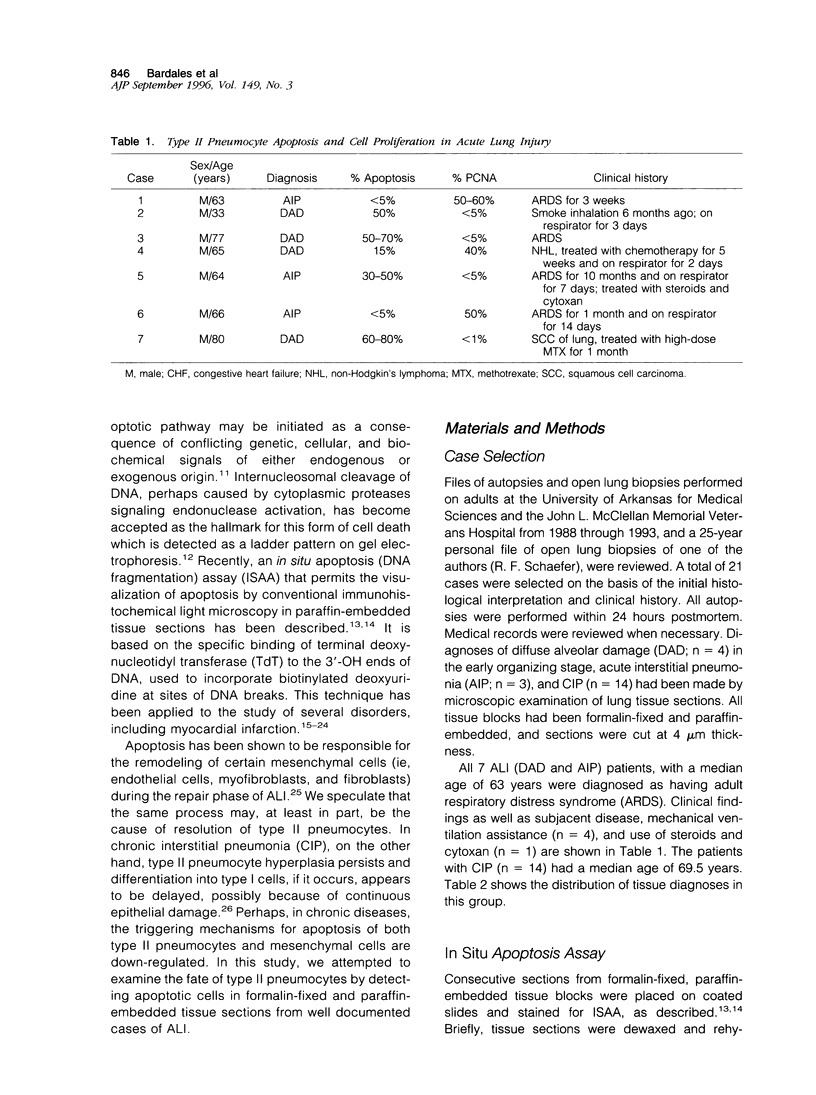
Abstract
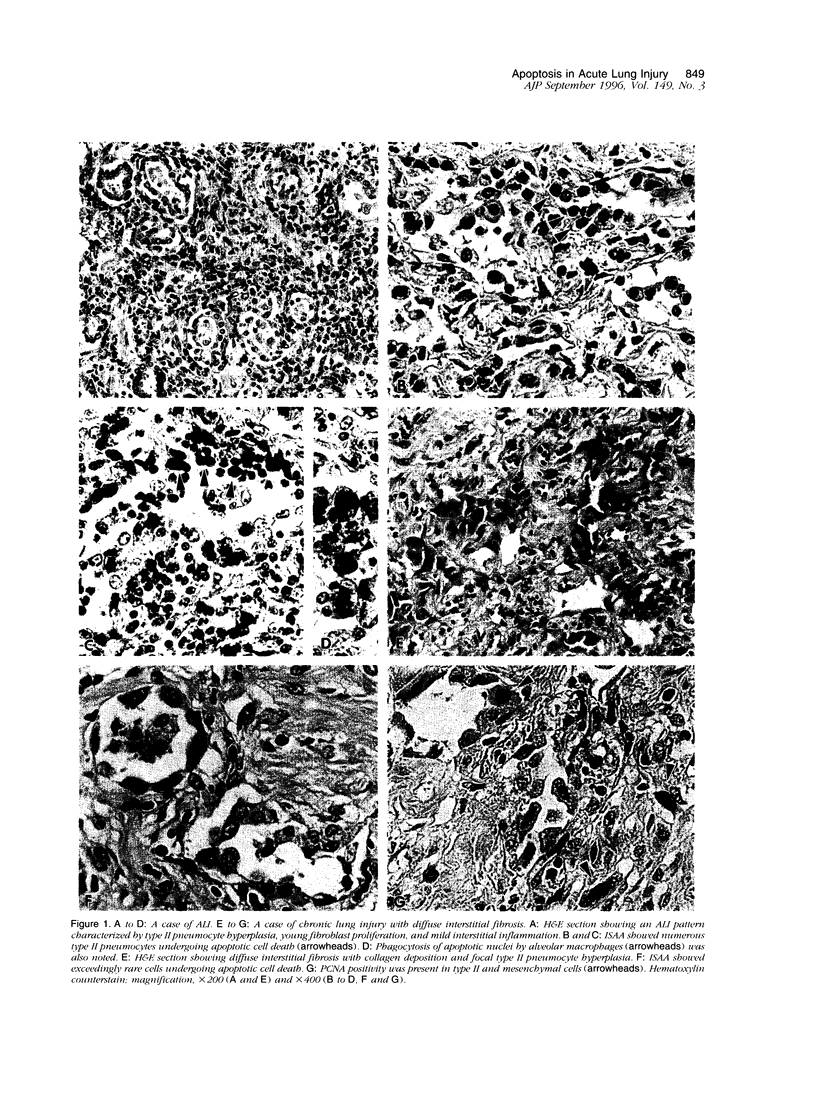
Proliferation of type II pneumocytes has been linked to a repair process during the early phase of acute lung injury, and it persists for a variable period. The mechanisms responsible for their dissolution and/or disappearance are not known, but we speculate that it may be partly due to apoptosis. Sections of lung tissue from patients with acute lung injury (n = 7) and chronic interstitial pneumonia (n = 14) were stained for detection of apoptotic cells via specific labeling of nuclear DNA fragmentation. Results were correlated with those of proliferating cell nuclear antigen (PCNA) staining for cell proliferation. Marked apoptosis of CD68-negative type II pneumocytes (30 to 80%) was detected in four of the seven (57%) cases of acute lung injury. In these cases, representing the resolution phase of acute lung injury, PCNA positivity in pneumocytes was extremely rare. In the 3 other cases in the acute/proliferative phase, apoptotic type II pneumocytes were rare whereas PCNA expression was quite evident in these cells. In chronic interstitial pneumonia, only rare type II pneumocytes (< 5%) exhibited apoptosis, and they showed variable staining for PCNA (up to 70%). We conclude that proliferation of type II pneumocytes occurs during the early phase of acute lung injury and is of variable extent and duration. In the resolution phase of acute lung injury, extensive apoptosis of type II pneumocytes is largely responsible for the disappearance of these cells. The time frame within which the apoptotic response occurs is variable and is likely to be dependent upon the specific etiology and extent of the injury. In chronic interstitial pneumonia, type II pneumocytes proliferate continuously, although to a much lesser degree than in the early phase of acute lung injury, and are minimally apoptotic.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson I. Y., Bowden D. H. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Lab Invest. 1974 Jan;30(1):35–42. [PubMed] [Google Scholar]
- Ansari B., Coates P. J., Greenstein B. D., Hall P. A. In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol. 1993 May;170(1):1–8. doi: 10.1002/path.1711700102. [DOI] [PubMed] [Google Scholar]
- Bardales R. H., Hailey L. S., Xie S. S., Schaefer R. F., Hsu S. M. In situ apoptosis assay for the detection of early acute myocardial infarction. Am J Pathol. 1996 Sep;149(3):821–829. [PMC free article] [PubMed] [Google Scholar]
- Brody J. S., Burki R., Kaplan N. Deoxyribonucleic acid synthesis in lung cells during compensatory lung growth after pneumonectomy. Am Rev Respir Dis. 1978 Feb;117(2):307–316. doi: 10.1164/arrd.1978.117.2.307. [DOI] [PubMed] [Google Scholar]
- Buch S., Han R. N., Liu J., Moore A., Edelson J. D., Freeman B. A., Post M., Tanswell A. K. Basic fibroblast growth factor and growth factor receptor gene expression in 85% O2-exposed rat lung. Am J Physiol. 1995 Mar;268(3 Pt 1):L455–L464. doi: 10.1152/ajplung.1995.268.3.L455. [DOI] [PubMed] [Google Scholar]
- Chess P. R., Ryan R. M., Finkelstein J. N. Tyrosine kinase activity is necessary for growth factor-stimulated rabbit type II pneumocyte proliferation. Pediatr Res. 1994 Oct;36(4):481–486. doi: 10.1203/00006450-199410000-00012. [DOI] [PubMed] [Google Scholar]
- Desmoulière A., Redard M., Darby I., Gabbiani G. Apoptosis mediates the decrease in cellularity during the transition between granulation tissue and scar. Am J Pathol. 1995 Jan;146(1):56–66. [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973 Feb;70(2):175–198. [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Cabral L. J., Stephens R. J., Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Exp Mol Pathol. 1975 Feb;22(1):142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Fukasawa Y., Ishikura H., Takada A., Yokoyama S., Imamura M., Yoshiki T., Sato H. Massive apoptosis in infantile myofibromatosis. A putative mechanism of tumor regression. Am J Pathol. 1994 Mar;144(3):480–485. [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Itoh G., Tamura J., Suzuki M., Suzuki Y., Ikeda H., Koike M., Nomura M., Jie T., Ito K. DNA fragmentation of human infarcted myocardial cells demonstrated by the nick end labeling method and DNA agarose gel electrophoresis. Am J Pathol. 1995 Jun;146(6):1325–1331. [PMC free article] [PubMed] [Google Scholar]
- James T. N. Normal and abnormal consequences of apoptosis in the human heart. From postnatal morphogenesis to paroxysmal arrhythmias. Circulation. 1994 Jul;90(1):556–573. [PubMed] [Google Scholar]
- James T. N., Terasaki F., Pavlovich E. R., Vikhert A. M. Apoptosis and pleomorphic micromitochondriosis in the sinus nodes surgically excised from five patients with the long QT syndrome. J Lab Clin Med. 1993 Sep;122(3):309–323. [PubMed] [Google Scholar]
- Kasagi N., Gomyo Y., Shirai H., Tsujitani S., Ito H. Apoptotic cell death in human gastric carcinoma: analysis by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling. Jpn J Cancer Res. 1994 Sep;85(9):939–945. doi: 10.1111/j.1349-7006.1994.tb02972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawano H., Okada R., Kawano Y., Sueyoshi N., Shirai T. Apoptosis in acute and chronic myocarditis. Jpn Heart J. 1994 Nov;35(6):745–750. doi: 10.1536/ihj.35.745. [DOI] [PubMed] [Google Scholar]
- Kressel M., Groscurth P. Distinction of apoptotic and necrotic cell death by in situ labelling of fragmented DNA. Cell Tissue Res. 1994 Dec;278(3):549–556. doi: 10.1007/BF00331373. [DOI] [PubMed] [Google Scholar]
- Lwebuga-Mukasa J. S. Matrix-driven pneumocyte differentiation. Am Rev Respir Dis. 1991 Aug;144(2):452–457. doi: 10.1164/ajrccm/144.2.452. [DOI] [PubMed] [Google Scholar]
- Majno G., Joris I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am J Pathol. 1995 Jan;146(1):3–15. [PMC free article] [PubMed] [Google Scholar]
- Nash J. R., McLaughlin P. J., Butcher D., Corrin B. Expression of tumour necrosis factor-alpha in cryptogenic fibrosing alveolitis. Histopathology. 1993 Apr;22(4):343–347. doi: 10.1111/j.1365-2559.1993.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Nash J. R., McLaughlin P. J., Hoyle C., Roberts D. Immunolocalization of tumour necrosis factor alpha in lung tissue from patients dying with adult respiratory distress syndrome. Histopathology. 1991 Nov;19(5):395–402. doi: 10.1111/j.1365-2559.1991.tb00228.x. [DOI] [PubMed] [Google Scholar]
- Panos R. J., Rubin J. S., Csaky K. G., Aaronson S. A., Mason R. J. Keratinocyte growth factor and hepatocyte growth factor/scatter factor are heparin-binding growth factors for alveolar type II cells in fibroblast-conditioned medium. J Clin Invest. 1993 Aug;92(2):969–977. doi: 10.1172/JCI116673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polunovsky V. A., Chen B., Henke C., Snover D., Wendt C., Ingbar D. H., Bitterman P. B. Role of mesenchymal cell death in lung remodeling after injury. J Clin Invest. 1993 Jul;92(1):388–397. doi: 10.1172/JCI116578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulford K. A., Rigney E. M., Micklem K. J., Jones M., Stross W. P., Gatter K. C., Mason D. Y. KP1: a new monoclonal antibody that detects a monocyte/macrophage associated antigen in routinely processed tissue sections. J Clin Pathol. 1989 Apr;42(4):414–421. doi: 10.1136/jcp.42.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaberg L., Nexø E., Buckley S., Luo W., Snead M. L., Warburton D. Epidermal growth factor transcription, translation, and signal transduction by rat type II pneumocytes in culture. Am J Respir Cell Mol Biol. 1992 Jan;6(1):44–49. doi: 10.1165/ajrcmb/6.1.44. [DOI] [PubMed] [Google Scholar]
- Ryan R. M., Mineo-Kuhn M. M., Kramer C. M., Finkelstein J. N. Growth factors alter neonatal type II alveolar epithelial cell proliferation. Am J Physiol. 1994 Jan;266(1 Pt 1):L17–L22. doi: 10.1152/ajplung.1994.266.1.L17. [DOI] [PubMed] [Google Scholar]
- Smith L. J. The effect of type 2 cell mitosis on the surfactant system of injured mouse lungs. J Lab Clin Med. 1983 Sep;102(3):434–443. [PubMed] [Google Scholar]
- Stahlman M. T., Orth D. N., Gray M. E. Immunocytochemical localization of epidermal growth factor in the developing human respiratory system and in acute and chronic lung disease in the neonate. Lab Invest. 1989 Apr;60(4):539–547. [PubMed] [Google Scholar]
- Stewart B. W. Mechanisms of apoptosis: integration of genetic, biochemical, and cellular indicators. J Natl Cancer Inst. 1994 Sep 7;86(17):1286–1296. doi: 10.1093/jnci/86.17.1286. [DOI] [PubMed] [Google Scholar]
- Ulich T. R., Yi E. S., Longmuir K., Yin S., Biltz R., Morris C. F., Housley R. M., Pierce G. F. Keratinocyte growth factor is a growth factor for type II pneumocytes in vivo. J Clin Invest. 1994 Mar;93(3):1298–1306. doi: 10.1172/JCI117086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman J. H., Jonker R. R., Keijzer R., van de Velde C. J., Cornelisse C. J., van Dierendonck J. H. A new method to detect apoptosis in paraffin sections: in situ end-labeling of fragmented DNA. J Histochem Cytochem. 1993 Jan;41(1):7–12. doi: 10.1177/41.1.7678025. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Morris R. G., Smith A. L., Dunlop D. Chromatin cleavage in apoptosis: association with condensed chromatin morphology and dependence on macromolecular synthesis. J Pathol. 1984 Jan;142(1):67–77. doi: 10.1002/path.1711420112. [DOI] [PubMed] [Google Scholar]




