Abstract
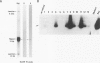
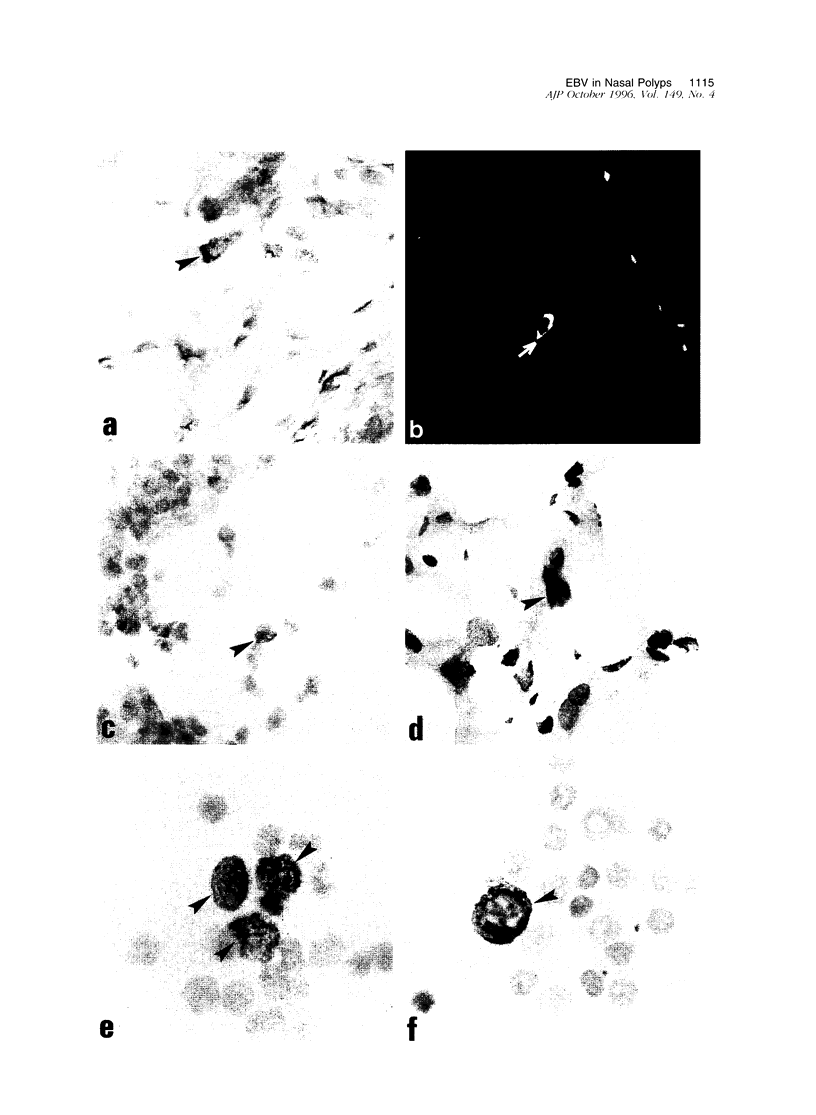
Primary nasal lymphomas of T or NK cell origin are known to be associated with Epstein-Barr virus (EBV). However, it is not known whether EBV is normally present in nasal mucosa as distinct to nasopharyngeal tissue. This study investigates the prevalence of EBV infection in 13 cases of nasal polyps. EBV DNA was detected in 2 of 13 (15%) by Southern blot hybridization and in 9 of 13 (69%) by polymerase chain reaction. In situ hybridization for EBV-encoded small nuclear RNAs (EBER) was positive in 11 of 13 (85%) cases; the virus was present in stromal lymphocytes only and not in the epithelial cells. Immunohistochemistry for EBV proteins in 7 cases revealed EBV nuclear antigen (EBNA)-2, latent membrane protein (LMP)-1, and ZEBRA (the switch protein encoded by gene BZLF1) expression in rare isolated stromal lymphocytes in 3 cases. Double immunostaining in 1 case showed that the LMP-1+ cells were B or T cells. Immunohistochemistry for EBV lytic proteins showed very rare viral capsid antigen (VCA)+ and membrane antigen (MA)+ cells in 1 case and very rare diffuse early antigen (EA-D)+ and VCA+ cells in 1 other case. The expression of ZEBRA, EA-D, VCA, and MA suggested a disruption of latency in very rare stromal lymphocytes leading to a productive cycle. Although the incidence of EBV positivity in nasal polyps in our population is high (85%), very low numbers of EBV+ cells are found in each case. Nevertheless, they indicate that nasal mucosa could be one of the sites of EBV persistence through a low level of infection of the resident lymphocytes and thereby provide a possible setting for the emergence of virally associated tumors in this site.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos I., Hummel M., Kreschel C., Stein H. Morphology, immunophenotype, and distribution of latently and/or productively Epstein-Barr virus-infected cells in acute infectious mononucleosis: implications for the interindividual infection route of Epstein-Barr virus. Blood. 1995 Feb 1;85(3):744–750. [PubMed] [Google Scholar]
- Cheung A., Kieff E. Long internal direct repeat in Epstein-Barr virus DNA. J Virol. 1982 Oct;44(1):286–294. doi: 10.1128/jvi.44.1.286-294.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung W. Y., Chan A. C., Loke S. L., Srivastava G., Pittaluga S., Lim L. Y., Ho F. C. Latent sites of Epstein-Barr virus infection. Am J Clin Pathol. 1993 Nov;100(5):502–506. doi: 10.1093/ajcp/100.5.502. [DOI] [PubMed] [Google Scholar]
- Deamant F. D., Albújar P. F., Chen Y. Y., Weiss L. M. Epstein-Barr virus distribution in nonneoplastic lymph nodes. Mod Pathol. 1993 Nov;6(6):729–732. [PubMed] [Google Scholar]
- Dunnette S. L., Hall M. M., Washington J. A., 2nd, Kern E. B., McDonald T. J., Facer G. W., Gleich G. J. Microbiologic analyses of nasal polyp tissue. J Allergy Clin Immunol. 1986 Jul;78(1 Pt 1):102–108. doi: 10.1016/0091-6749(86)90121-1. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Ho F. C., Choy D., Loke S. L., Kung I. T., Fu K. H., Liang R., Todd D., Khoo R. K. Polymorphic reticulosis and conventional lymphomas of the nose and upper aerodigestive tract: a clinicopathologic study of 70 cases, and immunophenotypic studies of 16 cases. Hum Pathol. 1990 Oct;21(10):1041–1050. doi: 10.1016/0046-8177(90)90254-3. [DOI] [PubMed] [Google Scholar]
- Ho F. C., Srivastava G., Loke S. L., Fu K. H., Leung B. P., Liang R., Choy D. Presence of Epstein-Barr virus DNA in nasal lymphomas of B and 'T' cell type. Hematol Oncol. 1990 Sep-Oct;8(5):271–281. doi: 10.1002/hon.2900080505. [DOI] [PubMed] [Google Scholar]
- Khanna R., Burrows S. R., Kurilla M. G., Jacob C. A., Misko I. S., Sculley T. B., Kieff E., Moss D. J. Localization of Epstein-Barr virus cytotoxic T cell epitopes using recombinant vaccinia: implications for vaccine development. J Exp Med. 1992 Jul 1;176(1):169–176. doi: 10.1084/jem.176.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak F. K., Mahony J. B., Chernesky M. A., Newhouse M. T., Dolovich J., Hitch D. A., Rossman C. M. Nasal polyposis: in search of a viral etiology using DNA hybridization. J Otolaryngol. 1991 Dec;20(6):404–407. [PubMed] [Google Scholar]
- Li Q. X., Young L. S., Niedobitek G., Dawson C. W., Birkenbach M., Wang F., Rickinson A. B. Epstein-Barr virus infection and replication in a human epithelial cell system. Nature. 1992 Mar 26;356(6367):347–350. doi: 10.1038/356347a0. [DOI] [PubMed] [Google Scholar]
- Murray R. J., Kurilla M. G., Brooks J. M., Thomas W. A., Rowe M., Kieff E., Rickinson A. B. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992 Jul 1;176(1):157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiper S. C. Angiocentric lymphoproliferative disorders of the respiratory system: incrimination of Epstein-Barr virus in pathogenesis. Blood. 1993 Aug 1;82(3):687–688. [PubMed] [Google Scholar]
- Settipane G. A., Chafee F. H. Nasal polyps in asthma and rhinitis. A review of 6,037 patients. J Allergy Clin Immunol. 1977 Jan;59(1):17–21. doi: 10.1016/0091-6749(77)90171-3. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Davis D. S., Young L. S., Hutt-Fletcher L., Tedder T. F., Rickinson A. B. Human epithelial cell expression of an Epstein-Barr virus receptor. J Gen Virol. 1987 Mar;68(Pt 3):805–811. doi: 10.1099/0022-1317-68-3-805. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Vesterinen E. H., Nedrud J. G., Raab-Traub N., Walton L. A., Pagano J. S. Replication of Epstein-Barr virus in human epithelial cells infected in vitro. Nature. 1983 Dec 1;306(5942):480–483. doi: 10.1038/306480a0. [DOI] [PubMed] [Google Scholar]
- Sixbey J. W., Yao Q. Y. Immunoglobulin A-induced shift of Epstein-Barr virus tissue tropism. Science. 1992 Mar 20;255(5051):1578–1580. doi: 10.1126/science.1312750. [DOI] [PubMed] [Google Scholar]
- Stoop A. E., van der Heijden H. A., Biewenga J., van der Baan S. Lymphocytes and nonlymphoid cells in human nasal polyps. J Allergy Clin Immunol. 1991 Feb;87(2):470–475. doi: 10.1016/0091-6749(91)90004-8. [DOI] [PubMed] [Google Scholar]
- Tao Q., Ho F. C., Loke S. L., Srivastava G. Epstein-Barr virus is localized in the tumour cells of nasal lymphomas of NK, T or B cell type. Int J Cancer. 1995 Jan 27;60(3):315–320. doi: 10.1002/ijc.2910600306. [DOI] [PubMed] [Google Scholar]
- Tao Q., Srivastava G., Chan A. C., Chung L. P., Loke S. L., Ho F. C. Evidence for lytic infection by Epstein-Barr virus in mucosal lymphocytes instead of nasopharyngeal epithelial cells in normal individuals. J Med Virol. 1995 Jan;45(1):71–77. doi: 10.1002/jmv.1890450114. [DOI] [PubMed] [Google Scholar]
- Tao Q., Srivastava G., Chan A. C., Ho F. C. Epstein-Barr-virus-infected nasopharyngeal intraepithelial lymphocytes. Lancet. 1995 May 20;345(8960):1309–1310. doi: 10.1016/s0140-6736(95)90960-5. [DOI] [PubMed] [Google Scholar]
- Tao Q., Srivastava G., Loke S. L., Chan E. Y., Ho F. C. Improved double immunohistochemical staining method for cryostat and paraffin wax sections, combining alkaline phosphatase anti-alkaline phosphatase and indirect immunofluorescence. J Clin Pathol. 1994 Jul;47(7):597–600. doi: 10.1136/jcp.47.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. C., Mann R. B., Epstein J. I., MacMahon E., Lee W. A., Charache P., Hayward S. D., Kurman R. J., Hayward G. S., Ambinder R. F. Abundant expression of EBER1 small nuclear RNA in nasopharyngeal carcinoma. A morphologically distinctive target for detection of Epstein-Barr virus in formalin-fixed paraffin-embedded carcinoma specimens. Am J Pathol. 1991 Jun;138(6):1461–1469. [PMC free article] [PubMed] [Google Scholar]