Abstract
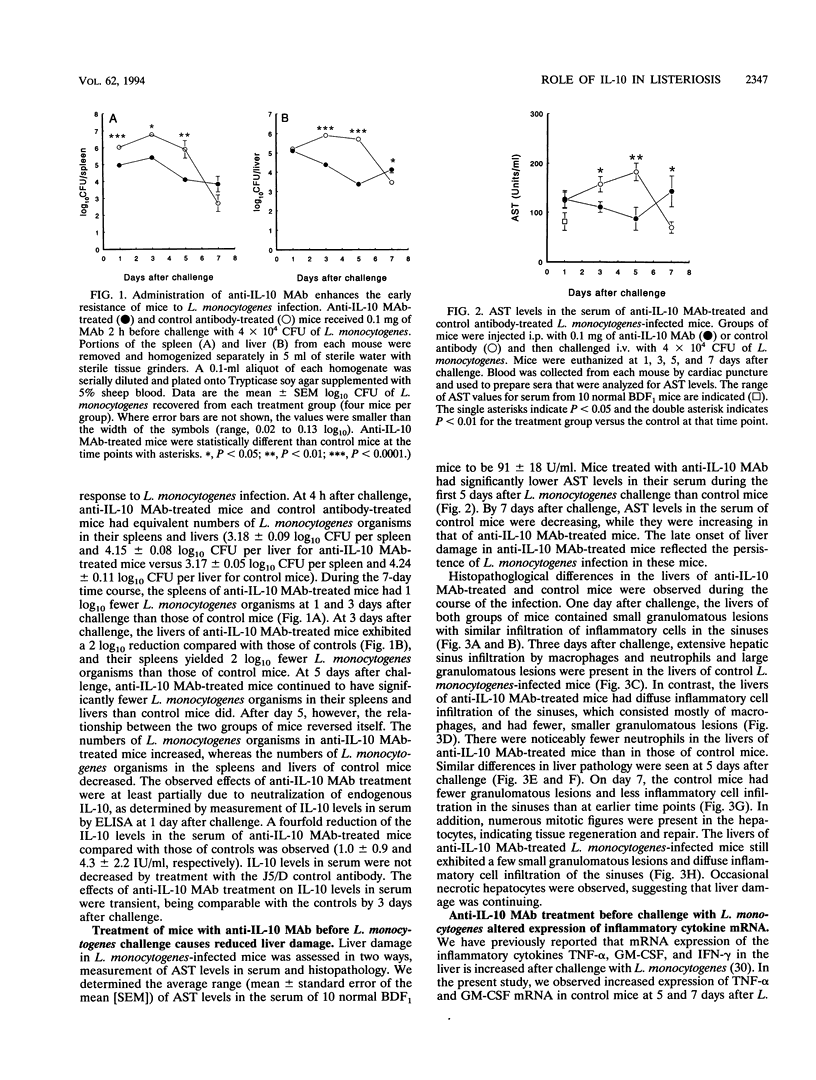
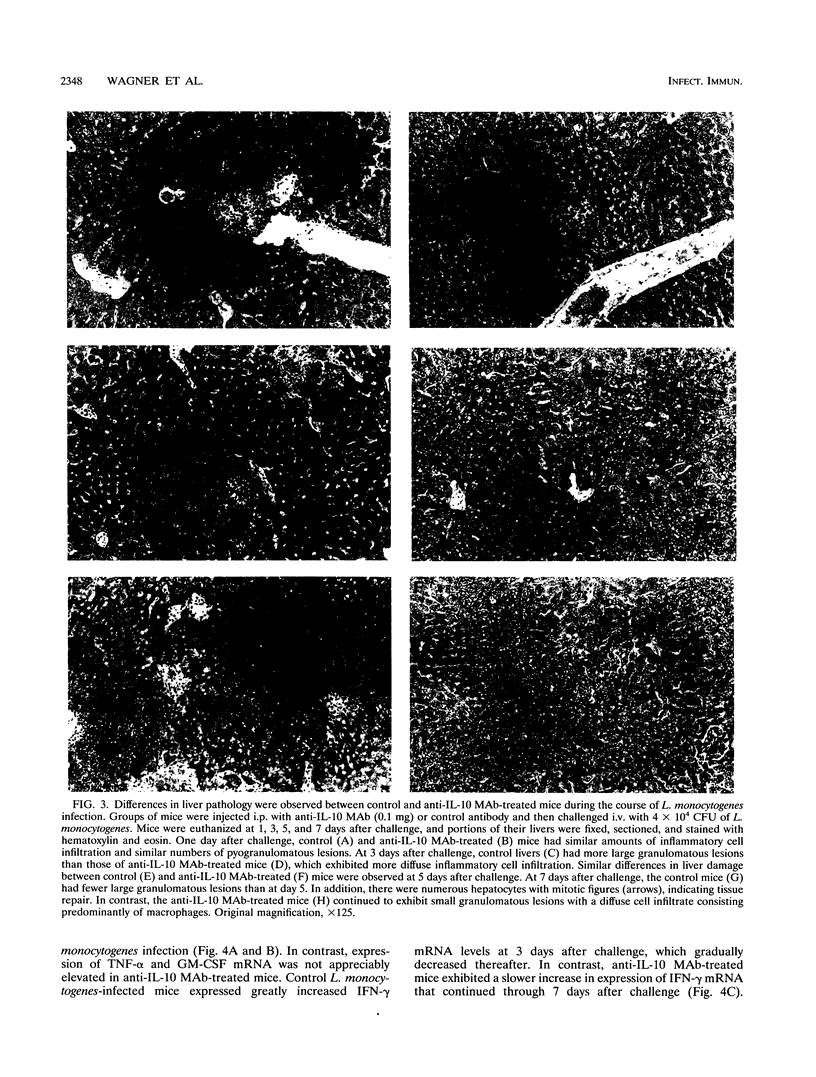
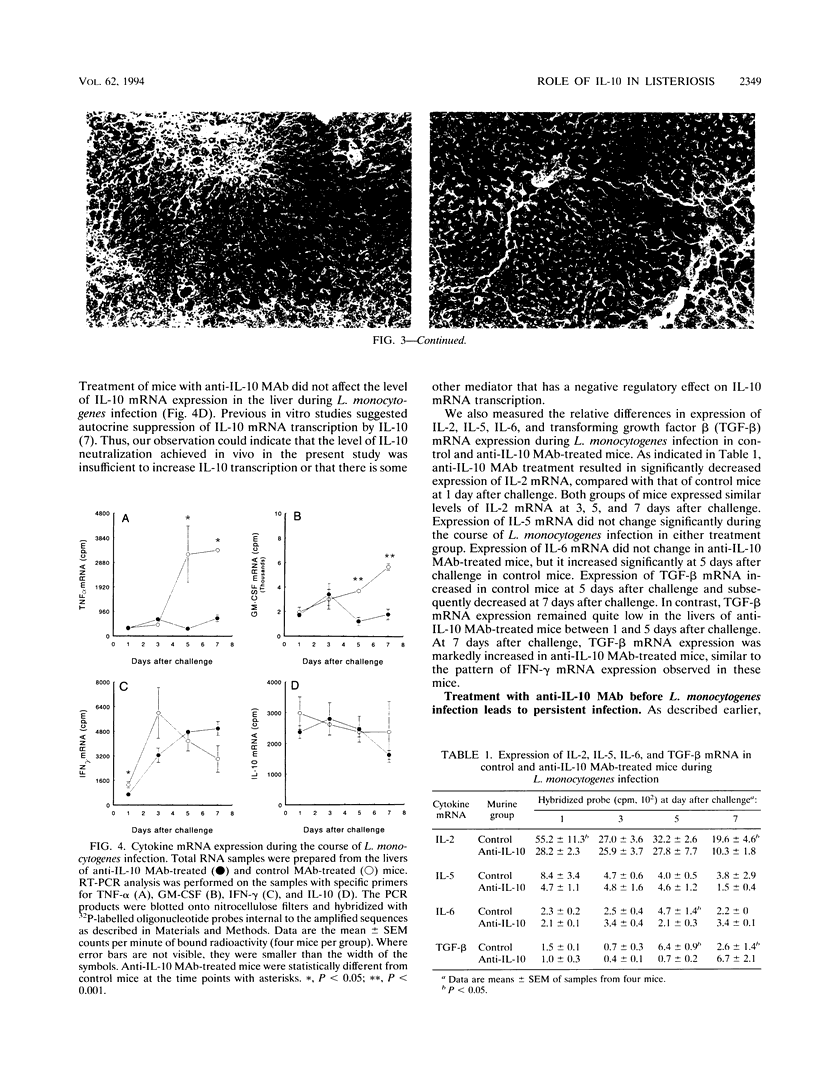
Mice that received an anti-interleukin-10 (anti-IL-10) neutralizing monoclonal antibody (MAb) (SXC-1) prior to infection with Listeria monocytogenes initially demonstrated resistance to the infection, as indicated by reduced recovery of L. monocytogenes from their spleens and livers during the first 5 days after challenge. Anti-IL-10 MAb-treated mice then demonstrated reduced resistance during the later stage of infection, as indicated by persistent infection with L. monocytogenes in their livers 11 days after challenge. Aspartate aminotransferase (AST) levels (a measure of liver damage) in the sera of control mice increased between 1 and 5 days after challenge, while anti-IL-10 MAb-treated mice maintained lower AST levels. At 7 days after challenge, AST levels in the sera of control mice decreased as the numbers of organisms declined. In contrast, AST levels increased as the infections persisted in anti-IL-10 MAb-treated mice. The AST levels in serum reflected liver histopathology as anti-IL-10 MAb-treated mice exhibited fewer granulomatous lesions and less necrosis of liver tissue than the control mice during the first 5 days after challenge. Anti-IL-10 MAb treatment altered the expression of inflammatory cytokine mRNAs during L. monocytogenes infection. Control MAb-treated mice exhibited increased expression of tumor necrosis factor alpha and granulocyte-macrophage colony-stimulating factor mRNA in their lives during L. monocytogenes infection, but this increase did not occur in anti-IL-10 MAb-treated mice. Gamma interferon mRNA expression in the livers of the control MAb-treated mice was increased between 1 and 5 days after L. monocytogenes challenge and then decreased at 7 days after challenge. In contrast, gamma interferon mRNA expression in the livers of anti-IL-10 MAb-treated mice was not decreased until 7 days after challenge. These results indicate that endogenous IL-10 has both beneficial and detrimental effects on the host response to L. monocytogenes infection in mice.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bermudez L. E., Champsi J. Infection with Mycobacterium avium induces production of interleukin-10 (IL-10), and administration of anti-IL-10 antibody is associated with enhanced resistance to infection in mice. Infect Immun. 1993 Jul;61(7):3093–3097. doi: 10.1128/iai.61.7.3093-3097.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdan C., Vodovotz Y., Nathan C. Macrophage deactivation by interleukin 10. J Exp Med. 1991 Dec 1;174(6):1549–1555. doi: 10.1084/jem.174.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cano R. J., Torres M. J., Klem R. E., Palomares J. C. DNA hybridization assay using ATTOPHOS, a fluorescent substrate for alkaline phosphatase. Biotechniques. 1992 Feb;12(2):264–269. [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Early pathogenesis of infection in the liver with the facultative intracellular bacteria Listeria monocytogenes, Francisella tularensis, and Salmonella typhimurium involves lysis of infected hepatocytes by leukocytes. Infect Immun. 1992 Dec;60(12):5164–5171. doi: 10.1128/iai.60.12.5164-5171.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlan J. W., North R. J. Neutrophil-mediated dissolution of infected host cells as a defense strategy against a facultative intracellular bacterium. J Exp Med. 1991 Sep 1;174(3):741–744. doi: 10.1084/jem.174.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis M., Ghadirian E. IL-10 neutralization augments mouse resistance to systemic Mycobacterium avium infections. J Immunol. 1993 Nov 15;151(10):5425–5430. [PubMed] [Google Scholar]
- Ding L., Linsley P. S., Huang L. Y., Germain R. N., Shevach E. M. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993 Aug 1;151(3):1224–1234. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Mosmann T. R., Howard M., O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991 Dec 1;147(11):3815–3822. [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- Goossens P. L., Jouin H., Milon G. Dynamics of lymphocytes and inflammatory cells recruited in liver during murine listeriosis. A cytofluorimetric study. J Immunol. 1991 Nov 15;147(10):3514–3520. [PubMed] [Google Scholar]
- Haak-Frendscho M., Brown J. F., Iizawa Y., Wagner R. D., Czuprynski C. J. Administration of anti-IL-4 monoclonal antibody 11B11 increases the resistance of mice to Listeria monocytogenes infection. J Immunol. 1992 Jun 15;148(12):3978–3985. [PubMed] [Google Scholar]
- Havell E. A. Production of tumor necrosis factor during murine listeriosis. J Immunol. 1987 Dec 15;139(12):4225–4231. [PubMed] [Google Scholar]
- Hsieh C. S., Heimberger A. B., Gold J. S., O'Garra A., Murphy K. M. Differential regulation of T helper phenotype development by interleukins 4 and 10 in an alpha beta T-cell-receptor transgenic system. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6065–6069. doi: 10.1073/pnas.89.13.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida H., Hastings R., Kearney J., Howard M. Continuous anti-interleukin 10 antibody administration depletes mice of Ly-1 B cells but not conventional B cells. J Exp Med. 1992 May 1;175(5):1213–1220. doi: 10.1084/jem.175.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langermans J. A., van der Hulst M. E., Nibbering P. H., van der Meide P. H., van Furth R. Intravenous injection of interferon-gamma inhibits the proliferation of Listeria monocytogenes in the liver but not in the spleen and peritoneal cavity. Immunology. 1992 Nov;77(3):354–361. [PMC free article] [PubMed] [Google Scholar]
- Macatonia S. E., Doherty T. M., Knight S. C., O'Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993 May 1;150(9):3755–3765. [PubMed] [Google Scholar]
- Mosmann T. R., Schumacher J. H., Fiorentino D. F., Leverah J., Moore K. W., Bond M. W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J Immunol. 1990 Nov 1;145(9):2938–2945. [PubMed] [Google Scholar]
- Nakane A., Numata A., Minagawa T. Endogenous tumor necrosis factor, interleukin-6, and gamma interferon levels during Listeria monocytogenes infection in mice. Infect Immun. 1992 Feb;60(2):523–528. doi: 10.1128/iai.60.2.523-528.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Garra A., Stapleton G., Dhar V., Pearce M., Schumacher J., Rugo H., Barbis D., Stall A., Cupp J., Moore K. Production of cytokines by mouse B cells: B lymphomas and normal B cells produce interleukin 10. Int Immunol. 1990;2(9):821–832. doi: 10.1093/intimm/2.9.821. [DOI] [PubMed] [Google Scholar]
- Oswald I. P., Gazzinelli R. T., Sher A., James S. L. IL-10 synergizes with IL-4 and transforming growth factor-beta to inhibit macrophage cytotoxic activity. J Immunol. 1992 Jun 1;148(11):3578–3582. [PubMed] [Google Scholar]
- Ralph P., Nakoinz I., Sampson-Johannes A., Fong S., Lowe D., Min H. Y., Lin L. IL-10, T lymphocyte inhibitor of human blood cell production of IL-1 and tumor necrosis factor. J Immunol. 1992 Feb 1;148(3):808–814. [PubMed] [Google Scholar]
- Sieling P. A., Abrams J. S., Yamamura M., Salgame P., Bloom B. R., Rea T. H., Modlin R. L. Immunosuppressive roles for IL-10 and IL-4 in human infection. In vitro modulation of T cell responses in leprosy. J Immunol. 1993 Jun 15;150(12):5501–5510. [PubMed] [Google Scholar]
- Street N. E., Mosmann T. R. Functional diversity of T lymphocytes due to secretion of different cytokine patterns. FASEB J. 1991 Feb;5(2):171–177. doi: 10.1096/fasebj.5.2.1825981. [DOI] [PubMed] [Google Scholar]
- Taga K., Tosato G. IL-10 inhibits human T cell proliferation and IL-2 production. J Immunol. 1992 Feb 15;148(4):1143–1148. [PubMed] [Google Scholar]
- Tripp C. S., Wolf S. F., Unanue E. R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R. D., Czuprynski C. J. Cytokine mRNA expression in livers of mice infected with Listeria monocytogenes. J Leukoc Biol. 1993 May;53(5):525–531. doi: 10.1002/jlb.53.5.525. [DOI] [PubMed] [Google Scholar]
- Wogensen L., Huang X., Sarvetnick N. Leukocyte extravasation into the pancreatic tissue in transgenic mice expressing interleukin 10 in the islets of Langerhans. J Exp Med. 1993 Jul 1;178(1):175–185. doi: 10.1084/jem.178.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., Roncarolo M. G., Spits H., de Vries J. E. Interleukin-10. Curr Opin Immunol. 1992 Jun;4(3):314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]