Abstract
Complement fragment-induced sequestration of neutrophils within the lungs may be mediated by stimulus-induced decreases in the deformability of neutrophils, prolonging their lung capillary transit times. As changes in deformability often occur through changes in cytoskeletal proteins, this study determined whether the distribution of actin within intracapillary neutrophils was altered by intravascular complement fragments and whether sequestered neutrophils were less deformed. Ultrathin cryosections of lung tissue from rabbits given an infusion of complement fragments or saline were immunolabeled with anti-actin antibodies. The number of gold particles/microvillus and the density of gold particles/microgram 2 cytoplasm in the submembrane and the central region of intracapillary neutrophils was quantitated. Neutrophil shape was evaluated using laser confocal microscopy. In control rabbits, the ratio of submembrane/central gold was always greater than one and most neutrophils were elongated, 97% having shape factors > 1.10. The ratio of submembrane/central gold was greater in complement-treated rabbits (5.1 +/- 0.9) than controls (2.6 +/- 0.4; P < 0.026). The number of gold particles/microvillus was also increased in complement-treated rabbits (3.9 +/- 0.5) compared with controls (2.3 +/- 0.5; P < 0.045). Neutrophils were more often spherical when rabbits received complement fragments for 1.5 minutes than in control lungs or after 15-minute infusions. These data suggest that complement fragments induce a rapid redistribution of actin from the central to the submembrane region and the microvilli and result in more round neutrophils. This redistribution may decrease the deformability of neutrophils by altering the stiffness of the submembrane region and/or by preventing the microvilli from flattening.
Full text
PDF


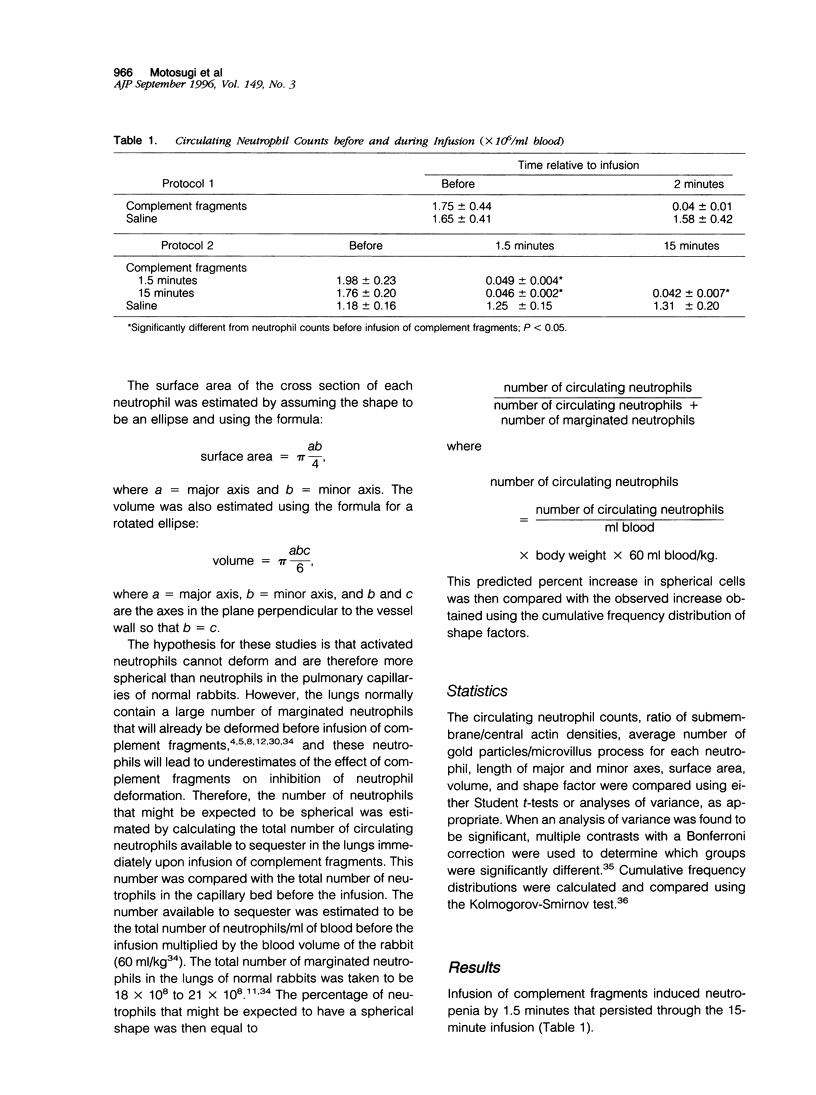

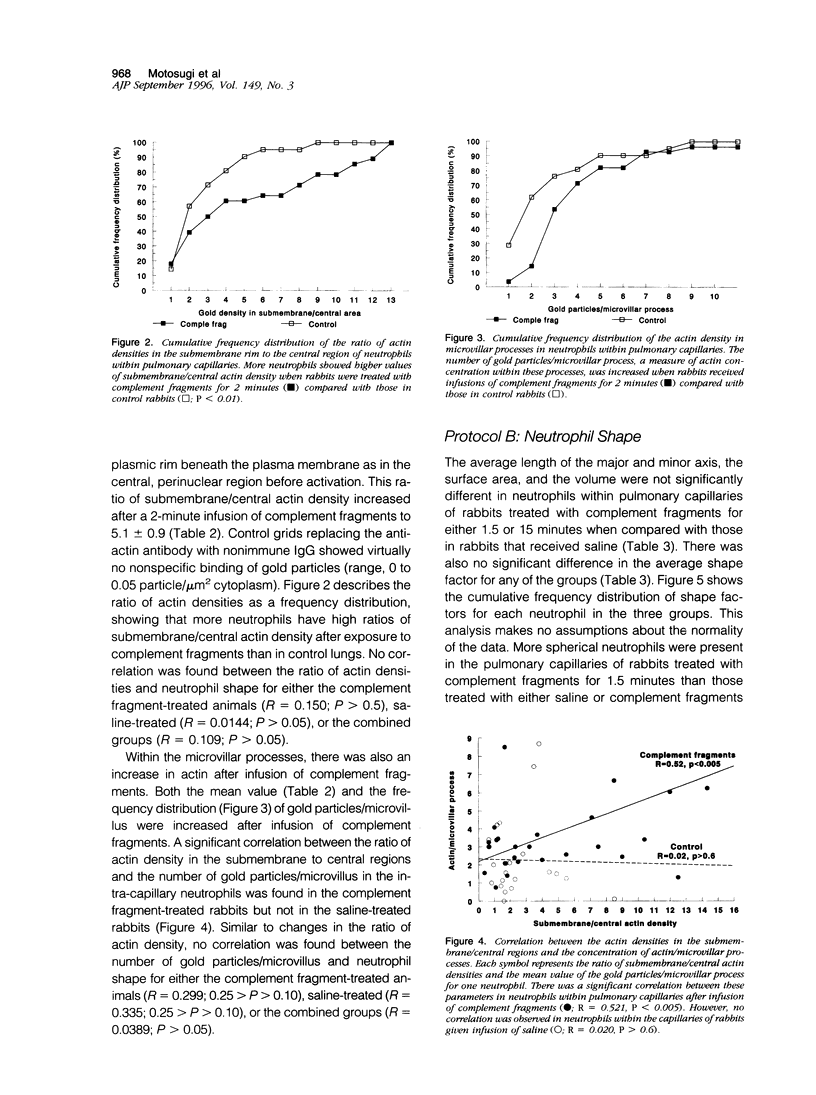
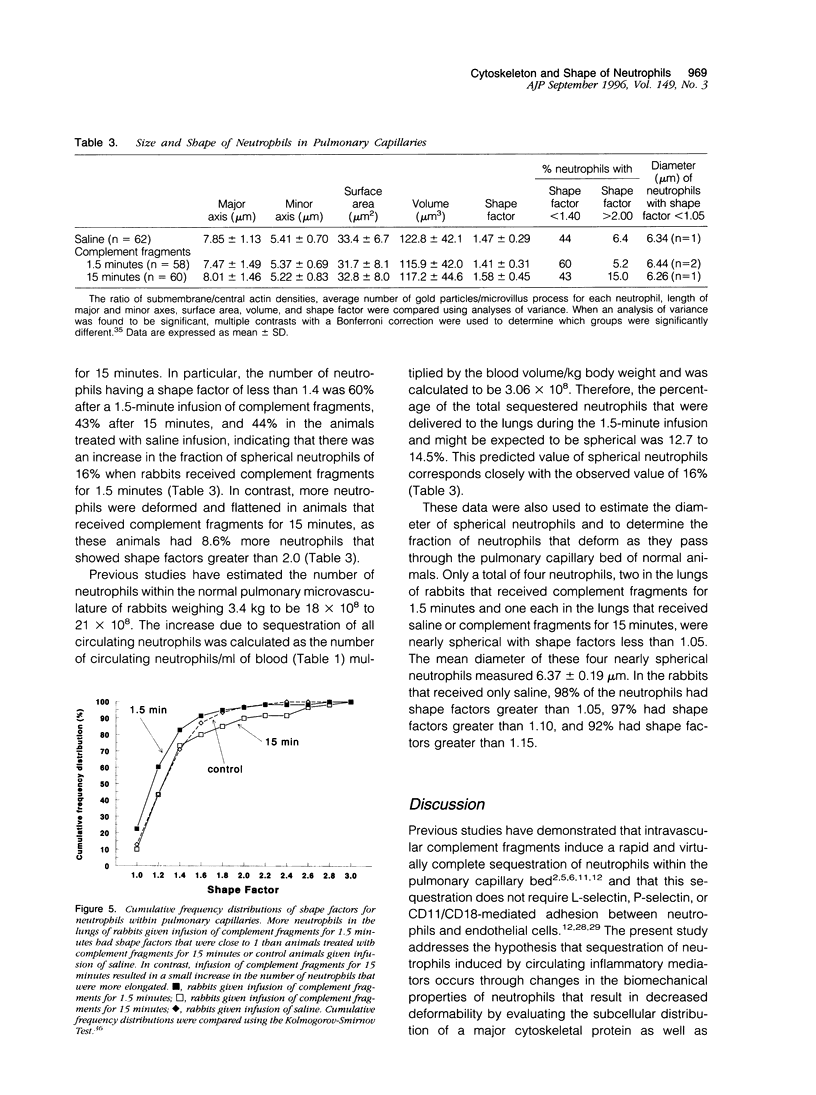




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertine K. H., Rosolia D. L., Schuhl R. A., Peters S. P., Gee M. H. Physical and cytochemical properties of neutrophils activated in situ in the lung during ZAP infusion in sheep. J Appl Physiol (1985) 1993 Mar;74(3):1361–1373. doi: 10.1152/jappl.1993.74.3.1361. [DOI] [PubMed] [Google Scholar]
- Doerschuk C. M., Allard M. F., Hogg J. C. Neutrophil kinetics in rabbits during infusion of zymosan-activated plasma. J Appl Physiol (1985) 1989 Jul;67(1):88–95. doi: 10.1152/jappl.1989.67.1.88. [DOI] [PubMed] [Google Scholar]
- Doerschuk C. M., Allard M. F., Martin B. A., MacKenzie A., Autor A. P., Hogg J. C. Marginated pool of neutrophils in rabbit lungs. J Appl Physiol (1985) 1987 Nov;63(5):1806–1815. doi: 10.1152/jappl.1987.63.5.1806. [DOI] [PubMed] [Google Scholar]
- Doerschuk C. M., Beyers N., Coxson H. O., Wiggs B., Hogg J. C. Comparison of neutrophil and capillary diameters and their relation to neutrophil sequestration in the lung. J Appl Physiol (1985) 1993 Jun;74(6):3040–3045. doi: 10.1152/jappl.1993.74.6.3040. [DOI] [PubMed] [Google Scholar]
- Doerschuk C. M. The role of CD18-mediated adhesion in neutrophil sequestration induced by infusion of activated plasma in rabbits. Am J Respir Cell Mol Biol. 1992 Aug;7(2):140–148. doi: 10.1165/ajrcmb/7.2.140. [DOI] [PubMed] [Google Scholar]
- Downey G. P., Worthen G. S. Neutrophil retention in model capillaries: deformability, geometry, and hydrodynamic forces. J Appl Physiol (1985) 1988 Oct;65(4):1861–1871. doi: 10.1152/jappl.1988.65.4.1861. [DOI] [PubMed] [Google Scholar]
- Erzurum S. C., Downey G. P., Doherty D. E., Schwab B., 3rd, Elson E. L., Worthen G. S. Mechanisms of lipopolysaccharide-induced neutrophil retention. Relative contributions of adhesive and cellular mechanical properties. J Immunol. 1992 Jul 1;149(1):154–162. [PubMed] [Google Scholar]
- Frank R. S. Time-dependent alterations in the deformability of human neutrophils in response to chemotactic activation. Blood. 1990 Dec 15;76(12):2606–2612. [PubMed] [Google Scholar]
- Gebb S. A., Graham J. A., Hanger C. C., Godbey P. S., Capen R. L., Doerschuk C. M., Wagner W. W., Jr Sites of leukocyte sequestration in the pulmonary microcirculation. J Appl Physiol (1985) 1995 Aug;79(2):493–497. doi: 10.1152/jappl.1995.79.2.493. [DOI] [PubMed] [Google Scholar]
- Hogg J. C. Neutrophil kinetics and lung injury. Physiol Rev. 1987 Oct;67(4):1249–1295. doi: 10.1152/physrev.1987.67.4.1249. [DOI] [PubMed] [Google Scholar]
- Howard T. H., Oresajo C. O. The kinetics of chemotactic peptide-induced change in F-actin content, F-actin distribution, and the shape of neutrophils. J Cell Biol. 1985 Sep;101(3):1078–1085. doi: 10.1083/jcb.101.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inano H., English D., Doerschuk C. M. Effect of zymosan-activated plasma on the deformability of rabbit polymorphonuclear leukocytes. J Appl Physiol (1985) 1992 Oct;73(4):1370–1376. doi: 10.1152/jappl.1992.73.4.1370. [DOI] [PubMed] [Google Scholar]
- Kawaoka E. J., Miller M. E., Cheung A. T. Chemotactic factor-induced effects upon deformability of human polymorphonuclear leukocytes. J Clin Immunol. 1981 Jan;1(1):41–44. doi: 10.1007/BF00915475. [DOI] [PubMed] [Google Scholar]
- Lessard J. L. Two monoclonal antibodies to actin: one muscle selective and one generally reactive. Cell Motil Cytoskeleton. 1988;10(3):349–362. doi: 10.1002/cm.970100302. [DOI] [PubMed] [Google Scholar]
- Lien D. C., Henson P. M., Capen R. L., Henson J. E., Hanson W. L., Wagner W. W., Jr, Worthen G. S. Neutrophil kinetics in the pulmonary microcirculation during acute inflammation. Lab Invest. 1991 Aug;65(2):145–159. [PubMed] [Google Scholar]
- Lundberg C., Wright S. D. Relation of the CD11/CD18 family of leukocyte antigens to the transient neutropenia caused by chemoattractants. Blood. 1990 Sep 15;76(6):1240–1245. [PubMed] [Google Scholar]
- Mulligan M. S., Varani J., Warren J. S., Till G. O., Smith C. W., Anderson D. C., Todd R. F., 3rd, Ward P. A. Roles of beta 2 integrins of rat neutrophils in complement- and oxygen radical-mediated acute inflammatory injury. J Immunol. 1992 Mar 15;148(6):1847–1857. [PubMed] [Google Scholar]
- Nash G. B., Jones J. G., Mikita J., Christopher B., Dormandy J. A. Effects of preparative procedures and of cell activation on flow of white cells through micropore filters. Br J Haematol. 1988 Oct;70(2):171–176. doi: 10.1111/j.1365-2141.1988.tb02459.x. [DOI] [PubMed] [Google Scholar]
- Packman C. H., Lichtman M. A. Activation of neutrophils: measurement of actin conformational changes by flow cytometry. Blood Cells. 1990;16(1):193–207. [PubMed] [Google Scholar]
- Pécsvárady Z., Fisher T. C., Fabók A., Coates T. D., Meiselman H. J. Kinetics of granulocyte deformability following exposure to chemotactic stimuli. Blood Cells. 1992;18(2):333–358. [PubMed] [Google Scholar]
- Roberts P. J., Yong K. L., Khwaja A., Johnson B. V., Pizzey A. R., Carver J. E., Addison I. E., Linch D. C. Pentoxifylline at clinically achievable levels inhibits FMLP-induced neutrophil responses, but not priming, upregulation of cell-adhesion molecules, or migration induced by GM-CSF. Eur J Haematol. 1993 Jan;50(1):1–10. doi: 10.1111/j.1600-0609.1993.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein G. W., Shih Y. Y., Chien S. Morphometry of human leukocytes. Blood. 1980 Nov;56(5):866–875. [PubMed] [Google Scholar]
- Selby C., MacNee W. Factors affecting neutrophil transit during acute pulmonary inflammation: minireview. Exp Lung Res. 1993 Jul-Aug;19(4):407–428. doi: 10.3109/01902149309064355. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till G. O., Johnson K. J., Kunkel R., Ward P. A. Intravascular activation of complement and acute lung injury. Dependency on neutrophils and toxic oxygen metabolites. J Clin Invest. 1982 May;69(5):1126–1135. doi: 10.1172/JCI110548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonnesen M. G., Smedly L. A., Henson P. M. Neutrophil-endothelial cell interactions. Modulation of neutrophil adhesiveness induced by complement fragments C5a and C5a des arg and formyl-methionyl-leucyl-phenylalanine in vitro. J Clin Invest. 1984 Nov;74(5):1581–1592. doi: 10.1172/JCI111574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace P. J., Wersto R. P., Packman C. H., Lichtman M. A. Chemotactic peptide-induced changes in neutrophil actin conformation. J Cell Biol. 1984 Sep;99(3):1060–1065. doi: 10.1083/jcb.99.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlin W. F., Kiely J. M., Gimbrone M. A., Jr Interleukin-8 induces changes in human neutrophil actin conformation and distribution: relationship to inhibition of adhesion to cytokine-activated endothelium. J Leukoc Biol. 1992 Jul;52(1):43–51. doi: 10.1002/jlb.52.1.43. [DOI] [PubMed] [Google Scholar]
- Wiggs B. R., English D., Quinlan W. M., Doyle N. A., Hogg J. C., Doerschuk C. M. Contributions of capillary pathway size and neutrophil deformability to neutrophil transit through rabbit lungs. J Appl Physiol (1985) 1994 Jul;77(1):463–470. doi: 10.1152/jappl.1994.77.1.463. [DOI] [PubMed] [Google Scholar]
- Windsor A. C., Mullen P. G., Fowler A. A., Sugerman H. J. Role of the neutrophil in adult respiratory distress syndrome. Br J Surg. 1993 Jan;80(1):10–17. doi: 10.1002/bjs.1800800106. [DOI] [PubMed] [Google Scholar]
- Wortel C. H., Doerschuk C. M. Neutrophils and neutrophil-endothelial cell adhesion in adult respiratory distress syndrome. New Horiz. 1993 Nov;1(4):631–637. [PubMed] [Google Scholar]
- Worthen G. S., Schwab B., 3rd, Elson E. L., Downey G. P. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989 Jul 14;245(4914):183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]