Abstract
We report direct experimental evidence that human αB-crystallin, a member of the small heat shock protein family, actively participates in the refolding of citrate synthase (CS) in vitro. In the presence of 3.5 mM ATP, CS reactivation by αB-crystallin was enhanced approximately twofold. Similarly, 3.5 mM ATP enhanced the chaperone activity of αB-crystallin on the unfolding and aggregation of CS at 45°C. Consistent with these findings, cell viability at 50°C was improved nearly five orders of magnitude in Escherichia coli expressing αB-crystallin. SDS/PAGE analysis of cell lysates suggested that αB-crystallin protects cells against physiological stress in vivo by maintaining cytosolic proteins in their native and functional conformations. This report confirms the action of αB-crystallin as a molecular chaperone both in vitro and in vivo and describes the enhancement of αB-crystallin chaperone functions by ATP.
Molecular chaperones of the large heat shock protein families not only suppress protein unfolding and aggregation in response to stress but also actively participate in the refolding of denatured proteins in vitro, often in an ATP-dependent manner (1, 2). αB-crystallin is a protein that shares sequence and functional similarities with small heat shock proteins (sHsps) from numerous species (3, 4). αB-crystallin is expressed constitutively at high levels in the lens of the eye and at lower levels in many nonlens cells and tissues and is up-regulated dramatically in response to stress and pathological conditions in vivo (3, 5). Chaperone-like activity has been described for αB-crystallin from humans and various other species in suppressing protein unfolding and aggregation in response to thermal or chemical stress (4, 6–9). Although the participation of α-crystallin and other sHsps in the refolding of proteins has been reported (4, 6, 10), the enhancement by ATP on the reactivation and refolding of proteins by αB-crystallin has not. To functionally characterize αB-crystallin as a molecular chaperone, its effects on the unfolding and refolding of citrate synthase (CS) in the presence and absence of ATP were studied in vitro. CS was chosen as a model protein for the characterization of unfolding and refolding reactions because it has been used extensively with molecular chaperones that include GroEL, Hsp90, and the sHsps (6, 11–14).
ATP is an abundant phosphorous metabolite in lenses from many species (15) that is present in lens cells at concentrations as high as 6.7 mM (16, 17), which is among the highest levels of any cell in the body (18). High concentrations of ATP (4–8 mM) are found in skeletal muscle (19), in which high levels of αB-crystallin are expressed (20). The activity of GroEL and other chaperones on unfolding and refolding of proteins has been characterized at high concentrations of ATP (6, 11, 21–24), which may reflect a functional relationship between high concentrations of ATP and chaperone activity in all cells. The functional experiments with αB-crystallin in this report used ATP at a concentration of 3.5 mM. Not only is this concentration comparable directly to that used in functional in vitro studies published for well characterized chaperones (6, 11, 21–24), but this is a concentration that is present in vivo in cells that express αB-crystallin (15–20).
The action of human αB-crystallin as a molecular chaperone was characterized in vivo by using thermal stress to Escherichia coli. Mouse αB-crystallin expression has been shown to confer protection to E. coli and NIH 3T3 cells against thermal stress (7, 25). Expression of recombinant human αB-crystallin in vivo protected cell viability nearly five orders of magnitude over control cultures.
The major finding in this report is that αB-crystallin functions as an ATP-enhanced molecular chaperone not only in suppression of the unfolding and aggregation of CS but also in mediating the proper refolding of CS to a fully functional conformation. Although the role of ATP in the reaction cycles of protein folding by GroEL/Hsp60 and DnaK/Hsp70 is well characterized, a detailed understanding at the molecular level of the functional interactions between αB-crystallin and ATP remains to be elucidated.
METHODS
Refolding and Reactivation of CS.
Human αB-crystallin was purified as described (8), and CS purified from porcine heart was purchased from Boehringer Mannheim. CS was denatured for 1.5 h at 23°C in 100 mM Tris⋅HCl (pH 8), 2 mM DTT, and 6 M guanidine hydrochloride. Reactivation was initiated by a 100-fold dilution into 100 mM Tris⋅HCl (pH 8) and 10 mM KCl. For testing the effects of ATP (or other nucleotides/nonhydrolyzable ATP analogs), the reaction buffer was equilibrated with 3.5 mM ATP (or other nucleotide/nonhydrolyzable ATP analogs) and 3.5 mM MgCl2 before addition of αB-crystallin or CS. Dilution of denatured CS into the reactivation buffer was performed with vigorous stirring in glass vessels at 23°C. Activity of CS was assayed as described (11) after indicated times of refolding at 23°C. Reactivation of chemically denatured CS is given as the percentage relative to a control sample of native 150 nM CS tested under identical conditions.
Unfolding and Aggregation of CS.
The apparent OD (relative light scattering at λ = 320 nm) was a direct measure of the thermal unfolding and aggregation of CS at 45°C over 60 min. Native CS (50 μM) was diluted 1:100 into a reaction buffer containing 40 mM Hepes, 20 mM KOH, 50 mM KCl, 10 mM (NH4)2SO4, and 2 mM potassium acetate (pH 7.8) equilibrated at 45°C in the presence and absence of αB-crystallin and/or ATP. For testing the effects of ATP (or nonhydrolyzable ATP analogs), the reaction buffer was equilibrated with 3.5 mM ATP (or nonhydrolyzable ATP analogs) and 3.5 mM MgCl2 before addition of αB-crystallin or CS. Concentrations listed for CS refer to the monomer and for αB-crystallin refer to the ≈20-mer complex as determined by dynamic light scattering (unpublished data) and size exclusion chromatography (8).
Intrinsic Tryptophan Fluorescence.
The emission spectra for 0.025 mg/ml human αB-crystallin in a reaction buffer consisting of 20 mM Tris⋅HCl (pH 7.5), 25 mM NaCl, 10 mM KCl, and 3.5 mM MgCl2 was monitored at 300 < λ < 450 nm by using an excitation λ = 290 nm. Spectra were recorded at 37°C by using a Perkin–Elmer Luminescence spectrometer LS50B in the presence and absence of 350 nM-3.5 mM ATP. Spectra were corrected for background emission Raman scattering. Spectra of the buffers (including ATP) were subtracted from the spectra of the protein samples. The effect of ATP on the intrinsic tryptophan fluorescence of human αB-crystallin was consistent with a previous study using 50–300 μM ATP with total bovine α-crystallin (26).
Cell Viability Experiments.
A cDNA sequence encoding human αB-crystallin (8) was inserted into the pET16b vector (Novagen) at the NcoI and XbaI restriction sites to produce pET16b-αB (data not shown). This plasmid was used to transform competent E. coli BL21 (DE3) cells (Novagen). For control cultures, pET16b lacking any inserted cDNA sequence (Control Culture 1) and pET16b containing a cDNA sequence encoding β-galactosidase (Control Culture 2) also were transformed into competent E. coli BL21 (DE3) cells (Novagen). For the heat shock experiment, equal numbers of cells transformed with pET16b–αB or control plasmids were inoculated into 50 ml of L broth that contained 100 μg/ml carbenicillin. Cells were grown at 37°C with vigorous shaking until they reached an OD of 0.5 at A = 600 nm, at which point protein expression was induced by the addition of isopropyl-β-d-thiogalactopyranoside to a final concentration of 1.0 mM (Sigma). Two hours after induction of protein expression, the cultures were shifted to 50°C for the remainder of the experiment. Cell viability of cultures was determined by counting the number of colony forming units (CFUs) formed at selected time points. Cell viability is plotted as the percentage of CFUs formed throughout heat shock relative to the starting number of CFUs formed in each culture before heat shock. Shown are the means and SD of triplicate cultures. The effect of heat shock on protein stability was analyzed at selected time points by electrophoresis on 4–12% Bis–Tris polyacrylamide electrophoretic gels run in the presence of 0.1% SDS (NOVEX, San Diego).
RESULTS
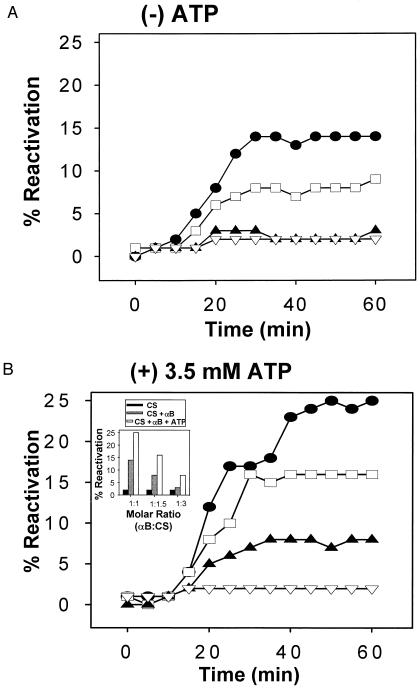
The effects of human αB-crystallin on the refolding and reactivation of CS in the presence and absence of ATP were determined in vitro. Upon dilution into a nondenaturing solution, chemically denatured CS aggregates rapidly upon refolding, achieving only a 1–2% reactivation yield in comparison to the enzymatic rate of the native enzyme (Fig. 1A and Table 1). However, when chemically denatured CS was diluted into a nondenaturing solution containing αB-crystallin, reactivation yields of enzymatically active CS were increased in a concentration-dependent manner (Fig. 1A). Similar to GroEL and DnaK (11, 21–24), 3.5 mM ATP enhanced the effect of αB-crystallin on refolding and reactivation of CS nearly twofold (Fig. 1B and Inset and Table 1). More specifically, in the absence of αB-crystallin and in the presence of 3.5 mM ATP, the reactivation yield of CS after 60 min was 1% (Fig. 1B and Inset and Table 1). In contrast, at a molar ratio of 1:1 (αB-to-CS) and in the absence of ATP, the reactivation yield of CS reached 14% whereas addition of 3.5 mM ATP increased the reactivation yield of CS to 25% (Fig. 1B and Inset and Table 1). ATP enhanced reactivation yields of CS by αB-crystallin at all concentrations of αB tested (Fig. 1B and Inset). The concentration of ATP tested with αB-crystallin was similar or identical to the concentrations used in several previous studies published for GroEL, DnaK, and sHsps (6, 11, 21–24). It is important to note that 3.5 mM ATP alone displayed no effect on the refolding and reactivation of CS (Fig. 1B and Table 1). ADP (3.5 mM) and the nonhydrolyzable ATP analogs ATP-γS, AMP-PCP, and AMP-PNP displayed no effect on the refolding and reactivation of CS alone (data not shown). In the presence of αB-crystallin, the ATP analogs did not enhance reactivation yield of CS (Table 1). Similar to published reports for GroEL and DnaK (11, 21–24), the enhanced chaperone effect appeared to be specific to ATP and not to chemically similar inactive ATP analogs. When BSA, maltose-binding protein, alcohol dehydrogenase, or bovine γ-d crystallin were substituted for αB-crystallin in the presence and absence of ATP in the refolding reactions, only minimal increases in CS refolding and reactivation were observed (Table 1). In the refolding reactions, αB-crystallin was 25 times more effective than γ-d crystallin and 8 times more effective than BSA, a control protein also used in GroEL experiments (Table 1) (11).
Figure 1.
ATP enhances the effects of human αB-crystallin on the refolding and reactivation of chemically denatured CS. Effects of human αB-crystallin on the reactivation of 150 nM chemically denatured CS in the absence (A) and presence (B) of 3.5 mM ATP. ▿, 0 nM αB; ▴, 50 nM αB (1:3 αB-to-CS molar ratio); □, 100 nM αB (1:1.5); and •, 150 nM αB (1:1). (Inset) Effects of 3.5 mM ATP on the reactivation of chemically denatured CS by human αB-crystallin shown as percent reactivation at t = 60 min for selected molar ratios of αB-to-CS.
Table 1.
Influence of proteins and nucleotides on the reactivation yields of CS*
| αB-crystallin
|
GroEL (11)
|
||
|---|---|---|---|
| Added component | Reactivation yield, % | Added component | Reactivation yield, % |
| None | 1 | None | 1 |
| ATP | 1 | ATP | 3 |
| αB | 14 | GroEL | 0 |
| GroEL + ATP | 16 | ||
| αB + ATP | 25 | GroEL + GroES + ATP | 28 |
| αB + ADP | 14 | GroEL + ADP | ND |
| αB + ATPγS | 17 | GroEL + ATPγS | ND |
| αB + AMP-PCP | 17 | GroEL + AMP-PCP | ND |
| αB + AMP-PNP | 9 | GroEL + AMP-PNP | ND |
| BSA + ATP | 3 | BSA + ATP | 7 |
| Lysozyme + ATP | ND | Lysozyme + ATP | 6 |
| γd-crystallin + ATP | 1 | γd-crystallin + ATP | ND |
| Alcohol dehydrogenase + ATP | 4 | Alcohol dehydrogenase + ATP | ND |
| Maltose-binding protein + ATP | 5 | Maltose-binding protein + ATP | ND |
Refolding and reactivation of CS in the presence of αB-crystallin was performed as described in Methods. The effects of various proteins and nucleotides on the reactivation of chemically denatured CS are shown as percent reactivation at t = 60 min for equimolar ratios of protein-to-CS. Results from refolding and reactivation of CS in the presence of GroEL are taken from Buchner et al. (11).
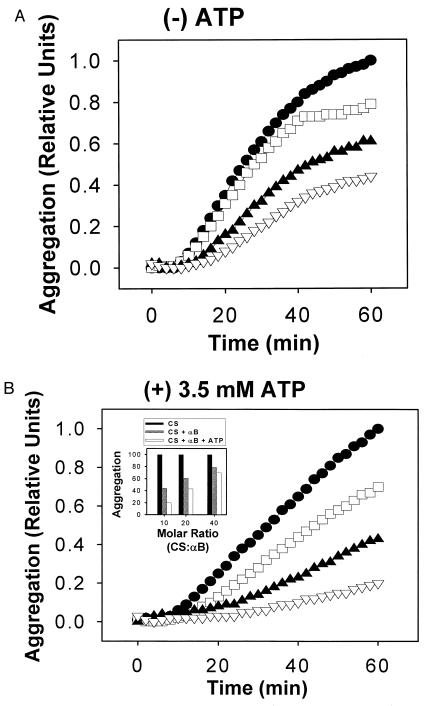
Next, we determined the effects of ATP on αB-crystallin in suppression of unfolding and aggregation of CS. CS unfolds rapidly and aggregates in solution at 45°C (Fig. 2A). In the absence of ATP, αB-crystallin suppressed the unfolding and aggregation of thermally denatured CS in a concentration-dependent manner (Fig. 2A). Addition of 3.5 mM ATP enhanced the suppression of CS aggregation by αB-crystallin at all concentrations of αB tested (Fig. 2B and Inset). More specifically, after 60 min at 45°C, aggregation of CS in the presence of αB-crystallin was reduced to 44% (Fig. 2A and Table 2). ATP alone had no effect on the aggregation kinetics of CS (Fig. 2B and Table 2). However, in the presence of αB-crystallin and 3.5 mM ATP, CS aggregation reached only 20% (Fig. 2B and Table 2). The concentration of ATP tested with αB-crystallin was similar or identical to the concentrations used in several previous studies published for GroEL, DnaK, and sHsps (6, 11, 21–24). The effects of the nonhydrolyzable ATP analogs ATP-γS, AMP-PCP, and AMP-PNP also were tested in the experimental system. All nucleotide analogs tested displayed no effect on CS aggregation alone (data not shown) or in the presence of αB-crystallin (Table 2). When BSA, lysozyme, and maltose-binding protein were substituted for αB-crystallin in the presence and absence of ATP in the unfolding reactions, no suppression of CS unfolding and aggregation was observed (Table 2).
Figure 2.
ATP enhances the suppression of CS unfolding and aggregation by human αB-crystallin. Effects of human αB-crystallin on the aggregation at 45°C of 500 nM CS in the absence (A) and presence (B) of 3.5 mM ATP. •, 0 nM αB; □, 12.5 nM αB (1:40 αB-to-CS molar ratio); ▴, 25 nM αB (1:20); and ▿, 50 nM αB (1:10). (Inset) Effects of 3.5 mM ATP on the suppression of CS unfolding and aggregation by human αB-crystallin shown as relative aggregation at t = 60 min for selected molar ratios of αB-to-CS.
Table 2.
Influence of proteins and nucleotides on the unfolding and aggregation of CS at 45°C
| Added component | CS aggregation, % |
|---|---|
| None | 100 |
| ATP | 100 |
| αB | 44 |
| αB + ATP | 20 |
| αB + ATPγS | 41 |
| αB + AMP-PCP | 41 |
| αB + AMP-PNP | 39 |
| BSA + ATP | 142 |
| Lysozyme + ATP | 157 |
| Maltose-binding protein + ATP | 149 |
The unfolding and aggregation of CS at 45°C was performed as described in Methods. The effects of various proteins and nucleotides on the unfolding and aggregation of CS are shown as percent aggregation at t = 60 min for equimolar ratios of protein-to-CS.
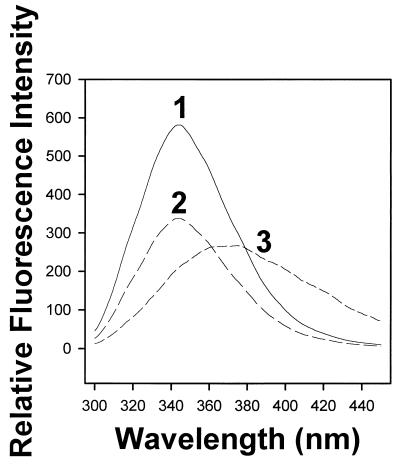
Intrinsic tryptophan fluorescence spectroscopy demonstrated an interaction between human αB-crystallin and ATP. The fluorescence emission spectra of αB-crystallin was monitored at 300 < λ < 450 nm in the absence and presence of varying concentrations of ATP (Fig. 3). In the presence of 350 μM ATP, the λ = 340 nm (tryptophan) peak fluorescence intensity decreased 42% compared with the peak fluorescence intensity observed in the absence of ATP (compare traces 2 and 1 in Fig. 3). A similar decrease in peak fluorescence intensity was observed for αB-crystallin in the presence of 350 nM-350 μM ATP (data not shown). In the presence of 3.5 mM ATP, both quenching (≈54%) and a pronounced red shift (≈31 nm) were observed in a solution of αB-crystallin (Fig. 3, trace 3).
Figure 3.
Fluorescence spectroscopy of human αB-crystallin in the presence and absence of ATP. Shown are emission spectra for human αB-crystallin in the absence of ATP (trace 1) and in the presence of 350 μM and 3.5 mM ATP (traces 2 and 3, respectively). A similar decrease in peak fluorescence intensity was observed for human αB-crystallin in the presence of 350 nM-350 μM ATP (data not shown). Quenching was observed at concentrations of ATP between 350 nM and 350 μM whereas 3.5 mM ATP resulted in both quenching and a red shift. Spectra were corrected for background emission Raman scattering. Spectra of the buffers (including ATP) were subtracted from the spectra of the protein samples.
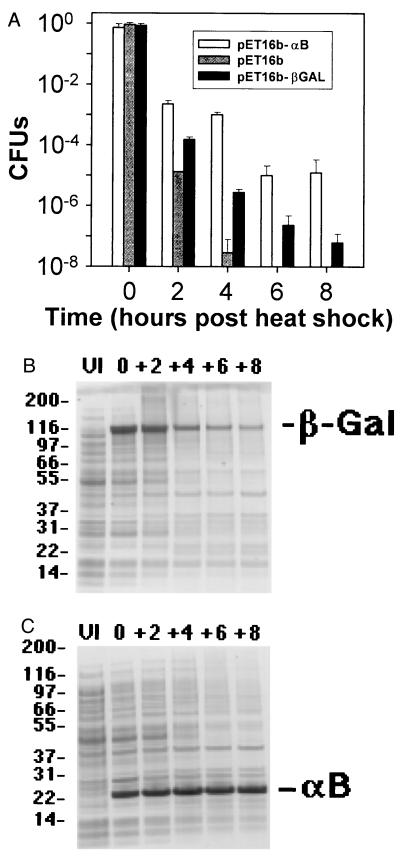
The protective effect of human αB-crystallin expression was evaluated in cells exposed to thermal stress in vivo. Bacterial cells were cultured for 4 h at 37°C and then transferred to 50°C, a temperature at which the bacterial cells rapidly undergo autolysis and concomitant necrotic death. Cell viability was measured by counting CFUs at selected time points during the course of the heat shock. Upon the shift to 50°C, un-induced control cultures (pET16b) and control cultures that overexpressed β-galactosidase (pET16b-β-gal) rapidly underwent cell autolysis as measured by the decrease in CFUs (Fig. 4A). Within 6 h of heat shock at 50°C no viable cells were observed in the un-induced control cultures (pET16b; Fig. 4A) whereas cultures that overexpressed αB-crystallin remained viable for 8 h (pET16b-αB; Fig. 4A). Viability of cultures that overexpressed β-galactosidase decreased seven orders of magnitude after 8 h of heat shock whereas viability of cultures that overexpressed αB-crystallin decreased five orders of magnitude (pET16b-βGAL and pET16b-αB, respectively; Fig. 4A). Although overexpression of β-galactosidase (pET16b-β-gal) appeared to provide a slight protective effect on cell viability, it should be noted that cultures that overexpressed αB-crystallin maintained between one and five orders of magnitude more viability than either control culture for the entire course of the experiment.
Figure 4.
Protective effect of human αB-crystallin expression on cell viability and protein stability at 50°C in vivo. (A) The protection of human αB-crystallin expression (pET16b–αB) vs. Control Culture 1 (pET16b; un-induced control) and Control Culture 2 (pET16b–β-galactosidase; overexpressed β-galactosidase) on CFUs at 0, 2, 4, 6, and 8 h after heat shock at 50°C. (B) SDS/PAGE analysis of crude cell lysates of bacterial cultures overexpressing β-galactosidase (Control Culture 2) or (C) human αB-crystallin before induction of protein expression (UI = un-induced cells) and at 0, 2, 4, 6, and 8 h after heat shock at 50°C. Expression of human αB-crystallin was associated directly with protection of protein stability (most evident at early time points) and cell viability in these experiments.
Protein degradation resulting from heat shock was examined in cell lysates by polyacrylamide gel electrophoresis. In cultures that overexpressed β-galactosidase (pET16b-β-gal), rapid protein degradation was observed after heat shock (Fig. 4B), which resembled the degradation of cellular proteins in uninduced control cultures (pET16b) (data not shown). In contrast, in cultures that overexpressed αB-crystallin, no degradation could be detected for αB-crystallin over the entire course of the heat shock experiment, consistent with the high thermostability reported for sHsps in vivo (27) (Fig. 4C). Overexpression of αB-crystallin appeared to delay degradation of most cellular proteins especially at early time points post-heat shock (compare Fig. 4 B and C).
DISCUSSION
Our results demonstrate that human αB-crystallin behaved as a molecular chaperone by suppressing the unfolding and aggregation of CS and by actively participating in the refolding and reactivation of CS. ATP enhanced the functions of αB-crystallin as a molecular chaperone in both in vitro assays and was shown to interact with αB-crystallin by intrinsic tryptophan fluorescence spectroscopy. Lastly, overexpression of αB-crystallin protected against cell death and protein degradation in E. coli during heat shock.
CS is a model substrate protein that has been studied extensively in refolding and reactivation experiments with many molecular chaperones including GroEL, Hsp90, and the sHsps (6, 11–14). In refolding experiments in this report, the reactivation yield of CS in the presence of αB-crystallin was 14%, a value that is approximately five times higher than what was observed in the presence of BSA (Table 1). Addition of ATP to αB-crystallin resulted in a CS reactivation yield of 25%, a value that is approximately eight times higher than the reactivation yield observed in the presence of BSA and ATP (Table 1). Buchner et al. (11) reported the reactivation yield of CS in the presence of GroEL, GroES, and ATP as 28%, a value that is only four times higher than what was observed under their experimental conditions with BSA and ATP (Table 1) (11). Hence, the chaperone activity of αB-crystallin was nearly identical to GroEL/GroES on the reactivation yield of CS in the presence of ATP (25% vs. 28%, respectively; Table 1) (11). Furthermore, the relative increase in CS reactivation yield in the presence of ATP for both chaperones was quite similar, from 14 to 25% for αB-crystallin and from 0 to 16% for GroEL (Table 1) (11). To control for the effect of ATP on the refolding and reactivation of CS by αB-crystallin, nonhydrolyzable ATP analogs were substituted for ATP in the refolding reactions. ATP-γS, AMP-PCP, and AMP-PNP displayed little or no enhancement of the CS reactivation yield in the presence of αB-crystallin beyond the level observed with αB-crystallin alone (Table 1). The absence of enhanced chaperone activity in the presence of nonhydrolyzable ATP analogs suggests that hydrolysis may be required for the action of ATP on the molecular chaperone function of αB-crystallin in assisting refolding and reactivation of proteins.
The action of αB-crystallin as a molecular chaperone in vitro also was tested in an assay measuring the unfolding and aggregation of CS at 45°C. After 60 min at 45°C, αB-crystallin alone suppressed CS aggregation to 44%. These results are consistent with prior reports demonstrating the suppression of protein unfolding and aggregation by αB-crystallin (4–9). Although ATP alone did not influence the kinetics of CS aggregation, when it was included with αB-crystallin, CS aggregation was suppressed to 20% (Table 2). To control for the effect of ATP on the suppression of CS unfolding and aggregation by αB-crystallin, nonhydrolyzable ATP analogs were substituted for ATP in the unfolding reactions. ATP-γS, AMP-PCP, and AMP-PNP displayed little or no enhancement of the suppression of CS aggregation by αB-crystallin (Table 2). The absence of enhanced chaperone activity in the presence of nonhydrolyzable ATP analogs suggests that hydrolysis may be required for the action of ATP on the molecular chaperone function of αB-crystallin in suppressing the unfolding and aggregation of proteins.
A functional relationship between ATP and chaperone-like activity for α-crystallin has been suggested by equilibrium binding studies, intrinsic tryptophan fluorescence, and 31P nuclear magnetic resonance spectroscopy that demonstrated an interaction between ATP and total bovine α-crystallin (26, 28). In the presence of 300 μM ATP, a decrease of 36% in the λ = 340 nm peak fluorescence intensity demonstrated that ATP binding induced conformational changes in total bovine α-crystallin (26). In the presence of 50 μM ATP, the peak fluorescence intensity of DnaK decreased 15% (29), and for GroEL in the presence of 50 μM AMP-PNP, the peak fluorescence intensity decreased 85% (30). In this report, the decrease in peak fluorescence intensity of human αB-crystallin in the presence of ATP concentrations between 350 nM and 350 μM resembled that of total α-crystallin, DnaK, and GroEL (26, 29–30). It was demonstrated recently by electron microscopy that, upon ATP binding, GroEL changes its conformation, causing large rotations of the apical domains containing the GroES and substrate protein-binding sites (31). Future experiments will be necessary to determine the specificity of the interaction between αB-crystallin and ATP and to investigate the effect of ATP on the conformation of αB-crystallin in greater detail.
Expression of the sHsp αB-crystallin in bacterial cells was associated directly with maintenance of cell viability under conditions of thermal stress. An association between cytosolic proteins and αB-crystallin under conditions of thermal stress may prevent both protein denaturation and degradation in vivo and may be responsible for protection against cell death.
Of interest, ATP levels in the lens of the eye are among the highest of any cells in the body (15–18). The high levels of ATP associated with chaperone activity in vitro may indicate that large lenticular pools of ATP participate in the chaperone activity of αB-crystallin in the folding of normal lens proteins during development and in protection against unfolding and aggregation, a primary cause of lens cell opacification and the leading cause of blindness in the world. This action of ATP is consistent with the concept of a functional relationship between common cell metabolites and molecular chaperones (32).
Although the molecular nature of the reaction cycle that αB-crystallin uses in the refolding of proteins remains to be determined, it is possible that αB-crystallin may share similar mechanisms with GroEL and/or other molecular chaperones. It has been suggested that sHsps are a class of ATP-independent chaperones (33); however the sHsps are the least well characterized of the Hsps. The reaction mechanism that GroEL uses in the folding of proteins, originally determined by biochemical approaches, has been substantiated by the recent solutions of its crystal structures (34, 35). In contrast, the atomic structure of αB-crystallin has not been accomplished. Future experiments will be required to define the reaction mechanism of αB-crystallin in the refolding of proteins and to determine whether the chaperone functions of αB-crystallin are unique among sHsps in the use of ATP. The results reported here for human αB-crystallin are consistent with previous studies demonstrating that bovine αB-crystallin possesses an autophosphorylation activity similar to GroEL, DnaK, and Hsp90 (36–40). Future investigations will be needed to determine how autophosphorylation of αB-crystallin is involved in the chaperone functions of αB-crystallin during unfolding and refolding reactions. In one report, the phosphorylated forms of αA- and αB-crystallins suppressed the unfolding and aggregation of β-crystallin at 60°C better than nonphosphorylated forms (41). In addition, αA- and αB-crystallins have been shown to prevent the cytochalasin-induced depolymerization of actin in a phosphorylation-dependent manner (42). Future experiments using site-directed mutants of αB-crystallin will establish the molecular sites responsible for ATP interactions, as well as the sites responsible for intra- and inter-molecular protein–protein interactions.
Acknowledgments
We thank Francois Baneyx, Wim Hol, Yoshihiko Igarashi, Krzysztof Palczewski, and John Saari for critically reviewing the manuscript and giving helpful insights and suggestions and Melissa Valdez for technical assistance. This work was supported by the following grants from the National Eye Institute: EY0452 (J.I.C.) and Vision Training Grant T 32 EY07031 (P.J.M.).
ABBREVIATIONS
- Hsp
heat shock protein
- sHsp
small Hsp
- CS
citrate synthase
- CFUs
colony forming units
References
- 1.Hartl F U. Nature (London) 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 2.Gething M J, Sambrook J. Nature (London) 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 3.de Jong W W, Leunissen J A, Voorter C E. Mol Biol Evol. 1993;10:103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]
- 4.Horwitz J. Proc Natl Acad Sci USA. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groenen P J, Merck K B, de Jong W W, Bloemendal H. Eur J Biochem. 1994;225:1–19. doi: 10.1111/j.1432-1033.1994.00001.x. [DOI] [PubMed] [Google Scholar]
- 6.Jakob U, Gaestel M, Engel K, Buchner J. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 7.Plater M L, Goode D, Crabbe M J C. J Biol Chem. 1996;271:28558–28566. doi: 10.1074/jbc.271.45.28558. [DOI] [PubMed] [Google Scholar]
- 8.Muchowski P J, Bassuk J A, Lubsen N H, Clark J I. J Biol Chem. 1997;272:2578–2582. doi: 10.1074/jbc.272.4.2578. [DOI] [PubMed] [Google Scholar]
- 9.Sun T X, Das B K, Liang J J N. J Biol Chem. 1997;272:6220–6225. doi: 10.1074/jbc.272.10.6220. [DOI] [PubMed] [Google Scholar]
- 10.Raman B, Ramakrishna T, Rao C M. J Biol Chem. 1995;270:19888–19892. doi: 10.1074/jbc.270.34.19888. [DOI] [PubMed] [Google Scholar]
- 11.Buchner J, Schmidt M, Fuchs M, Jaenicke J, Rudolph R, Schmid F X, Kiefhaber T. Biochemistry. 1991;30:1586–1591. doi: 10.1021/bi00220a020. [DOI] [PubMed] [Google Scholar]
- 12.Wiech H, Buchner J, Zimmermann R, Jakob U. Nature (London) 1992;358:169–170. doi: 10.1038/358169a0. [DOI] [PubMed] [Google Scholar]
- 13.Jakob U, Lilie H, Meyer I, Buchner J. J Biol Chem. 1995;270:7288–7294. doi: 10.1074/jbc.270.13.7288. [DOI] [PubMed] [Google Scholar]
- 14.Ehrnsperger S, Graber S, Gaestel M, Buchner J. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirie A. Exp Eye Res. 1962;1:427–435. doi: 10.1016/s0014-4835(62)80034-7. [DOI] [PubMed] [Google Scholar]
- 16.Greiner J V, Kopp S J, Glonek T. Invest Ophthalmic Vis Sci. 1985;26:537–544. [PubMed] [Google Scholar]
- 17.Greiner J V, Kopp S J, Sanders D R, Glonek T. Invest Ophthalmic Vis Sci. 1981;21:700–713. [PubMed] [Google Scholar]
- 18.Klethi A M, Mandel P. Nature (London) 1965;205:1114–1115. doi: 10.1038/2051114a0. [DOI] [PubMed] [Google Scholar]
- 19.Burt C T, Glonek T, Barany M. J Biol Chem. 1976;251:2584–2591. [PubMed] [Google Scholar]
- 20.Bennardini F, Wrzosek, Chiesi M. Circ Res. 1992;71:288–294. doi: 10.1161/01.res.71.2.288. [DOI] [PubMed] [Google Scholar]
- 21.Ayling A, Baneyx F. Prot Sci. 1996;5:478–487. doi: 10.1002/pro.5560050309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skowyra D, Georgopolous C, Zylicz M. Cell. 1990;62:939–944. doi: 10.1016/0092-8674(90)90268-j. [DOI] [PubMed] [Google Scholar]
- 23.Frydman J, Nimmesgern E, Ohtsuka K, Hartl F U. Nature (London) 1994;370:111–117. doi: 10.1038/370111a0. [DOI] [PubMed] [Google Scholar]
- 24.Martin J, Mayhew M, Langer T, Hartl F U. Nature (London) 1993;366:228–233. doi: 10.1038/366228a0. [DOI] [PubMed] [Google Scholar]
- 25.Aoyama A, Frohli E, Schafer R, Klemenz R. Mol Cell Biol. 1993;13:1824–1835. doi: 10.1128/mcb.13.3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmisano D V, Groth-Vaselli B, Farnsworth P N, Reddy M C. Biochim Biophys Acta. 1995;1246:91–97. doi: 10.1016/0167-4838(94)00176-h. [DOI] [PubMed] [Google Scholar]
- 27.Landry J, Chretien A, Laszlo A, Lambert H. J Cell Physiol. 1991;147:93–101. doi: 10.1002/jcp.1041470113. [DOI] [PubMed] [Google Scholar]
- 28.Reddy M C, Palmisano D V, Groth-Vaselli B, Farnsworth P N. Biochem Biophys Res Commun. 1992;189:1578–1584. doi: 10.1016/0006-291x(92)90256-k. [DOI] [PubMed] [Google Scholar]
- 29.Theyssen H, Schuster H P, Packschies L, Bukau B, Reinstein J. J Mol Biol. 1996;263:657–670. doi: 10.1006/jmbi.1996.0606. [DOI] [PubMed] [Google Scholar]
- 30.Gibbons D L, Hixson J D, Hay N, Lund P, Gorovits B M, Ybarra J, Horowitz P M. J Biol Chem. 1996;271:31989–31995. doi: 10.1074/jbc.271.50.31989. [DOI] [PubMed] [Google Scholar]
- 31.Roseman A M, Chen S, White H, Braig K, Saibil H R. Cell. 1996;87:241–251. doi: 10.1016/s0092-8674(00)81342-2. [DOI] [PubMed] [Google Scholar]
- 32.Clark J I, Huang Q L. Proc Natl Acad Sci USA. 1996;93:15185–15189. doi: 10.1073/pnas.93.26.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buchner J. FASEB J. 1996;10:10–19. [PubMed] [Google Scholar]
- 34.Braig K, Otwinowski Z, Hedge R, Boisvert D C, Joachimiak A, Horwich A L, Sigler P B. Nature (London) 1994;371:578–586. doi: 10.1038/371578a0. [DOI] [PubMed] [Google Scholar]
- 35.Xu Z, Horwich A L, Sigler P B. Nature (London) 1997;388:741–750. doi: 10.1038/41944. [DOI] [PubMed] [Google Scholar]
- 36.Kantorow M, Piatigorsky J. Proc Natl Acad Sci USA. 1994;91:3112–3116. doi: 10.1073/pnas.91.8.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kantorow M, Horwitz J, van Boekel M A M, de Jong W W, Piatigorsky J. J Biol Chem. 1995;270:17215–17220. doi: 10.1074/jbc.270.29.17215. [DOI] [PubMed] [Google Scholar]
- 38.Sherman M Yu, Goldberg A L. Nature (London) 1992;357:167–169. doi: 10.1038/357167a0. [DOI] [PubMed] [Google Scholar]
- 39.Zylicz M, LeBowitz J H, McMacken R, Georgopoulos C. Proc Natl Acad Sci USA. 1983;80:6431–6435. doi: 10.1073/pnas.80.21.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Csermely P, Kahn C R. J Biol Chem. 1991;266:4943–4950. [PubMed] [Google Scholar]
- 41.van Boekel, M. A. M., Hoogakker, SEA., Harding, J. J. & de Jong, W. W. (1996) Opthalmic Res. 28, Suppl. 1, 32–38. [DOI] [PubMed]
- 42.Wang K, Spector A. Eur J Biochem. 1996;242:56–66. doi: 10.1111/j.1432-1033.1996.0056r.x. [DOI] [PubMed] [Google Scholar]