Abstract
K-ras and p53 gene alterations are frequently found in human pancreatic carcinomas and cell lines. The aim of this study was to analyze for the presence of K-ras and p53 gene mutations in hamster pancreatic tumors and cell lines. Mutations at the first coding exon of the K-ras gene and in exons V to VIII of the hamster p53 gene were analyzed in six cell lines (H2T, PC1, PC1.2, PC1.0, WD, and PD) and in N-nitroso-bis(2-oxopropyl)amine-induced pancreatic (n = 9) and extra-pancreatic (n = 4) tumors. K-ras mutations were present in seven of the nine pancreatic tumors and in all extra-pancreatic tumors. No p53 mutations were detected in the tumors. All cell lines analyzed contained K-ras mutations. Moreover, four of the six cell lines contained single amino acid substitutions in the p53 gene. Cell lines derived from nitrosamine-induced pancreatic tumors in the hamster contained K-ras and p53 alterations similar to those found in cell lines derived from human pancreatic carcinomas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almoguera C., Shibata D., Forrester K., Martin J., Arnheim N., Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988 May 20;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- Barton C. M., Staddon S. L., Hughes C. M., Hall P. A., O'Sullivan C., Klöppel G., Theis B., Russell R. C., Neoptolemos J., Williamson R. C. Abnormalities of the p53 tumour suppressor gene in human pancreatic cancer. Br J Cancer. 1991 Dec;64(6):1076–1082. doi: 10.1038/bjc.1991.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budowle B., Chakraborty R., Giusti A. M., Eisenberg A. J., Allen R. C. Analysis of the VNTR locus D1S80 by the PCR followed by high-resolution PAGE. Am J Hum Genet. 1991 Jan;48(1):137–144. [PMC free article] [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., da Costa L. T., Redston M. S., Schutte M., Seymour A. B., Weinstein C. L., Hruban R. H., Yeo C. J., Kern S. E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994 Sep;8(1):27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- Capella G., Cronauer-Mitra S., Pienado M. A., Perucho M. Frequency and spectrum of mutations at codons 12 and 13 of the c-K-ras gene in human tumors. Environ Health Perspect. 1991 Jun;93:125–131. doi: 10.1289/ehp.9193125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella G., Matias-Guiu X., Ampudia X., de Leiva A., Perucho M., Prat J. Ras oncogene mutations in thyroid tumors: polymerase chain reaction-restriction-fragment-length polymorphism analysis from paraffin-embedded tissues. Diagn Mol Pathol. 1996 Mar;5(1):45–52. doi: 10.1097/00019606-199603000-00008. [DOI] [PubMed] [Google Scholar]
- Cerny W. L., Mangold K. A., Scarpelli D. G. Activation of K-ras in transplantable pancreatic ductal adenocarcinomas of Syrian golden hamsters. Carcinogenesis. 1990 Nov;11(11):2075–2079. doi: 10.1093/carcin/11.11.2075. [DOI] [PubMed] [Google Scholar]
- Cerny W. L., Mangold K. A., Scarpelli D. G. K-ras mutation is an early event in pancreatic duct carcinogenesis in the Syrian golden hamster. Cancer Res. 1992 Aug 15;52(16):4507–4513. [PubMed] [Google Scholar]
- Chang K. W., Laconi S., Mangold K. A., Hubchak S., Scarpelli D. G. Multiple genetic alterations in hamster pancreatic ductal adenocarcinomas. Cancer Res. 1995 Jun 15;55(12):2560–2568. [PubMed] [Google Scholar]
- Chang K. W., Mangold K. A., Hubchak S., Laconi S., Scarpelli D. G. Genomic p53 mutation in a chemically induced hamster pancreatic ductal adenocarcinoma. Cancer Res. 1994 Jul 15;54(14):3878–3883. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Egami H., Takiyama Y., Cano M., Houser W. H., Pour P. M. Establishment of hamster pancreatic ductal carcinoma cell line (PC-1) producing blood group-related antigens. Carcinogenesis. 1989 May;10(5):861–869. doi: 10.1093/carcin/10.5.861. [DOI] [PubMed] [Google Scholar]
- Egawa S., Tsutsumi M., Konishi Y., Kobari M., Matsuno S., Nagasaki K., Futami H., Yamaguchi K. The role of angiogenesis in the tumor growth of Syrian hamster pancreatic cancer cell line HPD-NR. Gastroenterology. 1995 May;108(5):1526–1533. doi: 10.1016/0016-5085(95)90703-3. [DOI] [PubMed] [Google Scholar]
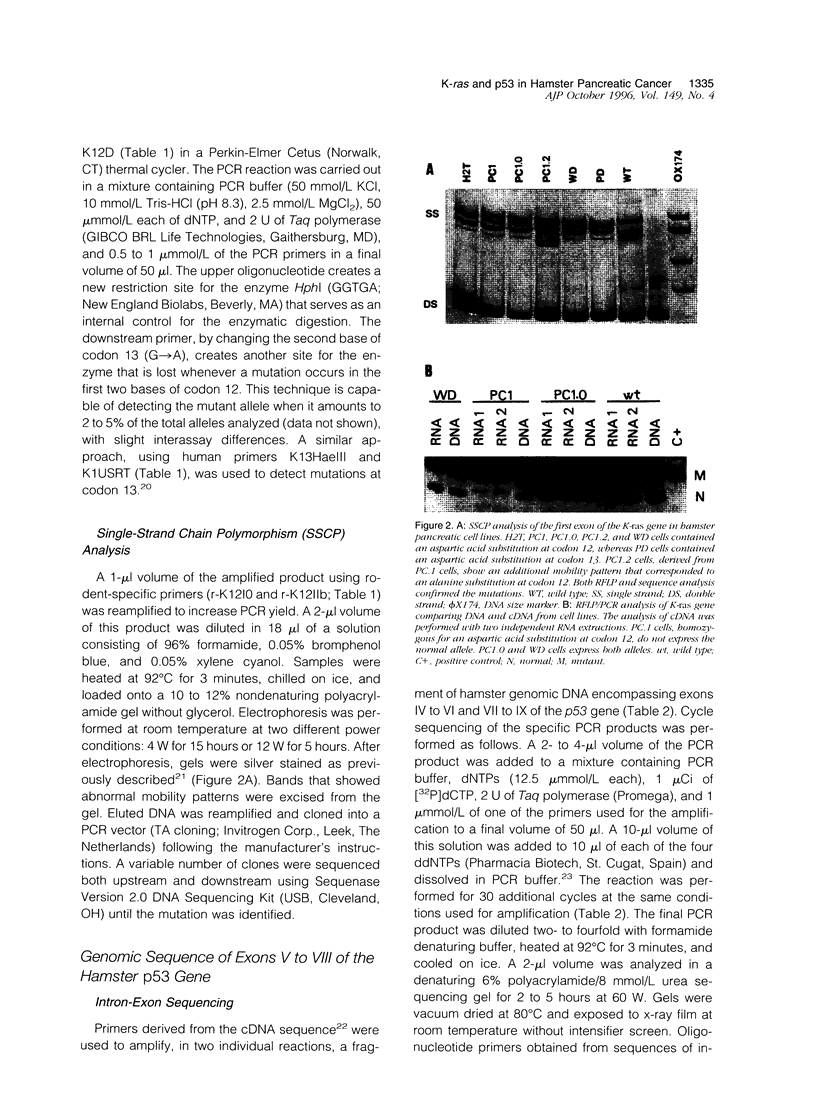
- Fujii H., Egami H., Chaney W., Pour P., Pelling J. Pancreatic ductal adenocarcinomas induced in Syrian hamsters by N-nitrosobis(2-oxopropyl)amine contain a c-Ki-ras oncogene with a point-mutated codon 12. Mol Carcinog. 1990;3(5):296–301. doi: 10.1002/mc.2940030510. [DOI] [PubMed] [Google Scholar]
- Hirota M., Egami H., Corra S., Fujii H., Chaney W. G., Rizzino A., Pour P. M. Production of scatter factor-like activity by a nitrosamine-induced pancreatic cancer cell line. Carcinogenesis. 1993 Feb;14(2):259–264. doi: 10.1093/carcin/14.2.259. [DOI] [PubMed] [Google Scholar]
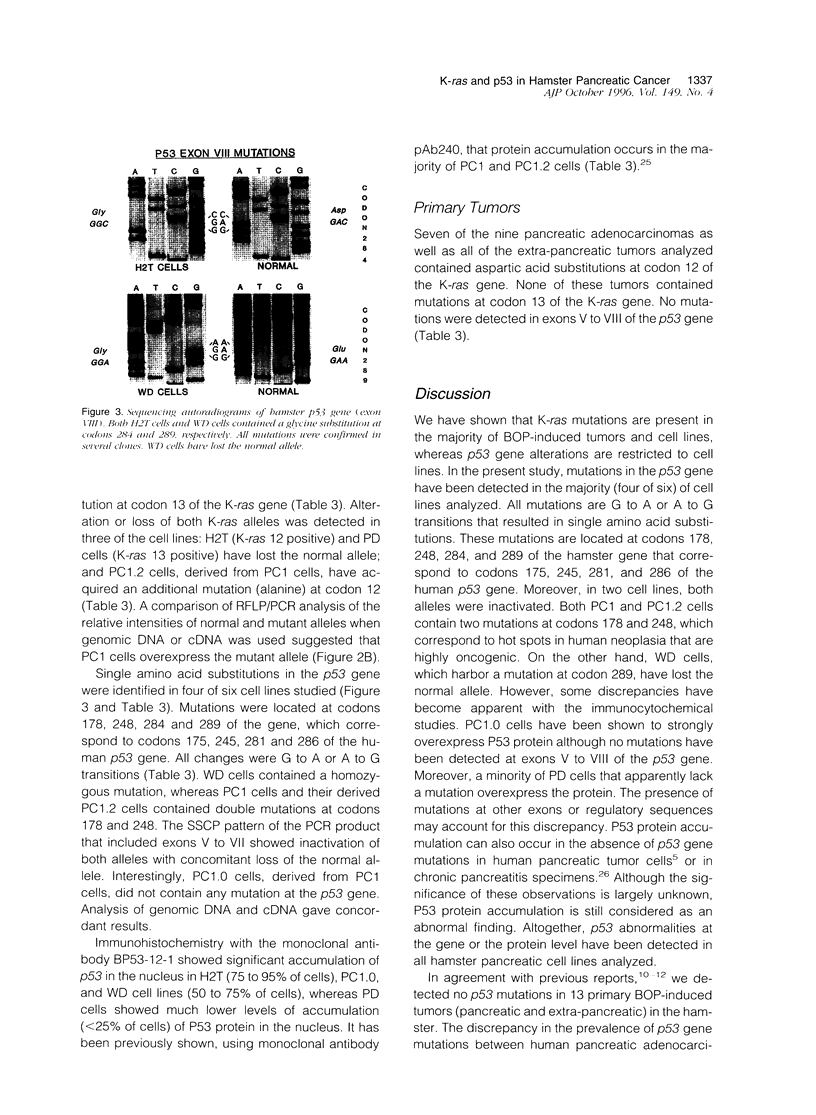
- Hruban R. H., van Mansfeld A. D., Offerhaus G. J., van Weering D. H., Allison D. C., Goodman S. N., Kensler T. W., Bose K. K., Cameron J. L., Bos J. L. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993 Aug;143(2):545–554. [PMC free article] [PubMed] [Google Scholar]
- Kalthoff H., Schmiegel W., Roeder C., Kasche D., Schmidt A., Lauer G., Thiele H. G., Honold G., Pantel K., Riethmüller G. p53 and K-RAS alterations in pancreatic epithelial cell lesions. Oncogene. 1993 Feb;8(2):289–298. [PubMed] [Google Scholar]
- Kress S., König J., Schweizer J., Löhrke H., Bauer-Hofmann R., Schwarz M. p53 mutations are absent from carcinogen-induced mouse liver tumors but occur in cell lines established from these tumors. Mol Carcinog. 1992;6(2):148–158. doi: 10.1002/mc.2940060210. [DOI] [PubMed] [Google Scholar]
- Legros Y., McIntyre P., Soussi T. The cDNA cloning and immunological characterization of hamster p53. Gene. 1992 Mar 15;112(2):247–250. doi: 10.1016/0378-1119(92)90384-2. [DOI] [PubMed] [Google Scholar]
- Matias-Guiu X., Cuatrecasas M., Musulen E., Prat J. p53 expression in anaplastic carcinomas arising from thyroid papillary carcinomas. J Clin Pathol. 1994 Apr;47(4):337–339. doi: 10.1136/jcp.47.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogaki M., Hirota M., Chaney W. G., Pour P. M. Comparison of p53 protein expression and cellular localization in human and hamster pancreatic cancer cell lines. Carcinogenesis. 1993 Dec;14(12):2589–2594. doi: 10.1093/carcin/14.12.2589. [DOI] [PubMed] [Google Scholar]
- Mori T., Tsutsumi M., Noguchi O., Horiguchi K., Hohnoki K., Okita S., Suzuki F., Konishi Y. Characterization of three cloned cell lines from a N-nitrosobis(2-hydroxypropyl)amine-induced transplantable hamster pancreatic ductal adenocarcinoma. Int J Pancreatol. 1994 Oct-Dec;16(2-3):171–177. doi: 10.1007/BF02944328. [DOI] [PubMed] [Google Scholar]
- Okamoto M., Ohtsu H., Miyaki M., Yonekawa H. No allelic loss at the p53 locus in 1,2-dimethylhydrazine-induced mouse colon tumours: PCR-SSCP analysis with sequence-tagged microsatellite site primers. Carcinogenesis. 1993 Jul;14(7):1483–1486. doi: 10.1093/carcin/14.7.1483. [DOI] [PubMed] [Google Scholar]
- Okita S., Tsutsumi M., Onji M., Konishi Y. p53 mutation without allelic loss and absence of mdm-2 amplification in a transplantable hamster pancreatic ductal adenocarcinoma and derived cell lines but not primary ductal adenocarcinomas in hamsters. Mol Carcinog. 1995 Aug;13(4):266–271. doi: 10.1002/mc.2940130409. [DOI] [PubMed] [Google Scholar]
- Oreffo V. I., Lin H. W., Gumerlock P. H., Kraegel S. A., Witschi H. Mutational analysis of a dominant oncogene (c-Ki-ras-2) and a tumor suppressor gene (p53) in hamster lung tumorigenesis. Mol Carcinog. 1992;6(3):199–202. doi: 10.1002/mc.2940060305. [DOI] [PubMed] [Google Scholar]
- Pour P. M., Egami H., Takiyama Y. Patterns of growth and metastases of induced pancreatic cancer in relation to the prognosis and its clinical implications. Gastroenterology. 1991 Feb;100(2):529–536. doi: 10.1016/0016-5085(91)90226-b. [DOI] [PubMed] [Google Scholar]
- Pour P. M. Experimental pancreatic cancer. Am J Surg Pathol. 1989;13 (Suppl 1):96–103. [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Shibata D., Capella G., Perucho M. Mutational activation of the c-K-ras gene in human pancreatic carcinoma. Baillieres Clin Gastroenterol. 1990 Mar;4(1):151–169. doi: 10.1016/0950-3528(90)90044-h. [DOI] [PubMed] [Google Scholar]
- Simon B., Weinel R., Höhne M., Watz J., Schmidt J., Körtner G., Arnold R. Frequent alterations of the tumor suppressor genes p53 and DCC in human pancreatic carcinoma. Gastroenterology. 1994 Jun;106(6):1645–1651. doi: 10.1016/0016-5085(94)90422-7. [DOI] [PubMed] [Google Scholar]
- Sugio K., Gazdar A. F., Albores-Saavedra J., Kokkinakis D. M. High yields of K-ras mutations in intraductal papillary mucinous tumors and invasive adenocarcinomas induced by N-nitroso(2-hydroxypropyl)(2-oxopropyl)amine in the pancreas of female Syrian hamsters. Carcinogenesis. 1996 Feb;17(2):303–309. doi: 10.1093/carcin/17.2.303. [DOI] [PubMed] [Google Scholar]
- Townsend C. M., Jr, Franklin R. B., Gelder F. B., Glass E., Thompson J. C. Development of a transplantable model of pancreatic duct adenocarcinoma. Surgery. 1982 Jul;92(1):72–78. [PubMed] [Google Scholar]
- van Kranen H. J., Vermeulen E., Schoren L., Bax J., Woutersen R. A., van Iersel P., van Kreijl C. F., Scherer E. Activation of c-K-ras is frequent in pancreatic carcinomas of Syrian hamsters, but is absent in pancreatic tumors of rats. Carcinogenesis. 1991 Aug;12(8):1477–1482. doi: 10.1093/carcin/12.8.1477. [DOI] [PubMed] [Google Scholar]