Abstract
Although vesicular stomatitis virus (VSV) neurovirulence and pathogenicity in rodents have been well studied, little is known about VSV pathogenicity in non-human primates. To address this question, we measured VSV viremia, shedding, and neurovirulence in macaques. Following intranasal inoculation, macaques shed minimal recombinant VSV (rVSV) in nasal washes for 1 day post-inoculation; viremia was not detected. Following intranasal inoculation of macaques, wild type (wt) VSV, rVSV, and two rVSV-HIV vectors showed no evidence of spread to CNS tissues. However, macaques inoculated intrathalamically with wt VSV developed severe neurological disease. One of four macaques receiving rVSV developed clinical and histological signs similar to the wt group, while the remaining three macaques in this group and all of the macaques in the rVSV-HIV vector groups showed no clinical signs of disease and reduced severity of histopathology compared to the wt group. The implications of these findings for rVSV vaccine development are discussed.
Keywords: Vesicular stomatitis virus, Pathogenicity, Neurovirulence, Viral vectors, Non-human primates, HIV vaccine, Intrathalamic
Introduction
Vesicular stomatitis virus (VSV) is a non-segmented, single-stranded, negative-sense RNA virus of the family Rhabdoviridae. Recombinant VSV (rVSV) is a promising vaccine vector because its simple genome can accommodate multiple foreign genes, it does not undergo either recombination or integration into host cell DNA, and it grows to high titers in many different cell types, facilitating vaccine manufacture. Moreover, rVSV generates potent cellular and humoral immune responses to abundantly expressed foreign antigens (for reviews, see Roberts and Rose, 1998, Rose and Whitt, 2001). Recombinant VSV-based vectors have been investigated preclinically as vaccine candidates for such infectious diseases as influenza virus (Roberts et al., 1999), respiratory syncytial virus (Kahn et al., 2001), measles virus (Schlereth et al., 2000, Schlereth et al., 2003), papillomavirus (Reuter et al., 2002, Roberts et al., 2004), severe acute respiratory syndrome-coronavirus (Kapadia et al., 2005), Ebola and Marburg viruses (Jones et al., 2005), as well as human immunodeficiency virus [HIV] (Egan et al., 2004, Rose et al., 2000, Rose et al., 2001). The efficacy of rVSV vectors was demonstrated for HIV vaccine candidates when macaques immunized with rVSVs expressing SIV Gag and HIV Env proteins were protected from disease following challenge with pathogenic SIV/HIV hybrid (SHIV) 89.6P (Egan et al., 2004, Rose et al., 2001). A more recent study has demonstrated enhanced immunogenicity and efficacy in macaques following a DNA-prime/rVSV-boost vaccination regimen (Egan et al., 2005). Based on these promising results, we chose to develop rVSVs expressing HIV proteins for clinical evaluation.
The prototypic rVSV vector is derived from San Juan and Mudd-Summers isolates of wild type (wt) Indiana serotype VSV (VSVIN) that were first adapted through multiple passages in cultured cells. A reverse genetics system allowed rescue of infectious virus from cDNA (Lawson et al., 1995, Whelan et al., 1995). The prototypic VSVIN cDNA clone was modified to allow foreign gene expression (Schnell et al., 1996a, Schnell et al., 1996b). To investigate immunogenicity and efficacy in an SHIV challenge model, separate rVSVIN vectors were engineered that encoded SIV gag and HIV env genes. To circumvent the high level of neutralizing antibodies directed against the VSV surface glycoprotein (G), rVSV vectors encoding SIV Gag and HIV Env were modified by exchanging the Indiana G gene with G genes of other vesiculovirus serotypes (Rose et al., 2000, Rose et al., 2001). Vectors incorporating heterologous glycoproteins were used to boost macaques that had been primed with G-Indiana serotype vectors (Egan et al., 2004, Rose et al., 2001). Following a high dose SHIV challenge, all vaccinated macaques were able to control the infection, maintain low viral loads and normal CD4 counts, and remain free from disease 5 years post-challenge (Egan et al., 2004, Rose et al., 2001).
VSV is a natural pathogen of livestock that causes disease in cattle, swine, and horses marked by febrile illness and vesicular lesions of the lips, mouth, nose, teats, and hooves that usually resolve in a few weeks without fatality [reviewed in Letchworth et al., 1999]. Transmission occurs through direct contact between infected animals, and it is thought that abrasion or scarification of skin or mucosal epithelium increases the virus's ability to infect at these sites (Letchworth et al., 1999). Furthermore, there is evidence that biting insects may serve as vectors for VSV (Brandly and Hanson, 1957, Letchworth et al., 1999, Tesh and Johnson, 1975).
Documented cases of human VSV infections are rare and tend to occur in agricultural, veterinary, and laboratory workers or in areas where VSV infection is endemic in its natural hosts (Brandly and Hanson, 1957, Brody et al., 1967, Fellowes et al., 1955, Ferris et al., 1955, Fields and Hawkins, 1967, Hanson et al., 1950, Heiny, 1945, Johnson et al., 1966, Reif et al., 1987, Webb et al., 1987). Human infection is typically subclinical or results in a mild flu-like disease that is self-limited (Brandly and Hanson, 1957, Fellowes et al., 1955, Patterson et al., 1958, Tesh and Johnson, 1975). While encephalitis is not a feature of VSV natural infection (Letchworth et al., 1999), VSV neurotropism and neurovirulence (NV) have been demonstrated in rodent models (Bi et al., 1995, Fournier et al., 1988, Fultz et al., 1981, Huneycutt et al., 1994, Miyoshi et al., 1971, Plakhov et al., 1995, Reiss et al., 1998, Sur et al., 2003, van den Pol et al., 2002). Furthermore, early studies involving experimental intracerebral inoculation of rhesus and cynomolgus macaques (Olitsky et al., 1934), horses, cattle, and sheep (Frank et al., 1945) also indicated the NV potential of VSV. Recently, Chandipura virus, a related vesiculovirus, was associated with an outbreak of encephalitis in children in India (Rao et al., 2004), although the etiologic role of Chandipura virus in human epidemic encephalitis has been questioned (John, 2004).
Replication competent viral vaccines have historically been subjected to NV testing, which usually involves direct inoculation of virus into the central nervous system of susceptible non-human primates (NHP). NHP NV tests have been developed for poliovirus (Nathanson and Horn, 1992, Wood and Macadam, 1997), measles virus (Sharova et al., 1979, Yamanouchi et al., 1976), mumps virus (Levenbook et al., 1979, Maximova et al., 1996, Rubin et al., 1999), and yellow fever virus (Levenbook et al., 1987, Marchevsky et al., 2003, WHO, 1998). In most cases, vaccine strains are inoculated directly into NHP brains and compared to reference controls. Clinical parameters are measured for a period of up to 30 days, at which time brain and spinal cord tissues are analyzed histologically for virus-induced lesions. The NHP NV test for yellow fever vaccine involves scoring systems for both clinical signs as well as histological lesions in CNS tissue; acceptability is based on a comparison of clinical and histological scores to those of a reference control (Levenbook et al., 1987). Similarly, a histological lesion scoring system is used to determine acceptability of mumps vaccine lots following direct inoculation into the thalamus of NHPs (Maximova et al., 1996).
The development of rVSV vaccines for human use requires appropriate attenuation to remove vector-associated pathogenesis. A previous study in mice indicated that a low dose of the tissue culture-adapted wt VSVIN San Juan isolate inoculated intranasally caused weight loss, paralysis, and death, while a similar dose of rVSVIN caused only weight loss in mice (Roberts et al., 1998). Similarly, an rVSVIN vector encoding an influenza virus hemagglutinin gene caused a transient weight loss following intranasal (IN) inoculation of mice at higher doses (Roberts et al., 1999). An rVSV vector containing a truncation in G (labeled CT1) did not cause weight loss following IN inoculation of mice (Roberts et al., 1999), indicating the ability to decrease vector pathogenicity with attenuating mutations. The well-established ability of VSV to cause NV in rodents following IN inoculation (Bi et al., 1995, Huneycutt et al., 1994, Lundh et al., 1987, Olitsky et al., 1934, Reiss et al., 1998, Sabin and Olitsky, 1937, Sur et al., 2003) and the weight loss observed in mice following IN inoculation with the rVSVIN vector (Roberts et al., 1998, Roberts et al., 1999) indicate that rodents are highly permissive for VSV infection. Since more than 150 rhesus macaques have been inoculated both intranasally and intramuscularly with high doses of rVSVIN vectors without adverse clinical events [(Egan et al., 2004, Rose et al., 2001), and unpublished observations], we decided to assess our rVSV-HIV variants for vector-associated pathogenicity in NHPs. In three separate exploratory studies, we measured viremia, viral shedding, and neuroinvasiveness following IN inoculation and NV following intrathalamic (IT) inoculation. In each study, macaques were inoculated with 107 PFU of virus, which is the highest dose previously tested in preclinical NHP studies (Egan et al., 2004, Egan et al., 2005). The neuroinvasiveness and NV studies were modeled on the stringent NV tests used to characterize live mumps virus and yellow fever virus vaccine preparations (Levenbook et al., 1979, Levenbook et al., 1987, Marchevsky et al., 2003, Maximova et al., 1996, Rubin et al., 1999, WHO, 1998).
Results
VSV shedding and lack of detectable viremia following IN inoculation with rVSVIN
A pilot study was performed to measure viremia and shedding of infectious virus following IN inoculation of three rhesus macaques with 107 PFU of rVSVIN (Fig. 1A). Blood and serum were collected pre-dose and then, along with nasal washes and saliva, were collected every day for the first 4 days following inoculation and at defined intervals thereafter (see Materials and methods). Using a standard plaque assay, infectious virus was observed in nasal wash samples of all three inoculated macaques at day 1 (Table 1 ). Only one macaque (number 23) had a low level (1–10 PFU/mL) of detectable infectious virus in its nasal wash at day 2. No infectious virus was detected in nasal wash samples from any macaques following day 2. The two macaques (numbers 48 and 62) that had the highest titers of virus in nasal wash samples at day 1 also shed low levels of infectious virus (10–100 PFU/mL) in their saliva at day 1. No virus was detected in saliva of the third macaque (number 23) on day 1. Infectious virus was not detected in saliva from any macaque following day 1. The unvaccinated macaques served as negative controls for all samples at all time points (Table 1).
Fig. 1.
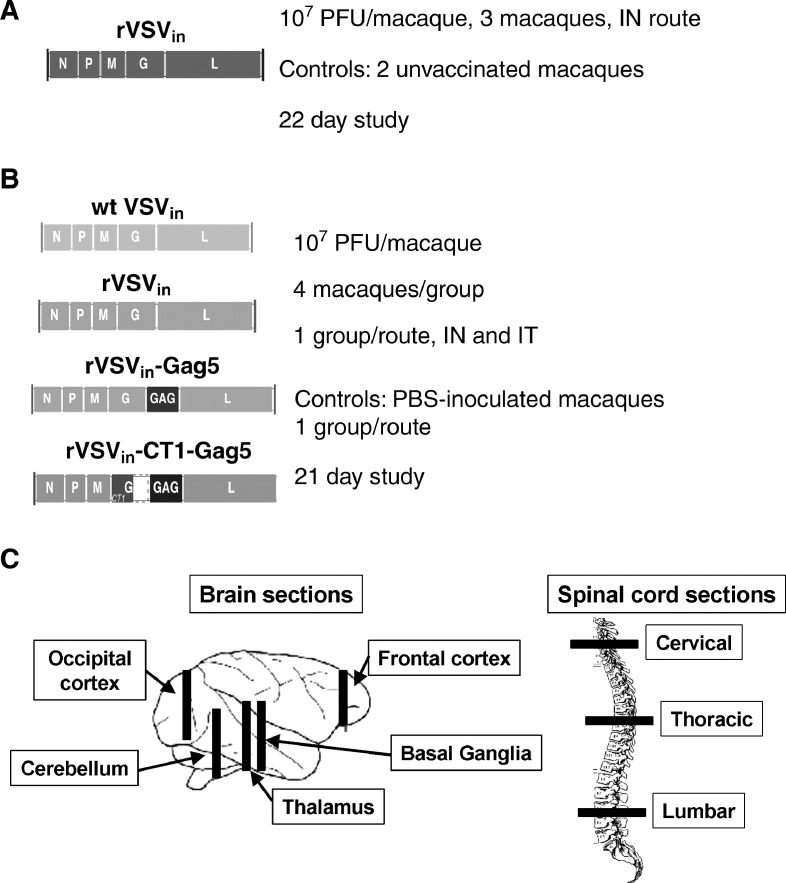
NHP safety study designs. (A) Rhesus macaque shedding study. The genome organization of the rVSVIN virus is depicted, and the dose, route of inoculation, and controls for the study are indicated. (B) Cynomolgus macaque NV and neuroinvasiveness studies. The genome organization of each virus or vector is depicted, along with the dose per macaque, route of inoculation, the number of macaques per group, and the day of scheduled euthanasia. (C) Bars indicate sections of cynomolgus macaque brain tissue and spinal cord sections collected at necropsy for histological analysis in the NV and neuroinvasiveness studies. Anatomical regions covered by collected sections are also indicated.
Table 1.
Shedding of infectious virus following IN inoculation of rhesus macaques
| Group | Macaque no.a | Nasal wash |
Saliva |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 1 | Day 2 | Day 3 | Day 4 | ||
| rVSVIN | 23 | +b | +/− | − | − | − | − | − | − |
| 48 | +++ | − | − | − | + | − | − | − | |
| 62 | +++ | − | − | − | + | − | − | − | |
| Control | 31 | − | − | − | − | − | − | − | − |
| 24 | − | − | − | − | − | − | − | − | |
Macaques 23, 48, and 62 were inoculated IN with 107 PFU of rVSVIN. Macaques 31 and 24 were un-inoculated controls.
Infectivity scale: −, no virus detected; +/−, 1–10 PFU/mL; +, 10–100 PFU/mL; ++, 100–1000 PFU/mL; +++, 1000–10,000 PFU/mL.
In addition to viral shedding at mucosal surfaces, we assayed plasma and peripheral blood mononuclear cells (PBMCs) for the presence of infectious virus following IN inoculation. Infectious virus was not detected in plasma or PBMCs of any macaque post-inoculation, indicating the absence of detectable viremia in vaccinated macaques.
Using a VSV neutralization assay, VSVIN-specific serum antibodies were detected in all three vaccinated macaques by day 14 (Table 2 ), indicating that a productive infection occurred following IN inoculation.
Table 2.
Anti-VSVIN serum neutralization antibodies
| Study | Route | Group | Day | Geometric mean titera |
|---|---|---|---|---|
| Shedding | IN | rVSVIN | 8 | < 40 |
| 14 | 1040 | |||
| 22 | 1890 | |||
| Control | 22 | < 40 | ||
| Neuroinvasiveness | IN | wt VSVIN | 21 | 1522 |
| rVSVIN | 21 | 2167 | ||
| rVSVIN-HIVGag5 | 21 | 1280 | ||
| rVSVIN–CT1-HIVGag5 | 21 | < 40 | ||
| PBS | 21 | < 40 | ||
| Neurovirulence | IT | wt VSVIN | 8 | 226 |
| rVSVIN | 8b | 40 | ||
| 21c | 1280 | |||
| rVSVIN-HIVGag5 | 21 | 4305 | ||
| rVSVIN-CT1-HIVGag5 | 21 | 80 | ||
| PBS | 21 | < 40 |
Titer expressed as reciprocal dilution required to neutralize 100 PFU VSV (limit of detection = 40).
One macaque in this group was euthanized by day 8.
Three macaques in this group survived 21 days.
No evidence of neuroinvasion following IN inoculation
Cynomolgus macaques were inoculated IN on day 0 with 107 PFU of VSVIN as shown in Fig. 1B. Macaques were clinically monitored for 21 days, at which time they were subjected to euthanasia, necropsy, and tissue harvest. Group mean clinical scores of macaques in all groups were ≤ 0.1 (out of 4); the wt VSVIN and all rVSVIN groups were indistinguishable from the scores of the negative control group.
To assess neuroinvasiveness, brain and spinal cord tissues were harvested at necropsy and analyzed by plaque assay, real-time, quantitative RT-PCR, and histopathologic examination. No virus or viral RNA was detected in frontal cortex, occipital cortex, olfactory bulb, CSF, or nasal turbinates from any of the macaques at day 21. Furthermore, histopathological analysis revealed that no treatment related lesions or changes were present in the brains or spinal cords of macaques given wt VSVIN or rVSVIN vectors compared with the control macaques given PBS. These results indicate that none of the rVSVIN vectors or even the wt VSVIN was capable of spreading to the CNS following IN inoculation. To demonstrate that the macaques inoculated IN with each virus were productively infected, we measured anti-VSVIN neutralizing antibodies (Table 2). Macaques in the wt VSVIN, rVSVIN, and rVSVIN-HIVGag5 groups all had neutralizing antibodies in the range of 600–6000 (geometric mean titers between 1280 and 2167, Table 2). Macaques in the rVSVIN-CT1-HIVGag5 and PBS groups did not have detectable neutralization titers.
Neurovirulence study clinical observations
Following IT inoculation with 107 PFU of virus, all four macaques in the wt VSVIN group and one of four in the rVSVIN backbone group were euthanized early because of declining health status, which included head tilt, irregular gait, tremors, and ataxia, signs consistent with neurological disease (Table 3 ). The onset of these signs generally occurred on day 6 or 7 post-inoculation. There were no significant virus related clinical signs in the remaining macaques in the IT groups (Table 3). One of the PBS control macaques, 22290F, had a head tilt, lethargy, and bilateral pupil dilation on days 1–21 (Table 3). Because the onset of these signs occurred earlier than those in the wt or rVSVIN groups, and because none of the other macaques in the control group displayed similar signs, it was concluded that these clinical features were procedure-related.
Table 3.
Daily and group mean clinical scores for cynomolgus macaques inoculated IT
| Group | Macaque no. | Clinical scorea |
|
|---|---|---|---|
| Daily mean | Group mean | ||
| wt VSVIN | 21545Mb | 3 | 3 |
| 22212Mb | 3 | ||
| 22190Fb | 3 | ||
| 16739Fb | 3 | ||
| rVSVIN | 21733M | 0 | 1 |
| 21725M | 1 | ||
| 21789F | 0 | ||
| 21392Fb | 3 | ||
| rVSVIN-HIVGag5 | 19439M | 0 | 0 |
| 22245M | 0 | ||
| 21275F | 0 | ||
| 21578F | 0 | ||
| rVSVIN-CT1-HIVGag5 | 22253M | 0 | 0 |
| 22235M | 0 | ||
| 21797F | 0 | ||
| 21576F | 0 | ||
| PBS | 21724M | 0 | 1 |
| 22238M | 0 | ||
| 22290F | 3c | ||
| 21765F | 0 | ||
Clinical scores based on a scale of 0–4, see Materials and methods.
Euthanized on day 8 post IT challenge and given a score of 4 for subsequent days.
Macaque had head tilt, lethargy, decreased activity, and bilateral pupil dilation on days 1–21, which were considered secondary to the surgical procedures (see Results).
Detection of VSV in tissues from IT groups
At the time of necropsy, samples were taken from the frontal and occipital cortices, as well as from CSF, and were assayed for infectious VSV by plaque assay and for viral RNA by real-time quantitative RT-PCR. All five macaques euthanized by day 8 had infectious virus in the frontal cortex, which was the brain section most proximal to the injection site. Viral titers were within the range of 102–107 PFU/g (Table 4 ). Similarly, viral genomic RNA within the range of 106–1010 copies/g was detected in the frontal cortex of all five of these macaques. None of the five macaques euthanized by day 8 had infectious virus in the occipital cortex, which was distal to the injection site. However, three of the four macaques in the wt group did have low levels (105 to 106 copies/g) of detectable viral RNA in their occipital lobes. In addition, low levels of infectious virus (56 PFU/mL) and viral genomes (5.8 × 105 copies/mL) were detected in the CSF of one macaque in the wt group, F22190F (Table 4). All other tissues and CSF from this group were negative for infectious virus and viral genomes. Viruses recovered from brain samples of macaques subjected to euthanasia by day 8 were sequenced across their entire genomes. Virus isolated from two out of four macaques in the wt group had no mutations relative to the inoculum. Of the viruses isolated from the remaining two wt-inoculated macaques, one had a single amino acid substitution in G and the other had two substitutions in L (Table 5 ). The virus isolated from the one rVSVIN macaque euthanized by day 8 was found to contain identical sequence relative to input virus (Table 5). Taken together, these results suggest that the clinical outcomes in these macaques were caused by the input viruses rather than by selection mutants with increased NV potential.
Table 4.
Viral loads in tissues from IT-inoculated cynomolgus macaques, day 8 post-inoculationa
| Virus | Macaque no. | Plaque assay (log10 PFU/g tissue or mL CSF) |
RT-PCR (log10 copies/g tissue or mL CSF) |
||||
|---|---|---|---|---|---|---|---|
| Frontal cortexb | Occipital lobeb | CSFb | Frontal cortex | Occipital lobe | CSF | ||
| wt VSVIN | F22190F | 6.74 | –c | 1.74 | 9.10 | 5.78 | 5.76 |
| F21545M | 2.16 | – | – | 6.61 | – | – | |
| F22212M | 2.87 | – | – | 8.40 | 5.05 | – | |
| F16739F | 5.67 | – | – | 8.64 | 5.01 | – | |
| rVSVIN | F21392F | 5.21 | – | – | 6.66 | – | – |
Viral loads were assayed for macaques in all groups; all of those that survived to day 21 had undetectable viral loads in both assays.
Tissues collected during necropsy, CSF collected immediately prior to necropsy.
“–” indicates below the limit of detection of the assay (plaque assay = 2 log10 PFU/g of tissue and 1 log10 PFU/mL of CSF; RT-PCR assay = 5 log10 copies/g of tissue and 5.3 log10 copies/mL of CSF).
Table 5.
Genomic sequence results of viruses isolated from macaque brain tissue at day 8
| Virus | Macaque no. | Sequence changes from input virus |
|---|---|---|
| wt VSVIN | F16739F | Q299R in G gene |
| F22212M | No changes | |
| F22190F | No changes | |
| F21545M | Q496H, D1316N in L gene | |
| rVSVIN | F21392F | No changes |
For the remaining macaques in the rVSVIN group and all the macaques in the rVSVIN-HIVGag5 and rVSVIN-CT1-HIVGag5 groups, no infectious virus or viral RNA was detected in any tissue sample collected at necropsy (day 21). Similarly, the tissues from the PBS group of macaques were negative in both assays.
To demonstrate that productive infections occurred in all groups, including those with no clinical scores or detectable virus, neutralizing antibodies were measured in the IT groups of macaques (Table 2). All macaques that were euthanized by day 8 had low levels of neutralizing antibodies (geometric mean titers between 40 and 230). All rVSVIN and rVSVIN-HIVGag5 macaques that survived to day 21 had neutralizing antibodies between 600 and 6000 (geometric mean titers between 1280 and 4305). With the exception of one macaque that had an undetectable neutralizing antibody titer, those in the rVSVIN-CT1-HIVGag5 group had neutralizing antibody titers of 80–160 (geometric mean = 80).
Histopathological analysis of brain and spinal cord tissues from macaques in IT groups
Slides of fixed brain and spinal cord tissues were analyzed for histological changes. For each tissue section, observed lesions were noted according to relative size, number, and type (e.g., inflammatory, necrotic, perivascular, periventricular). To summarize the histological findings, the following scoring system was used to quantify the observed lesion severities: 0 = no lesions, 1 = minimal, 2 = moderate, 3 = marked, 4 = severe (Table 6 ). Macaques inoculated with PBS had only minimal to moderate areas of malacia and inflammation localized to the injection site, which indicated tissue damage resulting from the needle. Macaque 22290F (PBS), which had an average clinical score of 3 for the length of the study (Table 3), displayed only needle track-associated tissue damage (Table 6), which supported the notion that the clinical features were procedure related. There were no notable lesions in other tissue sections in the PBS group (Fig. 2E, Table 6). Brain sections from macaques inoculated with wt VSVIN showed moderate to severe necrotizing meningoencephalitis characterized by malacia, hemorrhage, choroiditis, loss of ependymal cells, and moderate numbers of perivascular cuffs of mononuclear cells (Fig. 2A). Macaques inoculated with rVSVIN had lesions that were of similar severity to those in the wt VSVIN group; however, the lesions in the rVSVIN macaques were characterized by a predominant cellular inflammatory response with fewer areas of malacia than in the wt VSVIN inoculated macaques (Fig. 2B). The macaques in the rVSVIN-HIVGag5 and rVSVIN-CT1-HIVGag5 groups had lesions that were primarily inflammatory with minimal to moderate severity (Figs. 2C, D, F, and Table 6). Statistical analysis of the brain lesion scores (Table 6) revealed that the wt VSVIN and rVSVIN groups were significantly greater than those of the PBS control group (p < 0.01) whereas the rVSVIN-HIVGag5 and rVSVIN-CT1-HIVGag5 group scores did not differ significantly from those of the PBS control group.
Table 6.
Summary of histopathological scores in brain tissue of cynomolgus macaques following IT inoculation
| Virus | Macaque no. | Daya | Brain sectionb |
Spinal cord sectionc |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BG | T | C | FC | OC | Mean | Cerv | Thor | Lum | Mean | |||
| wt VSVIN | 22190F | 8 | 4.0d | 3.0 | 2.0 | 2.0 | 2.0 | 2.6 | NSe | NS | NS | naf |
| 22212M | 8 | 4.0 | 4.0 | 4.0 | 2.0 | 2.0 | 3.2 | 2.0 | 2.0 | 0.0 | 1.3 | |
| 16739F | 8 | 2.0 | 3.0 | 2.0 | 1.0 | 1.0 | 1.8 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 21545M | 8 | 2.0 | 3.0 | 2.0 | 0.0 | 0.0 | 1.4 | 0.0 | 0.0 | 0.0 | 0.0 | |
| mean | 3.0 | 3.3 | 2.5 | 1.3 | 1.3 | 2.3g | 0.7 | 0.7 | 0.0 | 0.5h | ||
| rVSVIN | 21392F | 8 | 2.0 | 4.0 | 2.0 | 2.0 | 2.0 | 2.4 | NS | NS | NS | na |
| 21733M | 21 | 2.0 | 2.0 | 2.0 | 1.0 | 1.0 | 1.6 | 2.0 | 3.0 | 4.0 | 3.0 | |
| 21789F | 21 | 4.0 | 3.0 | 2.0 | 2.0 | 1.0 | 2.4 | 2.0 | 3.0 | 4.0 | 3.0 | |
| 21725M | 21 | 2.0 | 3.0 | 2.0 | 1.0 | 1.0 | 1.8 | 1.0 | 3.0 | 2.0 | 2.0 | |
| mean | 2.5 | 3.0 | 2.0 | 1.5 | 1.3 | 2.1g | 1.7 | 3.0 | 3.3 | 2.7g | ||
| rVSVIN-HIVGag5 | 19439M | 21 | 2.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 |
| 21275F | 21 | 1.0 | 1.0 | 1.0 | 0.0 | 0.0 | 0.6 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 22245M | 21 | 1.0 | 1.0 | 0.0 | 1.0 | 1.0 | 0.8 | 2.0 | 1.0 | 2.0 | 1.7 | |
| 21578F | 21 | 2.0 | 4.0 | 2.0 | 1.0 | 1.0 | 2.0 | 1.0 | 2.0 | 3.0 | 2.0 | |
| mean | 1.5 | 1.8 | 0.8 | 0.5 | 0.5 | 1.0i | 0.8 | 0.8 | 1.3 | 1.0h | ||
| rVSVIN-CT1-HIVGag5 | 22253M | 21 | 1.0 | 2.0 | 1.0 | 0.0 | 0.0 | 0.8 | 0.0 | 2.0 | 0.0 | 0.7 |
| 21797F | 21 | 2.0 | 3.0 | 0.0 | 0.0 | 2.0 | 1.4 | 0.0 | 2.0 | 0.0 | 0.7 | |
| 22235M | 21 | 1.0 | 3.0 | 0.0 | 1.0 | 1.0 | 1.2 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 21576F | 21 | 1.0 | 3.0 | 2.0 | 1.0 | 1.0 | 1.6 | 1.0 | 0.0 | 1.0 | 0.7 | |
| mean | 1.3 | 2.8 | 0.8 | 0.5 | 1.0 | 1.3i | 0.3 | 1.0 | 0.3 | 0.5h | ||
| PBS | 21724M | 21 | 1.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| 22290F | 21 | 0.0 | 2.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 22238M | 21 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | |
| 21765F | 21 | 0.0 | 1.0 | 0.0 | 0.0 | 0.0 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | |
| mean | 0.3 | 1.0 | 0.0 | 0.0 | 0.0 | 0.3i | 0.0 | 0.0 | 0.0 | 0.0h | ||
Day of euthanasia.
Brain sections: BG, basal ganglia; T, thalamus; C, cerebellum; FC, frontal cortex; OC, occipital cortex (see Fig. 1C).
Spinal cord sections: cerv, cervical; thor, thoracic; lum, lumbar (see Fig. 1C).
Lesion severity scoring system: 0, no lesions; 1, minimal; 2, moderate; 3, marked; 4, severe.
NS, no slide available.
na, not applicable.
P < 0.01 vs. PBS.
P < 0.05 vs. rVSVIN.
P ≤ 0.05 vs. wt VSVIN.
Fig. 2.
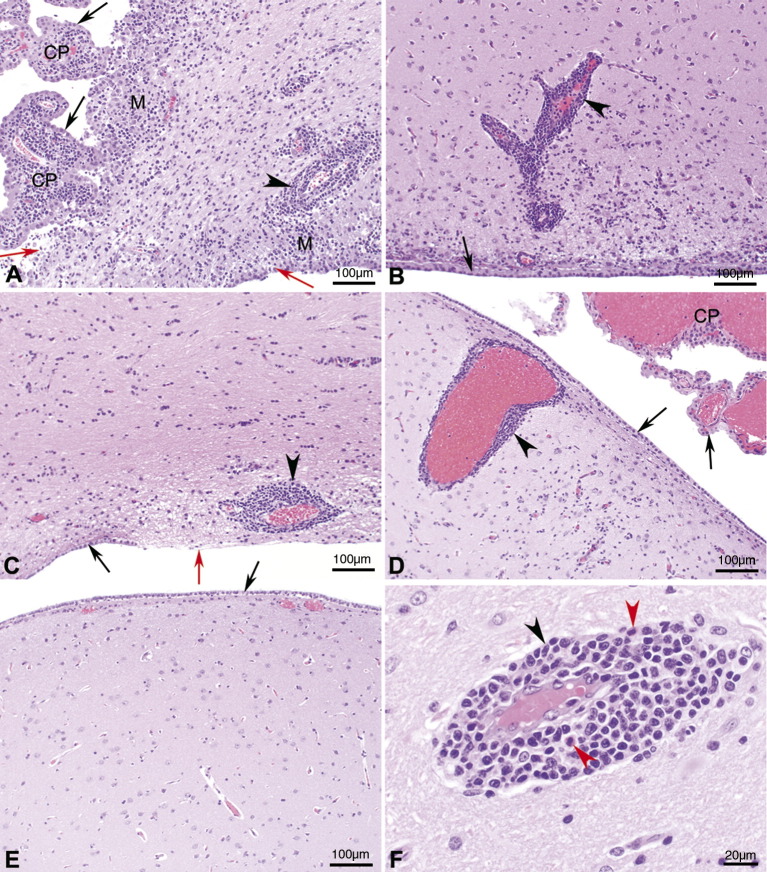
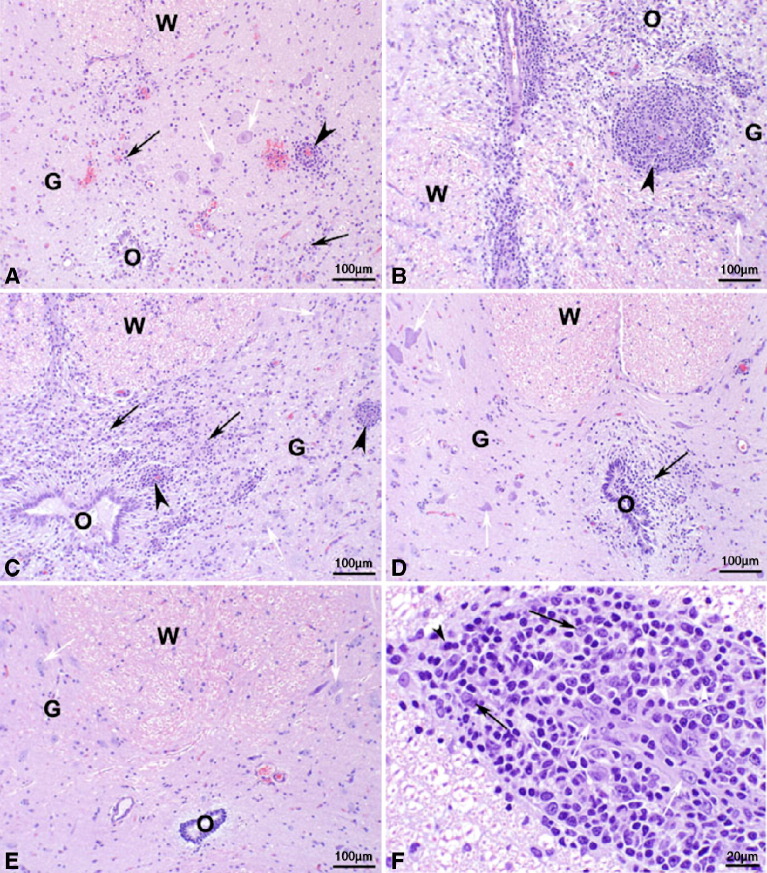
Brain histology. Photomicrographs of hematoxylin and eosin stained sections of lateral ventricle from the thalamic region of the brain of cynomolgus macaques following IT inoculation of: (A) wt VSVIN (macaque number 22212M) with severe periventricular inflammation and malacia, severe choroiditis, and loss of ependymal cells; (B) rVSVIN (macaque number 21789F) with moderate periventricular inflammation; (C) rVSVIN-HIVGag5 (macaque number 22245M) with moderate periventricular inflammation; (D) rVSVIN-CT1-HIVGag5 (macaque number 21797F) with minimal periventricular inflammation; (E) PBS (macaque number 22238M) with no significant lesions observed; (F) rVSVIN-CT1-HIVGag5 (macaque number 21797F, higher magnification), showing characteristic mononuclear inflammatory cell infiltrate composed primarily of lymphocytes, plasma cells, and mononuclear phagocytes along with scattered eosinophils. Black arrow = ependyma, red arrow = denuded ependyma, black arrow head = perivascular cuffs, red arrow head = eosinophil, M = malacia, and CP = choroids plexus. Magnification: A–E = 100 × and F = 400 ×.
It is noteworthy that the wt VSVIN inoculated macaques generally had fewer, less severe lesions in their spinal cords than the macaques in the rVSVIN group (Fig. 3 and Table 6). The mean severity score for spinal cord lesions in the wt VSVIN group (Fig. 3A) was 0.5 (minimal), while the mean score for the rVSVIN group (Fig. 3B) was 2.7 (marked). Macaques in both HIVGag5 vector groups had spinal cord lesions that were less severe than those in the rVSVIN group, with mean severity scores of 1.0 (minimal) and 0.5 (minimal) for rVSVIN-HIVGag5 and rVSVIN-CT1-HIVGag5, respectively (Figs. 3C, D, F, and Table 6). These observations may have resulted from the differences in days of necropsy; that is, viruses in macaques that survived to day 21 may have had more opportunity to spread to the spinal cord than those in macaques euthanized by day 8. Statistical analysis of the mean spinal cord lesion scores (Table 6) revealed that no significant differences existed between the PBS group, the wt group, or the two rVSV-HIV groups (p > 0.05); however, all four of these groups had significantly lower lesion scores than the rVSVIN group (p < 0.05).
Fig. 3.
Spinal cord histology. Photomicrographs of hematoxylin and eosin stained sections of lumbar spinal cord of cynomolgus macaques following IT inoculation of: (A) wt VSVIN (macaque number 22212M) with moderate multifocal gliosis, microhemorrhages, and mononuclear perivascular cuffs; (B) rVSVIN (macaque number 21789F) with severe multifocal coalescing gliosis and malacia, and prominent mononuclear perivascular cuffs; (C) rVSVIN-HIVGag5 (macaque number 21578F) with marked multifocal coalescing gliosis and malacia and prominent mononuclear perivascular cuffs; (D) rVSVIN-CT1-HIVGag5 (macaque number 21576F) with minimal focal gliosis; (E) PBS (macaque number 21724M) with no significant lesions observed; (F) rVSVIN-CT1-HIVGag5 (macaque number 21789F, higher magnification), showing characteristic mononuclear perivascular inflammatory cell infiltrate composed primarily of lymphocytes, plasma cells, and mononuclear phagocytes. Panels A–E: black arrow = gliosis, black arrowhead = mononuclear perivascular cuffs, white arrow = neurons, W = white matter, G = gray matter, O = central canal of spinal cord. Panel F: Black arrow = macrophage, black arrowhead = plasma cell, white arrow = endothelial cell, and white arrowhead = lymphocyte. Magnification: A–E = 100 × and F = 400 ×.
Discussion
Recombinant VSV as candidate HIV vaccine vectors have demonstrated excellent protective efficacy in SHIV challenge studies in macaques (Egan et al., 2004, Rose et al., 2001). However, the rVSVIN vector is both pathogenic and neurotropic in mice (Roberts et al., 1998, Roberts et al., 1999). Therefore, we designed exploratory studies to investigate the pathogenicity of rVSVIN vectors in NHP prior to advancing such vectors to clinical trials.
We did not observe any vector-associated pathogenicity following IN vaccination with the prototypic rVSVIN vector. In the neuroinvasiveness study, neither the wt VSV nor the rVSV vectors showed evidence of spread from nasal tissue to the CNS. Although no virus was detected in sampled brain tissues in any macaques at day 21, it is possible that replicating virus did spread to neuronal tissue proximal to the inoculation site and was simply undetectable by day 21. However, histological analysis revealed no evidence of VSV-related neuropathology in the brains of any of the macaques inoculated IN. In a separate study in rhesus macaques, rVSVIN was shed in nasal secretions and saliva at low levels following IN inoculation, and only on day 1 post-inoculation (Table 1); additionally, there was no evidence of viremia in these macaques at any time point after IN inoculation. Therefore, these results, as well as those from previous studies in NHP (Egan et al., 2004, Egan et al., 2005, Rose et al., 2001), indicate an absence of vector-associated pathogenicity and neuroinvasiveness for replication-competent rVSV vectors by the IN route. The low level of shedding at mucosal surfaces should not be a concern for early phase clinical trials, particularly because neither vertical nor horizontal transmission of VSV is easily achieved, either experimentally or in natural infection (Brandly and Hanson, 1957, Hanson, 1952, Reif et al., 1987, Tesh and Johnson, 1975, Webb et al., 1987).
Following IT inoculation of macaques, the wt VSVIN caused primarily moderate to marked neuropathology, as measured by both clinical and histological parameters. These results are consistent with the observation that VSV is neurovirulent in macaques (Olitsky et al., 1934) and livestock (Frank et al., 1945) following direct intracerebral inoculation.
Of the vector groups, rVSVIN was most similar to the wt group because one of four animals from this group displayed clinical features identical to those observed in the wt VSVIN group and because all four animals had histological lesion scores of similar severity to those in the wt group. Despite the small numbers in each group, statistical analysis of the mean brain lesion scores demonstrated that both the wt VSVIN group and the rVSVIN vector groups had significantly increased lesion scores with respect to the PBS control group, suggesting that the rVSVIN vector is insufficiently attenuated for clinical use. The rVSVIN-HIVGag5 and rVSVIN-CT1-HIVGag5 vectors were significantly more attenuated than the wt and rVSVIN backbone vector in this macaque model, particularly in the clinical analysis, where they showed no signs of disease and were most similar to the negative control group (Table 3). Histologically, these two rVSV-HIV vectors caused lesions that were, on average, less severe and more inflammatory in nature than both the wt and rVSVIN backbone vector groups (Fig. 2, Fig. 3, and Table 6); the mean brain lesion scores for the rVSV-HIV vector groups were statistically significantly lower than those of the wt group and were not statistically significantly higher than the PBS control group. The presence of the additional gene (HIV Gag) and the CT1 mutation was likely responsible for the observed attenuation of virulence.
The histology results (Table 6) indicated that the severity scores in the wt VSVIN inoculated macaques were inconsistent between the brain sections (mean score of 2.3) and the spinal cord sections (mean score of 0.5). These results are most likely due to the fact that all of the wt inoculated macaques were euthanized at day 8, which may have been too early to allow spread to the spinal cords. The detection of replicating virus in occipital lobes and CSF in some macaques in this group (Table 4) suggested that spread of virus to the spinal cord occurred via the CSF. Since the spinal cord lesion scores in the rVSVIN and rVSVIN-HIVGag5 macaques that survived to day 21 were higher than those in the wt group (Table 6), which were all euthanized by day 8, it apparently required more than 8 days for virus to cause spinal cord lesions. We have observed a similar phenomenon in mice inoculated intracranially with the wt and rVSVIN-CT1 viruses; the wt virus killed the mice so rapidly that their brain lesion scores were lower than those in the rVSVIN-CT1 inoculated mice that survived longer (unpublished results). It is also possible that slight variations in the inoculation procedure may have resulted in variation in the immediate distribution of the inoculum, with some macaques having increased initial contamination of the CSF with the virus and consequently increased neuropathology in sites distal to the injection site.
Histological evidence of neuropathology was not clearly reflected by clinical or virological parameters. For example, macaques in the rVSVIN-HIVGag5 and rVSVIN-CT1-HIVGag5 groups had clinical scores similar to the negative control group, and there was no detectable virus or viral RNA in tissues at day 21. However, the histological analysis of brain and spinal cord tissue indicated that productive infection had occurred in these macaques, causing lesions that were mostly minimal, but were moderate to marked in some tissues in some macaques (Table 6). Similar results were observed in the three macaques in the rVSVIN group that survived to day 21, in which the lack of clinical signs or detectable virus did not coincide with the relatively high lesion scores in brains and spinal cords. Therefore, the histological analysis appeared to be the more sensitive means of assessing NV in this model, as is the case in NHP NV tests of yellow fever and mumps vaccines (Levenbook et al., 1987, Maximova et al., 1996, Rubin et al., 1999). Furthermore, the viral load measurements were best suited to time points when viral replication occurred, as observed in the macaques that died or were euthanized by day 8. Based on the collective infectivity and RT-PCR data, it is clear that the wt virus and rVSVIN backbone vector replicated at early time points following inoculation and that detectable replication had ceased in all groups by day 21. Presumably, the rVSVIN-HIVGag5 and rVSVIN-CT1-HIVGag5 viruses also replicated at early time points because there was serologic evidence of viral replication (Table 2). Although some of the macaques inoculated with rVSVIN-CT1-HIVGag5 did not raise detectable neutralizing antibodies, the virus likely was able to establish an infection at the site of inoculation. It has previously been demonstrated in mice that the rVSVIN-CT1 vector generated low levels of antibodies after a single IN inoculation and that boosting was required to achieve high levels of anti-VSV neutralizing antibodies (Roberts et al., 1999). Subsequent studies in the NHP NV model should include sampling CNS tissues at earlier time points to more accurately measure viral replication within these tissues.
Regulatory agencies have historically required NV testing for live viral vaccines. In the case of yellow fever and mumps vaccines, both of which were originally approved under different, early regulatory standards, the vaccine strains must show a significant decrease in histopathology if the reference control is a wild type strain, or no significant increase in histopathology if the reference control is a vaccine strain. In this study, HIV vaccine candidates rVSVIN-HIVGag5 and rVSVIN-CT1-HIVGag5 generated histopathology severity scores that were not statistically different from those of the PBS group and that were statistically lower than those of the wt group. However, increased regulatory stringency and a lack of established safety record for rVSV vectors in humans as compared to such vaccines as measles, mumps, polio, and yellow fever (Arya, 2002) remain concerns for developing rVSV vectors as vaccine candidates. Therefore, we have proceeded to construct additional, further attenuated rVSV vector candidates [reviewed in Clarke et al., 2006] using such approaches as gene rearrangement (Ball et al., 1999, Flanagan et al., 2001, Flanagan et al., 2003, Novella et al., 2004, Wertz et al., 1998), ablation of expression of the M2 and M3 polypeptides encoded by the M gene (Jayakar and Whitt, 2002), temperature sensitive mutations (Flamand and Pringle, 1971, Pringle, 1970), and gene deletions or truncations (Jeetendra et al., 2003, Kahn et al., 2001, Roberts et al., 1999, Robison and Whitt, 2000).
There is an additional concern regarding the utility of the macaque model for rVSV NV testing. In this model, there was no statistically significant difference between the histological scores in the rVSVIN-HIVGag5 and rVSVIN-CT1-HIVGag5 groups (Table 6) despite the fact that the CT1 variant is measurably more attenuated than rVSVIN in vitro and in mice following IN inoculation (Roberts et al., 1999, Schnell et al., 1998). In addition, the wt VSVIN caused severe clinical illness that required euthanasia of macaques inoculated IT by day 8, which complicated the comparisons to other groups in which the macaques survived to day 21. The NHP NV model for VSV therefore requires additional development. Because of cost and procurement issues associated with NHP, we also intend to investigate alternate animal models for testing rVSV vector-associated pathogenicity.
Materials and methods
Viruses and vectors tested in NHP studies
The viruses tested in these studies included tissue culture-adapted wt VSVIN; the prototypic, attenuated vector rVSVIN; the rVSVIN vector encoding HIV Gag protein; and a further attenuated vector encoding HIV Gag, rVSVIN-CT1-HIVGag5 (Fig. 1). The tissue culture-adapted San Juan isolate of VSV Indiana serotype (wt VSVIN) was least attenuated in vitro and was highly pathogenic in mice (Roberts et al., 1998). The rVSVIN was derived from a cDNA encoding the entire genome of VSVIN (Lawson et al., 1995), which was constructed using RT-PCR products combined from the genomes of VSVIN, San Juan and Mudd-Summers isolates. The resulting virus contained multiple nucleotide sequence differences from the parental San Juan isolate that presumably accounted for the in vivo attenuation of rVSVIN relative to wt VSVIN (Roberts et al., 1998). This cDNA served as the “backbone” vector from which the two rVSV-HIV vectors were derived. The prototypic HIV vaccine vector, rVSVIN-HIVGag5, was similar to the vectors tested previously in SHIV challenge studies in macaques (Rose et al., 2001), except that this vector encoded an HIV-1 Gag protein rather than an SIV Gag. Finally, the rVSVIN-CT1-HIVGag5 contained a mutant G gene that encodes a protein in which the cytoplasmic tail domain is truncated from 29 amino acids to a single amino acid (CT1); this mutation has been shown to greatly reduce vector pathogenicity in mice following IN inoculation (Roberts et al., 1999, Schnell et al., 1998). Since PBS was the diluent for each virus, it was used as the negative control inoculum.
The wild type, rVSVIN, and rVSVIN-CT1-HIVGag5 viruses were provided by Dr. John K. Rose (Yale University, New Haven, CT). The rVSVIN-HIVGag5 virus was rescued from cDNA pVSVIN-HIVGag5 using standard reverse genetics procedures (Lawson et al., 1995, Schnell et al., 1996b). The cDNA pVSVIN-HIVGag5 was generated by PCR amplification of the ORF of the HIV-1 gag gene (HXB2 strain) and insertion into the VSV vector backbone pVSV-XN1 (Schnell et al., 1996b) via XhoI and NheI restriction sites as the fifth gene in the genome. All viruses were amplified on baby hamster kidney (BHK) cells at a multiplicity of infection (MOI) between 0.1 and 1, concentrated by ultracentrifugation through 10% sucrose (28,000 rpm in a Beckman SW28 rotor at 4 °C for 75–90 min) and resuspended in phosphate buffered saline (PBS).
Viral shedding study in rhesus macaques
The rhesus macaque study was performed at the University of Louisiana at Lafayette-New Iberia Research Center (New Iberia), LA, with adherence to the regulations outlined in the USDA Animal Welfare Act (9 CFR, parts 1, 2 and 3) and the conditions specified in the Guide for the Care and Use of Laboratory Animals (ILAR publication, 1996, National Academy Press). Any clinical signs of illness or distress were promptly reported to the responsible veterinarian, who recommended either treatment for minor ailments and injuries or euthanasia for profound effects such as severe emesis or convulsions.
Because of limited availability of macaques, three male rhesus macaques (3–4 years old) were vaccinated, and two un-inoculated macaques served as negative controls (Fig. 1A). Following light sedation with ketamine, macaques were inoculated IN with a total of 107 plaque-forming units (PFU) of rVSVIN in PBS, delivered to both nostrils in a total of 0.8 mL by micropipettor. Samples were collected as follows: saliva and nasal washes (in that order, ≥ 1.0 mL each) on days 1–4, 6, 8, 10, 13, 16, 19, and 22; serum (1.0 mL) pre-dose and on days 1–4, 8, 13, and 22; whole blood (including sodium heparin as an anti-coagulant, 10 mL) pre-dose and on days 8, 14, and 22.
Neuroinvasiveness and neurovirulence (NV) studies in cynomolgus macaques
Both the NV and neuroinvasiveness studies were performed at Charles River Laboratories, Inc.-Preclinical Services (Sparks, NV). The cynomolgus macaque studies adhered to the regulations outlined in the USDA Animal Welfare Act (9 CFR, Parts 1, 2, and 3) and the conditions specified in the Guide for the Care and Use of Laboratory Animals (ILAR publication, 1996, National Academy Press). Any clinical signs of illness or distress were promptly reported to the responsible veterinarian, who recommended either treatment for minor ailments and injuries or euthanasia for profound effects such as severe emesis or convulsions.
Cynomolgus macaques (2–3.5 years old) were inoculated either intranasally (IN) or intrathalamically (IT) with a total of 107 PFU of virus per macaque (Fig. 1B). Each group contained two females and two males. For IN inoculations, macaques were lightly sedated with ketamine. Each macaque received a total of 1.0 mL of inoculum, injected via syringe into both nostrils over the course of approximately 1 min. Non-anti-inflammatory analgesics were given at the discretion of the veterinarian to prevent post-inoculation discomfort.
For IT inoculations, macaques were anesthetized with a mixture of ketamine and valium; each macaque's head was shaved, and incisions were made through the skin at approximately 1.5 cm on either side of the sagittal suture and at approximately 0.5 cm from the coronal suture of the head of the macaque. A high-speed, general twist drill was used to make two 0.16 cm holes through the skull, corresponding to the positioned incisions described above. Subsequently, 0.1 mL of the virus or control inoculum was injected into the right and left thalamic regions for a total dose volume of 0.2 mL/macaque, using a 25 gauge × 1 1/2 in. needle (to a depth of approximately 1 in.) on a 1.0 mL tuberculin syringe, introduced in a medial–oral direction. After inoculation, the surgical area was closed with a subcuticular suture to minimize self-inflicted trauma by the macaque. Each macaque was observed until it recovered from anesthesia and was then returned to its cage. Non-anti-inflammatory analgesics were given at the discretion of the veterinarian to prevent post-surgical discomfort.
Macaques were monitored for 21 days after challenge for the following parameters: food consumption, body weight and temperature, serum chemistry, hematology, and general clinical parameters, the latter of which were scored using a system based on that used for yellow fever vaccine (Levenbook et al., 1987). Macaques were examined twice daily for possible clinical signs of encephalitis and were assigned numerical scores as follows: 0, no clinical signs of encephalitis; 1, rough coat, not eating; 2, high pitched voice, inactive, slow moving; 3, shaky movements, tremors, incoordination, limb weakness; 4, inability to stand, limb paralysis, moribundity, or death. The higher of the two daily individual macaque scores was assigned as the daily score. The mean daily clinical score for each macaque was calculated by averaging the 22 individual macaque daily scores, and the group mean daily scores were calculated for each study day by averaging the individual macaque scores for each group. At the time of euthanasia (scheduled or unscheduled), the macaques were exsanguinated while under deep anesthesia induced with ketamine and Beuthanasia®-D.
Tissue preparation
All tissues and fluids were collected at the time of euthanasia. For quantification of virus, frozen samples of occipital and frontal cortex were thawed, weighed, and suspended in PBS supplemented with 1 × sucrose, phosphate, glutamate buffer (0.2 M sucrose, 7.0 mM K2HPO4, 3.8 mM KH2PO4, 5.0 mM glutamic acid) to generate 10% (w:v) tissue suspensions. Samples were homogenized using an Omni Mixer ES with aerosol-sealed stainless steel chambers (Omni International). Tissue homogenates were centrifuged at 3000 rpm for 15 min at 4 °C and clarified supernatants used immediately for plaque assay or RT-PCR, as described below. Cerebral spinal fluid (CSF) samples were thawed and used directly in assays. PBMCs were assayed for the presence of infectious VSV by two methods: direct co-culture of the PBMCs with BHKs or following a freeze–thaw and clarification of the lysates.
Histopathological evaluation
Brain and spinal cord tissue sections were collected from each macaque (following gross necropsy) and fixed in neutral buffered 10% formalin. Samples were taken from five brain regions (Fig. 1C) including frontal cortex, occipital cortex, cerebellum, thalamus, basal ganglia, and from three spinal cord regions (cervical, thoracic, and lumbar). Fixed tissues were mounted onto slides and stained with eosin and hematoxylin for histological examinations, which were performed by Roger Price, D.V.M., Ph.D., D.A.C.V.P. (Veterinary Pathology Services, Houston, TX), and peer-reviewed by Andrew Lackner, D.V.M., D.A.C.V.P. (Tulane National Primate Research Center, Covington, LA) and Mary E.P. Goad, D.V.M., Ph.D. (Exploratory Drug Safety, Wyeth Research, Andover, MA).
Plaque assay
Ten-fold serial dilutions of tissue homogenates, PBMCs, PBMC lysates, or CSF were prepared in Dulbecco's Modified Eagle Medium (DMEM, Mediatech, Inc.) with 10% fetal bovine serum (FBS). Aliquots (100 μL) of each dilution were placed on confluent BHK or Vero cell monolayers in 6-well plates and allowed to absorb for 15 min at room temperature followed by incubation at 37 °C for 30 min. Inoculum was removed from each well, which was then overlaid with 0.8% agarose in DMEM + 10% FBS. Plates were incubated at 37 °C overnight. Agar overlays were removed, and plates were stained for 10–20 min at room temperature with 2 mL/well of crystal violet stain solution (1% crystal violet in 70% methanol).
Real-time quantitative RT-PCR
Aliquots of tissue homogenates (500 μL) were centrifuged at 14,000 rpm for 5 min. Viral RNA was extracted from clarified supernatants or CSF samples (140 μL) using QIAamp Viral Mini Kit (Qiagen, Inc.). An equivalent volume of buffer was extracted as a negative control, and an aliquot of the viral inoculum was extracted as a positive control.
Using Primer Express software (PE Applied Biosystems), primers (Qiagen) and probe (AB Applied Biosystems) for the VSV N gene were designed to amplify a 70-nucleotide fragment. The sequence of the forward primer was as follows: 5′-GATAGTACCGGAGGATTGACGACTA-3′ corresponding to nucleotide positions 1075–1099 in the N open reading frame. The reverse primer sequence was as follows: 5′-AACCATCCGAGCCATTCGA-3′ corresponding to nucleotide positions 1127–1145 in the N open reading frame. The fluorogenic probe was labeled at the 5′ end with the reporter dye 6-carboxyfluorescein (FAM) and at the 3′ end with the quencher dye 6-carboxytetramethylrhodamine (TAMRA), and its sequence was as follows: 5′-TGCACCGCCACAAGGCAGAGA-3′ corresponding to nucleotide positions 1101–1121 in the N open reading frame.
To determine the number of genome copies of VSV, a synthetic RNA oligonucleotide corresponding to the entire amplified region was synthesized and PAGE-purified (TriLink BioTechnologies). Following precipitation and centrifugation, the number of oligonucleotide copies per μL of solution was calculated using Avogadro's number, the molecular weight, and the extinction coefficient of the oligonucleotide. Serial 10-fold dilutions were made of this synthetic oligonucleotide and used to generate a standard curve.
The quantitative RT-PCR assay was developed as a two-step procedure to detect the genomic (negative sense) RNA of VSV. The initial reaction mixture for reverse transcription (25 μL) contained 7.5 μL purified RNA, 900 nM forward primer, reagents from the TaqMan® Reverse Transcription Reagents kit (Applied Biosystems), and 15 units/μL of ThermoScript™ Reverse Transcriptase (InVitrogen™ Life Technologies). The reaction was incubated at 60 °C for 30 min, which was followed by an inactivation step of heating at 95 °C for 15 min. Then, 30 μL of 2× Master mix buffer (TaqMan® One Step PCR Master Mix Reagent Kit; Applied Biosystems), 900 nM of reverse primer, and 200 nM of probe was added to the cDNA reaction mix to bring the final volume to 60 μL. Amplification and detection were performed with an ABI Prism 7700 Sequence Detection System under the following conditions: 10 min at 95 °C, and 40 cycles of 15 s at 95 °C and 1 min at 60 °C. All samples were tested in duplicate.
VSV serum neutralization assay
Sera were diluted with PBS in a volume of 50 μL in serial two-fold dilutions in 96-well plates. One hundred PFU of virus (rVSVIN) in 50 μL of serum-free DMEM was added to each well. Plates containing serum and virus were incubated at 37 °C for 1 h. Approximately 1500 BHK cells in 100 μL of DMEM–10% FBS were then added to each well. The plates were incubated at 37 °C and 5% CO2 for 2 to 3 days and assayed by microscopy for the presence of VSV-induced cytopathology. Each assay was performed in duplicate. Neutralizing titers were assigned as the highest dilutions that completely inhibited VSV-induced cytopathology.
Statistical analysis
Differences in histopathological scores among groups of macaques were analyzed by one-way analysis of variance using the Dunnett multiple comparisons post test (InStat version 2.02, GraphPad Software, San Diego, CA).
Acknowledgments
The authors wish to thank the following individuals for their assistance: Andrew Lackner and Mary E.P. Goad for peer-reviewing the histopathology slides in the NV and neuroinvasivenss studies, Eva Schadeck for assistance with the rhesus macaque shedding study, Sannyu Heron for assistance with generation of the rVSVIN-HIVGag5 virus, Eleanor Ogin-Wilson for assistance with the RT-PCR analyses, Roger French for assistance with the statistical analysis, and John K. Rose for providing the wt VSVIN, rVSVIN, and rVSVIN-CT1-HIVGag5 viruses and advice.
The authors would also like to acknowledge the staff at Charles River Laboratories-Sierra Division (under the supervision of K.D.) for their execution of the NV and neuroinvasiveness studies and the staff at University of Louisiana at Lafayette-New Iberia Research Center (under the supervision of Dr. D.L. Hasselschwert, M.S., D.V.M.) for their execution of the shedding study.
Finally, the authors acknowledge Dr. David Cooper and Dr. Robert O'Neill for helpful comments on the manuscript.
This work was supported by NIH Contract N01-AI-25458.
References
- Arya S.C. Yellow fever vaccine safety: a reality or a myth? Vaccine. 2002;20(31–32):3627–3628. doi: 10.1016/s0264-410x(02)00409-7. [DOI] [PubMed] [Google Scholar]
- Ball L.A., Pringle C.R., Flanagan B., Perepelitsa V.P., Wertz G.W. Phenotypic consequences of rearranging the P, M, and G genes of vesicular stomatitis virus. J. Virol. 1999;73(6):4705–4712. doi: 10.1128/jvi.73.6.4705-4712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Z., Barna M., Komatsu T., Reiss C.S. Vesicular stomatitis virus infection of the central nervous system activates both innate and acquired immunity. J. Virol. 1995;69(10):6466–6472. doi: 10.1128/jvi.69.10.6466-6472.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandly C.A., Hanson R.P. Epizootiology of vesicular stomatitis. Am. J. Public Health. 1957;47(2):205–209. doi: 10.2105/ajph.47.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody J.A., Fischer G.F., Peralta P.H. Vesicular stomatitis virus in Panama. Human serologic patterns in a cattle raising area. Am. J. Epidemiol. 1967;86(1):158–161. doi: 10.1093/oxfordjournals.aje.a120721. [DOI] [PubMed] [Google Scholar]
- Clarke D.K., Cooper D., Egan M.A., Hendry R.M., Parks C.L., Udem S.A. Springer Semin. Immunopathol. 2006. Recombinant vesicular stomatitis virus as an HIV-1 vaccine vector. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan M.A., Chong S.Y., Rose N.F., Megati S., Lopez K.J., Schadeck E.B., Johnson J.E., Masood A., Piacente P., Druilhet R.E., Barras P.W., Hasselschwert D.L., Reilly P., Mishkin E.M., Montefiori D.C., Lewis M.G., Clarke D.K., Hendry R.M., Marx P.A., Eldridge J.H., Udem S.A., Israel Z.R., Rose J.K. Immunogenicity of attenuated vesicular stomatitis virus vectors expressing HIV type 1 Env and SIV Gag proteins: comparison of intranasal and intramuscular vaccination routes. AIDS Res. Hum. Retrovir. 2004;20(9):989–1004. doi: 10.1089/aid.2004.20.989. [DOI] [PubMed] [Google Scholar]
- Egan M.A., Chong S.Y., Megati S., Montefiori D.C., Rose N.F., Boyer J.D., Sidhu M.K., Quiroz J., Rosati M., Schadeck E.B., Pavlakis G.N., Weiner D.B., Rose J.K., Israel Z.R., Udem S.A., Eldridge J.H. Priming with plasmid DNAs expressing interleukin-12 and simian immunodeficiency virus gag enhances the immunogenicity and efficacy of an experimental AIDS vaccine based on recombinant vesicular stomatitis virus. AIDS Res. Hum. Retrovir. 2005;21(7):629–643. doi: 10.1089/aid.2005.21.629. [DOI] [PubMed] [Google Scholar]
- Fellowes O.N., Dimopoullos G.T., Callis J.J. Isolation of vesicular stomatitis virus from an infected laboratory worker. Am. J. Vet. Res. 1955;16(61 Part 1):623–626. [PubMed] [Google Scholar]
- Ferris D.H., Hanson R.P., Dicke R.J., Roberts R.H. Experimental transmission of vesicular stomatitis virus by Diptera. J. Infect. Dis. 1955;96(2):184–192. doi: 10.1093/infdis/96.2.184. [DOI] [PubMed] [Google Scholar]
- Fields B.N., Hawkins K. Human infection with the virus of vesicular stomatitis during an epizootic. N. Engl. J. Med. 1967;277(19):989–994. doi: 10.1056/NEJM196711092771901. [DOI] [PubMed] [Google Scholar]
- Flamand A., Pringle C.R. The homologies of spontaneous and induced temperature-sensitive mutants of vesicular stomatitis virus isolated in chick embryo and BHK 21 cells. J. Gen. Virol. 1971;11(2):81–85. doi: 10.1099/0022-1317-11-2-81. [DOI] [PubMed] [Google Scholar]
- Flanagan E.B., Zamparo J.M., Ball L.A., Rodriguez L.L., Wertz G.W. Rearrangement of the genes of vesicular stomatitis virus eliminates clinical disease in the natural host: new strategy for vaccine development. J. Virol. 2001;75(13):6107–6114. doi: 10.1128/JVI.75.13.6107-6114.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan E.B., Schoeb T.R., Wertz G.W. Vesicular stomatitis viruses with rearranged genomes have altered invasiveness and neuropathogenesis in mice. J. Virol. 2003;77(10):5740–5748. doi: 10.1128/JVI.77.10.5740-5748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J.G., Robain O., Cerutti I., Tardivel I., Chany-Fournier F., Chany C. Detection of vesicular stomatitis virus (VSV) RNA in the central nervous system of infected mice by in situ hybridization. Acta Neuropathol. (Berl.) 1988;75(6):554–556. doi: 10.1007/BF00686199. [DOI] [PubMed] [Google Scholar]
- Frank A.H., Appleby A., Seibold H.R. Experimental intracerebral infection of horses, cattle, and sheep with the virus of vesicular stomatitis. Am. J. Vet. Res. 1945:28–38. (January) [Google Scholar]
- Fultz P.N., Shadduck J.A., Kang C.Y., Streilein J.W. Genetic analysis of resistance to lethal infections of vesicular stomatitis virus in Syrian hamsters. Infect. Immun. 1981;32(3):1007–1013. doi: 10.1128/iai.32.3.1007-1013.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R.P. The natural history of vesicular stomatitis. Bacteriol. Rev. 1952;16(3):179–204. doi: 10.1128/br.16.3.179-204.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R.P., Rasmussen A.F., Jr., Brandly C.A., Brown J.W. Human infection with the virus of vesicular stomatitis. J. Lab. Clin. Med. 1950;36(5):754–758. [PubMed] [Google Scholar]
- Heiny E. Vesicular stomatitis in cattle and horses in Colorado. North Am. Vet. 1945;26:726–730. [PubMed] [Google Scholar]
- Huneycutt B.S., Plakhov I.V., Shusterman Z., Bartido S.M., Huang A., Reiss C.S., Aoki C. Distribution of vesicular stomatitis virus proteins in the brains of BALB/c mice following intranasal inoculation: an immunohistochemical analysis. Brain Res. 1994;635(1–2):81–95. doi: 10.1016/0006-8993(94)91426-5. [DOI] [PubMed] [Google Scholar]
- Jayakar H.R., Whitt M.A. Identification of two additional translation products from the matrix (M) gene that contribute to vesicular stomatitis virus cytopathology. J. Virol. 2002;76(16):8011–8018. doi: 10.1128/JVI.76.16.8011-8018.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeetendra E., Ghosh K., Odell D., Li J., Ghosh H.P., Whitt M.A. The membrane-proximal region of vesicular stomatitis virus glycoprotein G ectodomain is critical for fusion and virus infectivity. J. Virol. 2003;77(23):12807–12818. doi: 10.1128/JVI.77.23.12807-12818.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John T.J. Chandipura virus, encephalitis, and epidemic brain attack in India. Lancet. 2004;364(9452):2175. doi: 10.1016/S0140-6736(04)17579-X. [DOI] [PubMed] [Google Scholar]
- Johnson K.M., Vogel J.E., Peralta P.H. Clinical and serological response to laboratory-acquired human infection by Indiana type vesicular stomatitis virus (VSV) Am. J. Trop. Med. Hyg. 1966;15(2):244–246. doi: 10.4269/ajtmh.1966.15.244. [DOI] [PubMed] [Google Scholar]
- Jones S.M., Feldmann H., Stroher U., Geisbert J.B., Fernando L., Grolla A., Klenk H.D., Sullivan N.J., Volchkov V.E., Fritz E.A., Daddario K.M., Hensley L.E., Jahrling P.B., Geisbert T.W. Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses. Nat. Med. 2005;11(7):786–790. doi: 10.1038/nm1258. (see comment) [DOI] [PubMed] [Google Scholar]
- Kahn J.S., Roberts A., Weibel C., Buonocore L., Rose J.K. Replication-competent or attenuated, nonpropagating vesicular stomatitis viruses expressing respiratory syncytial virus (RSV) antigens protect mice against RSV challenge. J. Virol. 2001;75(22):11079–11087. doi: 10.1128/JVI.75.22.11079-11087.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia S.U., Rose J.K., Lamirande E., Vogel L., Subbarao K., Roberts A. Long-term protection from SARS coronavirus infection conferred by a single immunization with an attenuated VSV-based vaccine. Virology. 2005;340(2):174–182. doi: 10.1016/j.virol.2005.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson N.D., Stillman E.A., Whitt M.A., Rose J.K. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. U.S.A. 1995;92(10):4477–4481. doi: 10.1073/pnas.92.10.4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth G.J., Rodriguez L.L., Del cbarrera J. Vesicular stomatitis. Vet. J. 1999;157(3):239–260. doi: 10.1053/tvjl.1998.0303. [DOI] [PubMed] [Google Scholar]
- Levenbook I.S., Nikolayeva M.A., Chigirinsky A.E., Ralf N.M., Kozlov V.G., Vardanyan N.V., Sliopushkina V.G., Kolomiets O.L., Rukhamina M.L., Grigoryeva L.V. On the morphological evaluation of the neurovirulence safety of attenuated mumps virus strains in monkeys. J. Biol. Stand. 1979;7(1):9–19. doi: 10.1016/s0092-1157(79)80033-5. [DOI] [PubMed] [Google Scholar]
- Levenbook I.S., Pelleu L.J., Elisberg B.L. The monkey safety test for neurovirulence of yellow fever vaccines: the utility of quantitative clinical evaluation and histological examination. J. Biol. Stand. 1987;15(4):305–313. doi: 10.1016/s0092-1157(87)80003-3. [DOI] [PubMed] [Google Scholar]
- Lundh B., Kristensson K., Norrby E. Selective infections of olfactory and respiratory epithelium by vesicular stomatitis and Sendai viruses. Neuropathol. Appl. Neurobiol. 1987;13(2):111–122. doi: 10.1111/j.1365-2990.1987.tb00175.x. [DOI] [PubMed] [Google Scholar]
- Marchevsky R.S., Freire M.S., Coutinho E.S., Galler R. Neurovirulence of yellow fever 17DD vaccine virus to rhesus monkeys. Virology. 2003;316(1):55–63. doi: 10.1016/s0042-6822(03)00583-x. [DOI] [PubMed] [Google Scholar]
- Maximova O., Dragunsky E., Taffs R., Snoy P., Cogan J., Marsden S., Levenbook I. Monkey neurovirulence test for live mumps vaccine. Biologicals. 1996;24(3):223–224. doi: 10.1006/biol.1996.0030. [DOI] [PubMed] [Google Scholar]
- Miyoshi K., Harter D.H., Hsu K.C. Neuropathological immunofluorescence studies of experimental vesicular stomatitis virus encephalitis in mice. J. Neuropathol. Exp. Neurol. 1971;30(2):266–277. doi: 10.1097/00005072-197104000-00008. [DOI] [PubMed] [Google Scholar]
- Nathanson N., Horn S.D. Neurovirulence tests of type 3 oral poliovirus vaccine manufactured by Lederle Laboratories, 1964–1988. Vaccine. 1992;10(7):469–474. doi: 10.1016/0264-410x(92)90396-2. [DOI] [PubMed] [Google Scholar]
- Novella I.S., Ball L.A., Wertz G.W. Fitness analyses of vesicular stomatitis strains with rearranged genomes reveal replicative disadvantages. J. Virol. 2004;78(18):9837–9841. doi: 10.1128/JVI.78.18.9837-9841.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olitsky P.K., Cox H.R., Syverton J.T. Comparative studies on the viruses of vesicular stomatitis and equine encephalomyelitis. J. Exp. Med. 1934;59:159–171. doi: 10.1084/jem.59.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson W.C., Mott L.O., Jenney E.W. A study of vesicular stomatitis in man. J. Am. Vet. Med. Assoc. 1958;133(1):57–62. [PubMed] [Google Scholar]
- Plakhov I.V., Arlund E.E., Aoki C., Reiss C.S. The earliest events in vesicular stomatitis virus infection of the murine olfactory neuroepithelium and entry of the central nervous system. Virology. 1995;209(1):257–262. doi: 10.1006/viro.1995.1252. [DOI] [PubMed] [Google Scholar]
- Pringle C.R. Genetic characteristics of conditional lethal mutants of vesicular stomatitis virus induced by 5-fluorouracil, 5-azacytidine, and ethyl methane sulfonate. J. Virol. 1970;5(5):559–567. doi: 10.1128/jvi.5.5.559-567.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao B.L., Basu A., Wairagkar N.S., Gore M.M., Arankalle V.A., Thakare J.P., Jadi R.S., Rao K.A., Mishra A.C. A large outbreak of acute encephalitis with high fatality rate in children in Andhra Pradesh, India, in 2003, associated with Chandipura virus. Lancet. 2004;364(9437):869–874. doi: 10.1016/S0140-6736(04)16982-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif J.S., Webb P.A., Monath T.P., Emerson J.K., Poland J.D., Kemp G.E., Cholas G. Epizootic vesicular stomatitis in Colorado, 1982: infection in occupational risk groups. Am. J. Trop. Med. Hyg. 1987;36(1):177–182. doi: 10.4269/ajtmh.1987.36.177. [DOI] [PubMed] [Google Scholar]
- Reiss C.S., Plakhov I.V., Komatsu T. Viral replication in olfactory receptor neurons and entry into the olfactory bulb and brain. Ann. N. Y. Acad. Sci. 1998;30:752–761. doi: 10.1111/j.1749-6632.1998.tb10655.x. [DOI] [PubMed] [Google Scholar]
- Reuter J.D., Vivas-Gonzalez B.E., Gomez D., Wilson J.H., Brandsma J.L., Greenstone H.L., Rose J.K., Roberts A. Intranasal vaccination with a recombinant vesicular stomatitis virus expressing cottontail rabbit papillomavirus L1 protein provides complete protection against papillomavirus-induced disease. J. Virol. 2002;76(17):8900–8909. doi: 10.1128/JVI.76.17.8900-8909.2002. (erratum appears in J Virol. 2003 Feb; 77 (4): 2799) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Rose J.K. Recovery of negative-strand RNA viruses from plasmid DNAs: a positive approach revitalizes a negative field. Virology. 1998;247(1):1–6. doi: 10.1006/viro.1998.9250. [DOI] [PubMed] [Google Scholar]
- Roberts A., Kretzschmar E., Perkins A.S., Forman J., Price R., Buonocore L., Kawaoka Y., Rose J.K. Vaccination with a recombinant vesicular stomatitis virus expressing an influenza virus hemagglutinin provides complete protection from influenza virus challenge. J. Virol. 1998;72(6):4704–4711. doi: 10.1128/jvi.72.6.4704-4711.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Buonocore L., Price R., Forman J., Rose J.K. Attenuated vesicular stomatitis viruses as vaccine vectors. J. Virol. 1999;73(5):3723–3732. doi: 10.1128/jvi.73.5.3723-3732.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A., Reuter J.D., Wilson J.H., Baldwin S., Rose J.K. Complete protection from papillomavirus challenge after a single vaccination with a vesicular stomatitis virus vector expressing high levels of L1 protein. J. Virol. 2004;78(6):3196–31499. doi: 10.1128/JVI.78.6.3196-3199.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison C.S., Whitt M.A. The membrane-proximal stem region of vesicular stomatitis virus G protein confers efficient virus assembly. J. Virol. 2000;74(5):2239–2246. doi: 10.1128/jvi.74.5.2239-2246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J.K., Whitt M.A. Rhabdoviridae: the viruses and their replication. In: Knipe D.M., Howley P.M., Griffin D.E., Lamb R.A., Martin M.A., Roizman B., Straus S.E., editors. 4th edition. vol. 1. Lippincott Williams & Wilkins; Philadelphia: 2001. (Fields' Virology). [Google Scholar]
- Rose N.F., Roberts A., Buonocore L., Rose J.K. Glycoprotein exchange vectors based on vesicular stomatitis virus allow effective boosting and generation of neutralizing antibodies to a primary isolate of human immunodeficiency virus type 1. J. Virol. 2000;74(23):10903–10910. doi: 10.1128/jvi.74.23.10903-10910.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose N.F., Marx P.A., Luckay A., Nixon D.F., Moretto W.J., Donahoe S.M., Montefiori D., Roberts A., Buonocore L., Rose J.K. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell. 2001;106(5):539–549. doi: 10.1016/s0092-8674(01)00482-2. [DOI] [PubMed] [Google Scholar]
- Rubin S.A., Snoy P.J., Wright K.E., Brown E.G., Reeve P., Beeler J.A., Carbone K.M. The mumps virus neurovirulence safety test in Rhesus monkeys: a comparison of mumps virus strains. J. Infect. Dis. 1999;180(2):521–525. doi: 10.1086/314905. [DOI] [PubMed] [Google Scholar]
- Sabin A.B., Olitsky P.K. Influence of host factors on neuroinvasivenss of vesicular stomatitis virus: I. effect of age on the invasion of the brain by virus instilled in the nose. J. Exp. Med. 1937;66:15–34. doi: 10.1084/jem.66.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlereth B., Rose J.K., Buonocore L., ter Meulen V., Niewiesk S. Successful vaccine-induced seroconversion by single-dose immunization in the presence of measles virus-specific maternal antibodies. J. Virol. 2000;74(10):4652–4657. doi: 10.1128/jvi.74.10.4652-4657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlereth B., Buonocore L., Tietz A., Meulen Vt V., Rose J.K., Niewiesk S. Successful mucosal immunization of cotton rats in the presence of measles virus-specific antibodies depends on degree of attenuation of vaccine vector and virus dose. J. Gen. Virol. 2003;84(Pt. 8):2145–2151. doi: 10.1099/vir.0.19050-0. [DOI] [PubMed] [Google Scholar]
- Schnell M.J., Buonocore L., Kretzschmar E., Johnson E., Rose J.K. Foreign glycoproteins expressed from recombinant vesicular stomatitis viruses are incorporated efficiently into virus particles. Proc. Natl. Acad. Sci. U.S.A. 1996;93(21):11359–11365. doi: 10.1073/pnas.93.21.11359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell M.J., Buonocore L., Whitt M.A., Rose J.K. The minimal conserved transcription stop–start signal promotes stable expression of a foreign gene in vesicular stomatitis virus. J. Virol. 1996;70(4):2318–2323. doi: 10.1128/jvi.70.4.2318-2323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell M.J., Buonocore L., Boritz E., Ghosh H.P., Chernish R., Rose J.K. Requirement for a non-specific glycoprotein cytoplasmic domain sequence to drive efficient budding of vesicular stomatitis virus. EMBO J. 1998;17(5):1289–1296. doi: 10.1093/emboj/17.5.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharova O.K., Rozina E.E., Shteinberg L.S., Gordienko N.M., Kolyanova I.S. Morphological characteristics of the pathological process in the central nervous system of monkeys infected with variants of measles virus strain L-16. Acta Virol. 1979;23(5):393–397. [PubMed] [Google Scholar]
- Sur J.H., Allende R., Doster A.R. Vesicular stomatitis virus infection and neuropathogenesis in the murine model are associated with apoptosis. Vet. Pathol. 2003;40(5):512–520. doi: 10.1354/vp.40-5-512. [DOI] [PubMed] [Google Scholar]
- Tesh R.B., Johnson K.M. Vesicular stomatitis. In: Hubbert W.T., McColloch W.F., Schnurrenberger P.R., editors. Diseases Transmitted from Animals to Man. C.C. Thomas; Springfield, IL: 1975. pp. 897–910. [Google Scholar]
- van den Pol A.N., Dalton K.P., Rose J.K. Relative neurotropism of a recombinant rhabdovirus expressing a green fluorescent envelope glycoprotein. J. Virol. 2002;76(3):1309–1327. doi: 10.1128/JVI.76.3.1309-1327.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb P.A., Monath T.P., Reif J.S., Smith G.C., Kemp G.E., Lazuick J.S., Walton T.E. Epizootic vesicular stomatitis in Colorado, 1982: epidemiologic studies along the northern Colorado front range. Am. J. Trop. Med. Hyg. 1987;36(1):183–188. doi: 10.4269/ajtmh.1987.36.183. [DOI] [PubMed] [Google Scholar]
- Wertz G.W., Perepelitsa V.P., Ball L.A. Gene rearrangement attenuates expression and lethality of a nonsegmented negative strand RNA virus. Proc. Natl. Acad. Sci. U.S.A. 1998;95(7):3501–3506. doi: 10.1073/pnas.95.7.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan S.P., Ball L.A., Barr J.N., Wertz G.T. Efficient recovery of infectious vesicular stomatitis virus entirely from cDNA clones. Proc. Natl. Acad. Sci. U.S.A. 1995;92(18):8388–8392. doi: 10.1073/pnas.92.18.8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Requirements for yellow fever vaccine. W.H.O. Tech. Rep. Ser. 1998;872:31–68. [Google Scholar]
- Wood D.J., Macadam A.J. Laboratory tests for live attenuated poliovirus vaccines. Biologicals. 1997;25(1):3–15. doi: 10.1006/biol.1997.0055. [DOI] [PubMed] [Google Scholar]
- Yamanouchi K., Uchida N., Katow S., Sato T.A., Kobune K. Growth of measles virus in nervous tissues. IV. Neurovirulence of wild measles and SSPE viruses in monkeys. Jpn. J. Med. Sci. Biol. 1976;29(4):177–186. doi: 10.7883/yoken1952.29.177. [DOI] [PubMed] [Google Scholar]