and GABA(A) receptors. J. Biol. Chem. 2003;278:49279–49285. doi: 10.1074/jbc.M304034200. [DOI] [PubMed] [Google Scholar]
- Janaky R, Saransaari P, Oja SS. Release of GABA from rat hippocampal slices: involvement of quisqualate/N-methyl-D-aspartate-gated ionophores and extracellular magnesium. Neuroscience. 1993;53:779–785. doi: 10.1016/0306-4522(93)90623-n. [DOI] [PubMed] [Google Scholar]
- Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J. Neural Transm. 2000;107:1027–1063. doi: 10.1007/s007020070051. [DOI] [PubMed] [Google Scholar]
- Klein J, Chatterjee SS, Löffelholz K. Phospholipid breakdown and choline release under hypoxic conditions: inhibition by bilobalide, a constituent of Ginkgo biloba. Brain Res. 1997;755:347–350. doi: 10.1016/s0006-8993(97)00239-4. [DOI] [PubMed] [Google Scholar]
- Klein J, Weichel O, Hilgert M, Rupp J, Chatterjee SS, Nawrath H. Excitotoxic hippocampal membrane breakdown and its inhibition by bilobalide: role of chloride fluxes. Pharmacopsychiatry. 2003;36(Suppl 1):S78–S83. doi: 10.1055/s-2003-40453. [DOI] [PubMed] [Google Scholar]
- Krieglstein J, Ausmeier F, El-Abhar H, Lippert K, Welsch M, Ruppalla K, HenrichNoack P. Neuroprotective effects of Ginkgo biloba constituents. Eur. J. Pharm. Sci. 1995;3:39–48. [Google Scholar]
- LoPachin RM, Gaughan CL, Lehning EJ, Weber ML, Taylor CP. Effects of ion channel blockade on the distribution of Na, K, Ca and other elements in oxygen-glucose deprived CA1 hippocampal neurons. Neuroscience. 2001;103:971–983. doi: 10.1016/s0306-4522(01)00035-5. [DOI] [PubMed] [Google Scholar]
- Luo Y, Smith JV, Paramavisam V, Burdick A, Curry KJ, Buford JP, Khan I, Netzer WJ, Xu H, Butko P. Inhibition of amyloid-ß aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA. 2002;99:12197–12202. doi: 10.1073/pnas.182425199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor DG, Avshalumov MV, Rice ME. Brain edema induced by in vitro ischemia: causal factors and neuroprotection. J. Neurochem. 2003;85:1402–1411. doi: 10.1046/j.1471-4159.2003.01772.x. [DOI] [PubMed] [Google Scholar]
- Muir JK, Lobner D, Monyer H, Choi DW. GABAA receptor activation attenuates excitotoxicity but exacerbates oxygen-glucose deprivation-induced neuronal damage in vitro. J. Cereb. Blood Flow Metab. 1996;16:1211–1218. doi: 10.1097/00004647-199611000-00015. [DOI] [PubMed] [Google Scholar]
- Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch. Neurol. 1998;55:1409–1415. doi: 10.1001/archneur.55.11.1409. [DOI] [PubMed] [Google Scholar]
- Olney JW, Price MT, Samson L, Labruyere J. The role of specific ions in glutamate neurotoxicity. Neurosci. Lett. 1986;65:65–71. doi: 10.1016/0304-3940(86)90121-7. [DOI] [PubMed] [Google Scholar]
- Rothman SM. The neurotoxicity of excitatory amino acids is produced by passive chloride influx. J. Neurosci. 1985;5:1483–1489. doi: 10.1523/JNEUROSCI.05-06-01483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki K, Hatta S, Wada K, Ohshika H, Haga M. Anticonvulsant activity of bilobalide, a sesquiterpene in Ginkgo biloba L. Leaves against chemical-induced and electric shock-induced convulsions in mice. Res. Commun. Biol. Psychol. Psych. 1995;20:145–156. [Google Scholar]
- Sasaki K, Oota I, Wada K, Inomata K, Ohshika H, Haga M. Effects of bilobalide, a sesquiterpene in Ginkgo biloba leaves, on population spikes in rat hippocampal slices. Comp. Biochem. Physiol. 1995;C 124:315–321. doi: 10.1016/s0742-8413(99)00082-1. [DOI] [PubMed] [Google Scholar]
- Schwartz-Bloom RD, Sah R. γ-Aminobutyric acidA neurotransmission and cerebral ischemia. J. Neurochem. 2001;77:353–371. doi: 10.1046/j.1471-4159.2001.00274.x. [DOI] [PubMed] [Google Scholar]
- Siklos L, Kuhnt U, Parducz A, Szerdahelyi P. Intracellular calcium redistribution accompanies changes in Na+, K+ and water during the first two hours of in vitro incubation of hippocampal slices. Neuroscience. 1997;79:1013–1022. doi: 10.1016/s0306-4522(97)00031-6. [DOI] [PubMed] [Google Scholar]
- Squires RF, Casida JE, Richardson M, Saederup E. [35S]t-Butylbicyclo-phosphorothionate binds with high affinity to brain-specific sites coupled to γ-aminobutyric acid-A and ion recognition sites. Mol. Pharmacol. 1983;23:326–336. [PubMed] [Google Scholar]
- Sun D, Murali SG. Stimulation of Na+-K+-2Cl− cotransporter in neuronal cells by excitatory neurotransmitter glutamate. Am. J. Physiol. 1998;275:C772–C779. doi: 10.1152/ajpcell.1998.275.3.C772. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Liou SY, Kunihara M. Ca2+ and Cl− dependent, NMDA receptor-mediated neuronal death induced by depolarization in rat hippocampal organotypic cultures. Brain Res. 1995;675:249–256. doi: 10.1016/0006-8993(95)00078-5. [DOI] [PubMed] [Google Scholar]
- Vogel HG, Vogel WH. E.3.1.7: [35S]-TBPS binding in rat cortical homogenates and sections. Springer electronic media; Heidelberg, Germany: 2002. Drug discovery and evaluation: Pharmacological Assays. [Google Scholar]
- Weichel O, Hilgert M, Chatterjee SS, Lehr M, Klein J. Bilobalide, a constituent of Ginkgo biloba, inhibits NMDA-induced phospholipase A2 activation and phospholipid breakdown in rat hippocampus. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:609–615. doi: 10.1007/s002109900131. [DOI] [PubMed] [Google Scholar]
- Weinges K, Bähr W. Kondensierte Ringsysteme II. Bilobalid, ein neues Sesquiterpen mit tertiärer Butylgruppe aus den Blättern von Ginkgo biloba L. Liebig's Ann. Chem. 1969;724:214–216. [Google Scholar]
- Weissman BA, Burke TR, Jr., Rice KC, Skolnick P. Alkyl-substituted γ-butyrolactones inhibit [35S]TBPS binding to a GABA linked chloride ionophor. Eur. J. Pharmacol. 1984;105:195–196. doi: 10.1016/0014-2999(84)90669-1. [DOI] [PubMed] [Google Scholar]