Abstract
Phytochemical-mediated modulation of cytochrome P450 enzymes (CYPs) may underlie many herb-drug interactions. This study’s purpose was to assess the effects of milk thistle and black cohosh supplementation on CYP3A activity and compare them to a clinically recognized inducer, rifampin, and inhibitor, clarithromycin. Healthy volunteers were randomly assigned to receive a standardized milk thistle (900 mg) or black cohosh (80 mg) supplement for 14 days. Subjects also received rifampin (600 mg) and clarithromycin (1000 mg) for 7 days as positive controls for CYP3A induction and inhibition, respectively. Midazolam was administered orally before and after each supplementation and control period. The effects of milk thistle, black cohosh, rifampin, and clarithromycin on midazolam pharmacokinetics were determined using noncompartmental techniques. Unlike those observed for rifampin and clarithromycin, midazolam pharmacokinetics were unaffected by milk thistle or black cohosh. Milk thistle and black cohosh appear to have no clinically relevant effect on CYP3A activity in vivo.
Keywords: Cytochrome P450s, botanical supplements, pharmacokinetics, drug interactions
Concomitant self-administration of botanical supplements with conventional medications is a growing trend in the United States. Approximately 20% of adults take prescription medications concurrently with botanical dietary supplements.1,2 This upsurge in botanical supplement usage has sparked an increased concern regarding herb-drug interactions.3,4 Supplement-derived phytochemicals appear to modulate various cytochrome P450 enzymes (i.e., CYP3A4) and drug transporters (i.e., P-glycoprotein [P-gp,) in vitro, and such activities may underlie many herb-drug interactions. Of the hundreds of botanical supplements sold in the United States, St. John’s wort (Hypericum perforatum) is the most noteworthy for producing clinically significant herb-drug interactions. Interactions involving St. John’s wort can be traced to hyperforin, a phytochemical component of H. perforatum and a potent ligand for the steroid xenobiotic receptor (SXR),5 which functions as a transcription factor for the CYP3A4 and ABCB1 genes. Chronic ingestion of St. John’s wort leads to up regulation of intestinal CYP3A4 and P-gp (the gene product of ABCB1) expression, a consequence of which is pronounced reduction in oral bioavailability of many conventional medications.6–8
Numerous in vitro studies suggest that other botanical supplements may be capable of modulating CYP activity;9–11 however, results from human in vivo studies have been less compelling. Besides St. John’s wort, only goldenseal,12 garlic oil,13,14 ginkgo biloba,15 and possibly echinacea,16 appear capable of significantly affecting human CYP activity in vivo. Results from clinical studies into the interaction potential of other popular supplements, particularly garlic powder and milk thistle, have been equivocal. Piscitelli et al. observed a significant reduction in saquinavir area-under-the-curve when garlic powder was administered concurrently, suggesting that garlic components were capable of inducing CYP3A activity.17 Conversely, Markowitz et al. found no significant effect of garlic powder on the pharmacokinetics of orally administered midazolam, a recognized CYP3A4/5 substrate.18 In a similar vein milk thistle appeared to have no effect on the pharmacokinetics of indinavir, a substrate for CYP3A4 and P-gp;19–21 however, its effect on metronidazole, another putative CYP3A/P-gp substrate, suggested significant induction of these proteins.22 To complicate matters further is the significant underreporting of both herb-drug interactions and adverse events associated with dietary supplements.23–25 Taken together, these issues highlight the need for more clinical investigations into the interaction potential of botanical supplements in vivo. Such studies are particularly desirable for drugs metabolized via CYP3A-mediated pathways.
Two popular botanicals, often taken with conventional medications, that may pose a risk for CYP3A-mediated herb-drug interactions, include milk thistle (Silybum marianum) and black cohosh (Cimicifuga racemosa). Milk thistle, promoted for its hepatoprotective properties,26 ranks among the top-selling botanical supplements in the United States.1 The purported “active” phytochemicals present in milk thistle are a series of flavanolignans, known collectively as “silymarin,” which include silibinin A, silibinin B, silidianin, silichristin, and taxifolin. Recent in vitro studies indicate that both silymarin and individual flavanolignans may function as substrates and/or inhibitors of human CYP3A4.27–30 However, as mentioned above, clinical studies with milk thistle have produced mixed results regarding its ability to modulate CYP3A4/P-gp in vivo.19–22
Black cohosh is a popular alternative therapy among women for treating menopausal symptoms,31 and recent in vitro studies suggest that triterpene glycosides present in black cohosh can inhibit CYP3A4 activity.32 To date, only one clinical study has addressed the drug interaction potential of black cohosh. Gurley et al. demonstrated that 28 days of black cohosh supplementation, failed to produce significant changes in phenotypic markers of CYP1A2, CYP2D6, CY2E1, and CYP3A4 activity among healthy volunteers, implying a low interaction potential for this supplement.12 While the use of phenotypic metabolic ratios appears to be a suitable method for simultaneously evaluating multiple CYPs and botanical supplements, a limitation of the approach lies in its ability to only provide estimates of CYP probe drug clearance.12–14,33 For probe drugs like caffeine (CYP1A2 probe), debrisoquine (CYP2D6 probe), and chlorzoxazone (CYP2E1 probe), single-time point phenotypic metabolic ratios appear to be adequate predictors of drug metabolism.34–36 However, for midazolam (CYP3A4 probe) there is some debate about the utility of using one-hour 1-hydroxymidazolam/midazolam ratios to predict midazolam clearance.37,38 In this report we describe, for the first time, the effects of milk thistle and black cohosh supplementation on the pharmacokinetics of midazolam, a recognized CYP3A4 substrate. In addition, we compare the effects of milk thistle and black cohosh to those of rifampin, an inducer of CYP3A4 expression,39 and clarithromycin, an inhibitor of CYP3A4 activity,40 as a means of gauging the clinical relevancy of supplement-mediated interactions.
Methods
Study subjects
The University of Arkansas for Medical Sciences Human Research Advisory Committee (Little Rock, AR) approved this study protocol and all participants provided written informed consent before commencing the study. Nineteen young adults (9 females) (age, mean ±SD = 28 ± 6 years; weight, 76.5 ± 16.4 kg) participated in the study and all subjects were in good health as indicated by medical history, routine physical examination, electrocardiography, and clinical laboratory testing. All subjects were nonsmokers, ate a normal diet, were not users of botanical dietary supplements, and were not taking prescription or nonprescription medications. All female subjects had a negative pregnancy test at baseline. All subjects were instructed to abstain from alcohol, caffeine, fruit juices, cruciferous vegetables, and charbroiled meat throughout each two-week phase of the study. Adherence to these restrictions was further emphasized five days before midazolam administration. Subjects were also instructed to refrain from taking prescription and nonprescription medications during supplementation periods, and any medication use during this time was documented. Documentation of compliance to these restrictions was achieved through the use of a food/medication diary.
Supplements and supplementation/medication regimens
The effect of milk thistle, black cohosh, rifampin and clarithromycin on midazolam pharmacokinetics was evaluated individually on four separate occasions in each subject. This was an open-label study randomized for supplementation/medication sequence. (“Supplementation/medication” refers either to milk thistle, black cohosh, rifampin, or clarithromycin.) Each supplementation phase (milk thistle or black cohosh) lasted 14 days while each medication phase (rifampin or clarithromycin) was of 7 days duration. Each supplementation/medication phase was followed by a 30-day washout period. This randomly assigned sequence of supplementation/medication followed by washout was repeated until each subject had received all four products. Single lots of milk thistle (lot # 41678) and black cohosh (lot #41924) were purchased from the same vendor (Enzymatic Therapy, Inc. Green Bay, WI.). (Enzymatic Therapy Inc. is a leader in the botanical supplement industry for providing products of high quality and consistency.) Rifampin (Rifadin®, Aventis Pharmaceuticals, Kansas City, MO.) and clarithromycin (Biaxin®, Abbott Laboratories, North Chicago, IL) were utilized as positive controls for CYP3A induction and inhibition, respectively. Product labels were followed regarding the administration of milk thistle (300 mg, three times daily, standardized to contain 80% silymarin); black cohosh extract (40 mg, twice daily, standardized to 2.5% triterpene glycosides); rifampin (300 mg, twice daily); and clarithromycin (500 mg, twice daily). Telephone and electronic mail reminders were used to facilitate compliance, while pill counts and supplementation usage records, were used to verify compliance.
Midazolam administration
Following an overnight fast, subjects reported to the University of Arkansas for Medical Sciences General Clinical Research Center for midazolam administration and blood sampling. Prior to midazolam administration subjects were weighed and questioned about their adherence to the dietary and medication restrictions. Female subjects were administered pregnancy tests and only those with negative test results were allowed to participate. Following the placement of a 20 gauge, indwelling catheter into a peripheral vein of the forearm, an oral dose of midazolam (8 mg, Bedford Laboratories, Bedford, OH) was administered with 240 ml of water. Throughout the study, midazolam doses were administered 24 hours before the start of each supplementation/medication phase (baseline) and again on the last day of each phase. Serial blood samples were obtained before and at 0.25, 0.50, 0.75, 1, 1.5, 2, 3, 4, 5, and 6 hours after midazolam administration. Blood samples were allowed to clot for 30 minutes then centrifuged at 2500 rpm. Serum was removed, placed in 3mL cryovials and stored at −70°C until analysis. Each subject’s blood pressure, heart rate, and respiration rate was monitored at 0.5, 1, and 2 hours post-midazolam administration. Four hours after dosing, subjects received identical meals consisting of a turkey sandwich, potato chips, carrot sticks, and water.
Determination of midazolam (MDZ) and 1′-hydroxymidazolam (HMDZ) serum concentrations
Standard solutions of MDZ and HMDZ (Sigma, St Louis, MO) were prepared in methanol to give stock solutions of 1.0 μg/mL and were stored at −20°C until use. The internal standard, MDZ-[15N3], (a gift from Roche Laboratories, Nutley, NJ) was prepared to yield a stock solution of 0.2 ng/mL in methanol. Working standards were prepared daily in drug-free serum from the stock solution to yield concentrations of 250, 100, 50, 25, 10, 5.0, 2.50, 1.0, 0.5, 0.25 and 0.1 ng/mL.
500 μL of 0.2M sodium acetate (pH 4.75) was added to each 250 μL serum aliquot. 250 μL of ice-cold β-glucuronidase (Sigma, St. Louis, MO) were then added and the sample gently agitated. Samples were incubated at 37°C for 2.5 hours. After incubation, 2 mL of 0.1% H3PO4 was added along with 50 μL of the internal standard (MDZ-[15N3]) and samples vortex-mixed for 30 seconds. A Strata X-C solid phase extraction (SPE) column (Phenomenex, Torrance, CA 90501) was used to extract MDZ and HMDZ from serum. SPE columns were preconditioned with methanol/water. Serum samples were then loaded onto SPE columns and washed with 2 mL of 0.1% H3PO4 in 50:50 methanol:acetonitrile followed by 1 mL of 50:50 methanol:acetonitrile. MDZ and HMDZ were eluted by gravity flow using 3 mL 6% ammonia in 50:50 methanol:acetonitrile. Eluents were dried under nitrogen and reconstituted in 75 μL LC/MS mobile phase A (30% acetonitrile, 5mM ammonium formate pH 3.0). Samples were then transferred to microcentrifuge tubes and centrifuged at 13,200 rpm.
Using a Model 10Avp modular high performance liquid chromatography (HPLC) system (Shimadzu Corp., Columbia, MD), 10 μL aliquots were injected onto a 10 × 0.2 cm Gemini 5om C18 column (Phenomenex, Torrance, CA). MDZ and HMDZ were eluted at a flow of 0.2 mL/min using a 4-min. linear gradient from 30% to 80% acetonitrile:ammonium formate (5 mM pH 3.0). Analytes were converted to gas-phase ions in a TurboIon Spray™ heated electrospray ionization (ESI) source (MDS Sciex, Concord, Ontario, Canada) operated at +4,400 volts and 425°C. Ions were resolved and detected in an API3000 triple-quadrupole mass spectrometer (MDS Sciex, Concord, Ontario, Canada) by multiple reaction monitoring. MDZ and HMDZ serum concentrations were quantified by a comparison of the ratio of their peak areas to that of the internal control, MDZ-[15N3].
Validation studies found that standard curves were linear over the range of 250 to 0.1 ng/mL and yielded correlation coefficients > 0.995. The limit of quantitation was 0.1 ng/mL and the limit of detection 0.02 ng/mL. Mean recoveries of MDZ and HMDZ were 92% and 85%, respectively. Relative standard deviations for interday accuracy and precision assessments for quality control standards at 120, 20, and 1.5 ng/mL were < 10%.
Supplement analysis
The phytochemical content of each supplement was independently analyzed for specific “marker compounds” by HPLC. Analytical standards of the flavanolignans taxifolin, silychristin, silydianin, silibinin A, and silibinin B were purchased from ChromaDex, Inc. (Santa Ana, CA, USA). Standard solutions of each flavanolignan were prepared in methanol covering a range of 0.01–1 μg/mL and used for quantitative purposes. Flavanolignan content of milk thistle was quantitated using a previously published method.41 Briefly, contents of six milk thistle capsules were placed in individual brown glass bottles and dissolved in 75 mL of ethanol. The contents of each vessel were agitated at 75 rpm for 4 hours at 60°C. One milliliter aliquots were removed, evaporated under nitrogen, and redissolved in 1 mL methanol. 10 μL were injected onto a Symmetry C18 column (150 mm × 4.6 mm, 5μm) (Waters Corp., Milford, MA, USA) using a Waters Alliance 2690 component HPLC system (Waters Corp., Milford, MA, USA). A gradient elution using a mobile phase of methanol:water (20:80, designated Solvent A and 80:20, designated Solvent B) was used to separate the flavanolignans. A mixture of 85:15 (Solvent A:B) was initiated for 5 minutes at a flow of 0.75 ml/min, followed by a linear gradient over 15 minutes to achieve a mixture of 45:55 (Solvent A:B) which was held constant for an additional 20 minutes. The gradient was then ramped down linearly over 10 minutes to the original concentration (85:15, A:B). Column eluent was monitored by a Model 996 photodiode array detector (Waters Corp., Milford, MA) at a wavelength of 290nm. Retention times for taxifolin, silichristin, silidianin, silibinin A, and silibinin B were 9.6, 16.5, 18.8, 23.7 and 24.7 minutes, respectively. The lower limit of quantitation for each analyte was 0.01 μg/mL. The interday accuracy and precision for silymarin components at 0.05, 0.1, and 0.5 μg/mL was < 8%.
Black cohosh was analyzed for triterpene glycosides (cimiracemosides, cimicifugoside, 27-deoxyactein, and actein) (Chromadex Inc., Santa Ana, CA, USA) using reversed phase HPLC with evaporative light scattering detection as described previously.42 Standard curves for each standard were linear over the range of 10 to 400 μg/mL. The limit of quantitation for cimiracemoside (the least prevalent component) was 10 μg/mL. Extraction recoveries exceeded 95% and relative standard deviations for interday accuracy and precision assessments were < 5%.
Pharmacokinetic analysis and phenotype assessment
MDZ pharmacokinetic parameters were determined using standard noncompartmental methods with the computer program WinNonlin (version 2.1; Pharsight, Mountain View, CA, USA). Area under the serum concentration-time curves from zero to 6 hours (AUC(0–6)) were determined by use of the trapezoidal rule. The terminal elimination rate constant (ke) was calculated using the slope of the log-linear regression of the terminal elimination phase. Area under the serum concentration versus time curve from zero to infinity (AUC0–∞) was calculated using the log-linear trapezoidal rule up to the last measured time concentration (Clast) with extrapolation to infinity using Clast/ke. The elimination half-life was calculated as 0.693/ke. The apparent oral clearance of midazolam (Cl/F) was calculated as dose/AUC0–∞. Peak MDZ concentrations (Cmax) and the times when they occurred (Tmax) were derived directly from the data.
The justification of specified time points for obtaining metabolite/parent serum ratios to estimate probe drug clearance has been previously addressed.13 Serum ratios of HMDZ/MDZ determined one hour after dosing were used to estimate CYP3A activity. Differences in pre- and post-supplementation/medication HMDZ/MDZ ratios obtained one hour after MDZ dosing were used to estimate the effects of rifampin, clarithromycin, milk thistle, and black cohosh on CYP3A phenotype.
Disintegration tests
An absence of botanical-mediated effects on midazolam pharmacokinetics could stem from products exhibiting poor disintegration and/or dissolution characteristics. To address this concern, each product was subjected to disintegration testing as outlined in the United States Pharmacopeia 28.43 The disintegration apparatus consisted of a basket-rack assembly operated at 29–32 cycles per minute with 0.1 N HCl (37°C) as the immersion solution. One dosage unit (uncoated tablet or soft gel capsule) of each supplement was placed into each of the six basket assembly tubes. The time required for the complete disintegration of six dosage units was determined. This process was repeated with an additional six dosage units to assure accuracy. Since there are no specifications for the disintegration time of the botanical supplements used in this study, the mean of six individual dosage units was taken as the disintegration time for that particular product. A product was considered completely disintegrated if the entire residue passed through the mesh screen of the test apparatus, except for capsule shell fragments, or if the remaining soft mass exhibited no palpably firm core.
Statistical Analysis
A repeated measures ANOVA model was fit for each pharmacokinetic parameter using SAS Proc Mixed software (SAS Institute, Inc. Cary, N.C., USA). Since pre-and post-supplementation/medication pharmacokinetic parameters were determined in each subject for all four of the study phases, a covariance structure existed for measurements within subjects. Sex, supplement/medication, and supplement/medication-by-sex terms were estimated for each parameter using a Huynh-Feldt covariance structure fit. If supplement/medication-by-sex interaction terms for a specific parameter measure were significant at the 5% level, the focus of the post-supplementation/medication minus pre-supplementation/medcation response was assessed according to sex. If the supplement/medication-by-sex interaction was not statistically significant, responses for both sexes were combined.
An identical approach was taken to evaluate the effects of the supplementation/medication scheme on 1-hour HMDZ/MDZ ratios. Additionally, a power analysis was performed to estimate the ability to detect significant post- minus pre-supplementation/medication effects. All four models obtained at least 80% power at the 5% level of significance to detect a Cohen effect size of 1.32 to 1.50 standard deviation units.44 A Pearson’s correlation analysis for 1-hour HMDZ/MDZ serum ratios and MDZ oral clearance was also performed using SAS Proc Corr software (SAS Institute, Inc. Cary, N.C., USA).
RESULTS
All nineteen subjects completed each phase of the study. Neither spontaneous reports from study participants or their responses to questions asked by study nurses regarding supplement/medication usage revealed any serious adverse events. Nausea, indigestion, and complaints of a metallic taste were frequently noted during clarithromycin phases. Mild indigestion and reddish discoloration of the urine were common conditions reported with rifampin use. Three subjects noted an increase in headaches while taking milk thistle and one subject associated black cohosh with the onset of “vivid dreams”. No clinically significant changes in blood pressure, heart rate, or respiratory rate were observed after MDZ administration. Examination of pill counts and food/medication diaries revealed no significant deviations from the study protocol.
During each pre-supplementation/medication phase, subjects slept for approximately 1–2 hours after receiving MDZ. Milk thistle and black cohosh had no noticeable effect on the duration of MDZ-induced sleep. After rifampin, however, most subjects either did not sleep or their sleep time was less than 0.5 hours. Clarithromycin increased the average sleep time for all subjects to 3–4 hours.
The effects of clarithromycin, rifampin, milk thistle, and black cohosh on serum MDZ concentration versus time profiles are depicted in Figure 1. Supplement/medication effects on MDZ pharmacokinetics are presented in Table I. No statistical differences were observed for MDZ pharmacokinetic parameters at the beginning of each supplementation/medication phase (baseline) (Table I). Statistically significant increases (p < 0.001) in MDZ AUC(0–∞), Cmax, and elimination half-life were observed after 7 days of clarithromycin ingestion (Figure. 1; Table I). Clarithromycin also decreased MDZ apparent oral clearance by 88% (1.32 ± 0.34 vs. 0.16 ± 0.07 L/hr/kg)(p < 0.001; Table I). Statistically significant reductions (p < 0.001) in MDZ AUC(0–∞), Cmax, and elimination half-life were noted following rifampin administration (Figure 1; Table I). Rifampin increased MDZ apparent oral clearance by more than 20 fold (1.44 ± 0.37 vs. 29.6 ± 15.2 L/hr/kg)(p < 0.001; Table I). A statistically significant reduction in Tmax was also observed following rifampin (p < 0.05; Table I). In contrast to rifampin and clarithromycin, no significant changes in MDZ pharmacokinetics were observed as a result of milk thistle or black cohosh supplementation (Figure 1; Table I).
Figure 1.
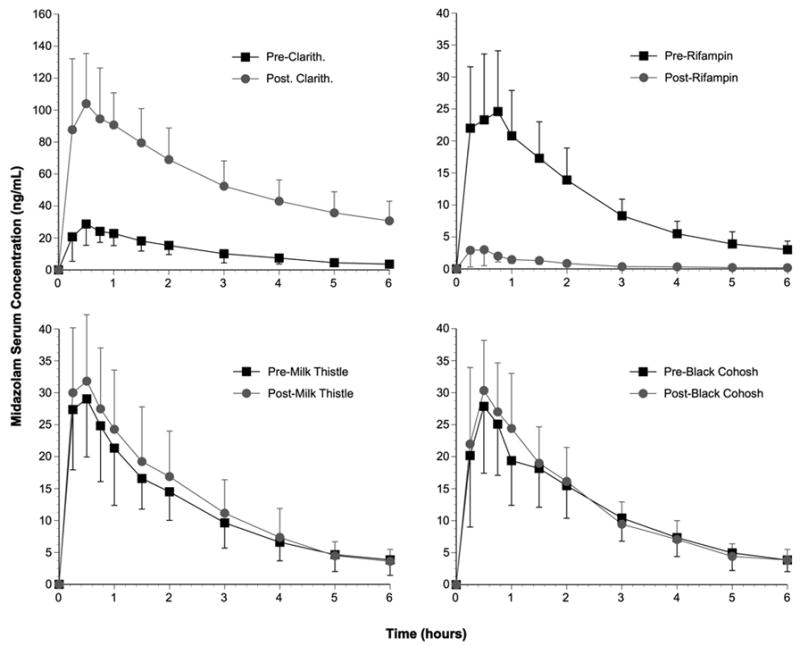
Midazolam concentration-time profiles before and after clarithromycin. rifampin, milk thistle and black cohosh. Black squares = baseline values; gray circles = post-supplementation/medication values. Error bars = standard deviations.
Table I.
Midazolam pharmacokinetic parameters before and after supplementation/drug phases (mean ± s.d.)
| Study Phase | AUC (0–∞) (ng/hr/mL) | Cl/F (L/hr) | Cl/F/kg (L/hr/kg) | T1/2 (hours) | Cmax (ng/mL) | Tmax (hours) |
|---|---|---|---|---|---|---|
| Pre-Clarithromycin | 89.6 ± 30.6 | 99.3 ± 25.2 | 1.32 ± 0.34 | 3.76 ± 0.81 | 31.8 ± 11.6 | 0.54 ± 0.25 |
| Post-Clarithromycin | 752 ± 398* | 12.8 ± 6.5* | 0.16 ± 0.07* | 8.64 ± 2.76* | 120.7 ± 29.3* | 0.52 ± 0.18 |
| Pre-Rifampin | 79.6 ± 23.2 | 110.8 ± 33.2 | 1.44 ± 0.37 | 3.93 ± 0.90 | 30.6 ± 14.6 | 0.64 ± 0.32 |
| Post-Rifampin | 4.55 ± 2.24* | 2256 ± 1168* | 29.6 ± 15.2* | 1.62 ± 0.74* | 3.7 ± 2.6* | 0.46 ± 0.20† |
| Pre-Milk Thistle | 96.2 ± 42.2 | 95.3 ± 33.0 | 1.30 ± 0.44 | 4.22 ± 1.22 | 37.2 ± 18.1 | 0.47 ± 0.24 |
| Post-Milk Thistle | 98.9 ± 40.5 | 91.2 ± 27.6 | 1.26 ± 0.33 | 3.73 ± 1.02 | 39.6 ± 18.9 | 0.48 ± 0.26 |
| Pre-Black Cohosh | 94.8 ± 29.2 | 93.3 ± 32.6 | 1.26 ± 0.39 | 3.89 ± 1.00 | 34.6 ± 15.5 | 0.64 ± 0.30 |
| Post-Black Cohosh | 94.0 ± 26.2 | 93.7 ± 26.9 | 1.29 ± 0.37 | 3.79 ± 0.86 | 33.9 ± 10.4 | 0.60 ± 0.29 |
AUC = area under concentration-time curve, Cl/F = apparent oral clearance, Cl/F/kg = apparent oral clearance normalized to body weight (kg), T1/2 = elimination half-life, Cmax = maximum serum concentration, Tmax = time to Cmax
= p < 0.0001 when compared to corresponding pre-phase;
= p < 0.05
A significant sex-related difference in baseline (e.g. pre-supplementation/medication) oral MDZ clearance was observed. The oral clearance of MDZ was significantly greater in female subjects (1.61 ± 0.49 versus 1.04 ± 0.30 L/hr/kg; p < 0.001) than in males. However, no sex-related differences in the extent of CYP3A induction (i.e., change in pre- versus post-rifampin CL/F/kg) or inhibition (i.e. change in pre- versus post-clarithromycin Cl/F/kg) were observed.
Comparisons of 1-hour HMDZ/MDZ serum concentration ratios before and after supplementation/medication mirrored the effects observed for MDZ concentration-time profiles (Figure 2; Table II). Like that observed for baseline values of MDZ oral clearance, no statistically significant differences occurred among mean values for 1-hour HMDZ/MDZ ratios at baseline (Table II). Again, as with MDZ oral clearance, a sex-related difference was noted for mean MDZ phenotype assessments. At each baseline determination, mean values for female 1-hour HMDZ/MDZ ratios were significantly greater (4.12 ± 1.3 versus 2.84 ± 0.82; p < 0.001) than those for males; however, no sex-related effect with regard to extent of change in MDZ phenotypes was noted after each treatment phase. Mean post-rifampin 1-hour HMDZ/MDZ serum ratios were significantly greater than pre-dose values indicating an induction of CYP3A activity (Figure 2A), while the opposite was true for clarithromycin treatment signifying CYP3A inhibition (Figure 2B). No significant changes in mean 1-hour HMDZ/MDZ serum ratios were noted after 14-day courses of either milk thistle or black cohosh (Figures 2C, 2D), suggesting that these supplements are not potent modulators of CYP3A in vivo. Correlation analysis revealed a significant positive correlation between 1-hour HMDZ/MDZ ratios and CL/F/kg (r2 = 0.82, p < 0.001; Figure 3).
Figure 2.
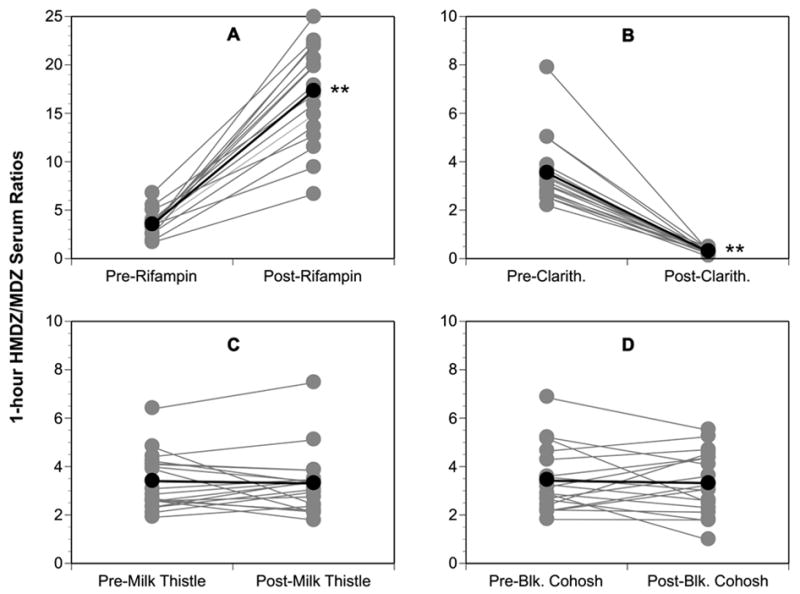
Comparison of pre- and post-supplementation phenotypic ratios (1-hydroxymidazolam/midazolam) for CYP3A4. (A) Rifampin, (B) Clarithromycin, (C) Milk thistle, (D) Black cohosh. Gray circles = individual values; Black circles = group means. Asterisks = statistically significant difference from baseline.
Table II.
Pre- and Postsupplementation/medication 1-hour Phenotypic Ratios
| Phenotypic Ratio | Supplement | Pre-administration (mean and 95% CI) | Post-administration (mean and 95% CI) | Difference (mean and 95% CI) | Post/Pre Ratios† (geometric mean and 90% CI) |
|---|---|---|---|---|---|
| HMDZ/MDZ (CYP3A4) | Clarithromycin | 3.54 (2.87 to 4.22) | 0.29 (0.24 to 0.34) | −3.25 (−2.58 to −3.92)* | 0.08 (0.07 to 0.10)* |
| Rifampin | 3.60 (3.06 to 4.14) | 18.0 (15.5 to 20.4) | 14.4 (16.7 to 12.1)* | 5.08 (4.46 to 5.78)* | |
| Milk Thistle | 3.38 (2.92 to 3.85) | 3.34 (2.80 to 3.88) | −0.04 (−0.44 to 0.51) | 0.98 (0.87 to 1.11) | |
| Black Cohosh | 3.42 (2.82 to 4.02) | 3.32 (2.73 to 3.90) | −0.10 (−0.53 to 0.73) | 0.95 (0.79 to 1.13) |
Difference = post-administration minus pre-administration, CI = confidence interval, HMDZ = 1-hydroxymidazolam, MDZ = midazolam
= geometric mean of postsupplementation/presupplementation ratios and 90% CI
= p < 0.001
Figure 3.
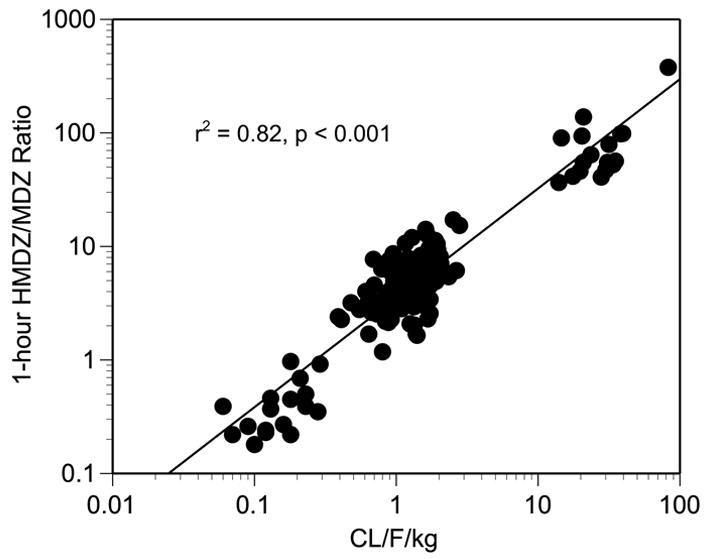
Plot of 1-hour 1′-hydroxymidazolam/midazolam phenotypic ratios versus midazolam oral clearance (CL/F/kg).
Results of phytochemical analyses and disintegration testing for milk thistle and black cohosh formulations are presented in Table III. Based on these findings, subjects ingested approximately 440 mg of silymarin and 3.0 mg of triterpene glycosides on a daily basis. In addition, both dosage forms disintegrated in less than 15 minutes.
Table III.
Phytochemical analysis and disintegration times for botanical dosage forms.
| Supplement (dosage form) | Compound | Content (mg/dosage form) | Daily Dose (mg) | Disintegration Time (minutes) |
|---|---|---|---|---|
| Milk Thistle (softgel capsule) | Silymarin | 12.6 | ||
| Silibinin A | 17.3 | 103.8 | ||
| Silibinin B | 30.5 | 183.0 | ||
| Silichristin | 18.6 | 111.6 | ||
| Silidianin | 5.4 | 32.4 | ||
| Taxifolin | 1.6 | 9.6 | ||
| Total | 73.4 | 440.4 | ||
| Black Cohosh (uncoated tablet) | Triterpene glycosides | 3.5 | ||
| Actein | 0.17 | 0.68 | ||
| 27-deoxyactein | 0.13 | 0.52 | ||
| Cimiracemosides A,C,E,F | 0.14 | 0.56 | ||
| Cimicifugoside | 0.21 | 0.84 | ||
| Triterpene A | 0.05 | 0.20 | ||
| Triterpene B | 0.03 | 0.12 | ||
| Total | 0.73 | 2.92 |
DISCUSSION
The present findings suggest that phytochemical components in the milk thistle and black cohosh formulations investigated in this study did not significantly affect the pharmacokinetics of MDZ in humans, and therefore are not likely to pose a significant interaction risk with other CYP3A substrates. This interpretation is bolstered by the significant changes in MDZ pharmacokinetics observed following the administration of clarithromycin, a known CYP3A4 inhibitor, and rifampin, a recognized inducer of CYP3A4 expression. In addition, our results do not support previous in vitro findings that silymarin (i.e., silibinin A, silibinin B silidianin, silichristin, and taxifolin) inhibits CYP3A4 activity, at least not in the context of the recommended supplementation regimen used in the study. This discrepancy may stem from the fact that milk thistle flavanolignans are practically insoluble in water and that in vitro studies demonstrating an inhibitory effect of silymarin on CYP3A4 activity have utilized solubilizing agents (e.g., dimethylsulfoxide27,29,30 or methanol28) to facilitate hepatocyte or microsomal membrane permeability. Depending on the in vitro model utilized, IC50 values for individual flavanolignans or silymarin extract have ranged from 25–250 μM,27–30 with some evidence that silibinin can act as a mechanism-based inhibitor of CYP3A.26 From the results of the present study, it would appear that local silymarin concentrations at intestinal enterocyte membrane interfaces were lower than the 25–250 μM necessary for in vitro inhibition of CYP3A,27–30 and the degree of mechanism-based inhibition, if any, was not comparable to that observed with clarithromycin.
The milk thistle product used in the present study was formulated with soybean oil, glycerin, and lecithin in a soft gelatin capsule, and upon disintegration the contents appeared to remain undissolved. Since flavanolignan serum concentrations were not measured, any indication as to their in vivo solubility and/or bioavailability status remains unknown. Nevertheless, bioavailability and dissolution characteristics for silymarin-containing products have been shown to vary widely. An evaluation of nine separate milk thistle products found that the amount of silibinin released over one hour into an aqueous buffered solution (pH 7.5, 37°C) ranged from 0–85%;45 while a comparative bioavailability study of three silibinin-containing dosage forms found that values for AUC and Cmax varied among products by factors of 3 and 6, respectively.46 Moreover, several studies have demonstrated that silymarin-containing products exhibit especially poor bioavailability and drug-release properties when not formulated with solubility-enhancing agents like phosphatidylcholine and polyethylene glycol.45,47–49 The bioavailability and drug-release characteristics of silymarin is significantly enhanced when silibinin is complexed with phosphotidylcholine or formulated as lipid-containing microspheres.50–53 Unlike many European products, the majority of milk thistle supplements sold in the United States do not appear to incorporate these technologies.52
With the multitude of supplement brands available on the market, discrepancies in product content, formulation, dissolution, and bioavailability can be the bane of any clinical safety, efficacy, or herb-drug interaction study—a problem not limited to milk thistle. Formulation differences could explain the disparity between a recent study performed in India22 and several conducted in the United States12,19–21 regarding the effects of milk thistle on the disposition of CYP3A substrates. Rajnarayana et al. found that 9 days of silymarin administration (Silybon™) increased the clearance of metronidazole, a substrate of CYP2C9, CYP3A, and P-gp, by almost 30%.22 The authors concluded that induction of CYP and/or P-gp might have accounted for the observed effects. Conversely, milk thistle interaction studies conducted in the United States and Canada found no statistically significant changes in the pharmacokinetics of indinavir, a CYP3A/P-gp substrate,19–21 although slight reductions (8–18%) in indinavir AUC were noted in each study. Collectively, the above studies hint at a possible mild inductive effect of silymarin on CYP3A and/or P-gp. In the only in vitro examination of milk thistle components on CYP3A induction, Raucy, using a reporter gene assay for the human pregnane X receptor and promoter regions of CYP3A transfected in HepG2 cells, found no evidence that silymarin, when solubilized with dimethylsulfoxide (DMSO), could induce the enzyme.54 Furthermore, in a previous study utilizing MDZ, we found that 28 days of milk thistle supplementation had no significant effect on 1-hour HMDZ/MDZ ratios (a finding confirmed by the current results) implying that conventional silymarin formulations are devoid of any clinically relevant CYP3A modulatory effects.12 Taken together, our results and those reported previously suggest that, when compared to potent inducers and inhibitors of CYP3A, milk thistle flavanolignans pose no clinically significant risks for pharmacokinetic herb-drug interactions involving CYP3A substrates.
Recent in vitro findings demonstrate that, in the presence of DMSO, black cohosh extracts or individual triterpene glycosides can inhibit CYP3A.32 However, the results reported here, and earlier,12 indicate that, when compared to clarithromycin and rifampin, recommended doses of black cohosh triterpene glycosides do not affect MDZ disposition and are therefore not effective modulators of CYP3A in vivo. Whether this lack of effect is a function of dose, solubility, bioavailability, or a combination of factors remains to be seen. Like many botanical supplements, the pharmacokinetic profile of black cohosh’s constituent phytochemicals has not been adequately investigated. Only one group has described an attempt at measuring mercapturate conjugates of black cohosh constituents (fukinolic acid, fukiic acid, caffeic acid, and cimiracemate B) in the urine of women after consuming 256 mg of a standardized black cohosh extract, and none of the target conjugates were detected.55 This finding alone brings into question the cellular permeability and bioavailability of these specific phytochemicals. Accordingly, the available in vivo evidence would seem to render black cohosh as an unlikely source of clinically important herb-drug interactions.
Poor systemic bioavailability of phytochemicals, however, may not always be associated with a lack of CYP3A modulation. This is best illustrated with grapefruit juice. Grapefruit juice, a well recognized mechanism-based inhibitor of intestinal CYP3A4 produces significant reductions in the oral clearance of many CYP3A4 substrates, yet the two phytochemicals primarily responsible for this effect, bergamottin and 6′,7′-dihydroxybergamottin, do not attain measurable concentrations in the plasma.56 From the results of the present study, however, it would appear that concentrations of specific milk thistle and black cohosh phytochemicals were insufficient to elicit any clinically noticeable effects on CYP3A.
Of particular interest was the close agreement between MDZ pharmacokinetic parameters described here and those reported earlier by Gorski et al.39,40 Like Gorski, we too noted a sex-related difference in oral MDZ clearance with women exhibiting higher values than men.40 Unlike Gorski, however, we failed to observe a sex-related effect in the response of CYP3A to induction by rifampin39 or inhibition by clarithromycin.40
Our findings also support the utility of 1-hour HMDZ/MDZ ratios as a practical method for identifying herb-drug interactions that involve CYP3A induction or inhibition. Previously, this approach had been used to document CYP3A induction following St. John’s wort supplementation,13,14 and its inhibition by goldenseal.12 The method also demonstrated that an absence of change in mean phenotypic ratios following botanical supplementation could be interpreted as a lack of effect on CYP activity. Such was the case with Citrus aurantium, Ginkgo biloba, Panax ginseng, and saw palmetto extracts13,14,33—the latter three examples being confirmed by other investigators using more conventional area-under-the-curve assessments.57–59 The list can now be extended to include milk thistle and black cohosh. Thus, a range of herb-mediated effects on CYP activity (e.g. induction, inhibition, or no effect) can be differentiated with single time-point phenotypic ratios. It must be emphasized, however, that single-time point phenotypic ratios simply provide estimates of probe drug clearance. The utility of these approximations have recently come under question, with some investigators finding the correlations unacceptable.38 We, however, found that the change, or lack thereof, in HMDZ/MDZ ratios provided a reasonable correlation with oral MDZ clearance (Figure 3). Our aim is not to propose that traditional means of determining MDZ clearance can be supplanted by metabolic phenotypic ratios; however, we do believe they provide a less labor-intensive, more subject-friendly means for evaluating multiple CYP enzymes and multiple botanical supplements in vivo while using a limited blood-sampling scheme.12–14,33 In short, single-time point metabolic phenotypic ratios may serve as a cost-effective in vivo screening method for assessing potential CYP-mediated herb-drug interactions. Once identified, candidate herbs may be evaluated further by using more traditional pharmacokinetic approaches.
In conclusion, when compared to the effects of rifampin and clarithromycin, the specific brand of milk thistle and black cohosh supplements utilized in this study produced no significant changes in the disposition of midazolam, a clinically recognized CYP3A substrate. Accordingly, these two products appear to pose no clinically significant risk for CYP3A-mediated herb-drug interactions. However, given the inter-product variability in phytochemical content, potency, and formulation among botanical supplements, these results may not extend to regimens utilizing higher dosages, longer supplementation periods, or brands with improved dissolution and/or bioavailability characteristics.
References
- 1.Committee on the Use of Complementary and Alternative Medicine by the American Public. Complementary and Alternative Medicine in the United States. The National Academies Press; Washington, DC: 2005. [PubMed] [Google Scholar]
- 2.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 3.Brazier NC, Levine MAH. Drug-herb interactions among commonly used conventional medicines: a compendium for health care professionals. Am J Ther. 2003;10:163–169. doi: 10.1097/00045391-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hu Z, Yang X, Ho PCL, Chan SY, Heng PWS, Chan E, Duan W, Kph HL, Zhou S. Herb-drug interactions: a literature review. Drugs. 2005;65:1239–1282. doi: 10.2165/00003495-200565090-00005. [DOI] [PubMed] [Google Scholar]
- 5.Wentworth JM, Agostini M, Love J, Schwabe JW, Chatterjee VKK. St. John’s wort, a herbal antidepressant, activates the steroid X receptor. J Endocrinol. 2000;166:R11–R16. doi: 10.1677/joe.0.166r011. [DOI] [PubMed] [Google Scholar]
- 6.Dürr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ, Fattinger K. St. John’s wort induces intestinal P-glycoprotein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther. 2000;68:598–604. doi: 10.1067/mcp.2000.112240. [DOI] [PubMed] [Google Scholar]
- 7.Sugimoto K, Ohmori M, Tsuruoka S, Nishiki K, Kawaguchi A, Harada K, Arakawa M, Sakamoto K, Masada M, Miyamori I, Fujimura A. Different effects of St. John’s wort on the pharmacokinetics of simvastatin and pravastatin. Clin Pharmacol Ther. 2002;70:518–524. doi: 10.1067/mcp.2001.120025. [DOI] [PubMed] [Google Scholar]
- 8.Dresser GK, Schwartz UI, Wilkinson GR, Kim RB. Coordinate induction of both cytochrome P4503A and MDR1 by St. John’s wort in healthy subjects. Clin Pharmacol Ther. 2003;73:41–50. doi: 10.1067/mcp.2003.10. [DOI] [PubMed] [Google Scholar]
- 9.Foster BC, Vandenhoek S, Hana J, Krantis A, Akhtar MH, Bryan M, Budzinski JW, Ramputh A, Arnason JT. In vitro inhibition of human cytochrome P450-mediated metabolism of marker substrates by natural products. Phytomed. 2003;10:334–342. doi: 10.1078/094471103322004839. [DOI] [PubMed] [Google Scholar]
- 10.Strandell J, Neil A, Carlin G. An approach to the in vitro evaluation of potential for cytochrome P450 enzyme inhibition from herbals and other natural remedies. Phytomed. 2004;11:98–104. doi: 10.1078/0944-7113-00379. [DOI] [PubMed] [Google Scholar]
- 11.Zou L, Harkey MR, Henderson GL. Effects of herbal components on cDNA-expressed cytochrome P450 enzyme catalytic activity. Life Sci. 2002;71:1579–1589. doi: 10.1016/s0024-3205(02)01913-6. [DOI] [PubMed] [Google Scholar]
- 12.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Khan IA, Shah A. In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes. Clin Pharmacol Ther. 2005;77:415–426. doi: 10.1016/j.clpt.2005.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, Ang CYW. Cytochrome P450 phenotypic ratios for predicting herb-drug interactions in humans. Clin Pharmacol Ther. 2002;72:276–287. doi: 10.1067/mcp.2002.126913. [DOI] [PubMed] [Google Scholar]
- 14.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, Ang CYW. Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St. John’s wort, garlic oil, panax ginseng, and ginkgo biloba. Drugs Aging. 2005;22:525–539. doi: 10.2165/00002512-200522060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin OQP, Tomlinson B, Waye MMY, Chow AHL, Chow MSS. Pharmacogenetics and herb-drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics. 2004;14:841–850. doi: 10.1097/00008571-200412000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Gorski JC, Huang S-M, Pinto A, Hamman MA, Hilligoss JK, Zaheer NA, Desai M, Miller M, Hall SD. The effect of Echinacea (Echinacea purpurea root) on cytochrome P450 activity in vivo. Clin Pharmacol Ther. 2004;75:89–100. doi: 10.1016/j.clpt.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Piscitelli SC, Burstein AH, Welden N, Gallicano KD, Falloon J. The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin Infect Dis. 2002;34:234–238. doi: 10.1086/324351. [DOI] [PubMed] [Google Scholar]
- 18.Markowitz JS, DeVane CL, Chavin KD, Taylor RM, Ruan Y, Donovan JL. Effects of garlic (Allium sativum L.) supplementation on cytochrome P450 2D6 and 3A4 activity in healthy volunteers. Clin Pharmacol Ther. 2003;74:170–177. doi: 10.1016/S0009-9236(03)00148-6. [DOI] [PubMed] [Google Scholar]
- 19.Piscitelli SC, Formentini E, Burstein AH, Alfaro R, Jagannatha S, Falloon J. Effect of milk thistle on the pharmacokinetics of indinavir in healthy volunteers. Pharmacotherapy. 2002;22:551–556. doi: 10.1592/phco.22.8.551.33205. [DOI] [PubMed] [Google Scholar]
- 20.DiCenzo R, Shelton M, Jordan K, Koval C, Forrest A, Reichman R, Morse G. Coadministration of milk thistle and indinavir in healthy subjects. Pharmacotherapy. 2003;23:866–870. doi: 10.1592/phco.23.7.866.32723. [DOI] [PubMed] [Google Scholar]
- 21.Mills E, Wilson K, Clarke M, Foster B, Walker S, Rachlis B, DeGroot N, Montori VM, Gold W, Phillips E, Myers S, Gallicano K. Milk thistle and indinavir: a randomized controlled pharmacokinetics study and meta analysis. Eur J Clin Pharmacol. 2005;61:1–7. doi: 10.1007/s00228-004-0843-z. [DOI] [PubMed] [Google Scholar]
- 22.Rajnarayana K, Reddy MS, Vidyasagar Krishna DR. Study on the influence of silymarin pretreatment on metabolism and disposition of metronidazole. Arzneim Forsch. 2004;54:109–113. doi: 10.1055/s-0031-1296944. [DOI] [PubMed] [Google Scholar]
- 23.Hensrud DD, Engle DD, Scheitel SM. Underreporting the use of dietary supplements and nonprescription medications among patients undergoing a periodic health examination. Mayo Clin Proc. 1999;74:443–447. doi: 10.4065/74.5.443. [DOI] [PubMed] [Google Scholar]
- 24.Barnes J, Mills SY, Abbot NC, Willoughby M, Ernst E. Different standards for reporting ADRs to herbal remedies and conventional OTC medicines: face-to-face interviews with 515 users of herbal remedies. Br J Clin Pharmacol. 1998;45:496–500. doi: 10.1046/j.1365-2125.1998.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yessian MR, Greenleaf JM. Adverse event reporting for dietary supplements: an inadequate safety valve. Department of Health and Human Services, Office of Inspector General; Washington DC: 2001. [accessed 9/14/2005]. www.dhhs.gov/oig/oei. [Google Scholar]
- 26.Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–2063. doi: 10.2165/00003495-200161140-00003. [DOI] [PubMed] [Google Scholar]
- 27.Sridar C, Goosen TC, Kent UM, Williams JA, Hollenberg PF. Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases. Drug Metab Dispos. 2004;32:587–594. doi: 10.1124/dmd.32.6.587. [DOI] [PubMed] [Google Scholar]
- 28.Zuber R, Modriansky M, Dvorak Z, Rohovsky P, Ulrichova J, Simanek V, Anzenbacher P. Effect of silybin and its congeners on human liver microsomal cytochrome P450 activities. Phytother Res. 2002;16:632–638. doi: 10.1002/ptr.1000. [DOI] [PubMed] [Google Scholar]
- 29.Venkataramanan R, Ramachandran V, Komoroski BJ, Zhang S, Schiff PL, Strom SC. Milk thistle, a herbal supplement, decreases the activity of CYP3A4 and uridine diphosphoglucuronsyl transferse in human hepatocyte cultures. Drug Metab Dispos. 2000;28:1270–1273. [PubMed] [Google Scholar]
- 30.Beckmann-Knopp S, Rietbrock S, Weyhenmeyer R, Böcker RH, Beckurts T, Lang W, Hunz M, Fuhr U. Inhibitory effects of silibinin on cytochromes P-450 enzymes in human liver microsomes. Pharmacol Toxicol. 2000;86:250–256. doi: 10.1111/j.0901-9928.2000.860602.x. [DOI] [PubMed] [Google Scholar]
- 31.Borrelli F, Izzo AA, Ernst E. Pharmacological effects of Cimicifuga racemosa. Life Sci. 2003;73:1215–1229. doi: 10.1016/s0024-3205(03)00378-3. [DOI] [PubMed] [Google Scholar]
- 32.Tsukamoto S, Aburatani M, Ohta T. Isolation of CYP3A4 inhibitors from the black cohosh (Cimicifuga racemosa) eCAM. 2005;2:223–226. doi: 10.1093/ecam/neh086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry B, Carrier J, Khan IA, Edwards DJ, Shah A. In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, saw palmetto. Clin Pharmacol Ther. 2004;76:428–440. doi: 10.1016/j.clpt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Kivisto KT, Kroemer HK. Use of probe drugs as predictors of drug metabolism in humans. J Clin Pharmacol. 1997;37:40S–48S. doi: 10.1177/009127009703700121. [DOI] [PubMed] [Google Scholar]
- 35.Streetman DS, Bertino JS, Nafziger AN. Phenotyping of drug metabolizing enzymes in adults: a review of in-vivo cytochrome P450 phenotyping probes. Pharmacogenetics. 2000;10:187–216. doi: 10.1097/00008571-200004000-00001. [DOI] [PubMed] [Google Scholar]
- 36.Bachman KA. Genotyping and phenotyping the cytochrome P-450 enzymes. Am J Ther. 2002;9:309–316. doi: 10.1097/00045391-200207000-00008. [DOI] [PubMed] [Google Scholar]
- 37.Zhu B, Ou-Yang D-S, Cheng Z-N, Huang S-L, Zhou H-H. Single plasma sampling to predict oral clearance of CYP3A probe midazolam. Acta Pharmacol Sin. 2001;22:634–638. [PubMed] [Google Scholar]
- 38.Rogers JF, Nafziger AN, Kashuba ADM, Streetman DS, Rocci ML, Choo EF, Wilkinson GR, Bertino JS. Single plasma concentrations of 1′-hydroxymidazolam or the ratio of 1′-hydroxymidazolam:midazolam do not predict midazolam clearance in healthy subjects. J Clin Pharmacol. 2002;42:1079–1082. doi: 10.1177/009127002401382614. [DOI] [PubMed] [Google Scholar]
- 39.Gorski JC, Vannaprasaht S, Hamman MA, Ambrosius WT, Bruce MA, Haehner-Daniels B, Hall SD. The effect of age, sex, and rifampin administration on intestinal and hepatic cytochrome P450 3A activity. Clin Pharmacol Ther. 2003;74:275–287. doi: 10.1016/S0009-9236(03)00187-5. [DOI] [PubMed] [Google Scholar]
- 40.Gorski JC, Jones DR, Haehner-Daniels BD, Hamman MA, O’Mara EM, Hall SD. The contribution of intestinal and hepatic CP3A to the interaction between midazolam and clarithromycin. Clin Pharmacol Ther. 1998;64:133–143. doi: 10.1016/S0009-9236(98)90146-1. [DOI] [PubMed] [Google Scholar]
- 41.Wallace SN, Carrier DJ, Clausen EC. Extraction of nutraceuticals from milk thistle. Appl Biochem Biotech. 2003;105–108:891–903. doi: 10.1385/abab:108:1-3:891. [DOI] [PubMed] [Google Scholar]
- 42.Ganzera M, Bedir E, Khan IA. Separation of Cimicifuga racemosa triterpene glycosides by evaporative light scattering detection. Chromatographia. 2000;52:301–304. [Google Scholar]
- 43.The Pharmacopeia of the United States Twenty-eighth Revision, and the National Formulary. 23. United States Pharmacopeial Convention, Inc; Rockville, MD: 2004. Disintegration and dissolution of dietary supplements; pp. 2778–2779. [Google Scholar]
- 44.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Hillsdale (NJ): Lawrence Erlbaum Associates Publishers; 1988. pp. 19–42. [Google Scholar]
- 45.Schulz H-U, Schurer M, Krumbiegel G, Wachter W, Weyhenmeyer R, Seidel G. Investigation of dissolution and bioequivalence of silymarin products. Arzneim-Forsch. 1995;45:61–64. [PubMed] [Google Scholar]
- 46.Kim YC, Kim EJ, Lee ED, Kim JH, Jang SW, Kim YG, Kwon JW, Kim WB, Lee MG. Comparative bioavailability of silibinin in healthy male volunteers. Int J Clin Pharmacol Ther. 2003;41:593–596. doi: 10.5414/cpp41593. [DOI] [PubMed] [Google Scholar]
- 47.Schandalik R, Gatti G, Perucca E. Pharmacokinetics of silybin in bile following administration of silipide and silymarin in cholecystectomy patients. Arzneim-Forsch. 1992;42:964–968. [PubMed] [Google Scholar]
- 48.Savio D, Harrasser PC, Basso G. (1998) Softgel capsule technology as an enhancer device for the absorption of natural principles in humans: a bioavailability cross-over randomized study on silybin. Arzneim-Forsch. 1998;48:1104–1106. [PubMed] [Google Scholar]
- 49.Li F-Q, Hu J-H. Improvement of the dissolution rate of silymarin by means of solid dispersions. Chem Pharm Bull. 2004;52:972–973. doi: 10.1248/cpb.52.972. [DOI] [PubMed] [Google Scholar]
- 50.Orlando R, Fragasso A, Lampertico M, Marena C. Silybin kinetics in patients with liver cirrhosis: a comparative study of a silybin-phosphatidylcholine complex and silymarin. Med Sci Res. 1990;18:861–863. [Google Scholar]
- 51.Gatti G, Perucca E. Plasma concentrations of free and conjugated silybin after oral intake of a silybin-phosphatidylcholine complex (silipide) in healthy volunteers. Int J Clin Pharmacol Ther. 1994;32:614–617. [PubMed] [Google Scholar]
- 52.Kidd P, Head K. A review of the bioavailability and clinical efficacy of milk thistle phytosome: a silybin-phosphatidylcholine complex (Siliphos) Altern Med Rev. 2005;10:193–203. [PubMed] [Google Scholar]
- 53.Abrol S, Trehan A, Katare OP. Formulation, characterization, and in vitro evaluation of silymarin-loaded lipid microspheres. Drug Deliv. 2004;11:185–191. doi: 10.1080/10717540490433958. [DOI] [PubMed] [Google Scholar]
- 54.Raucy JL. Regualtion of CYP3A4 expression in human hepatocytes by pharmaceuticals and natural products. Drug Metab Dispos. 2003;31:533–539. doi: 10.1124/dmd.31.5.533. [DOI] [PubMed] [Google Scholar]
- 55.Johnson BM, van Breemen RB. In vitro formation of quinoid metabolites of the dietary supplement Cimicifuga racemosa (Black cohosh) Chem Res Toxicol. 2003;16:838–846. doi: 10.1021/tx020108n. [DOI] [PubMed] [Google Scholar]
- 56.Goosen TC, Cillie D, Bailey DG, et al. Bergamottin contribution to the grapefruit juice-felodipine interaction and disposition in humans. Clin Pharmacol Ther. 2004;76:607–617. doi: 10.1016/j.clpt.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 57.Markowitz JS, Donovan JL, DeVane CL, Sipkes L, Chavin KD. Multiple-dose administration of Ginkgo biloba did not affect cytochrome P-450 2D6 or 3A4 activity in normal volunteers. J Clin Psychopharmacol. 2003;23:576–581. doi: 10.1097/01.jcp.0000095340.32154.c6. [DOI] [PubMed] [Google Scholar]
- 58.Anderson GD, Rosito G, Mohustsy MA, Elmer GW. Drug interaction potential of soy extract and Panax ginseng. J Clin Pharmacol. 2003;43:643–648. [PubMed] [Google Scholar]
- 59.Markowitz JS, Donovan JL, DeVane CL, Taylor RM, Ruan Y, Wang J-S, Chavin KD. Multiple doses of saw palmetto (Serenoa repens) did not alter cytochrome P450 2D6 and 3A4 activity in normal volunteers. Clin Pharmacol Ther. 2003;74:536–542. doi: 10.1016/j.clpt.2003.08.010. [DOI] [PubMed] [Google Scholar]


