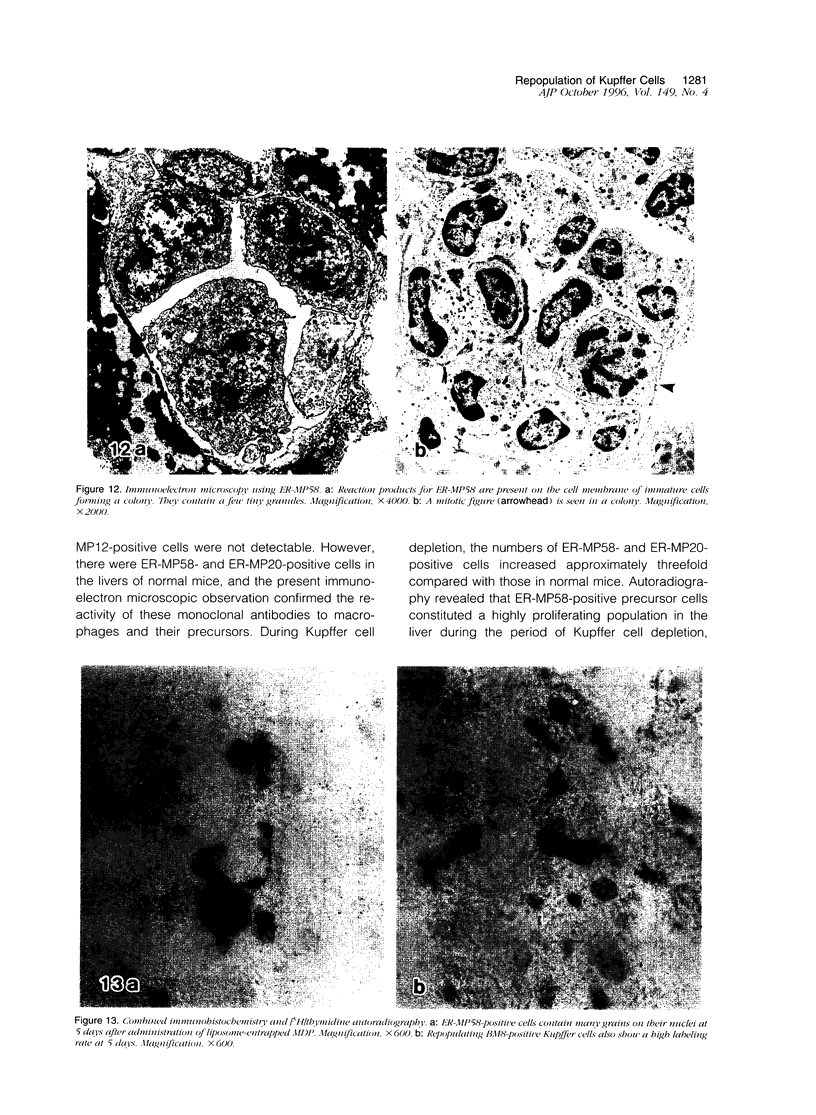
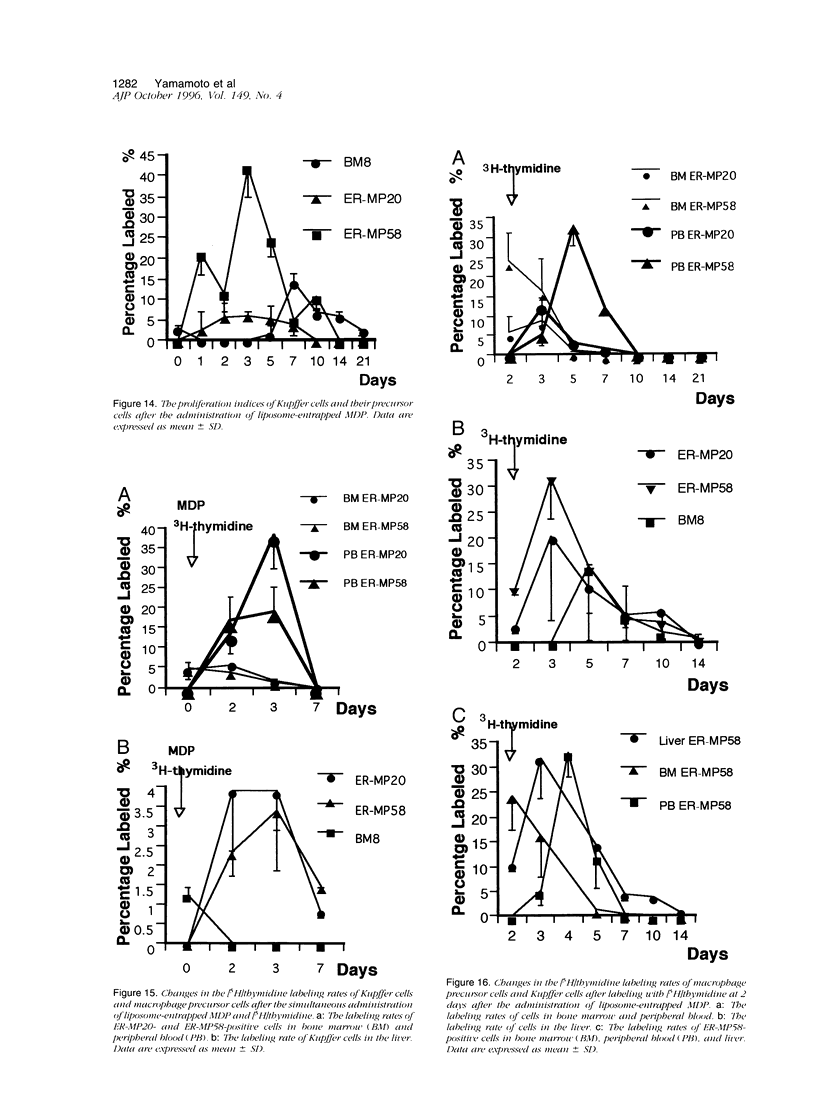
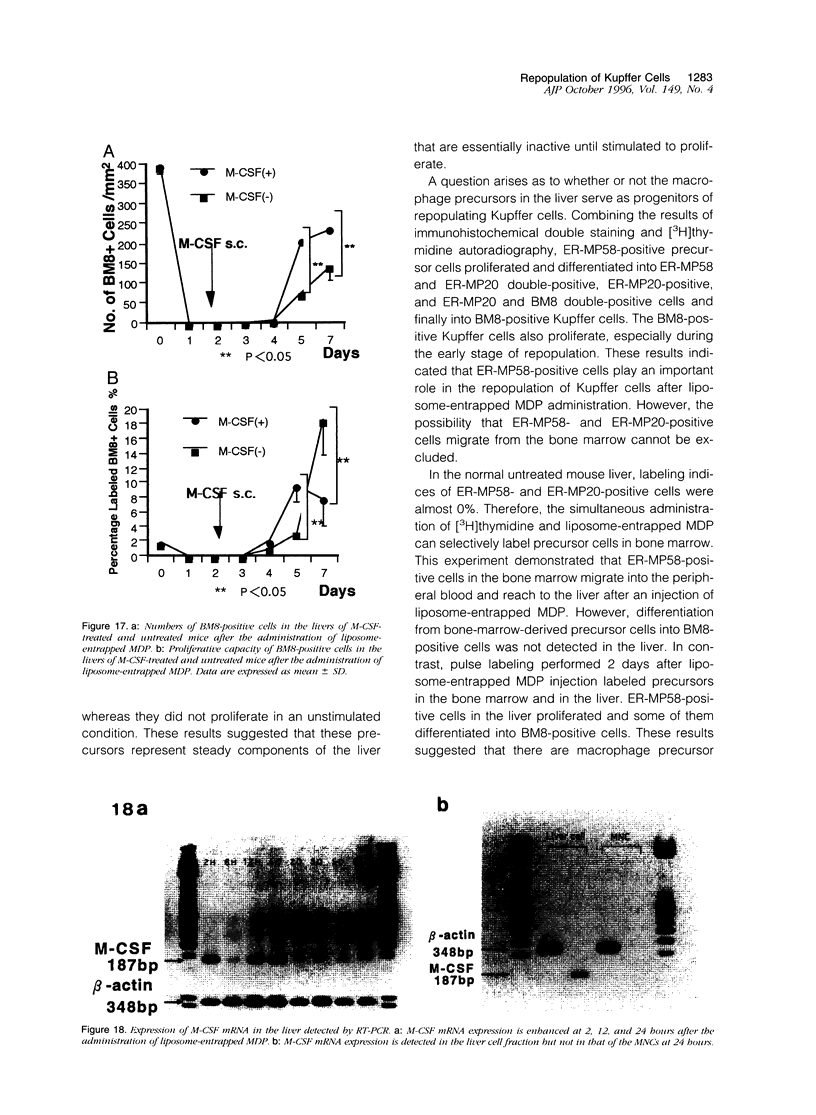
Abstract
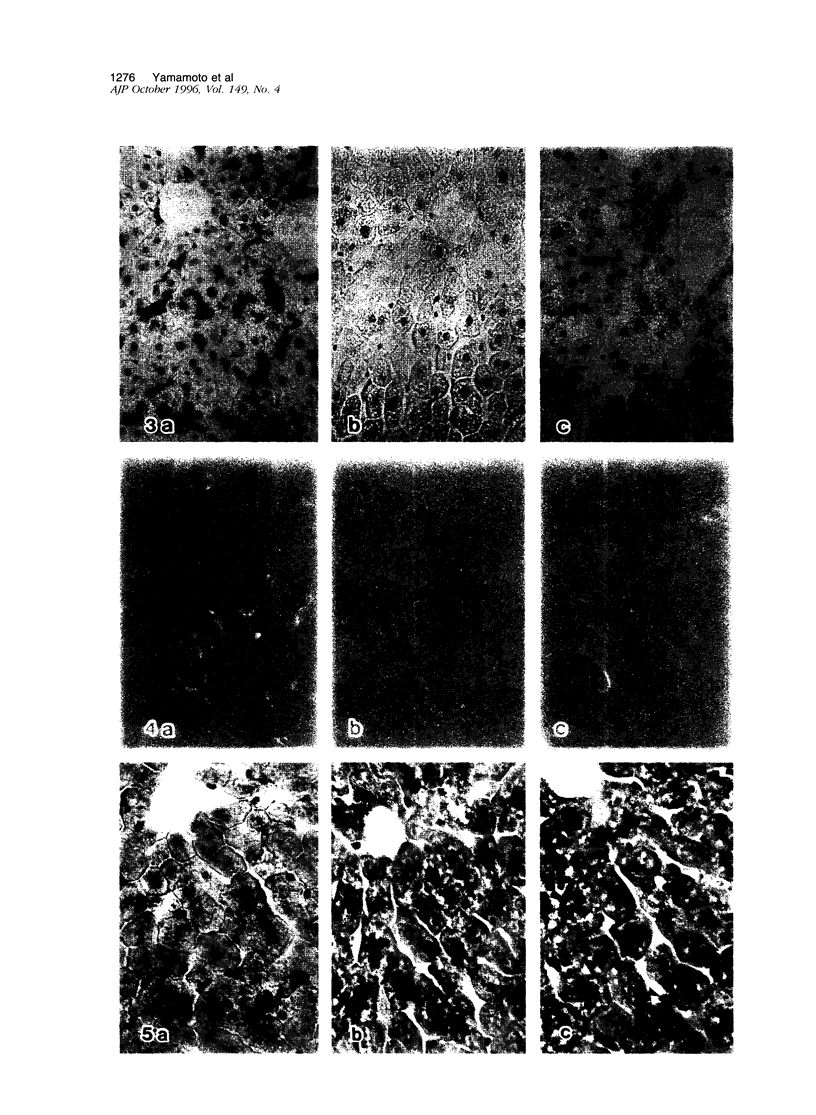
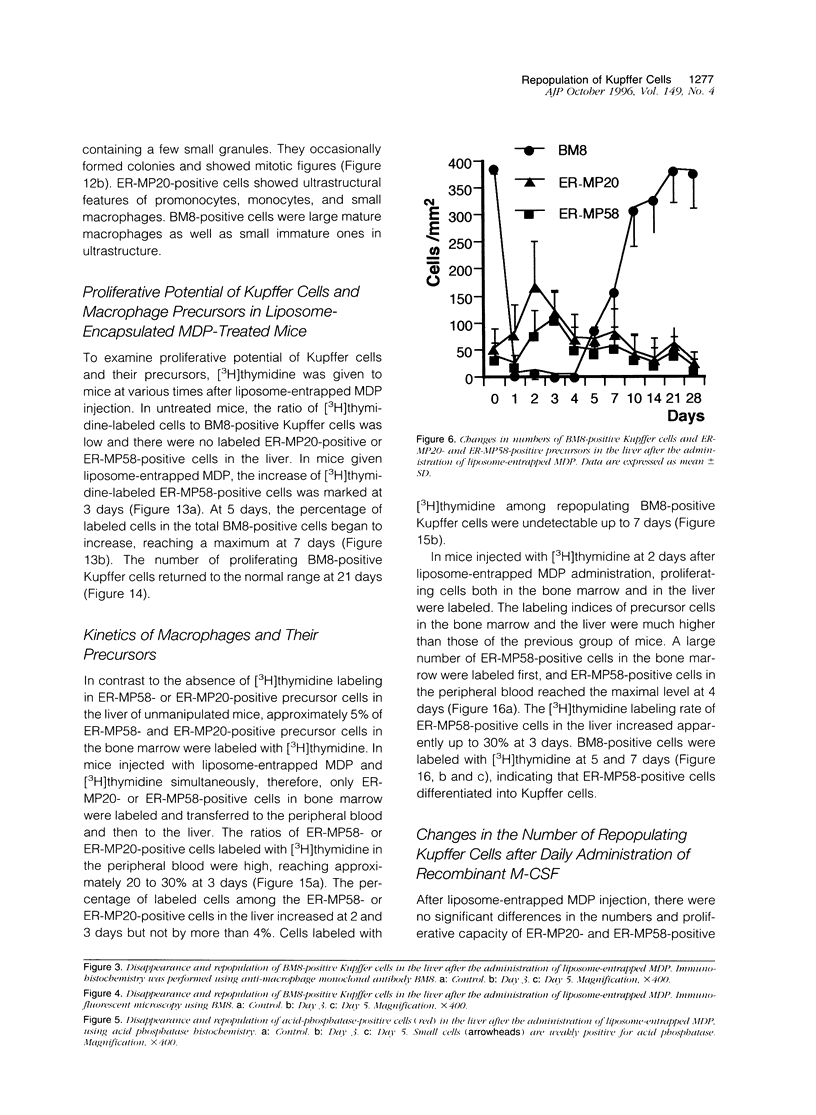
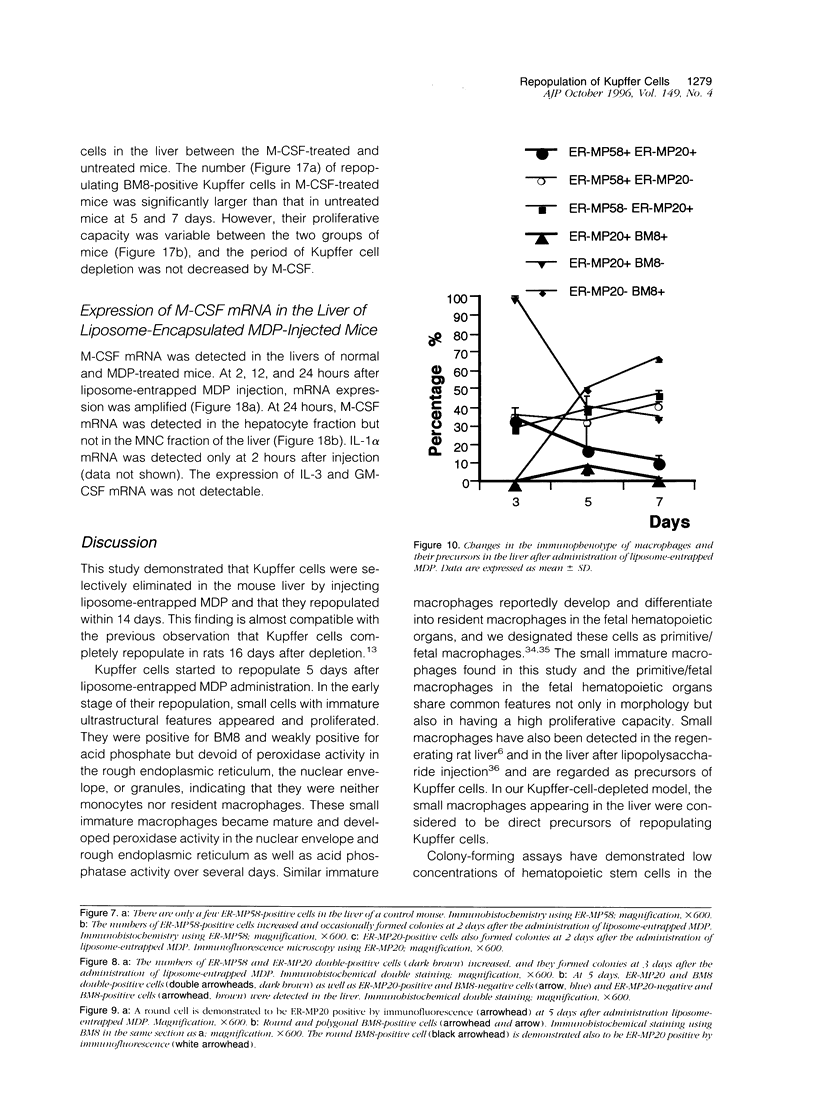
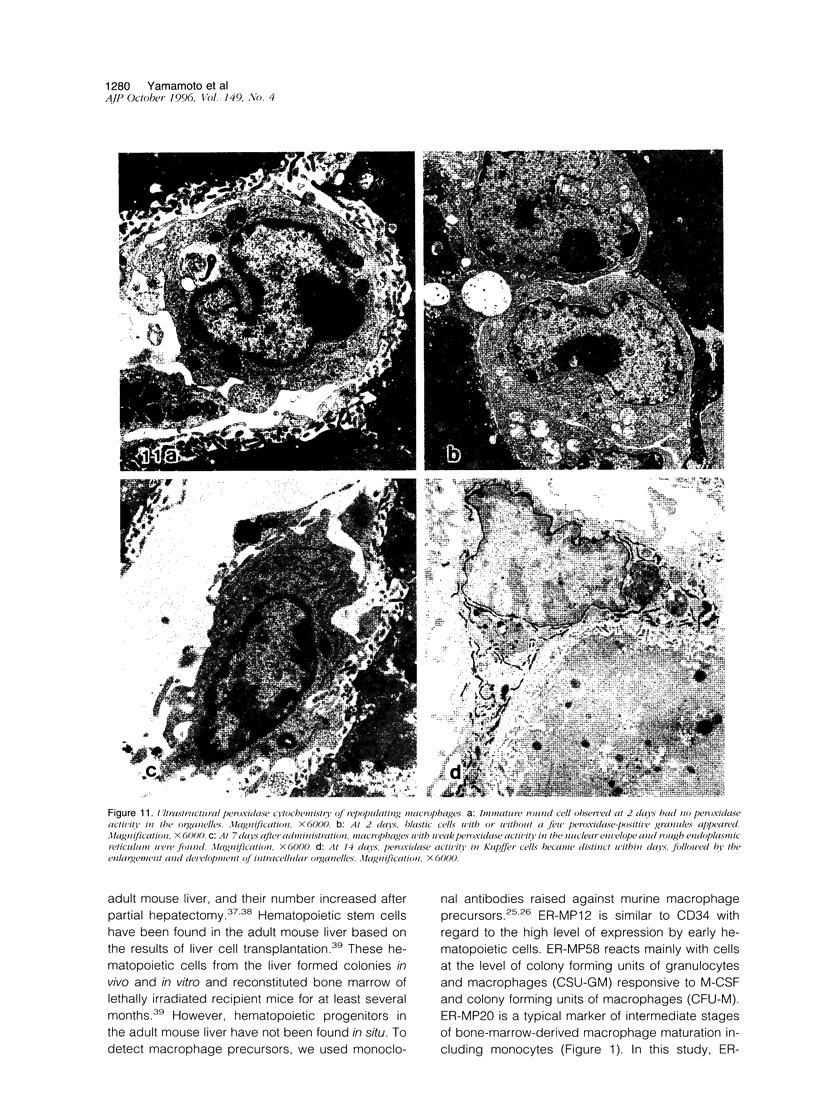
Kupffer cells were selectively eliminated in mice by the intravenous administration of liposome-entrapped dichloromethylene diphosphonate. At 5 days, small peroxidase-negative and acid-phosphatase-weakly-positive macrophages appeared, increased in number, and differentiated into peroxidase- and acid-phosphatase-positive Kupffer cells. Repopulating small macrophages actively proliferated, and the number of Kupffer cells returned to the normal level by day 14. The numbers of macrophage precursors in the liver as detected by the monoclonal antibodies ER-MP20 and ER-MP58 increased after liposome-entrapped dichloromethylene diphosphonate injection. ER-MP58-positive cells proliferated and differentiated into ER-MP20-positive cells and eventually into BM8-positive Kupffer cells in the liver. Bone-marrow-derived ER-MP58-positive cells were also detectable in the liver and differentiated into ER-MP20-positive cells, but they did not become BM8-positive macrophages. Macrophage colony-stimulating factor mRNA expression was enhanced in the liver 1 day after injection. The administration of macrophage colony-stimulating factor did not shorten the period of Kupffer cell depletion but increased the number and the proliferative capacity of repopulating Kupffer cells. These findings implied that repopulating Kupffer cells are derived from a macrophage precursor pool in the liver rather than from bone-marrow-derived monocytes. Local production of macrophage colony-stimulating factor in the liver plays a crucial role in the differentiation, maturation, and proliferation of Kupffer cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azoulay M., Webb C. G., Sachs L. Control of hematopoietic cell growth regulators during mouse fetal development. Mol Cell Biol. 1987 Sep;7(9):3361–3364. doi: 10.1128/mcb.7.9.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwens L., Wisse E. Proliferation, kinetics, and fate of monocytes in rat liver during a zymosan-induced inflammation. J Leukoc Biol. 1985 May;37(5):531–543. doi: 10.1002/jlb.37.5.531. [DOI] [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. Hepatic granulomas induced by glucan. An ultrastructural and peroxidase-cytochemical study. Lab Invest. 1980 Aug;43(2):172–181. [PubMed] [Google Scholar]
- Deimann W., Fahimi H. D. Induction of focal hemopoiesis in adult rat liver by glucan, a macrophage activator. A cytochemical and ultrastructural study. Lab Invest. 1980 Feb;42(2):217–224. [PubMed] [Google Scholar]
- Delemarre F. G., Kors N., Kraal G., van Rooijen N. Repopulation of macrophages in popliteal lymph nodes of mice after liposome-mediated depletion. J Leukoc Biol. 1990 Mar;47(3):251–257. doi: 10.1002/jlb.47.3.251. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Higashi K., Naito M., Takeya M., Ando M., Araki S., Takahashi K. Ontogenetic development, differentiation, and phenotypic expression of macrophages in fetal rat lungs. J Leukoc Biol. 1992 May;51(5):444–454. doi: 10.1002/jlb.51.5.444. [DOI] [PubMed] [Google Scholar]
- Hoedemakers R. M., Scherphof G. L., Daemen T. Proliferation of rat liver macrophages in vitro: influence of hemopoietic growth factors. Hepatology. 1994 Mar;19(3):666–674. doi: 10.1002/hep.1840190318. [DOI] [PubMed] [Google Scholar]
- Honda Y., Takahashi K., Naito M., Fujiyama S. The role of macrophage colony-stimulating factor in the differentiation and proliferation of Kupffer cells in the liver of protein-deprived mice. Lab Invest. 1995 Jun;72(6):696–706. [PubMed] [Google Scholar]
- Kiwada H., Niimura H., Fujisaki Y., Yamada S., Kato Y. Application of synthetic alkyl glycoside vesicles as drug carriers. I. Preparation and physical properties. Chem Pharm Bull (Tokyo) 1985 Feb;33(2):753–759. doi: 10.1248/cpb.33.753. [DOI] [PubMed] [Google Scholar]
- Leenen P. J., Melis M., Slieker W. A., Van Ewijk W. Murine macrophage precursor characterization. II. Monoclonal antibodies against macrophage precursor antigens. Eur J Immunol. 1990 Jan;20(1):27–34. doi: 10.1002/eji.1830200105. [DOI] [PubMed] [Google Scholar]
- Leenen P. J., de Bruijn M. F., Voerman J. S., Campbell P. A., van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. J Immunol Methods. 1994 Sep 14;174(1-2):5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A., Gough N. M., Elliott M., McArthur G., Li M. Synergistic suppression: anomalous inhibition of the proliferation of factor-dependent hemopoietic cells by combination of two colony-stimulating factors. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2819–2823. doi: 10.1073/pnas.89.7.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A. The clonal proliferation of normal mouse hematopoietic cells: enhancement and suppression by colony-stimulating factor combinations. Blood. 1992 Jun 1;79(11):2861–2866. [PubMed] [Google Scholar]
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989 May 4;339(6219):27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Morioka Y., Naito M., Sato T., Takahashi K. Immunophenotypic and ultrastructural heterogeneity of macrophage differentiation in bone marrow and fetal hematopoiesis of mouse in vitro and in vivo. J Leukoc Biol. 1994 May;55(5):642–651. doi: 10.1002/jlb.55.5.642. [DOI] [PubMed] [Google Scholar]
- Naito M., Hayashi S., Yoshida H., Nishikawa S., Shultz L. D., Takahashi K. Abnormal differentiation of tissue macrophage populations in 'osteopetrosis' (op) mice defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1991 Sep;139(3):657–667. [PMC free article] [PubMed] [Google Scholar]
- Naito M., Takahashi K. The role of Kupffer cells in glucan-induced granuloma formation in the liver of mice depleted of blood monocytes by administration of strontium-89. Lab Invest. 1991 May;64(5):664–674. [PubMed] [Google Scholar]
- Naito M., Yamamura F., Nishikawa S., Takahashi K. Development, differentiation, and maturation of fetal mouse yolk sac macrophages in cultures. J Leukoc Biol. 1989 Jul;46(1):1–10. doi: 10.1002/jlb.46.1.1. [DOI] [PubMed] [Google Scholar]
- Ohteki T., Seki S., Abo T., Kumagai K. Liver is a possible site for the proliferation of abnormal CD3+4-8- double-negative lymphocytes in autoimmune MRL-lpr/lpr mice. J Exp Med. 1990 Jul 1;172(1):7–12. doi: 10.1084/jem.172.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parwaresch M. R., Wacker H. H. Origin and kinetics of resident tissue macrophages. Parabiosis studies with radiolabelled leucocytes. Cell Tissue Kinet. 1984 Jan;17(1):25–39. doi: 10.1111/j.1365-2184.1984.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Mabuchi A., Kuriya S., Sudo T., Aida T., Asano G., Shoji T., Yokomuro K. Production of granulocyte-macrophage colony-stimulating factor by adult murine parenchymal liver cells (hepatocytes). Reg Immunol. 1990;3(5):260–267. [PubMed] [Google Scholar]
- Sato T., Shinzawa H., Abe Y., Takahashi T., Arai S., Sendo F. Inhibition of Corynebacterium parvum-primed and lipopolysaccharide-induced hepatic necrosis in rats by selective depletion of neutrophils using a monoclonal antibody. J Leukoc Biol. 1993 Feb;53(2):144–150. doi: 10.1002/jlb.53.2.144. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Naito M., Umeda S., Shultz L. D. The role of macrophage colony-stimulating factor in hepatic glucan-induced granuloma formation in the osteopetrosis mutant mouse defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1994 Jun;144(6):1381–1392. [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Yamamura F., Naito M. Differentiation, maturation, and proliferation of macrophages in the mouse yolk sac: a light-microscopic, enzyme-cytochemical, immunohistochemical, and ultrastructural study. J Leukoc Biol. 1989 Feb;45(2):87–96. doi: 10.1002/jlb.45.2.87. [DOI] [PubMed] [Google Scholar]
- Taniguchi H., Toyoshima T., Fukao K., Nakauchi H. Evidence for the presence of hematopoietic stem cells in the adult liver. Transplant Proc. 1995 Feb;27(1):196–199. [PubMed] [Google Scholar]
- Tsukui T., Kikuchi K., Mabuchi A., Sudo T., Sakamoto T., Sato N., Tsuneoka K., Shikita M., Aida T., Asano G. Production of macrophage colony-stimulating factor by adult murine parenchymal liver cells (hepatocytes). J Leukoc Biol. 1992 Oct;52(4):383–389. doi: 10.1002/jlb.52.4.383. [DOI] [PubMed] [Google Scholar]
- Ukai K., Terashima K., Imai Y., Shinzawa H., Okuyama Y., Takahashi T., Ishikawa M. Proliferation kinetics of rat Kupffer cells after partial hepatectomy. Immunohistochemical and ultrastructural analysis. Acta Pathol Jpn. 1990 Sep;40(9):623–634. doi: 10.1111/j.1440-1827.1990.tb01610.x. [DOI] [PubMed] [Google Scholar]
- Van Bossuyt H., Wisse E. Structural changes produced in Kupffer cells in the rat liver by injection of lipopolysaccharide. Cell Tissue Res. 1988 Jan;251(1):205–214. doi: 10.1007/BF00215466. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N., Kors N., vd Ende M., Dijkstra C. D. Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue Res. 1990 May;260(2):215–222. doi: 10.1007/BF00318625. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N., Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994 Sep 14;174(1-2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Van Rooijen N. The liposome-mediated macrophage 'suicide' technique. J Immunol Methods. 1989 Nov 13;124(1):1–6. doi: 10.1016/0022-1759(89)90178-6. [DOI] [PubMed] [Google Scholar]
- Volkman A. Disparity in origin of mononuclear phagocyte populations. J Reticuloendothel Soc. 1976 Apr;19(4):249–268. [PubMed] [Google Scholar]
- Wacker H. H., Radzun H. J., Parwaresch M. R. Kinetics of Kupffer cells as shown by parabiosis and combined autoradiographic/immunohistochemical analysis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(2):71–78. doi: 10.1007/BF02899017. [DOI] [PubMed] [Google Scholar]
- Widmann J. J., Fahimi H. D. Proliferation of mononuclear phagocytes (Kupffer cells) and endothelial cells in regenerating rat liver. A light and electron microscopic cytochemical study. Am J Pathol. 1975 Sep;80(3):349–366. [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A. W., Jr, Ahmed-Ansari A., Sell K. W., Pollard J. W., Stanley E. R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W. In vivo role of macrophage growth factors as delineated using CSF-1 deficient op/op mouse. Leukemia. 1993 Aug;7 (Suppl 2):S117–S121. [PubMed] [Google Scholar]
- Wisse E. Observations on the fine structure and peroxidase cytochemistry of normal rat liver Kupffer cells. J Ultrastruct Res. 1974 Mar;46(3):393–426. doi: 10.1016/s0022-5320(74)90064-1. [DOI] [PubMed] [Google Scholar]
- Xiong H., Kawamura I., Nishibori T., Mitsuyama M. Cytokine gene expression in mice at an early stage of infection with various strains of Listeria spp. differing in virulence. Infect Immun. 1994 Sep;62(9):3649–3654. doi: 10.1128/iai.62.9.3649-3654.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Naito M., Takahashi K. Kupffer cell proliferation and glucan-induced granuloma formation in mice depleted of blood monocytes by strontium-89. J Leukoc Biol. 1990 Mar;47(3):195–205. [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., Kors N., Kraal G. Macrophage subset repopulation in the spleen: differential kinetics after liposome-mediated elimination. J Leukoc Biol. 1989 Feb;45(2):97–104. doi: 10.1002/jlb.45.2.97. [DOI] [PubMed] [Google Scholar]
- van Rooijen N., van Nieuwmegen R. Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell Tissue Res. 1984;238(2):355–358. doi: 10.1007/BF00217308. [DOI] [PubMed] [Google Scholar]