Abstract
The basal ganglia are thought to participate in implicit sequence learning. However, the exact nature of this role has been difficult to determine in light of the conflicting evidence on implicit learning in subjects with Parkinson’s disease (PD). We examined the performance of PD subjects using a modified form of the serial reaction time task, which ensured that learning remained implicit. Subjects with predominantly right-sided symptoms were trained on a 12-element sequence using the right hand. Although there was no evidence of sequence learning on the basis of response time savings, the subjects showed knowledge of the sequence when performance was assessed in terms of the number of errors made. This effect transferred to the left (untrained) hand as well. Thus, these data demonstrate that PD patients are not impaired at implicitly learning sequential order, but rather at the translation of sequence knowledge into rapid motor performance. Furthermore, the results suggest that the basal ganglia are not essential for implicit sequence learning in PD.
Keywords: sequence learning, parkinson’s disease, implicit, serial reaction time
1. Introduction
Implicit learning is characterized by a lack of awareness for the learning process and its content (cf. Cleeremans, 1993; Reber, 1993). Several investigators have used the serial reaction time (SRT) task to study implicit motor learning (Nissen and Bullemer, 1987; Willingham et al., 1989; Keele et al., 1995; Grafton et al., 1998; Willingham et al., 1997, Seidler et al., 2002). Typically, in the SRT task subjects respond to one of four illuminated display boxes by pressing the corresponding button on a keypad. A repeating sequence is presented without the subject being aware of it. With practice, the subjects show learning of the sequence as evidenced by a progressively shorter response time (RT) compared to randomly presented stimuli.
The basal ganglia and their cortical projections have been implicated as neural substrates in implicit learning (Rauch et al., 1997; Doyon et al., 1996; Willingham et al., 2002; Seidler et al., 2002, 2005). However, inconsistency of impairments observed in patients with Parkinson’s disease (PD), who have significant dysfunction of the basal ganglia, have raised the issue of whether these structures play a crucial role in implicit sequence learning or merely modulate the expression of the learning. Although some studies have found these patients to have profound implicit learning deficits (Pascual-Leone et al., 1993; Jackson et al., 1995; Doyon et al., 1997; Stefanova et al., 2000), others observed either no (Smith et al., 2001) or only mild to moderate impairments (Pascual-Leone et al., 1993; Sommer et al., 1999; Ferraro et al., 1993; Shin and Ivry, 2003).
Investigators have studied the factors that could explain these disparate findings. The first issue is whether the motor deficits in PD somehow preclude the manifestation of motor learning rather than interfere with the learning itself. This position is supported by the finding that patients with PD may show evidence of learning in the serial reaction time (SRT) task on the basis of errors, without a concomitant decrease in RT (Sommer et al., 1999). However, the fact that neither the degree of cognitive impairment (Ferraro et al., 1993; Stefanova et al., 2000) nor the clinical stage of the disease (Stefanova et al., 2000) appears to influence the rate or the magnitude of implicit learning in PD suggests that this overall conclusion is tenuous.
Another factor that may have contributed to the conflicting results was the potentially different levels of implicit and explicit knowledge acquired by the subjects. For example, many of the studies used an approach that tended to favor the development of explicit awareness of the sequence, such as polling the subjects repeatedly on the presence of the sequence (Pascaul-Leone et al., 1993; Sommer et al., 1999), or using shorter sequence lengths containing predictable chunks (Shin and Ivry, 2003). Indeed, one study reports that both the PD patients and the control subjects could recall a substantial proportion of the sequence elements before completing the study, suggesting that most of the learning was explicit in nature (Sommer et al., 1999). Therefore, the issue of implicit motor learning in PD remains unresolved. This has implications for the significance of the role of the basal ganglia in motor learning.
Thus, to address this question we studied patients with PD who had mild to moderate disease that was primarily unilateral using a variant of the SRT task (Seidler et al., 2002, 2005). We have found this behavioral task to be associated with a low level of explicit knowledge, and more importantly, the sequence can be learned under conditions that do not require a change in performance. This last point ensures we are testing motor learning per se rather than the motor abilities of the patients with PD. Additionally, we have a good understanding of the neural bases associated with this task from prior experiments (Seidler et al., 2002, 2005).
2. Results
Eight PD patients (5 women, 3 men, mean age 57.4 years, SD = 8.0 years) and six age-matched control subjects (4 women, 2 men, mean age 59.2 years, SD = 7.4 years) volunteered for this study. PD patients were tested after withholding their morning dose of medication. The subject characteristics are presented in Table 1. Subjects performed a variant of the serial reaction time sequence learning task concurrently with a secondary distractor task. Subjects were instructed to press a key-press device with their fingers in response to stimuli presented on the screen. There was a separate response button for each finger, excluding the thumb. The subjects were instructed to press the appropriate button as fast as possible when an “X” appeared in one of the stimulus boxes. For some trial blocks the stimuli were presented in a repeating fashion and for others the stimuli were presented in a pseudorandom fashion. Subjects performed a concurrent distractor task for some of the blocks (Seidler et al., 2002, 2005). This task required subjects to watch a square placed centrally and directly above the other stimuli, and to report the number of times that a target color appeared. Following a training period with the right hand, subjects performed both random and sequence test blocks without the distractor, using either the right or the left hand to make responses.
Table 1.
Subject demographics.
| PD Patients | Controls | |
|---|---|---|
| Gender | 5F, 3M | 4F, 2M |
| Age (years) | 57.4 | 59.2 |
| UPDRS, right hand | 14.6 | |
| UPDRS, left hand | 7.0 | |
| UPDRS, total* | 48.6 (14.5) | |
| Mini-Mental | 28 | 29.6 |
| # Errors on distractor task** | 1.31 (.9) | .95 (.9) |
| Right hand recall score*** | 4.4 (.9) | 3.5 (2.1) |
| Left hand recall score | 4.6 (.6) | 3.3 (2.1) |
Total possible UPDRS points is 159.
There is no group difference for distractor task performance.
There are no group differences, nor hand effects, for sequence recall score.
On average, the control subjects missed .95 distractor targets per block while the PD patients missed 1.31. These rates were not significantly different between the two groups (t-test, P =.49). Both groups recalled less than 40% of the presented sequences, as assessed using a free recall test for both the right and the left hand at the end of the experiment. Furthermore, recall rates were not significantly different between the two groups (t-test, P =.58 left hand, P =.45 right hand).
Blocks 1–6 were the sequence encoding phase of the experiment, in which the sequence was acquired. Blocks 7–9 and 10–12 were the expression phase of the experiment, in which expression of the previously learned sequence was tested for both the right and the left hands. The RT data are presented in Fig. 1 for both groups across the course of the study. There was a significant group x block interaction for RT (F1, 11 = 38.0, P <.05). Follow-up contrasts performed across the sequence encoding phase (blocks 1–6) revealed no effects; that is, there was no group difference in RT nor any change in RT across blocks during this phase of the experiment (P >0.10 in both cases). Both subject groups exhibited a drop in RT when going from blocks 1–6 (with distractor) to blocks 7–12 (without distractor) (F1,12 = 19.8, P<0.01). There was no difference in RT between the sequence and the random blocks for the PD patients during the expression of learning phase (repeated contrasts comparing adjacent categories P >0.10). However, the control subjects showed a significant difference in RT between the random and the sequence blocks at expression for both hands, supporting the conclusion that not only did they learn the sequence, but also that they were able to transfer this sequence knowledge from the right to the left hand (repeated contrasts comparing adjacent categories resulted in P<0.01 for all sequence versus random comparisons).
Figure 1.
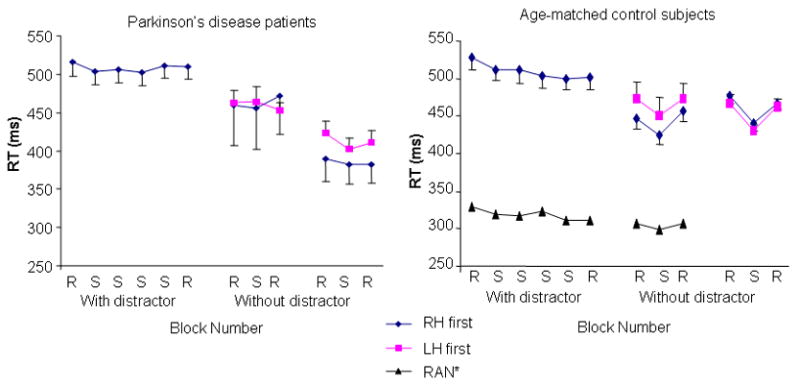
The mean and standard deviation response time (RT) is plotted for PD patients (left panel) and control subjects (right panel) across the experiment. There were no significant changes in RT across the blocks in which the subjects performed the concurrent distractor task. Both groups had faster RTs when performing without the distractor. The control subjects showed a significant response time advantage for both hands when performing sequence versus random blocks without the distractor. This was not true for the PD patients. *Data for the RAN subject group were presented in Seidler et al. (2005). These subjects performed random tapping for all of the blocks during the “learning” phase with the distractor task.
Data from the control group of Seidler et al. (2005) are presented in Figure 1 for comparison. These young adult subjects (n=9) performed random tapping during the “training” period with the concurrent distractor task, and were then tested for expression of learning. As can be seen, RT savings (difference in RT between sequence and random blocks at expression, see Table 2) at expression for this group was greatly suppressed in comparison to the control subjects of the current study. However, the most important aspect of presenting these additional control data in the current context is to address the potential criticism that subjects in the current study may have learned the sequence during the single block of trials presented without the distractor. Therefore, this comparison indicates that subjects in the current study were learning the sequence during the training period, as opposed to acquiring sequence knowledge during the expression phase of the study.
Table 2.
Performance savings at expression.
| Group | Condition | RT Savings | Error Savings |
|---|---|---|---|
| PD Patients | Right hand first | 10 ms | 3.5 |
| Right hand second | 3 ms | 3.4 | |
| Left hand first | 6 ms | 2.1 | |
| Left hand second | 15 ms | 2.5 | |
| Controls | Right hand first | 21 ms | 3.2 |
| Right hand second | 33 ms | 2.8 | |
| Left hand first | 27 ms | 1.5 | |
| Left hand second | 33 ms | −0.3 | |
| Random practice | Right hand | 9 ms | 0.6 |
The error data are shown in Fig. 2 for both groups. There was a main effect of block on the error data (F3,10 = 49.3, P <0.01). Follow-up testing without the distractor revealed that the PD patients exhibited a difference in the number of errors made between the random and sequence blocks at expression, regardless of which hand was used (repeated contrasts comparing adjacent categories resulted in P<0.05 for all sequence versus random comparisons). Likewise, the control subjects demonstrated differences in the number of errors made between the sequence and random blocks at expression. However, controls did not necessarily manifest a reduced error rate when the left hand was tested for expression second after the right hand (repeated contrasts resulted in P>0.10). The right hand when tested first or second and the left hand when only tested first demonstrated expression of learning (repeated contrasts comparing adjacent categories resulted in P<0.05). This suggests that transfer did not occur from the right to the left hand for control subjects when evaluated with this measure, except when the left hand was tested for expression immediately following the encoding phase.
Figure 2.
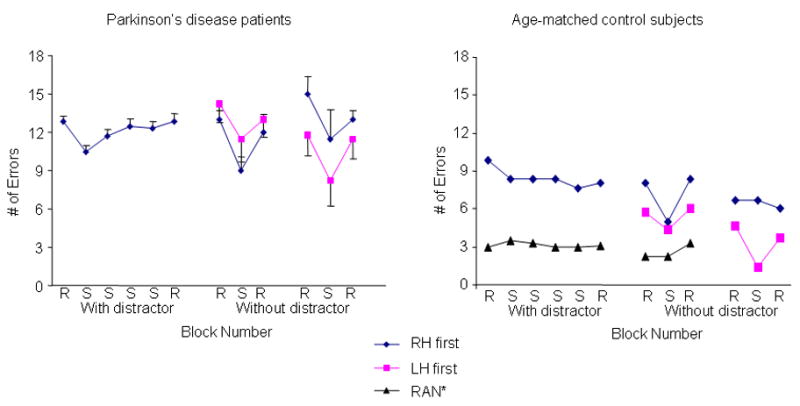
The mean and standard deviation error performance is plotted for PD patients (left panel) and control subjects (right panel) across the experiment. The PD patients showed a significant performance savings when performing sequence versus random blocks without the distractor. In contrast, for the control subjects, the right hand when tested first or second and the left hand when only tested first showed a sequence-specific savings in number of errors.
3. Discussion
Our data show that subjects with Parkinson’s disease are capable of implicit motor learning, as measured in terms of errors, and are able to transfer that learning between hands. We had been motivated to examine the issue of implicit learning in Parkinson’s disease because of the inconsistency in the results of published studies. In designing the experiment, we were particularly concerned with two factors that we felt might have contributed to the lack of uniformity in other data: uncertainty about the extent of explicit knowledge acquired by the subjects and the difficulty of separating motor performance and learning in this specific population.
The issue of the extent of explicit knowledge has arisen in many of the studies that have documented a behavioral improvement in sequence learning tasks. For example, an early study (Pascual-Leone et al., 1993) demonstrated an improvement in both reaction time and errors during SRT learning in PD subjects, albeit at a slower rate than controls, while another showed an improvement in errors despite the lack of a RT change (Sommer et al., 1999). However, levels of explicit knowledge in the subjects studied varied from 50% to 80% making data on implicit procedural learning difficult to interpret. In other work documenting an improvement in reaction time in PD subjects, there was no formal assessment of explicit knowledge (Ferraro et al., 1993). In an effort to minimize the possibility of acquiring explicit knowledge, we used a 12-element sequence that was specifically constructed for this purpose (Willingham et al., 1997). In addition, we employed a variant of the standard SRT task that uses a concomitant distractor (Seidler et al., 2002, 2005). We have previously shown that this concurrent distractor suppresses the performance changes that typically occur during learning, without affecting the learning itself; subjects show a response time advantage for tapping in response to sequential stimuli as opposed to random stimuli once the distractor task is removed. This response time savings is greater than the amount seen when subjects perform only random tapping during the “training” period, indicating that the benefit is not simply acquired during the single sequence test block (Seidler et al., 2005).
Distractors are known to diminish explicit knowledge (Shanks and Channon, 2002). We succeeded in reducing the amount of explicit knowledge acquired by the subjects in the current study. On the basis of probing the subjects after the completion of the training portion of the study, we showed that both PD and control groups recalled less than 40% of the sequence with either the left or right hand after the study was completed. Thus despite the low level of explicit knowledge, we demonstrated that PD subjects did indeed learn the sequence when we used error rate as an index of learning.
The other factor of concern was ensuring that the design of the experiment did not put undue emphasis on the inherent motor impairment of the PD subjects. The fact that these subjects have a primary movement problem may lead to spurious results. For example, some improvement in performance may be necessary for learning to occur and the inability to move more quickly may mask learning that had actually occurred. In an effort to address the role of inherent motor expression difficulties, PD subjects have been tested by using a verbal version of the SRT task. As in many other studies, the results have not been consistent, with one group of subjects showing learning (Smith et al., 2001), while in another group there was no effect (Westwater et al., 1998). Such mixed results across studies, combined with the finding that the clinical stage of the disease does not seem to influence the rate or the magnitude of implicit learning in PD (Stefanova et al., 2000), imply that the extent of motor symptoms is not related to the amount of implicit learning that occurs. Indeed, it has been shown that PD patients are impaired at implicit learning in nonmotor tasks as well (Knowlton et al., 1996). Nevertheless, our task makes it possible for subjects to learn without any decrease in RT during the learning phase (Seidler et al., 2002, 2005).
We found evidence of learning in the PD subjects, but this was on the basis of errors rather than RT changes. However, we noted that paradoxically PD patients could improve RT under some circumstances, such as during both random and sequence blocks after elimination of the distractor. This response time improvement was non sequence-specific and suggests that in addition to sequence learning, there was also a more general form of motor learning (stimulus-response mapping) in our subjects as a result of practice that was capable of being manifested in response time. The improvement with practice resulted in the PD patients having faster RTs at the end of the study than the control subjects, which was offset by a greater number of errors in the patient group, however. Similar speed-accuracy tradeoff differences have been observed for PD patients performing a comparable task (Kelly et al., 2004).
The other finding of note in the current study was on the transfer of learning. Previous work has demonstrated that SRT learning is not effector-specific (Keele et al., 1995; Grafton et al., 1998; Grafton et al., 2002; Willingham et al., 2000; Japikse et al., 2003) and our data would corroborate this. The control subjects showed transfer of learning between the two hands both in terms of RT and error, except in the case where the left hand was tested after the right hand, inducing a delay prior to left hand performance. In this case, inter-manual transfer was not observed in terms of errors made. It may be that more extensive practice or time is required to consolidate learning before equal transfer can occur for the pattern of spatial errors. However, these error data arebased on only three subjects (one half of each group was tested on the right hand first then the left, the other half tested left hand first then right). Therefore, more extensive testing would be required before making any firm conclusions about this finding and its underlying mechanisms. The PD subjects also exhibited transfer of sequence learning from the right hand, used during training, to the left hand, in terms of the number of errors made. These data indicate that a sequence-specific decline in RT is not a necessary condition for inter-manual transfer of implicit learning and that transfer still occurs in basal ganglia disease.
How can we place our findings in the context of what we currently understand about the role of the basal ganglia in motor learning? Using the same task, we have shown that an assortment of cortical and subcortical areas, including the basal ganglia, were active during the learning (Seidler et al., 2002, 2005), but that the cerebellum was the structure engaged primarily in modulating changes in performance (Seidler et al., 2002). However, though the basal ganglia may be important in implicit learning, our results suggest that neither the extent nor the distribution of basal ganglia dysfunction in PD is generally sufficient to impair implicit learning itself. What is impaired is the ability to translate the learning into a consistent improvement in response time; though there may also be other specific impairments (see Shin and Ivry, 2003). We propose that what is most often disrupted in Parkinson’s disease is the connection between the basal ganglia and cerebellum which prevents the normal facilitation of performance through improvement in response time that one would expect as a result of learning.
In summary, we have demonstrated that PD patients are able to learn a sequence of actions under implicit conditions, when learning is assessed in terms of the number of errors made. While these subjects are not able to express learning by decreasing their response times, they are able to transfer learning from the trained to the untrained hand. Although PD patients have been shown to be impaired at other types of procedural learning (Krebs et al., 2001; Laforce & Doyon, 2001), our findings suggest that the basal ganglia are not sufficiently damaged in mild to moderate PD to prevent implicit sequence learning, and thus this is not likely to be an important factor in the manifestation of the motor syndrome associated with the disease.
4. Experimental Procedure
Subjects
All subjects signed a Human Subjects Committee approved consent form. Subjects from both groups received a neurological exam and were evaluated by a neurologist using the Unified Parkinson’s Disease Rating scale (UPDRS, Fahn and Elton, 1987) and the Mini-Mental State Exam (Folstein et al., 1975).
Experimental setup and procedure
Subjects were seated in front of a computer monitor and were instructed to press a key-press device with their fingers in response to stimuli presented on the screen. There was a separate response button for each finger, excluding the thumb. There were four visual stimulus boxes corresponding to each of the four response buttons. The subjects were instructed to press the appropriate button as fast as possible when an “X” appeared in one of the stimulus boxes. For some trial blocks the stimuli were presented in a repeating fashion and for others the stimuli were presented in a pseudorandom fashion. In the repeating stimulus blocks, subjects were presented with a 12-element sequence (cf. Willingham et al., 1997). Within these 12 elements, each of the four possible stimulus locations was presented three times. Furthermore, in order to prevent explicit awareness of the sequence, it was constrained such that a stimulus could not be presented twice in a row, there could be no runs of four (e.g., 1234), and there could be no trills of four (e.g., 2424). If subjects did not respond by pushing the correct button, then the same stimulus location would be presented again on the next trial. Subjects were not informed about the presence of the sequence. Each block consisted of 94 trials spaced by a constant inter-stimulus interval of 1000 ms (for control subjects and 5 PD patients) or 1200 ms (3 PD patients). Thus, depending on the number of errors made by the subject, each sequence block would consist of between 7 and 8 repetitions of the sequence. Each time the sequence was repeated, the presentation would start at another random point within the sequence with the additional criterion that the other sequence requirements continue to be met. The random blocks were made up of different 12-element sequences appended together.
Subjects performed a concurrent distractor task for some of the blocks (Seidler et al., 2002, 2005). This task required subjects to watch a square placed centrally and directly above the other stimuli. The square changed color among four possible colors at a rate of 3 Hz. Subjects were instructed to watch for a target color and to keep track of the number of times that it occurred within one block. Following each block the subjects were requested to verbally report the number of occasions the distractor was observed. This resulted in a rest period between each block of approximately one minute. The target color was presented between 1% and 3% of the cases within a block, with the other three colors equally distributed. All subjects performed the same protocol.
The first 6 blocks were performed with the right hand by all subjects (cf. Fig. 1). The first block consisted of random stimuli in combination with the distractor task. Blocks 2–5 comprised sequence blocks performed concurrently with the distractor task. Block 6 was another random block with the distractor. Blocks 7–12 were performed without the distractor task and consisted of random, sequence, random, random, sequence, and then random presentations of the stimuli. Half of the subjects in each group performed blocks 7–9 with the right hand and blocks 10–12 with the left hand. The other half started with the left hand and then switched to the right hand.
Data Analysis
The median response time was computed for each subject for each repetition of the sequence. Then a mean RT across repetitions was computed for each subject within each block. These mean values were averaged across subjects for presentation purposes. The acquisition of explicit awareness of the sequence was probed at the end of the experiment. Subjects were first asked whether they had noticed anything about the manner in which the stimuli were presented. They were then informed that there was a sequential presentation for some of the blocks, and were asked to perform a free recall of the sequence on the same key-press device as during the experiment. Rather than responding to a stimulus, however, the stimuli were presented as feedback each time that they pressed a button. Subjects performed the generate task with both the right and the left hands, in a counterbalanced order. Subjects reporting greater than five stimuli (in a minimum run of 3 elements) correct were considered to have explicit awareness of the sequence (cf. Willingham et al., 1997).
The RT and error data were analyzed using a group (2) by block (12) repeated measures ANOVA, with repeated measures on block. Significant interactions were followed up with planned group x block contrasts. These compared the group difference for the amount of savings seen upon expression of learning, measured as the difference in RT or error for the sequence block 8 in comparison to random blocks 7 and 9, and for the sequence block 11 compared to random blocks 10 and 12.
Acknowledgments
Supported by NIH NS40106.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cleeremans A. Mechanisms of implicit learning. MIT Press; Cambridge: 1993. [Google Scholar]
- Doyon J, Owen AM, Petrides M, Sziklas V, Evans AC. Functional anatomy of visuomotor skill learning in human subjects using positron emission tomography. European Journal of Neuroscience. 1996;8:637–648. doi: 10.1111/j.1460-9568.1996.tb01249.x. [DOI] [PubMed] [Google Scholar]
- Doyon J, Gaudreau D, Laforce R, Castonguay M, Bedard PJ, Bedard F, Bouchard JP. Role of the striatum, cerebellum, and frontal lobes in the learning of a visuomotor sequence. Brain and Cognition. 1997;34:218–245. doi: 10.1006/brcg.1997.0899. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton RL. UPDRS Development Committee. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne CD, editors. Recent Developments in Parkinson’s Disease. MacMillan; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- Ferraro RF, Balota DA, Connor LT. Implicit memory and the formation of new associations in nondemented Parkinson’s disease individuals and individuals with senile dementia of the Alzheimer type: A serial reaction time (SRT) investigation. Brain and Cognition. 1993;21:163–180. doi: 10.1006/brcg.1993.1013. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB. Abstract and effector-specific representations of motor sequences identified with PET. J Neurosci. 1998;18:9420–9428. doi: 10.1523/JNEUROSCI.18-22-09420.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry RB. Motor sequence learning with the nondominant left hand. A PET functional imaging study. Exp Brain Res. 2002;146:369–378. doi: 10.1007/s00221-002-1181-y. [DOI] [PubMed] [Google Scholar]
- Jackson GM, Jackson SR, Harrison J, Henderson L, Kennard C. Serial reaction time learning and Parkinson’s disease: Evidence for a procedural learning deficit. Neuropsychologia. 1995;33:577–593. doi: 10.1016/0028-3932(95)00010-z. [DOI] [PubMed] [Google Scholar]
- Japikse KC, Negash S, Howard JH, Howard DV. Intermanual transfer of procedural learning after extended practice of probabilistic sequences. Exp Brain Res. 2003;148:38–49. doi: 10.1007/s00221-002-1264-9. [DOI] [PubMed] [Google Scholar]
- Keele SW, Jennings P, Jones S, Caulton D, Cohen A. On the modularity of sequence representation. J Mot Behav. 1995;27:17–30. [Google Scholar]
- Kelly SW, Jahanshahi M, Dirnberger G. Learning of ambiguous versus hybrid sequences by patients with Parkinson’s disease. Neuropsychologia. 2004;42:1350–1357. doi: 10.1016/j.neuropsychologia.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Krebs HI, Hogan N, Hening W, Admovich SV, Poizner H. Procedural motor learning in Parkinson’s disease. Exp Brain Res. 2001;141:425–437. doi: 10.1007/s002210100871. [DOI] [PubMed] [Google Scholar]
- Laforce R, Doyon J. Distinct contribution of the striatum and cerebellum to motor learning. Brain & Cogn. 2001;45:189–211. doi: 10.1006/brcg.2000.1237. [DOI] [PubMed] [Google Scholar]
- Nissen MJ, Bullemer P. Attentional requirements of learning: Evidence from performance measures. Cogn Psychol. 1987;19:1–32. [Google Scholar]
- Pascual-Leone A, Grafman J, Clark K, Stewart M, Massaquoi S, Lou JS, Hallett M. Procedural learning in Parkinson’s disease and cerebellar degeneration. Annals of Neurology. 1993;34:594–602. doi: 10.1002/ana.410340414. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Savage CR, Curran T, Kendrick A, Brown HD, Bush G, Breiter HC, Rosen BR. Striatal recruitment during an implicit sequence learning task as measured by functional magnetic resonance imaging. Hum Brain Map. 1997;5:124–132. [PubMed] [Google Scholar]
- Reber AS. Implicit learning and tacit knowledge: An essay on the cognitive unconsciousness. Oxford University Press; NY: 1993. [Google Scholar]
- Seidler RD, Purushotham A, Kim SG, Ugurbil K, Willingham D, Ashe J. Cerebellum activation associated with performance change but not motor learning. Science. 2002;296:2043–2046. doi: 10.1126/science.1068524. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Purushotham A, Kim SG, Ugurbil K, Willingham D, Ashe J. Neural correlates of encoding and expression in implicit sequence learning. Exp Brain Res. 2005;165:114–124. doi: 10.1007/s00221-005-2284-z. [DOI] [PubMed] [Google Scholar]
- Shanks DR, Channon S. Effects of a secondary task on “implicit” sequ ence learning: learning or performance? Psychol Res. 2002;66:99–109. doi: 10.1007/s00426-001-0081-2. [DOI] [PubMed] [Google Scholar]
- Shin JC, Ivry RB. Spatial and temporal sequence learning in patients with Parkinson’s disease or cerebellar lesions. Journal of Cognitive Neuroscience. 2003;15:1232–1243. doi: 10.1162/089892903322598175. [DOI] [PubMed] [Google Scholar]
- Smith J, Siegert RJ, McDowall J. Preserved implicit learning on both the serial reaction time task and artificial grammar in patients with Parkinson’s disease. Brain and Cognition. 2001;45:378–391. doi: 10.1006/brcg.2001.1286. [DOI] [PubMed] [Google Scholar]
- Sommer M, Grafman J, Clark K, Hallett M. Learning in Parkinson’s disease: eyeblink conditioning, declarative learning, and procedural learning. J Neurol Neurosurg Psychiatry. 1999;67:27–34. doi: 10.1136/jnnp.67.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova ED, Kostic VS, Ziropadja L, Markovic M, Ocic GG. Visuomotor skill learning on serial reaction time task in patients with early Parkinson’s disease. Movement Disorders. 2000;15:1095–1103. doi: 10.1002/1531-8257(200011)15:6<1095::aid-mds1006>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Westwater H, McDowall J, Siegert R, Mossman S, Abernethy D. Implicit learning in Parkinson’s disease: evidence from a verbal version of the serial reaction time task. J Clin Exp Neuropsychol. 1998;20:413–418. doi: 10.1076/jcen.20.3.413.826. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Greenberg AR, Thomas RC. Response-to-stimulus interval does not affect implicit motor sequence learning, but does affect performance. Mem & Cogn. 1997;25:534–542. doi: 10.3758/bf03201128. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Salidis J, Gabrieli JD. Direct comparison of neural systems mediating conscious and unconscious skill learning. J Neurophysiol. 2002;88:1451–1460. doi: 10.1152/jn.2002.88.3.1451. [DOI] [PubMed] [Google Scholar]
- Willingham DB, Wells LA, Farrell JM, Stemwedel ME. Implicit motor sequence learning is represented in response locations. Mem Cognit. 2000;28:366–375. doi: 10.3758/bf03198552. [DOI] [PubMed] [Google Scholar]


