Abstract
Cancer chemotherapy drugs, such as cisplatin, are extremely potent for producing nausea and vomiting. The acute effects of these treatments are partly controlled using anti-emetic drugs, but the delayed effects (> 24 h), especially nausea, are much more difficult to treat. Furthermore, cisplatin induces a long-term (up to 48 h) increase in pica in rats. Pica is manifested as an increase in consumption of kaolin (clay) and is used as a measure of visceral sickness. It is unknown what brain pathways might be responsible for this sickness associated behavior. As a first attempt to define this neural system, rats were injected (i.p.) with 3, 6, or 10 mg/kg cisplatin (doses reported to produce pica) and sacrificed at 6, 24, or 48 h to determine brain Fos expression. The primary results indicate: 1) increasing the dose of cisplatin increased the magnitude and duration of brain Fos expression, 2) most excitatory effects on hindbrain nucleus of the solitary tract (NTS) and area postrema (AP) Fos expression occurred within 24 h after cisplatin injection, 3) 6 and 10 mg/kg cisplatin treatment produced large increases in Fos expression in the central amygdala (CeA) and bed nucleus of the stria terminalis (BNST), including 48 h after injection, and 4) cisplatin treatment produced little effect on Fos expression in the paraventricular and supraoptic nuclei of the hypothalamus. These results indicate that cisplatin activates a neural system that includes the dorsal vagal complex (NTS and AP), CeA, and BNST.
Keywords: nausea, vomiting, vagus, c-Fos, rat, amygdala
1. Introduction
Nausea and vomiting are commonly encountered in clinical practice as adverse effects of a wide range of drug treatments and diseases. Some cancer chemotherapy drugs, such as cisplatin, are extremely potent agents for inducing nausea and vomiting (e.g., Hesketh, 1996; Martin, 1996). The acute emetic effect of these treatments are partly controlled using anti-emetic drugs, however the delayed effects (> 24 h), particularly nausea, are much more difficult to treat (e.g., Rudd and Andrews, 2005). Additionally, there is little understanding of the neural systems that are involved in the long-term side effects of chemotherapy and the underlying neural systems that are responsible for nausea (for review see Andrews and Horn, 2006). This lack of information has hampered the development of treatments that might target these systems to more completely manage the distress produced by nausea and emesis.
Several animal models have been used to study the delayed (> 24 h) emesis and malaise occurring after treatment with chemotherapy agents. Ferrets, pigs, dogs, and the house musk shrew show delayed phases of emesis after injection with chemotherapy agents (Fukui and Yamamoto, 1999; Milano et al., 1995; Rudd and Naylor, 1994; Sam et al., 2003). The rat, a species without a vomiting reflex, shows a delayed pica response when injected with cisplatin (e.g., Rudd et al., 2002; Vera et al., 2006). Pica is the consumption of a non-nutritive material, such as clay, and is probably an adaptive response to toxicosis because clay can bind toxins and limit the systemic effects of a poison (Phillips et al., 1995; Phillips, 1999). Delayed emesis in animals possessing an emetic reflex, and pica in rats, is partially inhibited by drug treatments that suppress chemotherapy-induced emesis in humans, including dexamethasone, 5-HT3 (serotonin type 3) receptor antagonists, and NK1 (neurokinin type 1) receptor antagonists (Fukui and Yamamoto, 1999; Rudd et al., 2002; Sam et al., 2003).
Despite the use of animal models for studies of delayed emesis very little work has been conducted to determine the neural systems of the brain responsible for the delayed adverse effects of chemotherapy agents. One approach to define activation of neural systems is labeling Fos protein in neuronal cells (e.g., Sagar et al., 1988). The contribution of the caudal hindbrain to the emetic reflex was shown by Fos expression in the nucleus of the solitary tract (NTS) and area postrema (AP) of the ferret and cat after treatment with cisplatin (Ariumi et al., 2000; Miller and Ruggiero, 1994; Reynolds et al., 1991; Van Sickle et al., 2003). The rat also shows increased cFos mRNA in the NTS and AP for up to 6 h after an injection of 10 mg/kg cisplatin (Endo et al., 2004). In contrast to the focus on hindbrain Fos expression after treatment with cisplatin forebrain Fos expression that might be induced by injection of chemotherapy agents has not been reported. Nausea is likely the result of activation of forebrain systems and elucidating the neural systems of the forebrain activated by cisplatin might prove important for delineating the neural substrates for the perception of acute and delayed nausea. Furthermore, little is known about the neural systems responsible for delayed emesis or malaise (> 24 h) produced by chemotherapy treatments.
In the current investigation brain Fos-like immunoreactivity (Fos expression) was used to investigate the involvement of hindbrain and forebrain neural pathways in the short- (6 and 24 h) and long-term (48 h) responses to the highly emetic agent cisplatin. Rats were injected intraperitoneally (i.p.) with 3, 6, or 10 mg/kg cisplatin and sacrificed at 6, 24, or 48 h to determine caudal hindbrain and forebrain Fos expression. These doses of cisplatin and time points chosen represent those used in studies of cisplatin-induced pica in the rat (Rudd et al., 2002; Saeki et al., 2001; Takeda et al., 1993; Takeda et al., 1995; Yamamoto et al., 2002).
2. Materials and methods
2.1. Subjects
Ninety adult male Sprague–Dawley rats (Charles River, Kingston, NY, USA) were housed individually in a temperature-controlled (22°C) vivarium maintained on a 12:12 h light–dark cycle (lights on at 0700 h). Rats were maintained in the animal facility for at least 2 weeks before testing and weighed 400–500 g at the time of sacrifice. Unless otherwise noted, standard rodent chow and tap water were available ad libitum throughout the experiment. Rats were weighed frequently to habituate them to handling, and were adapted to test procedures by giving them at least two mock trials prior to testing, in which the injection needle was inserted (i.p.) with no injection. All experiments conformed to established standards of the National Institutes of Health and the Monell Center’s Institutional Animal Care and Use Committee.
2.2. Injection and tissue collection
Three separate experiments were conducted at different times. In each experiment (N = 30) a different concentration of cisplatin (3, 6, or 10 mg/kg) was compared to a saline control group at three time points (6, 24, or 48 h), i.e., each experiment contained a total of 6 conditions (n = 5 in each). The thirty animals in each experiment were received as a single shipment from the supplier and were tested together. The sacrifice of all animals within each experiment occurred over six separate days (five animals were sacrificed each day). Animals were randomly assigned to an experimental condition and a time point for sacrifice.
Animals were injected (ip) with saline (0.15 M NaCl) or cisplatin at 1000–1100 h. Water bottles and food cups were removed 4 h prior to sacrifice in order to eliminate the possibility of increased brain Fos expression by drinking and eating behaviors. Cisplatin was dissolved in saline (0.15 M NaCl) and vortexed immediately before injection. For the 3 and 6 mg/kg cisplatin experiments the injected volume of cisplatin solution was 1.5 ml/kg, which equaled a concentration of 2 and 4 mg/ml, respectively. For the 10 mg/kg experiment, because of the difficulty to dissolve high concentrations of cisplatin, cisplatin was injected at a volume of 5 ml/kg with a concentration of 2 mg/ml. For all experiments control animals were injected with saline using the same volumes as cisplatin injected animals.
At the time of sacrifice, rats were deeply anesthetized by injection of 50 mg of sodium pentobarbital (i.p.). The thoracic cavity was opened and, to assure a thorough fixation of the brain, rats were given 0.3 ml of heparin (1000 IU/ml) intracardially. This was followed by transcardial perfusion with 300 ml of 0.2 M phosphate buffered saline (PBS; pH 7.4) and then 500 ml of 2% acrolein–4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Animals were then perfused with an additional 150 ml of PBS to remove excess fixative. Brains were removed, blocked into forebrain and brainstem, and placed in 10% sucrose/PBS followed by 20% and 30% sucrose/PBS, each for 24 h. After cryoprotection in sucrose, brains were frozen on dry ice and cut into 30-μm sections using a cryostat.
2.3. Fos Immunohistochemistry
Sections were collected from two locations based on previous work and knowledge of the viscerosensory pathways (e.g., Horn et al., 1999; Horn et al., 2001); from caudal hindbrain (approximately −14.5 to −12.5 mm bregma) and forebrain (approximately 0.2 to −3.6 mm bregma) (Paxinos and Watson, 2005). Sections were placed serially into six culture plate wells containing cryoprotectant (Watson, Jr. et al., 1986), and stored at −20°C for 1–2 weeks followed by immunohistochemical processing.
Brain sections were initially rinsed in PBS to remove cryoprotectant. A sequence of incubation steps was done in 1% sodium borohydride in PBS (20 min), 0.3% hydrogen peroxide in PBS (30 min), and 5% normal goat serum (NGS) in PBS containing 0.2% triton X-100 (PBS-TX), with rinses between each step. Sections were then incubated at room temperature with gentle agitation in 1:40,000 polyclonal anti-Fos (Santa Cruz Biotechnology, Santa Cruz, CA, USA; lot no. D172) containing 1% NGS in PBS–TX for 20 h. Following rinses in PBS–TX, sections were placed in 1:400 biotinylated rabbit anti-rat (Elite kit, Vector Laboratories) for 3 h at room temperature with gentle agitation. This was followed by rinses in PBS and a 3-h incubation in avidin–biotin (4.5 μl of avidin and biotin per ml PBS; Elite kit, Vector Laboratories, Burlingame, CA) with gentle agitation. Sections were then rinsed twice in PBS and twice in 175 mM acetate–10 mM imidazole buffer (A/I buffer, pH 7.4). Sections were placed in 3,3′-diaminobenzidine (DAB; 5 mg/ml in A/I buffer) with nickel sulfate (25 mg/ml) for 1–3 min for the chromogen reaction. Finally, sections were rinsed twice in A/I buffer and twice in PBS. Tissue sections were mounted on gelatin-coated slides and cover-slipped.
2.4. Cell counting and analysis
Fos cell staining was determined by the presence of a blue–black reaction product in the cell nuclei. Tissue sections were viewed with a Zeiss microscope (Axiostar Plus) equipped with a digital camera (Scion CFW-1312C). Brain regions and cells expressing Fos were imaged and the number of cells expressing Fos was manually counted using ImageJ (NIH; http://rsb.info.nih.gov/ij) software.
Based on previous examinations of brain sections from rats treated with cisplatin, cell counts were made in areas that consistently showed Fos expression. To standardize the analyses, cells from each area were counted in coronal sections of brain from each animal at approximately the same level relative to bregma (Paxinos and Watson, 2005). The brain areas analyzed were: 1) hindbrain: NTSc (nucleus of the solitary tract, caudal), NTSm (middle), NTSr (rostral), AP (area postrema), 2) forebrain: PVNp (paraventricular nucleus of the hypothalamus, parvocellular division), PVNm (magnocellular division), SON (supraoptic nucleus), CeA (central nucleus of the amygdala), and BNST (bed nucleus of the stria terminalis). The position of each counted brain area relative to bregma is shown in Figures 1 and 3. Cell counts were obtained from 1 to 3 (consecutive and averaged) sections for each brain area and, because Fos expression was not lateralized in any of the bilateral structures examined, cell counts reflect the totals for both sides in these areas. Although the nickel enhanced DAB chromagen staining provided some architectural details, some brain areas, like the viscerosensory thalamus and cortex, were not counted for Fos expression because it was difficult to delineate the boundaries for these regions. It was sometimes difficult to find appropriate brain sections for group comparisons, and therefore the sample size varied from 4 to 5 animals for each brain area analyzed. Brains were processed in batches and each batch also included a positive control. Positive controls for Fos expression consisted of brains from rats treated with cholecystokinin (100 μMol/kg, i.p.) 1 h prior to sacrifice (e.g., Olson et al., 1992). These positive controls consistently showed a large number of Fos cell counts in the hindbrain and forebrain.
Fig. 1.
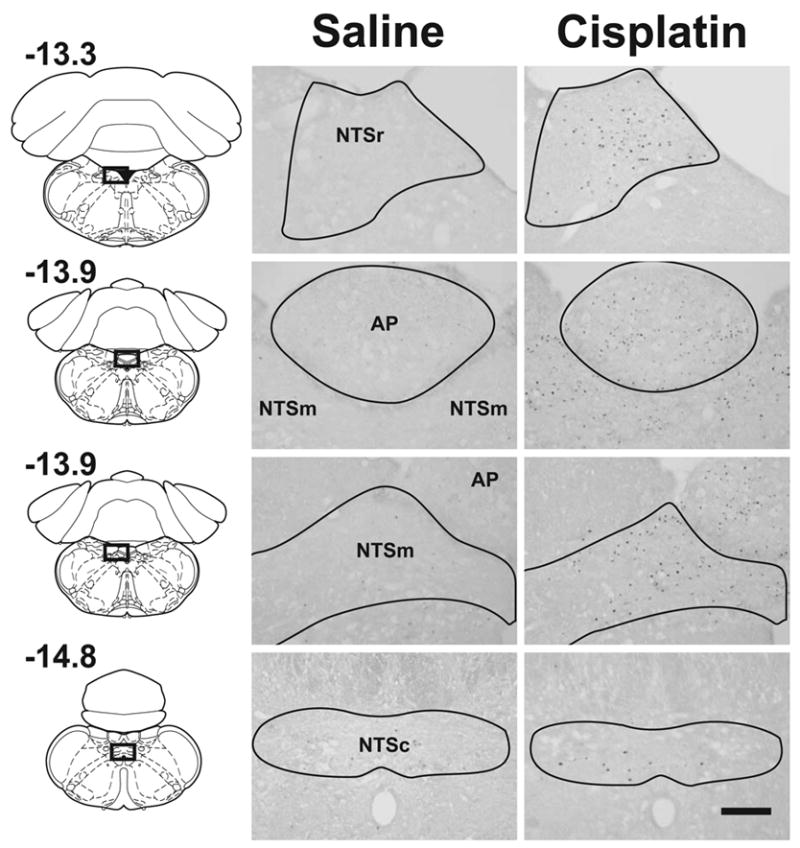
Photomicrographs from representative hindbrains showing Fos expression at 48 h after intraperitoneal injection with saline (0.15 M NaCl) or cisplatin (10 mg/kg). The left column shows the location of cell counts in slices of the whole brain (values represent position, mm, relative to bregma; reprinted from “The rat brain in stereotaxic coordinates,” G. Paxinos and C. Watson, pages 144, 149, and 156, Copyright, 2005, with permission from Elsevier). The Calibration bar equals 200 μm. See text for key of abbreviations.
Fig. 3.
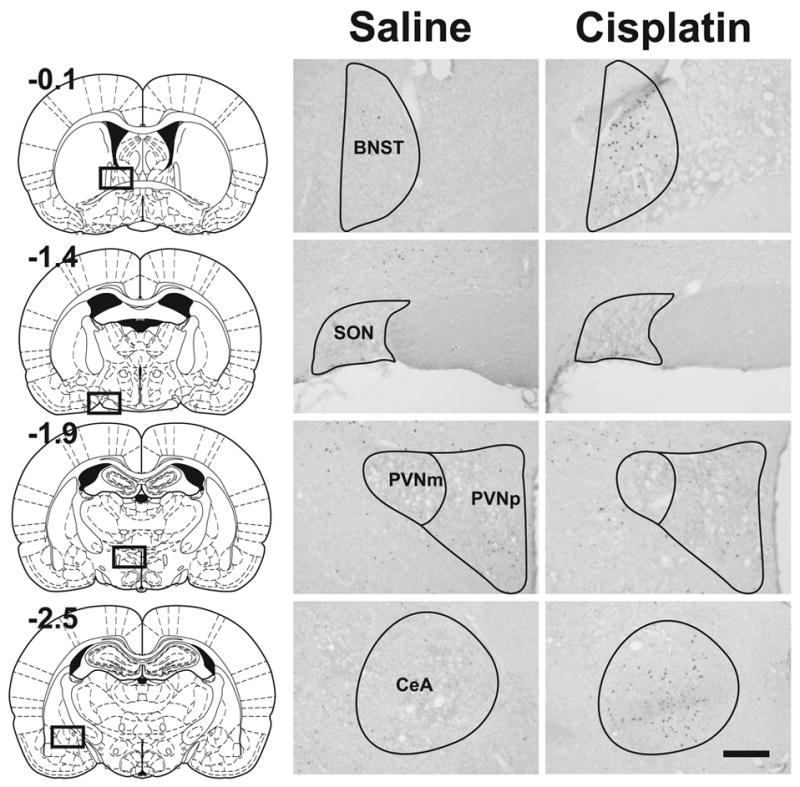
Photomicrographs from representative forebrains showing Fos expression at 48 h after intraperitoneal injection with saline (0.15 M NaCl) or cisplatin (10 mg/kg). The left column shows the location of cell counts in slices of the whole brain (values represent position, mm, relative to bregma; reprinted from “The rat brain in stereotaxic coordinates,” G. Paxinos and C. Watson, pages 34, 45, 49, and 54, Copyright, 2005, with permission from Elsevier). Calibration bar equals 200 μm. See text for key of abbreviations.
The raw Fos cell counts were analyzed using 2 x 3 analysis of variance (ANOVA) for each brain area, treatment (saline, cisplatin) by time (6, 24, and 48 h). When ANOVA showed a statistically significant effect for the interaction (treatment by time) or the main effect of cisplatin treatment planned comparisons of group means were conducted (least significant difference tests, LSD-tests). For all analyses, a level of p < 0.05 was used to determine statistical significance. The percentage change in Fos expression was calculated by the following equation: the percent difference between saline and cisplatin conditions; mean of the 3 time points of each brain area and then the sum of all areas in region of interest (e.g., hindbrain, hypothalamus, or CeA/BNST).
3. Results
3.1. Hindbrain Fos expression
See Figure 1 for representative images comparing hindbrain Fos expression in saline and cisplatin (10 mg/kg) treated animals at 48 h after injection. The mean cell counts in Figure 2 show Fos expression in the hindbrain occurring primarily in the middle and rostral levels of the NTS and AP with little expression in the NTSc following cisplatin treatment. Most cisplatin-induced Fos expression appeared at 6 and 24 h, but injection with 10 mg/kg cisplatin produced a prolonged response at 48 h (Fig. 2). The percentage increase in hindbrain Fos expression induced by cisplatin compared to saline was 96% for 3 mg/kg, 106% for 6 mg/kg, and 356% for 10 mg/kg over the 48 h period.
Fig. 2.
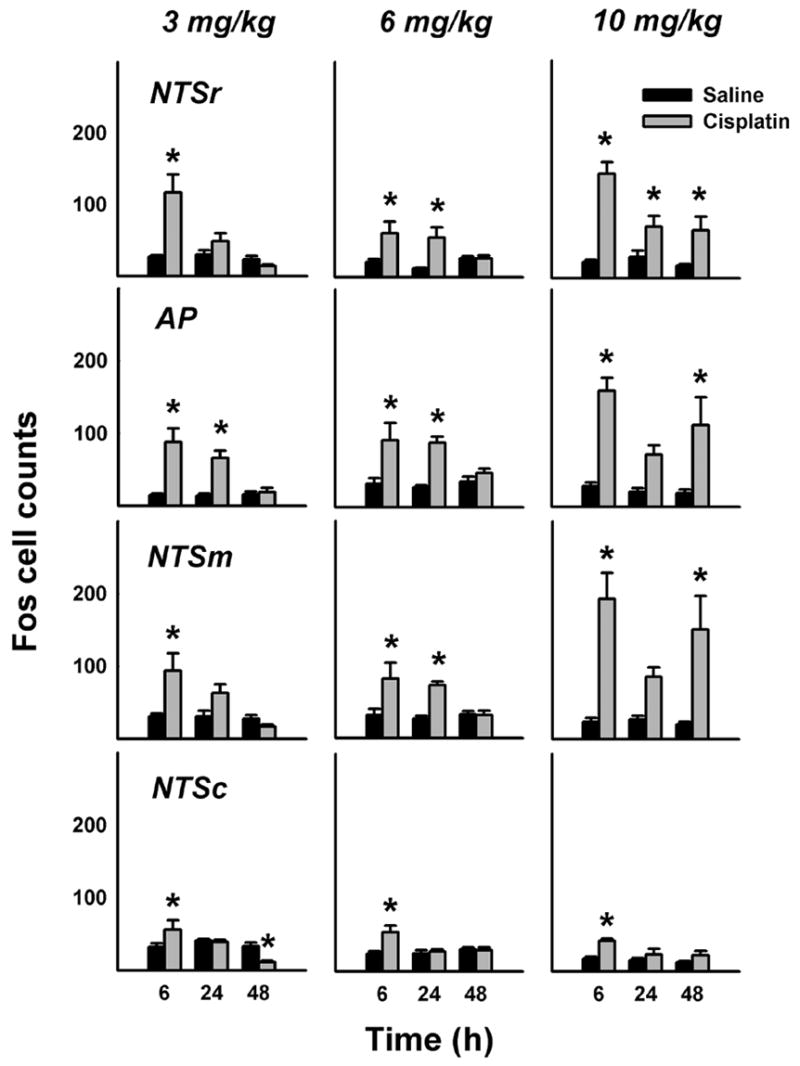
Number of Fos cells in the caudal hindbrain at 6, 24, and 48 h after intraperitoneal treatment with saline or cisplatin (3, 6, or 10 mg/kg). = p < 0.05, LSD-test, saline versus cisplatin. See text for key of abbreviations.
3 mg/kg cisplatin
Time of sacrifice significantly affected 3 mg/kg cisplatin-induced Fos expression in the NTSc, NTSm, AP, and NTSr [ps < 0.05, ANOVAs, treatment by time interaction effects], with significantly increased Fos expression produced by cisplatin treatment at the 6 h time points (Fig. 2, ps < 0.5, LSD-tests). The only significant cisplatin-induced Fos expression beyond 6 h occurred in the AP at 24 h (Fig. 2, p < 0.05, LSD-test). Interestingly, 3 mg/kg cisplatin significantly decreased Fos expression in the NTSc at 48 h [Fig. 2, LSD-test, p < 0.05], but this effect was not reproduced by injection of higher doses of cisplatin (see below).
6 mg/kg cisplatin
Time of sacrifice significantly affected 6 mg/kg cisplatin-induced Fos expression in the NTSc and NTSm [ps < 0.05, ANOVAs, treatment by time interaction effects]. Cisplatin also significantly affected Fos expression in the AP and NTSr [ps < 0.005, ANOVAs, main effects of cisplatin treatment]. Planned comparisons revealed that 6 mg/kg cisplatin significantly induced Fos expression at 6 and 24 h in the NTSm, AP, and NTSr, but only at 6 h in the NTSc (ps < 0.05, LSD-tests; Fig. 2).
10 mg/kg cisplatin
Time of sacrifice significantly affected 10 mg/kg cisplatin-induced Fos expression in the NTSr [p < 0.01, ANOVA, treatment by time interaction effect]. Additionally, cisplatin affected Fos expression in the NTSc, NTSm, and AP [ps < 0.001, ANOVAs, main effects of cisplatin treatment]. Fos expression in the NTSc, NTSm, AP, and NTSr at 6 h was significantly increased by cisplatin treatment (ps < 0.05, LSD-tests; Fig. 2). Although cisplatin produced a significant increase Fos expression at 24 h in the NTSr, this did not reach statistical significance for the NTSm and AP (p = 0.07 and 0.08, respectively; Fig. 2). 10 mg/kg Cisplatin produced a significant long-term, 48 h, increase in Fos expression in the NTSm, AP, and NTSr (ps < 0.05, LSD-tests; Fig. 2).
3.2. Forebrain Fos expression
See Figure 3 for representative images comparing forebrain Fos expression in saline and cisplatin (10 mg/kg) treated animals at 48 h after injection. The mean cell counts show that cisplatin treatment primarily produced no increase in PVNp and PVNm Fos expression (Fig. 4). In contrast, cisplatin treatment produced a robust increase in Fos expression in the CeA and BNST (Fig. 5). There were only a few Fos cells observed in the SON, with several small, but statistically significant effects of cisplatin treatment (Fig. 4). The percentage reduction in hypothalamic Fos expression induced by cisplatin compared to saline was 40% for 3 mg/kg, 10% for 6 mg/kg, and 4% for 10 mg/kg over 48 h. In contrast, cisplatin treatment increased Fos expression in the CeA plus BNST by 156% for 3 mg/kg, 316% for 6 mg/kg, and 557% for 10 mg/kg over 48 h.
Fig. 4.
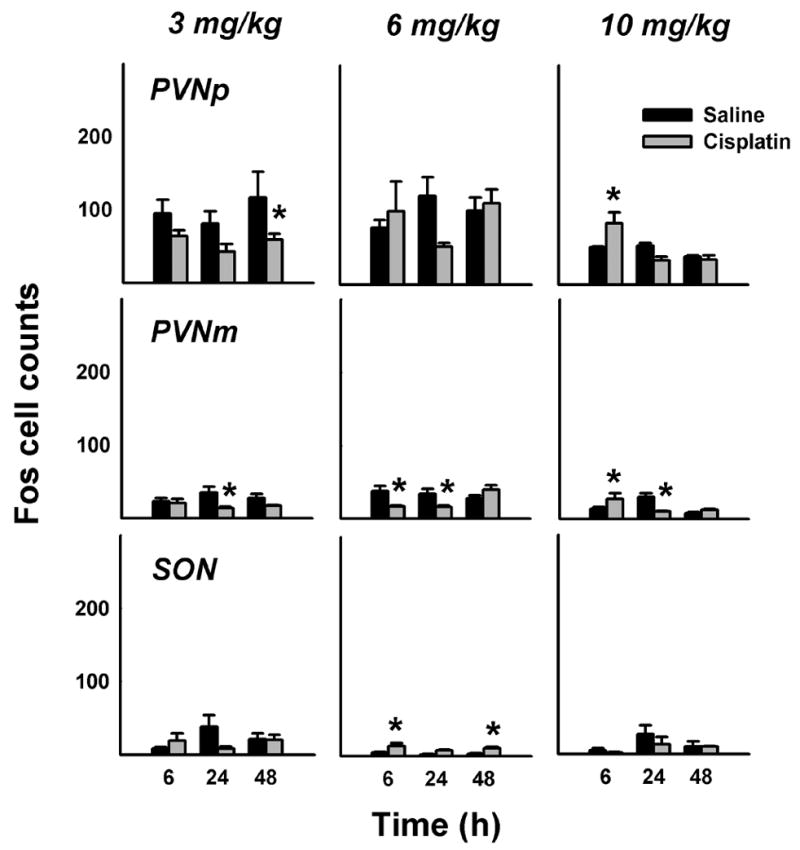
Number of Fos cells in the PVN (parvocellular and magnocellular regions) and SON at 6, 24, and 48 h after intraperitoneal treatment with saline or cisplatin (3, 6, or 10 mg/kg). = p < 0.05, LSD-test, saline versus cisplatin. See text for key of abbreviations.
Fig. 5.
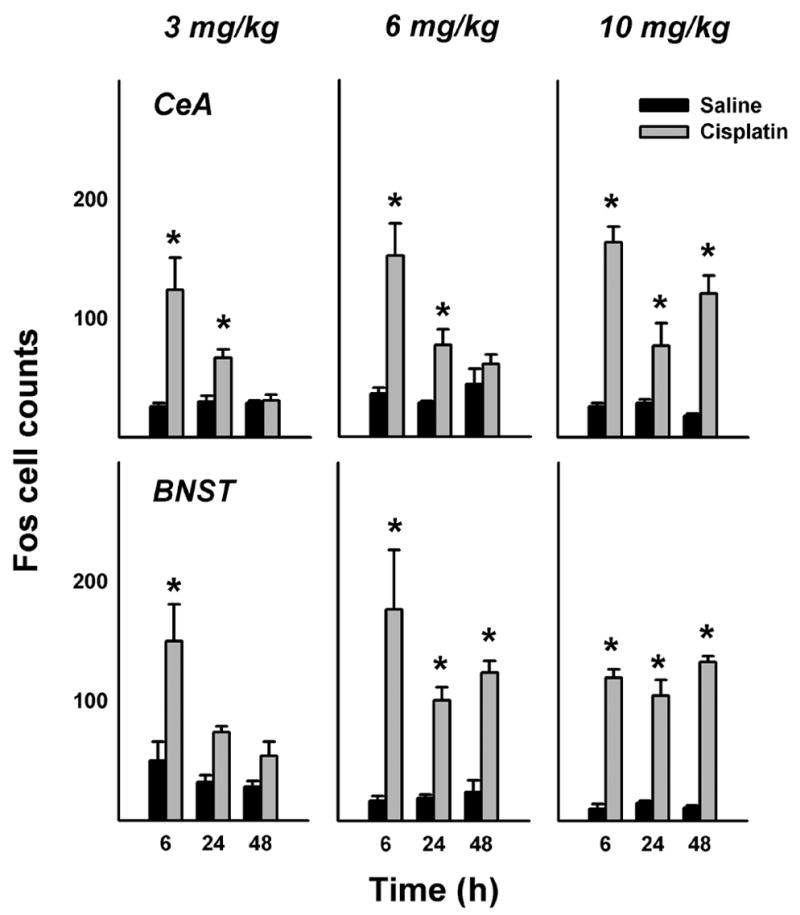
Number of Fos cells in the CeA and BNST at 6, 24, and 48 h after intraperitoneal treatment with saline or cisplatin (3, 6, or 10 mg/kg). = p < 0.05, LSD-test, saline versus cisplatin. See text for key of abbreviations.
3 mg/kg cisplatin
Time of sacrifice significantly affected 3 mg/kg cisplatin-induced Fos expression in the CeA [p < 0.01, ANOVA, treatment by time interaction effect]. Cisplatin also affected Fos expression in the PVNp, PVNm, and BNST [ps < 0.05, ANOVAs, main effects of cisplatin treatment]. Planned comparisons revealed that cisplatin suppressed Fos expression at in the PVNp at 48 h and in the PVNm at 24 (ps < 0.05, LSD-tests; Fig. 4).
6 mg/kg cisplatin
Time of sacrifice significantly affected 6 mg/kg cisplatin-induced Fos expression in the PVNm and CeA [ps < 0.01, ANOVAs, treatment by time interaction effects]. Cisplatin also affected Fos expression in the SON and BNST [ps < 0.001, ANOVAs, main effects of cisplatin treatment]. Planned comparisons revealed that cisplatin induced Fos expression at 6 and 48 h in the SON (ps < 0.05, LSD-tests; Fig. 4), but this effect of very small (only a difference of 9 and 8 Fos-positive cells between saline and cisplatin conditions, respectively). The suppression of Fos expression at 6 and 24 h was also small but statistically significant (ps < 0.05, LSD-tests; Fig. 4; a difference of 21 and 18 Fos-positive cells between saline and cisplatin conditions, respectively). In contrast, 6 mg/kg cisplatin produced large increases in Fos expression in the CeA at 6 and 24 h and in the BNST at all time points (ps < 0.05, LSD-tests; Fig. 5).
10 mg/kg cisplatin
Time of sacrifice significantly affected 10 mg/kg cisplatin-induced Fos expression in the PVNp, PVNm, and CeA [ps < 0.01, ANOVAs, treatment by time interaction effects]. Cisplatin also affected Fos expression in the BNST [p < 0.001, ANOVA, main effect of cisplatin treatment]. Planned comparisons revealed that cisplatin induced Fos expression at 6 h in the PVNm and PVNp and reduced expression in the PVNm at 24 h (ps < 0.05, LSD-tests; Fig. 4). In contrast, 10 mg/kg cisplatin produced large increases in Fos expression at all time points in the CeA and BNST (ps < 0.05, LSD-tests; Fig. 5).
4. Discussion
The present study showed the pattern of Fos expression over 48 h in the hindbrain and forebrain in response to three doses of cisplatin (3, 6, and 10 mg/kg). The key findings were: 1) increasing the dose of cisplatin increased the magnitude and duration of brain Fos expression, 2) most excitatory effects on hindbrain Fos expression occurred within 24 h after cisplatin injection, 3) 6 and 10 mg/kg cisplatin increased Fos expression in the CeA and BNST by 316% and 557%, respectively, during 48 h, and 4) cisplatin treatment produced little effect on Fos expression in the PVN and SON, and an overall analysis suggests an inhibition of Fos expression in these hypothalamic areas.
In the hindbrain (NTS and AP), cisplatin treatment produced increased Fos expression by 96% for 3 mg/kg, 106% for 6 mg/kg, and 356% for 10 mg/kg over the 48 h period. Although a direct action of cisplatin on the hindbrain cannot be ruled out, it is possible that cisplatin produced Fos expression in hindbrain nuclei via an afferent vagal input from the gastrointestinal tract. The NTS and AP receive extensive projections from vagal afferent fibers innervating the GI tract (e.g., Norgren and Smith, 1988). Many chemotherapy agents, such as cisplatin, cause the release of serotonin (5-HT) from entero-endocrine cells in the GI tract, which leads to the activation of vagal afferent fibers containing 5-HT3 receptors (Endo et al., 2002; Horn et al., 2004). Importantly, the acute effect of cisplatin on cFos mRNA in the NTS of the rat is reduced by pretreatment with a 5-HT3 receptor antagonist (Endo et al., 2004). Hindbrain Fos expression during the acute and delayed phases closely corresponds to the acute phase distribution of cFos mRNA produced by cisplatin treatment in the rat (Endo et al., 2004). These results might suggest that a core component of acute and delayed nausea produced by cisplatin treatment is activation of the NTS and AP.
Hindbrain Fos expression induced by cisplatin showed a characteristic temporal pattern, with significantly increased Fos expression by 6 h, and this response was much decreased by 48 h with 3 or 6 mg/kg cisplatin (Fig. 2). Cisplatin treatment at 10 mg/kg produced a more prominent hindbrain Fos response that persisted for 48 h (Fig. 2). Cisplatin-induced hindbrain Fos expression in rats in the current study is similar to previous reports showing Fos expression after cisplatin treatment in the hindbrains of vomiting species, e.g., cat and ferret (Ariumi et al., 2000; Miller and Ruggiero, 1994; Reynolds et al., 1991; Van Sickle et al., 2003). There was also a trend for cisplatin (at all doses) to produce more Fos expression in the rostral portions of the NTS (NTSm and NTSr) compared to the caudal part (NTSc) (Fig. 2).
An important result was the lack of a prominent effect of cisplatin on Fos expression in the PVN and SON. The PVN is considered to be an important component of the neural circuitry for the control of food intake (e.g., Wang et al., 2000) and cisplatin produces a prominent inhibition of feeding (Liu et al., 2006; Malik et al., 2006; Vera et al., 2006). There were small, but significant, increases in Fos expression at 6 h in the PVNp and PVNm using 10 mg/kg cisplatin (Fig. 4). An overall calculation indicates that cisplatin reduced Fos expression in the PVN and SON by 40% with a dose of 3 mg/kg, 10% for 6 mg/kg, and 4% for 10 mg/kg over 48 h. This diminished Fos response in the PVN and SON after cisplatin treatment is in sharp contrast to the enhanced Fos expression observed with different stressors, like osmotic challenge, immobilization, and pain (for review see Pacak and Palkovits, 2001). It is also notable that in a recent study in rats, treatment with 6 mg/kg cisplatin, despite causing a profound reduction in food intake, produced little effect on hypothalamic gene expression related to food intake (Malik et al., 2006). In this report, no changes were documented in hypothalamic neuropeptide Y, orexin, or agouti-related peptide mRNA, factors that have been implicated in the control of food intake (Billington and Levine, 1992; Sakurai et al., 1998; Wirth and Giraudo, 2000), or corticotrophin releasing factor mRNA levels, implicated in stress responses (e.g., Reul and Holsboer, 2002). However, tryptophan hydroxylase mRNA was downregulated after cisplatin treatment (Malik et al., 2006), which might relate to the important role of the serotonergic system in the malaise produced by chemotherapy agents. Overall, cisplatin appears to activate the hypothalamus in a way that is markedly different from agents, acting on the gut-brain axis, that produce stress, satiation of feeding, and pain, and therefore might provide insight into the neural systems responsible for nausea and emesis. Based on the current results, the brainstem and CeA/BNST areas seem to play different roles than the hypothalamus in the neural system for the detection of cisplatin.
Cisplatin treatment produced a large Fos response in the CeA and BNST; Fos expression was increased by 156% for 3 mg/kg, 316% for 6 mg/kg, and 557% for 10 mg/kg over 48 h. Increasing the dosage of cisplatin increased the duration of the Fos response; 3 mg/kg produced only an acute phase of Fos expression, but 10 mg/kg and, to a lesser extent, 6 mg/kg generated both acute and delayed components of Fos expression. This might be connected to the well known relationship between dosage of cisplatin and longevity of emesis and nausea in humans and animals with an emetic reflex (e.g., Hesketh, 1996; Rudd and Naylor, 1994). The BNST receives extensive projections from the CeA, shares similar neurotransmitter profiles as the CeA and has been considered to be a key component of an extended amygdala (de Olmos and Heimer, 1999; Heimer and Van Hoesen, 2006; Swanson, 2003). The CeA and BNST are highly differentiated nuclei that receive inputs from cortical areas involved in conscious perception, e.g., prefrontal, chemosensory (i.e., olfactory, gustatory), and viscerosensory cortices. Therefore, the CeA and BNST are strategically located to act as important relays for autonomic, emotional, and neuroendocrine signals involved in feeding, anorexia, and probably nausea. Studies suggest that the amygdala is an important integrator for feeding behavior, including conditioned flavor aversion (Holland and Gallagher, 1999; Kadohisa et al., 2005), and the BNST is involved in functions such as mediating the anorexia assoiciated with stress and cancer (Ciccocioppo et al., 2003; Konsman and Blomqvist, 2005).
The profile of Fos expression in the acute phase did not differ from the delayed phase. However, the NTSm, AP, and CeA showed biphasic responses using 10 mg/kg cisplatin treatment. In these brain regions there were high levels of Fos expression at the 6 h time point and reduced responses by 24 h, but the 48 h amounts of Fos expression returned the level of the 6 h response. These biphasic Fos responses might be related to the well known differential action of anti-emetic drugs to inhibit acute versus delayed emesis in humans and other emetic species and pica in the rat (e.g., Rudd et al., 2002; Rudd and Andrews, 2005).
The Fos response observed at 48 h post-cisplatin injection might reflect the neural substrates involved in mediating a delayed pica response in rats after cisplatin injection. The present study is the only report of Fos expression in the forebrain after cisplatin treatment, and suggests that the lateral extended amygdala might provide an important role in processing viscerosensory input for the production of nausea. These findings show that cisplatin treatment activates the hindbrain, produces a recruitment of limbic forebrain regions (the extended amygdala) and has little excitatory affect on the PVN and SON. This neural system, possibly involved in detecting toxins, might be responsible for the acute and delayed inhibition of food intake and increased consumption of kaolin (pica) that results from treatment with cisplatin.
Acknowledgments
This work was supported by NIH funding (DK065971).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andrews PL, Horn CC. Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton Neurosci. 2006;125:100–115. doi: 10.1016/j.autneu.2006.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariumi H, Saito R, Nago S, Hyakusoku M, Takano Y, Kamiya H. The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett. 2000;286:123–126. doi: 10.1016/s0304-3940(00)01113-7. [DOI] [PubMed] [Google Scholar]
- Billington CJ, Levine AS. Hypothalamic neuropeptide Y regulation of feeding and energy metabolism. Curr Opin Neurobiol. 1992;2:847–851. doi: 10.1016/0959-4388(92)90144-a. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Fedeli A, Economidou D, Policani F, Weiss F, Massi M. The bed nucleus is a neuroanatomical substrate for the anorectic effect of corticotropin-releasing factor and for its reversal by nociceptin/orphanin FQ. J Neurosci. 2003;23:9445–9451. doi: 10.1523/JNEUROSCI.23-28-09445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- Endo T, Hamaue N, Ihira E, Teramoto Y, Liu Y, Hirafuji M, Minami M. Effects of granisetron, a 5-HT3 receptor antagonist, on 5-hydroxytryptamine (5-HT) release from the isolated ileum in a delayed-emesis rat model. Res Commun Mol Pathol Pharmacol. 2002;111:55–68. [PubMed] [Google Scholar]
- Endo T, Minami M, Nakayasu M, Hirafuji M, Hamaue N, Omae N, Kang Y, Iwanaga T. Effects of granisetron and vagotomy on c-fos mRNA expression in the rat medulla oblongata as assessed by in situ hybridization. Biomedical Research. 2004;25:229–235. [Google Scholar]
- Fukui H, Yamamoto M. Methotrexate produces delayed emesis in dogs: a potential model of delayed emesis induced by chemotherapy. Eur J Pharmacol. 1999;372:261–267. doi: 10.1016/s0014-2999(99)00219-8. [DOI] [PubMed] [Google Scholar]
- Heimer L, Van Hoesen GW. The limbic lobe and its output channels: implications for emotional functions and adaptive behavior. Neurosci Biobehav Rev. 2006;30:126–147. doi: 10.1016/j.neubiorev.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hesketh P. Management of cisplatin-induced delayed emesis. Oncology. 1996;53(Suppl 1):73–77. doi: 10.1159/000227644. [DOI] [PubMed] [Google Scholar]
- Holland PC, Gallagher M. Amygdala circuitry in attentional and representational processes. Trends Cogn Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- Horn CC, Addis A, Friedman MI. Neural substrate for an integrated metabolic control of feeding behavior. Am J Physiol. 1999;276:R113–R119. doi: 10.1152/ajpregu.1999.276.1.R113. [DOI] [PubMed] [Google Scholar]
- Horn CC, Richardson EJ, Andrews PL, Friedman MI. Differential effects on gastrointestinal and hepatic vagal afferent fibers in the rat by the anti-cancer agent cisplatin. Auton Neurosci. 2004;115:74–81. doi: 10.1016/j.autneu.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Horn CC, Tordoff MG, Friedman MI. Role of vagal afferent innervation in feeding and brain Fos expression produced by metabolic inhibitors. Brain Res. 2001;919:198–206. doi: 10.1016/s0006-8993(01)02963-8. [DOI] [PubMed] [Google Scholar]
- Kadohisa M, Verhagen JV, Rolls ET. The primate amygdala: Neuronal representations of the viscosity, fat texture, temperature, grittiness and taste of foods. Neuroscience. 2005;132:33–48. doi: 10.1016/j.neuroscience.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Konsman JP, Blomqvist A. Forebrain patterns of c-Fos and FosB induction during cancer-associated anorexia-cachexia in rat. Eur J Neurosci. 2005;21:2752–2766. doi: 10.1111/j.1460-9568.2005.04102.x. [DOI] [PubMed] [Google Scholar]
- Liu YL, Malik NM, Sanger GJ, Andrews PL. Ghrelin alleviates cancer chemotherapy-associated dyspepsia in rodents. Cancer Chemother Pharmacol. 2006:1–8. doi: 10.1007/s00280-005-0179-0. [DOI] [PubMed] [Google Scholar]
- Malik NM, Moore GB, Smith G, Liu YL, Sanger GJ, Andrews PL. Behavioural and hypothalamic molecular effects of the anti-cancer agent cisplatin in the rat: A model of chemotherapy-related malaise? Pharmacol. Biochem Behav. 2006;83:9–20. doi: 10.1016/j.pbb.2005.11.017. [DOI] [PubMed] [Google Scholar]
- Martin M. The severity and pattern of emesis following different cytotoxic agents. Oncology. 1996;53(Suppl 1):26–31. doi: 10.1159/000227637. [DOI] [PubMed] [Google Scholar]
- Milano S, Blower P, Romain D, Grelot L. The piglet as a suitable animal model for studying the delayed phase of cisplatin-induced emesis. J Pharmacol Exp Ther. 1995;274:951–961. [PubMed] [Google Scholar]
- Miller AD, Ruggiero DA. Emetic reflex arc revealed by expression of the immediate-early gene c-fos in the cat. J Neurosci. 1994;14:871–888. doi: 10.1523/JNEUROSCI.14-02-00871.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren R, Smith GP. Central distribution of subdiaphragmatic vagal branches in the rat. J Comp Neurol. 1988;273:207–223. doi: 10.1002/cne.902730206. [DOI] [PubMed] [Google Scholar]
- Olson BR, Hoffman GE, Sved AF, Stricker EM, Verbalis JG. Cholecystokinin induces c-fos expression in hypothalamic oxytocinergic neurons projecting to the dorsal vagal complex. Brain Res. 1992;569:238–248. doi: 10.1016/0006-8993(92)90635-m. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- Phillips TD. Dietary clay in the chemoprevention of aflatoxin-induced disease. Toxicol Sci. 1999;52:118–126. doi: 10.1093/toxsci/52.suppl_1.118. [DOI] [PubMed] [Google Scholar]
- Phillips TD, Sarr AB, Grant PG. Selective chemisorption and detoxification of aflatoxins by phyllosilicate clay. Nat Toxins. 1995;3:204–213. doi: 10.1002/nt.2620030407. [DOI] [PubMed] [Google Scholar]
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Reynolds DJ, Barber NA, Grahame-Smith DG, Leslie RA. Cisplatin-evoked induction of c-fos protein in the brainstem of the ferret: the effect of cervical vagotomy and the anti-emetic 5-HT3 receptor antagonist granisetron (BRL 43694) Brain Res. 1991;565:231–236. doi: 10.1016/0006-8993(91)91654-j. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Andrews PLR. Mechanisms of acute, delayed, and anticipatory emesis induced by anticancer therapies. In: Hesketh PJ, editor. Management of Nausea and Vomiting in Cancer and Cancer Treatment. Jones and Bartlett; Sudbury, MA: 2005. pp. 15–65. [Google Scholar]
- Rudd JA, Naylor RJ. Effects of 5-HT3 receptor antagonists on models of acute and delayed emesis induced by cisplatin in the ferret. Neuropharmacology. 1994;33:1607–1608. doi: 10.1016/0028-3908(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Rudd JA, Yamamoto K, Yamatodani A, Takeda N. Differential action of ondansetron and dexamethasone to modify cisplatin-induced acute and delayed kaolin consumption ("pica") in rats. Eur J Pharmacol. 2002;454:47–52. doi: 10.1016/s0014-2999(02)02472-x. [DOI] [PubMed] [Google Scholar]
- Saeki M, Sakai M, Saito R, Kubota H, Ariumi H, Takano Y, Yamatodani A, Kamiya H. Effects of HSP-117, a novel tachykinin NK1-receptor antagonist, on cisplatin-induced pica as a new evaluation of delayed emesis in rats. Jpn J Pharmacol. 2001;86:359–362. doi: 10.1254/jjp.86.359. [DOI] [PubMed] [Google Scholar]
- Sagar SM, Sharp FR, Curran T. Expression of c-fos protein in brain: metabolic mapping at the cellular level. Science. 1988;240:1328–1331. doi: 10.1126/science.3131879. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(02)09256-5. [DOI] [PubMed] [Google Scholar]
- Sam TS, Cheng JT, Johnston KD, Kan KK, Ngan MP, Rudd JA, Wai MK, Yeung JH. Action of 5-HT3 receptor antagonists and dexamethasone to modify cisplatin-induced emesis in Suncus murinus (house musk shrew) Eur J Pharmacol. 2003;472:135–145. doi: 10.1016/s0014-2999(03)01863-6. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The amygdala and its place in the cerebral hemisphere. Ann N Y Acad Sci. 2003;985:174–184. doi: 10.1111/j.1749-6632.2003.tb07081.x. [DOI] [PubMed] [Google Scholar]
- Takeda N, Hasegawa S, Morita M, Horii A, Uno A, Yamatodani A, Matsunaga T. Neuropharmacological mechanisms of emesis. II. Effects of antiemetic drugs on cisplatin-induced pica in rats. Methods Find. Exp Clin Pharmacol. 1995;17:647–652. [PubMed] [Google Scholar]
- Takeda N, Hasegawa S, Morita M, Matsunaga T. Pica in rats is analogous to emesis: an animal model in emesis research. Pharmacol Biochem Behav. 1993;45:817–821. doi: 10.1016/0091-3057(93)90126-e. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Oland LD, Mackie K, Davison JS, Sharkey KA. Delta9-tetrahydrocannabinol selectively acts on CB1 receptors in specific regions of dorsal vagal complex to inhibit emesis in ferrets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G566–G576. doi: 10.1152/ajpgi.00113.2003. [DOI] [PubMed] [Google Scholar]
- Vera G, Chiarlone A, Martin MI, Abalo R. Altered feeding behaviour induced by long-term cisplatin in rats. Auton Neurosci. 2006 doi: 10.1016/j.autneu.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Wang L, Barachina MD, Martinez V, Wei JY, Tache Y. Synergistic interaction between CCK and leptin to regulate food intake. Regul Pept. 2000;92:79–85. doi: 10.1016/s0167-0115(00)00153-1. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Giraudo SQ. Agouti-related protein in the hypothalamic paraventricular nucleus: effect on feeding. Peptides. 2000;21:1369–1375. doi: 10.1016/s0196-9781(00)00280-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Matsunaga S, Matsui M, Takeda N, Yamatodani A. Pica in mice as a new model for the study of emesis. Methods Find Exp Clin Pharmacol. 2002;24:135–138. doi: 10.1358/mf.2002.24.3.802297. [DOI] [PubMed] [Google Scholar]


